1. Introduction
Inflammation is closely related to cancer, and
inflammatory conditions increase the risk of cancer. To respond to
the inflammation caused by a variety of factors, such as toxic
chemical exposure, cells secrete the soluble pro-inflammatory
cytokine tumour necrosis factor-α (TNF-α), which binds to TNF
receptors I and II (TNFRI/II) on the cell surface, leading to the
activation and phosphorylation of cytoplasmic inhibitor of κBα
(IκBα) kinase. This binding allows nuclear factor-κB (NF-κB) to
undergo nuclear translocation, thereby activating the transcription
and expression of TIPE family members (1,2).
This family includes four members: TNF-α-induced protein 8
(TNFAIP8), TIPE1 (TNFAIP8L1), TIPE2 (TNFAIP8L2) and TIPE3
(TNFAIP8L3). These four members are similar in structure but appear
to play diverse roles in the progression of cancer and inflammatory
responses (1). TNFAIP8, also
known as SCC-S2, was the first member of the TIPE family to be
discovered; it not only promotes tumour cell proliferation,
survival and autophagy, and induces drug resistance, but also
promotes tumour invasion, migration and angiogenesis, which are
closely related to oncogenesis and tumour progression (3). Meanwhile, TNFAIP8 participates in
the regulation of various other diseases; for example, it inhibits
the inflammation response after injury and regulates cell apoptosis
in inflammatory diseases. TNFAIP8 is also a risk factor for
bacterial infection. This review will comprehensively summarize
TNFAIP8-associated expression, regulation, functions and signalling
pathways in diverse diseases.
2. TNFAIP8 structure
The novel gene TNFAIP8 was identified by analysing
differentially expressed transcripts in head and neck squamous cell
carcinoma (HNSCC) cell lines using mRNA differential display PCR
(4). Importantly, the analysis of
the TNFAIP8 open reading frame revealed a putative death effector
domain (DED) at the amino terminus, which is significantly >25%
similar to DED II in Fas-associated death domain-like interleukin
(IL)-1β-converting enzyme-inhibitory protein and caspase homologous
protein in humans and mice. DEDs play important roles in
protein-protein interactions (2,5).
For example, the death receptor TNFR1 interacts with
TNFR-associated death domain, the adaptor molecules Fas-associated
death domain protein (FADD) and FADD-like interleukin-1β-converting
enzyme leading to apoptosis (5).
However, analysis of the TIPE2 crystal structure contradicts this
assumption. Structural analysis shows that TIPE2 is composed of six
antiparallel α helices, which differs from the known topological
structure of DEDs by being a mirror image (6). Notably, the TNFAIP8 family has
relatively high structural and sequence homology. Analysis of the
human TIPE3 crystal structure (7)
and TNFAIP8 from Mus musculus
(mTNFAIP8)-phosphatidylethanolamine (PE) (8) revealed that in addition to the six α
helices (α1-α6), mTNFAIP8-PE and TIPE3 have unique N-terminal
α0-helices. A flexible short hinge motif exists between α0 and α1,
while the remaining α helices (α1-α6) are folded into the TIPE2
homologous (TH) domain that is shared among TNFAIP8 family members.
A highly conserved hydrophobic cylindrical cavity exists in the
centre, and two long electron-dense regions exist in the cavity,
which may be binding sites for phospholipid molecules. The studies
on the crystal structures of TNFAIP8, TIPE2 and TIPE3 revealed that
the TH domain of mouse TIPE family members could sufficiently bind
to lipid messengers such as phosphatidylinositol.
Phosphatidylinositol insert their lipid tails in the cavity, while
the negatively charged head group forms a hydrogen bond with the
positively charged amino acid residues on the cavity surface
(7,9,10).
Numerous hydrophobic cofactors or substrates are expected to bind
inside the cavity in this way.
3. TNFAIP8 expression
The TNFAIP8 gene is located in the q23 region of
chromosome 5, and its full-length cDNA clone was completely
extracted and found to encode a cytoplasmic protein with a relative
molecular weight of 21 kDa (1).
Expression of TNFAIP8 was detected in the majority of normal
tissues and cells, such as the spleen, thymus, lymph node, lung,
gastrointestinal tract, uterus and prostate gland, but could not be
detected in the brain (5). In
particular, TNFAI8 was upregulated in malignant tumours, such as
lung cancer, gastric cancer (GC) and chronic myelogenous leukemia
(5). Compared with that in normal
tissues and cells, differential expression of this protein in
cancer is association with tumour development and progression. In
recent years, several transcriptional variants of TNFAIP8 have been
found. These variants are highly conserved in the C-terminus, but
variable in the N-terminus. Among them, TNFAIP8 variant 2 (v2) is
upregulated in a range of human cancer types and has been found to
modulate cancer progression. The v1 variant is downregulated in the
majority of cancer types, and its molecular weight differs from
that of v2. The expression levels of v3-v6 are very low in normal
tissues and cancer cells, which indicates that v1 and v2 of TNFAIP8
are differentially expressed in tumours, which might be related to
their functions (8,11).
Expression of TNFAIP8 is regulated by the presence
of a number of factors, including NF-κB, the p53 mutant K120R,
liver insulin, chicken ovalbumin upstream promoter transcription
factor (COUP-TFI), hypoxia-inducible factor-1α (Hif-1α), androgen,
methylation and microRNAs (miRNAs/miRs).
As aforementioned, TNF-α binds to TNFRI/II, thereby
activating the NF-κB pathway and inducing TNFAIP8 transcription.
The K120R mutant of p53 binds to the TNFAIP8 locus on the p53
response element, activating the transcription of TNFAIP8 (1). Liver insulin can temporarily
increase the expression of mTNFAIP8 (8). Genome-wide microarray analysis
revealed that TNFAIP8 was the target gene of COUP-TFI, a
transcription factor that participates in numerous biological
processes. The TNFAIP8 promoter is co-occupied by the COUP-TFI
complex, which is composed of nuclear receptor corepressors,
transcriptional intermediary factor 1b and deleted in breast cancer
1. These factors mediate the transcriptional repression of TNFAIP8.
TNF, which regulates the apoptotic pathway, alleviates the
repression of the TNFAIP8 promoter by downregulating COUP-TFI
expression (12). It is
hypothesized that NF-κB can also interact with the COUP-TFI
promoter through NF-κB-binding sites and suppress its expression,
thereby weakening the inhibition of the TNFAIP8 promoter and
ultimately inhibiting apoptosis by inhibiting caspase-8 activity
(12). In addition, the
expression of TNFAIP8 is important in the transcription factor
Hif-1α signalling pathway, which is involved in cell survival,
proliferation and migration (13). The TNFAIP8 promoter region
possesses an androgen response element that can be induced by
androgens synthesized by hormone-responsive prostate cells
(14). Whole-gene analysis of
androgen receptor (AR) in a long-term androgen-deprived prostate
cancer cell line (LNCaP-AI) indicated that the TNFAIP8 gene was
altered and potentially regulated by AR (15). GG2-1 (TNFAIP8) has been identified
as a methylation target in a prostate epithelial cancer cell line
(267B1) (16). In GC and
osteosarcoma cells (MG-63 and U2OS), TNFAIP8 is a direct target of
miRNAs, such as miR-9, miR-138 and miR-99a, which can decrease the
expression of TNFAIP8 by interacting with its 3′-untranslated
region (3′-UTR) (17-19).
4. Current studies of TNFAIP8 in
tumours
TNFAIP8 is a crucial antiapoptotic and carcinogenic
molecule, which is highly expressed in the cytoplasm of most tumour
cells to mediate the occurrence and development of tumours.
Specifically, TNFAIP8 plays an important role in promoting tumour
cell proliferation, migration, invasion and angiogenesis, and
inducing tolerance to chemotherapeutic drugs (20-23). Research on the link between
inflammation and cancer has been ongoing for several years. Recent
studies have shown that G protein-coupled receptors (GPCRs) are at
the core of these two processes, regulating the two main pathways
of NF-κB and STAT3 (23-26). NF-κB is regulated by a variety of
GPCR signals and is associated with tumour metastasis by affecting
cell migration. In addition, GPCR can also activate Ras, thereby
activating the phosphoinositide 3-kinase (PI3K). This kinase can
convert TIPE-anchored phosphatidylinositol 4,5-bisphosphate (PIP2)
to TIPE-anchored phosphatidylinositol 3,4,5-trisphosphate (PIP3)
(27). The lipid second messenger
conversion was shown to be related to TNFAIP8 (9,10),
which promotes the leading-edge formation of cells and recruits
downstream molecules, such as Akt, which plays a major role in
tumourigenesis (28), and
Rac-GTP. TNFAIP8 in the cytoplasm inhibits Rac-GTP migration to the
cell membrane to prevent leading-edge formation. In summary,
TNFAIP8 participates in the directionality of cell migration by
regulating phosphoinositide signaling and Rac (10). Furthermore, PI3K also activates
the STAT3 pathway, which is closely related to inflammation and
cancer. However, the role of TNFAIP8 in the pathway for
inflammation and tumour progression requires further research in
specific diseases (23). In
addition, previous studies showed that TNFAIP8 is not limited to
regulating the proliferation and migration of tumours, but that it
also mediated immune functions of CD4+ T lymphocytes,
promoting tumour progression. The expression level of TNFAIP8 in
tumour-infiltrating CD4+ T cells and CD8+ T
cells in patients with non-small cell lung cancer (NSCLC) was
significantly lower than that in CD4+ T cells and
CD8+ T cells in the peripheral tissues. Moreover, the
expression of TNFAIP8 in advanced tumour-infiltrating
CD8+ T cells was lower than that in these cells at
primary stages (22,29). TNFAIP8 may regulate the
development of NSCLC by affecting the function of immune cells. The
TNFAIP8 expression levels were increased in peripheral blood
CD4+ T lymphocytes and CD8+ T lymphocytes in
papillary thyroid cancer tissues, but there were no changes in the
expression in monocytes or natural killer T cells. Additionally,
the expression level of TNFAIP8 in tumour-infiltrating
CD4+ T lymphocytes and CD8+ T lymphocytes was
increased, indicating that TNFAIP8 may regulate tumour development
by affecting immune cells (22,30). These results imply that TNFAIP8
often serves as a prognostic marker and potential therapeutic
target for various malignant tumours. However, the research into
TNFAIP8 in immunity is still limited, and further studies are
required.
TNFAIP8 is a prognostic marker for
tumours
Elevated expression of TNFAIP8 was found in patients
with different cancer types, such as NSCLC (31), colon cancer (32), GC (33), epithelial ovarian cancer (EOC)
(34), endometrial cancer (EC)
(35), invasive ductal breast
carcinoma (36), oesophageal
squamous cell carcinoma (37),
pN0 oesophageal squamous cell carcinoma (38), hepatocellular carcinoma (HCC)
(39), diffuse large B-cell
lymphoma (DLBCL) (40), clear
cell renal cell carcinoma (ccRCC) (41) and papillary thyroid carcinoma
(30), which are often
significantly associated with poor prognostic features; for
example, advanced tumour stage, lymph node metastasis, increased
histological grade, poor survival time and tumour recurrence, thus
implying its potential diagnostic and prognostic value.
Recent multivariate Cox regression studies showed
that high TNFAIP8 expression is also an independent predictor.
TNFAIP8 overexpression was correlated with lymphatic metastatic
recurrence in patients without lymph node metastasis (pN0) who
underwent Ivor Lewis oesophagectomy (38). Increased expression level of both
TNFAIP8 and Ki-67 were independent factors of disease-free survival
rates in patients with NSCLC and EC (31,35). Similarly, high expression of
TNFAIP8 was associated with epithelial growth factor receptor
(EGFR) expression levels and revealed the recurrence of pancreatic
cancer (42). In gastric
adenocarcinoma, TNFAIP8 is associated with a poor prognosis for
intestinal-type gastric adenocarcinoma, but not for diffuse-type
gastric adenocarcinomas, and is closely related to tumour invasion
and the Lauren classification (43). In addition, serum CA72-4, a tumour
marker, is associated with TNFAIP8 expression and is presumed to be
associated with the early diagnosis and prognostic evaluation of
gastric adenocarcinoma (44).
Increased nuclear TNFAIP8 expression, which is regulated by
karyopherin α2 (importin-α1), is an independent prognostic marker
for recurrent prostate cancer (14).
Cancer susceptibility and tumour progression may be
influenced by a single nucleotide polymorphism (SNP) of TNFAIP8,
which further confers prognostic value to TNFAIP8. SNP analysis of
TNFAIP8 revealed that the rs11064 variant of the GG genotype was
associated with an increased risk of cervical cancer compared with
that of the AA and AG genotypes, and G was identified as the risk
allele (45). Given that the
rs11064 SNP is located at the TNFAIP8 3′-UTR, the ability of miR-22
to target TNFAIP8 is decreased, which increases the expression of
TNFAIP8 and upregulates the risk of cervical cancer and cisplatin
resistance (45). The TNFAIP8
rs11064 and rs1045242 minor alleles were shown to be highly
associated with the risk of EC in women in Heilongjiang Province,
China; in particular, the G allele variation increased the risk of
EC. In addition, rs11064 was associated with an advanced
International Federation of Gynecology and Obstetrics stage, deep
myometrial invasion and lymph node metastasis, suggesting that
these SNPs are associated with the expression level of TNFAIP8
(46). The rs1045241 SNP in
TNFAIP8 contributes to non-Hodgkin's lymphoma susceptibility in the
Chinese population, and the T allele of the rs1045241 variant is
associated with the risk of DLBCL and follicular lymphoma (47). With respect to these findings,
TNFAIP8 can be used as a powerful prognostic marker and a potential
therapeutic target for cancer.
TNFAIP8 regulates tumour growth,
proliferation and drug resistance
Compared with that in adjacent non-cancerous
tissues, TNFAIP8 is highly expressed in tumour tissues and promotes
cell proliferation and tumour growth, which plays an important role
in tumour progression, thereby affecting patient survival (33,48-50).
In a previous study, TNFAIP8 blocked TNF-α-induced
apoptosis by inhibiting the enzymatic activity, but not the
processing, of caspase-8 in a human fibrosarcoma cell line
(HT1080I). The suppression of caspase-8 inhibited the cleavage of
Bid (a substrate of caspase-8) and the activation of caspase-3.
Crystallographic structural analysis of the TNFAIP8 family showed
that the antiapoptotic effect may be achieved via the interaction
between the hydrophobic cavity and the TH domain. Since the
etoposide-induced apoptotic pathway is caspase-8-independent, it is
not regulated by TNFAIP8 (2,9)
(Fig. 1). TNFAIP8-silenced GC
cells (BGC823) were treated with an anti-death receptor 5
monoclonal antibody to achieve antitumour effects by activating
caspase signalling (51). Another
study found that a mutation at the non-hotspot K120 of the tumour
suppressor gene p53 deprived p53 of its ability to regulate tumour
cell apoptosis, but resulted in the acquisition of novel oncogenic
properties by inducing the transcription of TNFAIP8. The K120R
mutant binds to the TNFAIP8 locus on the p53 response element to
induce TNFAIP8 gene transcription and thereby promote cell
survival. TNFAIP8 enable tumours expressing the K120R mutant to
evade apoptosis by inhibiting caspase-8 enzymatic activity
(52). Apart from caspase
signalling, in Balb/c-3T3 fibroblasts, the coupling of Gαi and the
short dopamine D2 (D2S) receptor stimulates cell transformation and
regulates cell proliferation, but is not involved in the
MAPK-induced cell proliferation pathway (Fig. 2) (53). TNFAIP8 is a novel Gαi effector
that may rely on the interaction between the TH domain and Gαi,
suggesting that Gαi-TNFAIP8 coupling to the D2S receptor mediates
transformation, and TNFAIP8 depletion does not affect other
D2S-induced pathways, such as cAMP inhibition. D2S receptor
signalling inhibits TNF-α-induced caspase-3/7 activation, but
TNFAIP8 mediates Gαi-dependent D2S receptor signalling to prevent
TNF-α-induced cell death via caspase-3/7-independent pathways. In
summary, D2S/Gαi-TNFAIP8-induced signalling enhances the survival
of oncogenic cells and promotes oncogenic transformation, and it
may be possible to reduce tumour progression by blocking this
caspase-independent pathway (53). Furthermore, the upregulated
TNFAIP8 v2 in multiple human cancer types can interact with p53 to
promote tumour development, promote DNA synthesis by maintaining
the proliferating cell nuclear antigen levels in A549 cells, and
inhibit p53-dependent cell cycle arrest by inhibiting p21 (the
target of p53); however, the v2 variant inhibits cell cycle arrest
only in cells such as U2OS and HCT116 cells, indicating that the v2
variant can be specifically regulated according to the cell type
(11).
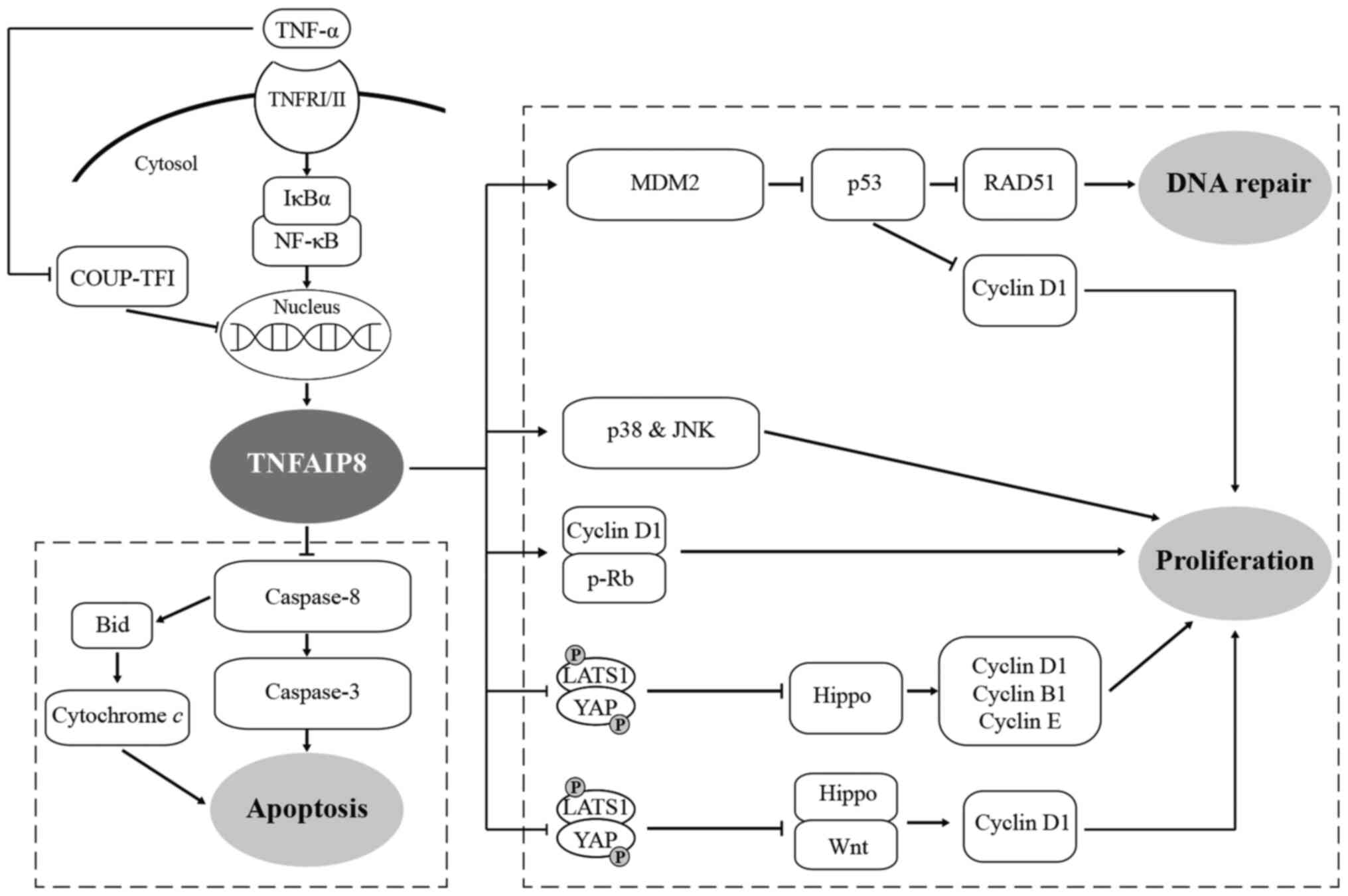 | Figure 1TNF-α induces the expression of
TNFAIP8, activating proliferation and inhibiting the apoptosis of
tumour cells through various signalling pathways, ultimately
facilitating tumourigenesis and the development of tumours. ⊥,
inhibition; TNFAIP8, tumour necrosis factor-α-inducible protein 8;
TNFRI/II, TNF receptors I and II; NF-κB, nuclear factor-κB; LATS1,
large tumour suppressor; YAP, yes-associated protein; COUP-TF1,
chicken ovalbumin upstream promoter transcription factor; MDM2,
murine double minute 2. |
The hippo signalling pathway plays a crucial role in
cell proliferation and apoptosis, and has become a hotspot of
research. TNFAIP8 inhibits the phosphorylation of large tumour
suppressor (LATS1) and yes-associated protein (YAP), and promotes
YAP nuclear localization and TEA domain family member protein
binding, which leads to the inhibition of the Hippo signalling
pathway (39,54,55). Suppression of Hippo signalling can
promote cell proliferation in lung cancer (54), EOC (55) and HCC (39). Inhibition of this pathway in lung
cancer cells (H460 and H1299) leads to increased expression of the
downstream target genes cyclin D1 and cyclin-dependent kinases 6,
and to decreased p27 expression. Similarly, inhibition of the Hippo
pathway increases the cyclin B1 and cyclin D1 levels in EOC cells
(A2780s), increases cyclin D1 and cyclin E levels, and
downregulates p27 expression in HCC cells (HepG2 and SK-Hep1).
Additionally, TNFAIP8 interacting with LATS1 may inhibit Hippo
signalling, elevate YAP protein expression and subsequently
activate Wnt signalling in colorectal cancer (CRC; H6T116), leading
to increased expression of downstream cyclin D1 and promoting cell
proliferation (56) (Fig. 1), suggesting that TNFAIP8 might be
a new target for the prevention and treatment of cancer. By
contrast, knockdown of TNFAIP8 in colon cancer cell lines (CACO2
and HCT116) results in reduced cell proliferation and inhibition of
cell cycle progression by downregulating cyclin D1 and
phosphorylated retinoblastoma (32) (Fig.
1). Knockdown of the TNFAIP8 gene affects antiproliferative and
apoptotic genes such as IL-24, FAT tumour suppressor homolog 3
(Drosophila), latrophilin 2 and EPH receptor A3 in prostate
cancer (PC-3), breast cancer (LM2-4175) and pancreatic cancer
(PANC-1), indicating that new signalling pathways may be discovered
in the future (13).
Elevated expression of TNFAIP8 promotes tumour cell
proliferation-induced resistance of chemotherapeutic drugs, which
results in a highly refractory nature and poor prognosis.
Upregulated TNFAIP8 increases the risk of platinum resistance in
EOC (57) and can interact with
TAF-Iα to regulate cisplatin resistance in oesophageal cancer cells
(EC-109/DDP and OE19/DDP) (58).
Conversely, treatment with ionizing radiation or docetaxel
following the decrease in expression of TNFAIP8 by an antisense
TNFAIP8 oligonucleotide (LE-AS5) in a mouse model (14) and treatment with CDDP in
TNFAIP8-downregulated oesophageal squamous cell carcinoma (37) resulted in increased apoptosis,
inhibition of tumour growth and reversal of TNFAIP8-induced
antitumour drug resistance. A similar result was observed in the
chemotherapy treatment of tumour cells, which upregulated p53 and
induced TNFAIP8 v2 expression, while v2-associated negative
feedback regulation enhanced the p53-mediated resistance to
apoptosis (11). In NSCLC cell
lines (A549/cDDP), TNFAIP8 promoted tumour cell proliferation in
vivo and in vitro, and increased cisplatin resistance,
which is regulated by the murine double minute 2 (MDM2)/p53
pathway. TNFAIP8 upregulates the oncoprotein MDM2, increases p53
ubiquitination, and promotes p53 protein degradation, leading to
the upregulation of RAD51 and cyclin D1 expression, and increased
DNA damage repair and cell proliferation (59) (Fig.
1). Cisplatin treatment of TNFAIP8-silenced cervical carcinoma
cells (HeLa) enhanced the activation of caspase-3/8 and
mitogen-activated protein kinase phosphorylation, but inhibited the
expression of B-cell lymphoma-2. These results suggest that TNFAIP8
promotes cell proliferation, colony formation and cisplatin
resistance by negatively regulating the p38 MAPK signalling pathway
(60). Furthermore, Liu et
al (61) also found that an
increased expression level of TNFAIP8 in RAW264.7 and EL4 cells,
and exposure to ultraviolet irradiation or cisplatin inhibited the
activation of caspase-3/9 and RARP, which revealed that TNFAIP8
plays an antiapoptotic role. In addition, the TNFAIP8
overexpression-induced activation of c-Jun N-terminal kinase and
p38 kinase contributes to cell survival and facilitates tumour
formation, but whether TNFAIP8 regulates the mitochondrial-mediated
apoptotic pathway and the role of MEK remain to be investigated
(61) (Fig. 1). Neoadjuvant chemotherapy (NACT)
is an alternative therapy that can improve the efficacy of advanced
OC surgical treatment. In a previous study, NACT treatment of
tumour cells significantly decreased the expression of TNFAIP8,
inhibiting the growth, proliferation and cell cycle of OVCAR-3
cells. Also, this treatment increased the level of LDH (indicating
the degree of cell damage) and increased the sensitivity of cells
to cisplatin, which improved the survival rates of the patients
with OC (62). These findings
might offer potential therapeutic targets for reversing the
resistance of chemotherapeutic drugs by downregulating TNFAIP8
expression in patients with cancer.
TNFAIP8 also regulates cell survival and apoptosis
through autophagy. Highly expressed TNFAIP8 mediates autophagy
through the Akt/mTOR pathway and by targeting autophagosome protein
3 (ATG3) and ATG7, and regulates lipidation of LC3, thereby
affecting cell survival and drug resistance (62-64). TNFAIP8 upregulation in PC-3
prostate cancer cells dysregulates the expression of multiple cell
cycle-related proteins; it does not directly affect cell cycle
progression but is associated with autophagy (63). Experiments have demonstrated that
TNFAIP8 interacts with ATG3 to induce autophagy, increase autophagy
effectors, such as microtubule-associated protein 1A/1B-light chain
3β (LC3βI/II), eukaryotic translation initiation factor 4E-binding
protein 1 (4E-BP1) and Beclin-1, and stabilize the expression of
sirtuin 1 and p62, which are related to autophagy regulation. TNF-α
induces the expression of the autophagy marker LC3βI/II, which
indicates that TNFAIP8 is involved in regulating TNF-α-induced
autophagy, inhibits TNF-α-induced apoptosis by attenuating
poly(ADP-ribose) polymerase (PARP) and caspase-3 cleavage, promotes
the survival of prostate cancer cells, increases the drug
resistance of cancer cells to the anticancer drugs docetaxel and
doxorubicin, and promotes the progression of prostate cancer
(63). Additionally,
TNFAIP8-induced autophagy may contribute to neuroendocrine
differentiation biomarkers in prostate cancer cells. However, the
specific regulatory mechanisms need further study (63). Ectopic expression of TNFAIP8 in
HCC induces the expression of the autophagy markers LC3βI/II, ATG3,
4E-BP1 and Beclin-1, and treatment of HCC cells (HepG2 and SK-Hep1)
with TNF-α induces ATG3, ATG7 and LC3βII expression, and p-Akt and
p-mTOR inhibition (64). These
phenomena indicate that TNF-α induces the expression of TNFAIP8,
inhibits the Akt/mTOR pathway, promotes the interaction of TNFAIP8
with ATG3 and ATG7 proteins, induces LC3 lipidation and autophagy,
and promotes autophagy and cell survival of hepatoma cells. This
results in the increased drug resistance of hepatoma cells to
anticancer drugs, such as sorafenib and regorafenib, decreased PARP
and caspase-3 cleavage, and decreased patient survival rates. This
suggests that TNFAIP8 can increase autophagy and drug resistance in
HCC cells and mediate the development of HCC, suggesting it as a
new target for the diagnosis of early liver disease (64). Furthermore, TNFAIP8-knockdown in
cisplatin-treated OVCAR-3 cells increased the expression of the
autophagy marker proteins Beclin-1 and LCII, suggesting that
TNFAIP8 modulates the response of OC cells to cisplatin by
regulating the expression of autophagy-related proteins (62).
miRNAs interact with the 3′-UTRs of specific target
mRNAs to regulate their expression at the post-transcriptional
level. Studies have found that miR-9 is expressed at low levels in
GC tissues and cell lines (MKN45 and MGC803), and that it targets
the 3′-UTR sequence of TNFAIP8 and plays an antitumour role by
negatively regulating TNFAIP8 expression, resulting in reduced cell
proliferation and increased sensitivity to antitumour drugs
(17) (Fig. 1). miR-99a and miR-138 (18,19) in osteosarcoma (MG-63 and U2OS) and
miR-155 in multiple myeloma (RPMI-8226 and MM.1S) also play the
same roles (65), highlighting
that miRNAs targeting TNFAIP8 represent a promising potential
therapeutic target in the prevention and treatment of tumours.
Contrary to the aforementioned effect of promoting
cell proliferation, glucocorticoids bind and activate
glucocorticoid receptors, which bind to glucocorticoid response
elements and regulate the de novo expression of genes,
leading to the activation of apoptosis in thymocytes, thus
promoting glucocorticoid-mediated cell death (Fig. 3). The upregulated gene expression
of TNFAIP8 plays a functional role in glucocorticoid-mediated
thymocyte apoptosis. Knockdown of TNFAIP8 expression has been show
to protect thymocytes from glucocorticoid-induced cell death. These
results indicate that the effect of TNFAIP8 on apoptosis depends on
different environmental stimuli (66).
TNFAIP8 regulates tumour migration,
invasion and angiogenesis
Tumour metastasis and recurrence are the leading
causes of death in patients with cancer. As well as inhibiting
apoptosis, elevated expression of TNFAIP8 in tumours promotes cell
migration, invasion and angiogenesis by regulating the expression
of the matrix metalloproteinase (MMP) family and vascular
endothelial growth factor receptor 2 (VEGFR-2). This indicates the
role of TNFAIP8 in tumour development and provides a basis for
TNFAIP8-associated target cancer gene therapy. TNFAIP8 upregulation
in breast cancer increased the expression of MMP-1 and MMP-9, but
not MMP-2, which promoted cell migration and invasion. However,
inhibition of endogenous TNFAIP8 expression with LE-AS5 resulted in
the downregulation of VEGFR-2, which exerted the opposite effect
(67) (Fig. 4). TNFAIP8 upregulation in EC
increased MMP-9 expression (35).
TNFAIP8-knockdown in pN0 oesophageal squamous cell carcinoma
(Eca109) inhibited the expression of MMP-1 and MMP-9, but did not
affect VEGFR-2 expression (38).
TNFAIP8-knockdown in osteosarcoma cells downregulated MMP-2 and
MMP-9 expression (18). TNFAIP8
silencing in hormone-refractory PC-3 prostate cancer cells caused
the downregulation of MMP-1, MMP-2, MMP-9, membrane type 1 MMP and
VEGFR-2. Additionally, microarray analysis indicated that TNFAIP8
and GDNF family receptor α1 might be involved in the regulation of
tumour invasion (14). However,
the invasion ability of NSCLC cells (A549 and H1299) was not
regulated by the VEGFR-2, MMP-1 and MMP-9 pathways, but other
regulatory pathways may exist (31). Furthermore, certain studies showed
that an abnormal Hippo pathway is closely related to the recurrence
and metastasis of cancer. TNFAIP8 promotes cell invasion in lung
cancer (54) and HCC (39) by inhibiting the phosphorylation of
LATS1 and YAP, promoting the nuclear localization of YAP and
inhibiting the Hippo signalling pathway. This pathway leads to
increased expression of the downstream target gene MMP-7 in lung
cancer and HCC. TNFAIP8 inhibits Hippo signalling and thus
activates Wnt signalling in CRC and promotes MMP-7 expression
(56) (Fig. 4), suggesting that the effects of
TNFAIP8 on the Hippo pathway influence tumour development and
reveal the molecular mechanism involved. There are few studies on
the regulation of TNFAIP8 with regard to invasion-associated gene
expression. TNFAIP8 was silenced in oesophageal cancer cells
(OE19), which resulted in the upregulation of TAF-Iα, while
knockdown of TAF-Iα had the opposite effect, indicating that TAF-Iα
is negatively correlated with TNFAIP8. TAF-Iα is a cytoplasmic
protein that can bind to the mRNA encoding TNFAIP8. Silencing
TAF-Iα in TNFAIP8-knockdown ECa cell lines reversed the TNFAIP8
downregulation-mediated inhibition of cell invasion and migration,
suggesting that these factors can mutually regulate each other and
that TNFAIP8 regulates cell migration and invasion through TAF-Iα
(58). Additionally, TNFAIP8 is a
multipronged target downstream of TNF-α, which regulates epidermal
growth factor (EGF)- and IGF-1-stimulated migration through
receptor tyrosine kinase signalling pathways in NSCLC cells (A549).
EGFR is one of the most common growth factors implicated in
numerous malignancies. A previous study showed that
TNFAIP8-knockdown enhances the expression of sorted nexin 1 and
upregulates the endosomal/lysosomal transport pathway, resulting in
decreased EGFR expression, decreased EGF-induced phosphorylated
extracellular signal-regulated kinase expression, reduced cell
migration and increased sensitivity to EGFR mutation-selective
tyrosine kinase inhibitors (68).
In TNFAIP8-knockdown cells, the expression of IGF-1 binding protein
3 (IGFBP3) was shown to be increased, leading to a decrease in the
IGF-1-induced expression of pIGF1R and p-Akt, decreased cell
migration, and enhanced sensitivity to PI3K and Akt inhibitors,
suggesting that targeting TNFAIP8 can inhibit adaptive responses
and provided a rational strategy for the management of aggressive
NSCLC (68).
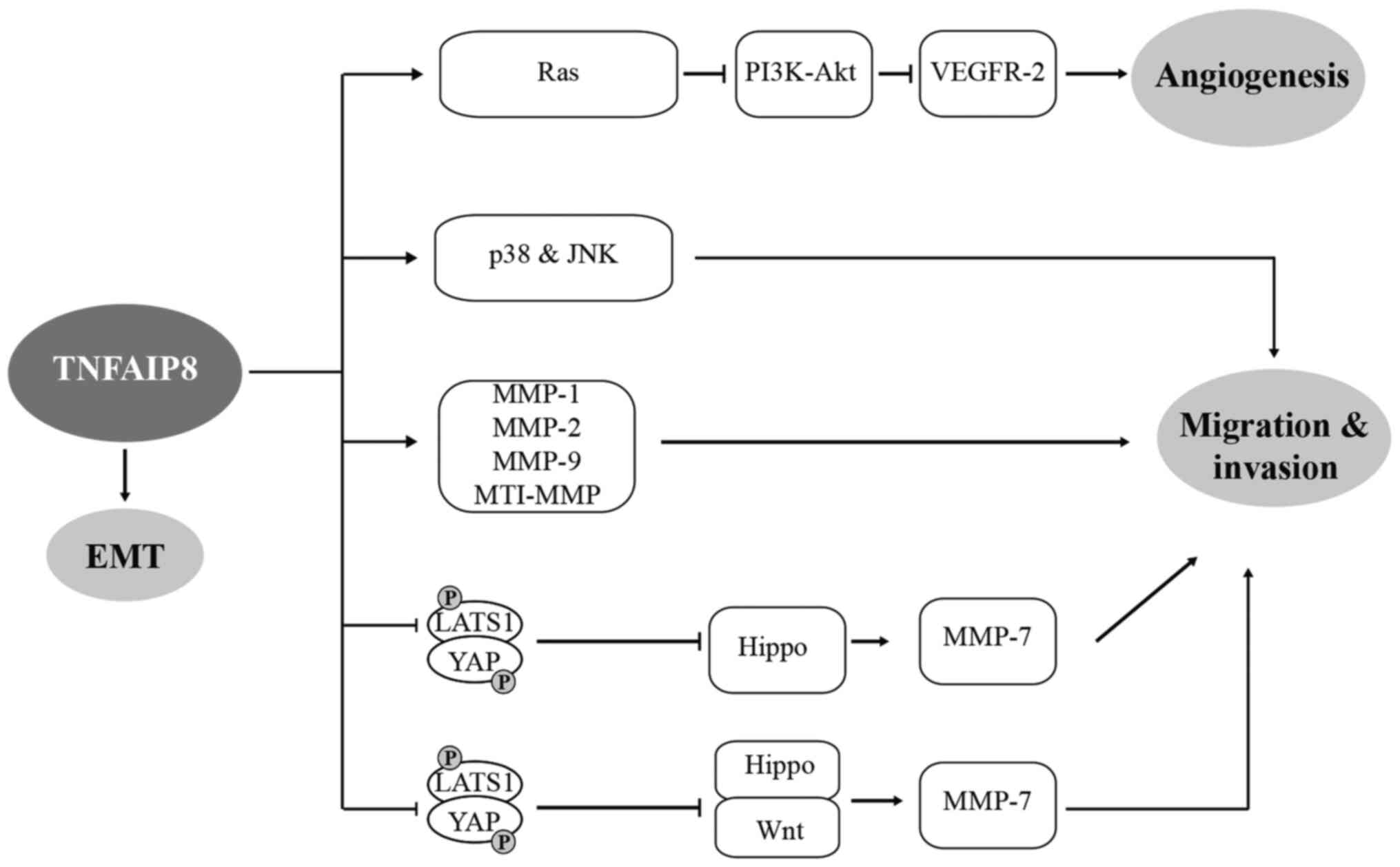 | Figure 4Role of TNFAIP8 in cell migration,
invasion, EMT and angiogenesis through various signalling pathways,
ultimately facilitating tumourigenesis and development of tumour.
⊥, inhibition; TNFAIP8, tumour necrosis factor-α-inducible protein
8; EMT, epithelial-mesenchymal transition; MMP, matrix
metalloproteinase. LATS1, large tumour suppressor; YAP,
yes-associated protein; VEGFR-2, vascular endothelial growth factor
receptor; MT1-MMP, membrane type 1 metalloprotease. |
Angiogenesis is an important step in tumour
progression that relies on the release of angiogenic molecule. VEGF
binds to VEGFR-2 to induce tumour angiogenesis and regulate CRC
metastasis, which involves the PI3K-Akt signalling pathway
(Fig. 4). Experiments showed that
VEGFR-2 and TNFAIP8 were highly expressed in CRC tissues, and
TNFAIP8-knockdown (HCT116) decreased the expression of VEGFR-2, the
distribution of cell membrane microfilaments, migration and
angiogenesis (69). Similar
results were also observed in chicken chorioallantoic membranes and
nude mouse models. TNFAIP8-knockdown was also found to inhibit the
phosphorylation of Ras and PDK1, suggesting that TNFAIP8 regulates
VEGFR2-mediated angiogenesis through the PI3K-Akt signalling
pathway (69).
Epithelial-mesenchymal transition (EMT) is known to drive tumour
migration and metastasis. Silencing TNFAIP8 in ccRCC significantly
reduced cell migration and invasion. Further experiments showed
that high expression of TNFAIP8 decreased the expression of the
epithelial markers E-cadherin and zonula occludens-1, and increased
the expression of the mesenchymal markers N-cadherin and vimentin
in ccRCC cells (769-P and ACHN). In addition, when TNFAIP8 was
increased, TGF-β, zinc finger E-box-binding homeobox 1 and Slug
expression was upregulated, while Snail expression was decreased.
The opposite effect was observed when TNFAIP8 was silenced. This
finding indicates that TNFAIP8 induces the migration and invasion
of ccRCC by regulating EMT and is a potential therapeutic target
for ccRCC (41).
5. Regulatory effects of TNFAIP8 in other
conditions
Previous studies on TNFAIP8 have mainly focused on
tumour-associated functions, but TNFAIP8 has also been shown to be
involved in the regulation of other conditions, such as
susceptibility to bacterial infection and suppression of
inflammatory responses to injury. TNFAIP8 also modulates cell
apoptosis in the resistance to inflammatory diseases. However, the
research into TNFAIP8 in other diseases is still very limited, and
further studies are required.
TNFAIP8 regulates bacterial
infections
In a previous study of Staphylococcus
aureus-induced sepsis, A/J mice were more susceptible than
C57BL/6J mice, and this susceptibility was related to loci on
chromosomes 8, 11 and 18. TNFAIP8 was differentially expressed
between S. aureus-infected A/J and C57BL/6J mice, and this
gene was shown to be closely associated with S. aureus
susceptibility, as determined by quantitative trait locus analysis
of chromosome 18 (70). Moreover,
TNFAIP8-knockdown in S. aureus-infected RAW264.7 macrophages
induced a decrease in cytokine IL-1β expression and an increase in
granulocyte-macrophage colony-stimulating factor (GM-CSF). Similar
changes were also observed in peritoneal macrophages from CSS18
mice, but not C57BL/6J mice, suggesting that TNFAIP8 is a strong
candidate gene that contributes to S. aureus susceptibility
in vivo and in vitro via the cytokines IL-1β and
GM-CSF (70). Similarly, TNFAIP8
regulates the TNF-α-induced host cell apoptosis defence to
Listeria monocytogenes infection through a Ras-related C3
botulinum toxin substrate 1 (Rac1) GTPase-dependent pathway
(71). TNFAIP8-knockout mice
resisted L. monocytogenes infection and exhibited a
decreased bacterial load; however, unlike the heightened immunity
of TIPE2, TNFAIP8-knockout mice showed no resistance to
extracellular pathogens and there was little difference in
immunity, which suggested that the non-immune cells played an
important role in protecting the host cells. TNFAIP8-knockdown in
non-immune HCC cells (Hepa1-6) with TNF-α treatment increased the
apoptosis of infected hepatocytes and induced the resistance to
bacterial invasion. In addition, TNFAIP8-knockdown induced
increased levels of p-Akt, Rac1-GTP and F-actin, and partially
inhibited TNF-α-induced apoptosis by introducing a
dominant-negative mutation of Rac1, indicating that TNF-α induces
Rac1 and NF-κB activation; Rac1 activates the NADPH oxidase complex
to produce reactive oxygen species (ROS), leading to apoptosis,
while NF-κB promotes TNFAIP8 expression to inhibit ROS production
by inhibiting Rac1 activation (71).
TNFAIP8 regulates inflammatory
disease
TNFAIP8 regulates cell apoptosis to maintain colonic
homeostasis. When TNFAIP8-knockout mice were administered water
containing dextran sodium sulphate (DSS), they developed more
severe colitis than wild-type mice, which manifested as weight
loss, increased mortality rate, shorter colon lengths and enhanced
inflammatory responses. TNFAIP8-deficient mice showed increased
sensitivity to DSS-induced colitis, increased apoptosis of colonic
epithelial cells and decreased cell proliferation, leading to the
destruction of epithelial integrity. Increased transmission of
symbiotic bacteria also caused a decrease in Akt activation,
possibly mediating cell death by downregulating the PI3K-Akt
signalling pathway, and the lack of TNFAIP8 in non-haematopoietic
cells played a key role. It has been suggested that TNFAIP8 plays
an important role in inflammatory diseases and can prevent colitis
and maintain colonic homeostasis (72,73). Similarly, TNFAIP8-knockout mice
are resistant to intestinal injury but have regeneration defects.
This injury inhibition is regulated by a number of signalling
pathways, such as that of PI3K/Akt/β-catenin. Ischaemia,
ischaemia-reperfusion (I/R) and radiation-induced intestinal injury
in TNFAIP8-deficient mice showed that their resistance to
intestinal injury was mediated by non-immune cells, which also led
to hyperproliferation, dedifferentiation and regenerative deficits
in enterocytes. The regenerative programme requires YAP signalling,
which activates Sca-1+ (Ly6a) fatal-like cells and
transiently induces Clu+ revival stem cells (revSCs) in
the intestinal epithelium (74).
The dysregulation of regeneration was also observed in the
TNFAIP8-knockout mice during DSS colitis. TNFAIP8-deficient mice
lost the ability to modulate membrane phospholipid abundance and
exhibited microbiome-dependent Akt activation and β-catenin
accumulation, resulting in injury resistance, but decreased
Sca-1+ and revSC induction. Similar results were
obtained in the colorectal cancer CMT-93 cell line (74). TNFAIP8 was also found to inhibit
symbiotic microorganism-induced Akt activation by extracting PIP2
from cell membranes, reducing the amount of PIP3 and inhibiting
PIP3-dependent signalling. It has been suggested that TNFAIP8 may
play important roles in the treatment of colitis and colon cancer
by regulating dynamic signalling to mediate the intestinal injury
response and regenerative plasticity (74).
The high expression of TNFAIP8 in chronic
pancreatitis tissues leads to the growth of inflammatory cells and
enhances cell proliferation in inflammatory tissues (42). Plantar fascia lesions, such as
those in plantar fasciitis and plantar fibromatosis, are associated
with chr5:118704153:D in TNFAIP8 and with increased risks of
disease development (75).
Inhalation of the phosphodiesterase-4 inhibitor CHF6001 by patients
with chronic obstructive pulmonary disease and chronic bronchitis
after triple therapy effectively modulates pathophysiological
pathways and downregulates pro-inflammatory cytokine genes, such as
TNFAIP8, in sputum (76). The
TNF-inducible protein TNFAIP8 is highly expressed in rheumatoid
arthritis synovial fibroblasts and regulates apoptosis,
proliferation and MMP-1 expression as an antiapoptotic gene
(77).
TNFAIP8 regulates the inflammatory
response
TNFAIP8 is highly expressed in both in vivo
and in vitro mouse models of spinal cord injury (SCI) and
time-dependently increases the expression of proinflammatory
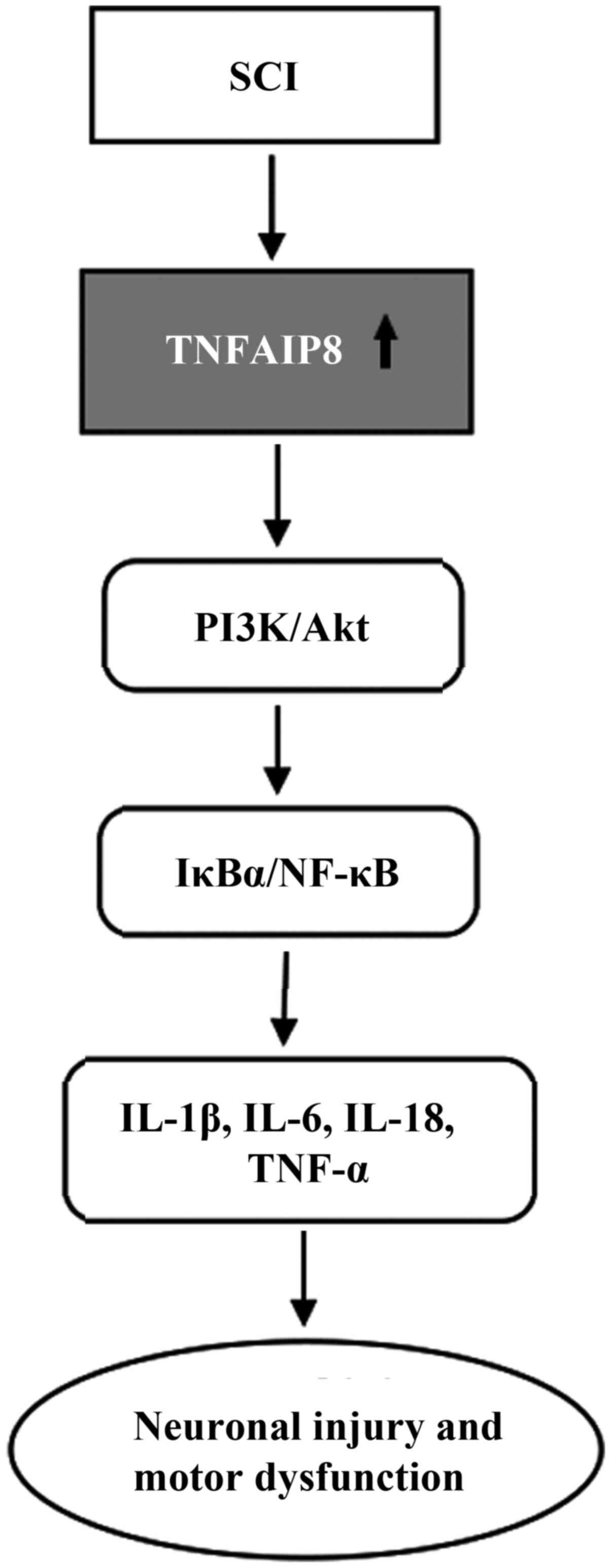
cytokines such as IL-1β, IL-6, IL-18 and TNF-α (Fig. 5). Suppression of TNFAIP8
expression alleviated the inflammatory response and cell viability
by inhibiting the activation of the IκBα/NF-κB and PI3K/Akt
signalling pathways in LPS-stimulated microglial BV2 cells, while
pretreatment of these cells with SC-79 (an Akt activator) reversed
the inhibition of NF-κB signalling and pro-inflammatory cytokines
(78). Similarly, TNFAIP8
deletion also ameliorated the inflammatory response, neuronal
injury and motor dysfunction by inhibiting these signalling
pathways in TNFAIP8-knockout mice after SCI, indicating that
TNFAIP8 can regulate SCI through the Akt signalling pathway and
that the regulatory effect of TNFAIP8 on inflammation was largely
dependent on the PI3K-Akt signalling pathway. Multiple interactions
may exist between TNFAIP8 and NF-κB depending on the environmental
stimulus (78), and TNFAIP8 may
be a novel target for developing effective treatment.
Allogeneic stem cell transplantation (aSCT) causes
chronic graft-versus-host disease (cGVHD), and TNFAIP8 is a
candidate molecular target of cGVHD and aSCT (79). In a previous study, allogeneic
haematopoietic cell transplantation caused acute GVHD in the
gastrointestinal tract, and TNFAIP8-knockout mice exhibited
significantly exacerbated GVHD, weight loss and increased mortality
rates (80). TNFAIP8 deficiency
induced the downregulation of Ki-67 in non-haematopoietic and
haematopoietic cells, increased epithelial cell apoptosis,
destroyed the epithelial barrier integrity and increased symbiotic
bacterial transmission, resulting in enhanced leukocyte
infiltration and inflammatory responses, increased levels of
proinflammatory cytokines such as IL-17A, TNF and interferon γ, and
increased expression of chemokine (C-X-C motif) ligand 1, which
contributes to exacerbating GVHD. This finding suggests that
TNFAIP8 plays an important role in maintaining intestinal
homeostasis and preventing complications associated with allograft
transplantation (80). Controlled
overexpression of TNFAIP8 might be an innovative therapeutic for
GVHD progression. Diosgenin (DIO) has pharmacological effects on
cerebral I/R. Proteomics analysis of brain tissues from DIO-treated
rats with I/R showed that TNFAIP8 was a potential target and
associated with autophagy and the inflammatory response. DIO
treatment upregulated the I/R-induced decrease in TNFAIP8
expression, mediated the effect of TNFAIP8 on the regulation of
autophagic activity and synergized with autophagy proteins in
response to I/R injury (81).
6. Conclusions and prospects
TNFAIP8 is a novel protein that was discovered in
the last decade; its family shares similar sequences and
structures, and can bind to phosphatidylinositol through a
conserved hydrophobic cavity, although each family member plays a
different role. When considering the two long electron-dense
regions inside the hydrophobic cylindrical cavity and positively
charged residues near the entrance of the cavity, determining the
manner of phosphatidylinositol binding will help in the further
exploration of specific ligands or substrates. Moreover, the
molecular mechanism of interaction, including the TNFAIP8-mediated
antiapoptotic effect in the TH domain, still needs further
elucidation. In addition, little research has been done on TNFAIP8
transcript variants, such as TNFAIP8 v2 and v1, which are known to
be differentially expressed in most tumours, and whether they play
different roles in different tumours. In recent years, TNFAIP8 has
been shown to regulate not only apoptosis, autophagy, tumour
migration and invasion, but also the proliferative activity of T
lymphocytes, immune cell migration and other immune functions,
thereby contributing to the development of new T lymphocyte-based
therapeutic strategies. However, the specific mechanism of action
is not as clear as that of TIPE2. Further studies are needed on how
TNFAIP8 controls immune functions, whether immunoregulatory
crosstalk exists among TIPE family members, and how T
lymphocyte-targeted interventions affect tumours and inflammatory
diseases. Additionally, the role of TNFAIP8 in cells depends on the
cell type and the environment in which the cells are located.
Although TNFAIP8 is known to regulate tumours and inflammatory
diseases, the specific molecular mechanisms of their interactions
and whether crosstalk exists between TNFAIP8 and signalling
pathways, such as the Hippo, Wnt and PI3K/Akt/β-catenin pathways,
remain unclear and need further elucidation. In conclusion, more
in-depth studies on TNFAIP8 are needed to determine its prognostic,
diagnostic and therapeutic roles in the clinic.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
Conceptualization and manuscript preparation were
performed by JH. GZ and ZQ provided supervision and direction, and
revised the paper. All authors have read and approved the
manuscript. Data authentication is not applicable to this
article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Key R&D
Program of China (no. 2018YFA0108304) and the National Natural
Science Foundation of China (no. 81771271).
References
|
1
|
Niture S, Moore J and Kumar D: TNFAIP8:
Inflammation, immunity and human diseases. J Cell Immunol. 1:29–34.
2019.PubMed/NCBI
|
|
2
|
You Z, Ouyang H, Lopatin D, Polver PJ and
Wang CY: Nuclear factor-kappa B-inducible death effector
domain-containing protein suppresses tumor necrosis factor-mediated
apoptosis by inhibiting caspase-8 activity. J Biol Chem.
276:26398–26404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu Z, Cui X, Sun P and Wang X: Regulatory
roles of tumor necrosis factor-α-induced protein 8 like-protein 2
in inflammation, immunity and cancers: A review. Cancer Manag Res.
12:12735–12746. 2020. View Article : Google Scholar :
|
|
4
|
Patel S, Wang FH, Whiteside TL and Kasid
U: Identification of seven differentially displayed transcripts in
human primary and matched metastatic head and neck squamous cell
carcinoma cell lines: Implications in metastasis and/or radiation
response. Oral Oncol. 33:197–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene, SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like
interleukin-1beta-converting enzyme-inhibitory protein. J Biol
Chem. 275:2973–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar
|
|
7
|
Fayngerts SA, Wu J, Oxley CL, Liu X,
Vourekas A, Cathopoulis T, Wang Z, Cui J, Liu S, Sun H, et al:
TIPE3 is the transfer protein of lipid second messengers that
promote cancer. Cancer Cell. 26:465–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JS, Park J, Kim MS, Ha JY, Jang YW,
Shin DH and Son JH: The Tnfaip8-PE complex is a novel upstream
effector in the anti-autophagic action of insulin. Sci Rep.
7:62482017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antony P, Baby B and Vijayan R: Molecular
insights into the binding of phosphoinositides to the TH domain
region of TIPE proteins. J Mol Model. 22:2722016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun H, Lin M, Zamani A, Goldsmith JR,
Boggs AE, Li M, Lee CN, Chen X, Li X, Li T, et al: The TIPE
molecular pilot that directs lymphocyte migration in health and
inflammation. Sci Rep. 10:66172020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lowe JM, Nguyen TA, Grimm SA, Gabor KA,
Peddada SD, Li L, Anderson CW, Resnick MA, Menendez D and Fessler
MB: The novel p53 target TNFAIP8 variant 2 is increased in cancer
and offsets p53-dependent tumor suppression. Cell Death Differ.
24:181–191. 2017. View Article : Google Scholar :
|
|
12
|
Zhang LJ, Liu X, Gafken PR, Kioussi C and
Leid M: A chicken ovalbumin upstream promoter transcription factor
I (COUP-TFI) complex represses expression of the gene encoding
tumor necrosis factor alpha-induced protein 8 (TNFAIP8). J Biol
Chem. 284:6156–6168. 2009. View Article : Google Scholar :
|
|
13
|
Day TF, Mewani RR, Starr J, Li X,
Chakravarty D, Ressom H, Zou X, Eidelman O, Pollard HB, Srivastava
M and Kasid UN: Transcriptome and proteome analyses of TNFAIP8
knockdown cancer cells reveal new insights into molecular
determinants of cell survival and tumor progression. Methods Mol
Biol. 1513:83–100. 2017. View Article : Google Scholar
|
|
14
|
Zhang C, Kallakury BV, Ross JS, Mewani RR,
Sheehan CE, Sakabe I, Luta G, Kumar D, Yadavalli S, Starr J, et al:
The significance of TNFAIP8 in prostate cancer response to
radiation and docetaxel and disease recurrence. Int J Cancer.
133:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng Y, Yu P, Duan X, Liu C, Xu S, Chen
Y, Tan Y, Qiang Y, Shen J and Tao Z: Genome-wide analysis of
androgen receptor binding sites in prostate cancer cells. Exp Ther
Med. 9:2319–2324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J,
Adamson E, Mercola D and McClelland M: Survey of differentially
methylated promoters in prostate cancer cell lines. Neoplasia.
7:748–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao HY, Huo FC, Wang HY and Pei DS:
MicroRNA-9 inhibits the gastric cancer cell proliferation by
targeting TNFAIP8. Cell Prolif. 50:e123312017. View Article : Google Scholar
|
|
18
|
Zhou Z, Li Z, Shen Y and Chen T:
MicroRNA-138 directly targets TNFAIP8 and acts as a tumor
suppressor in osteosarcoma. Exp Ther Med. 14:3665–3673. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing B and Ren C: Tumor-suppressive
miR-99a inhibits cell proliferation via targeting of TNFAIP8 in
osteosarcoma cells. Am J Transl Res. 8:1082–1090. 2016.PubMed/NCBI
|
|
20
|
Zhang L, Liu R, Luan YY and Yao YM: Tumor
necrosis factor-α induced protein 8: Pathophysiology, clinical
significance, and regulatory mechanism. Int J Biol Sci. 14:398–405.
2018. View Article : Google Scholar :
|
|
21
|
Padmavathi G, Banik K, Monisha J, Bordoloi
D, Shabnam B, Arfuso F, Sethi G, Fan L and Kunnumakkara AB: Novel
tumor necrosis factor-α induced protein eight (TNFAIP8/TIPE)
family: Functions and downstream targets involved in cancer
progression. Cancer Lett. 432:260–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niture S, Dong X, Arthur E, Chimeh U,
Niture SS, Zheng W and Kumar D: Oncogenic role of tumor necrosis
factor α-induced protein 8 (TNFAIP8). Cells. 8:92018. View Article : Google Scholar
|
|
23
|
Goldsmith JR, Fayngerts S and Chen YH:
Regulation of inflammation and tumorigenesis by the TIPE family of
phospholipid transfer proteins. Cell Mol Immunol. 14:482–487. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vogt PK and Hart JR: PI3K and STAT3: A new
alliance. Cancer Discov. 1:481–486. 2011. View Article : Google Scholar
|
|
26
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasaki AT, Chun C, Takeda K and Firtel RA:
Localized Ras signaling at the leading edge regulates PI3K, cell
polarity, and directional cell movement. J Cell Biol. 167:505–518.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Servant G, Weiner OD, Herzmark P, Balla T,
Sedat JW and Bourne HR: Polarization of chemoattractant receptor
signaling during neutrophil chemotaxis. Science. 287:1037–1040.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Song Y and Men X: Variance of
TNFAIP8 expression between tumor tissues and tumor-infiltrating
CD4+ and CD8+ T cells in non-small cell lung
cancer. Tumour Biol. 35:2319–2325. 2014. View Article : Google Scholar
|
|
30
|
Duan D, Zhu YQ, Guan LL and Wang J:
Upregulation of SCC-S2 in immune cells and tumor tissues of
papillary thyroid carcinoma. Tumour Biol. 35:4331–4337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX,
Jiang GY, Dong XJ, Cui QZ and Wang EH: Overexpression of SCC-S2
correlates with lymph node metastasis and poor prognosis in
patients with non-small-cell lung cancer. Cancer Sci.
101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J
and Xu H: SCC-S2 is overexpressed in colon cancers and regulates
cell proliferation. Tumour Biol. 33:2099–2106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Jing C, Chen Y, Wang J, Zhou M, Liu
X, Sun D, Mu L, Li L and Guo X: Expression of tumor necrosis factor
α-induced protein 8 is upregulated in human gastric cancer and
regulates cell proliferation, invasion and migration. Mol Med Rep.
12:2636–2642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Gao H, Chen X, Lou G, Gu L, Yang M,
Xia B and Yin H: TNFAIP8 as a predictor of metastasis and a novel
prognostic biomarker in patients with epithelial ovarian cancer. Br
J Cancer. 109:1685–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Gao H, Yang M, Zhao T, Liu Y and
Lou G: Correlation of TNFAIP8 overexpression with the
proliferation, metastasis, and disease-free survival in endometrial
cancer. Tumour Biol. 35:5805–5814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao M, Xu Q, Lou C, Qin Y, Ning X, Liu T,
Zhao X, Jia S and Huang Y: Overexpression of TNFAIP8 is associated
with tumor aggressiveness and poor prognosis in patients with
invasive ductal breast carcinoma. Hum Pathol. 62:40–49. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hadisaputri YE, Miyazaki T, Suzuki S,
Yokobori T, Kobayashi T, Tanaka N, Inose T, Sohda M and Kuwano H:
TNFAIP8 overexpression: Clinical relevance to esophageal squamous
cell carcinoma. Ann Surg Oncol. 19(Suppl 3): S589–S596. 2012.
View Article : Google Scholar
|
|
38
|
Sun Z, Liu X, Song JH, Cheng Y, Liu Y, Jia
Y, Meltzer SJ and Wang Z: TNFAIP8 overexpression: A potential
predictor of lymphatic metastatic recurrence in pN0 esophageal
squamous cell carcinoma after Ivor Lewis esophagectomy. Tumour
Biol. 37:10923–10934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong Q, Fu L, Zhao Y, Xie C, Li Q and Wang
E: TNFAIP8 interacts with LATS1 and promotes aggressiveness through
regulation of hippo pathway in hepatocellular carcinoma.
Oncotarget. 8:15689–15703. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carreras J, Hamoudi R and Nakamura N:
Artificial intelligence analysis of gene expression data predicted
the prognosis of patients with diffuse large B-cell lymphoma. Tokai
J Exp Clin Med. 45:37–48. 2020.PubMed/NCBI
|
|
41
|
Zhong M, Zhu M, Liu Y, Lin Y, Wang L, Ye
Y, Chen H, Yang Y, Zhuang G and Huang J: TNFAIP8 promotes the
migration of clear cell renal cell carcinoma by regulating the EMT.
J Cancer. 11:3061–3071. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu K, Qin CK, Wang ZY, Liu SX, Cui XP and
Zhang DY: Expression of tumor necrosis factor-alpha-induced protein
8 in pancreas tissues and its correlation with epithelial growth
factor receptor levels. Asian Pac J Cancer Prev. 13:847–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang M, Zhao Q, Wang X, Liu T, Yao G, Lou
C and Zhang Y: TNFAIP8 overexpression is associated with lymph node
metastasis and poor prognosis in intestinal-type gastric
adenocarcinoma. Histopathology. 65:517–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Yang X, Yang X, Fan K, Xiao P,
Zhang J and Wang X: Association between the expression levels of
tumor necrosis factor-α-induced protein 8 and the prognosis of
patients with gastric adenocarcinoma. Exp Ther Med. 12:238–244.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi TY, Cheng X, Yu KD, Sun MH, Shao ZM,
Wang MY, Zhu ML, He J, Li QX, Chen XJ, et al: Functional variants
in TNFAIP8 associated with cervical cancer susceptibility and
clinical outcomes. Carcinogenesis. 34:770–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu T, Jiang L, Yu L, Ge T, Wang J and Gao
H: Association of TNFAIP8 gene polymorphisms with endometrial
cancer in northern Chinese women. Cancer Cell Int. 19:1052019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Chen MB, Zhou XY and Hong XN:
Lymphotoxin alpha (LTA) polymorphism is associated with prognosis
of non-Hodgkin's lymphoma in a Chinese population. PLoS One.
8:e664112013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deeb SJ, Tyanova S, Hummel M,
Schmidt-Supprian M, Cox J and Mann M: Machine learning-based
classification of diffuse large B-cell lymphoma patients by their
protein expression profiles. Mol Cell Proteomics. 14:2947–2960.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eisele L, Klein-Hitpass L, Chatzimanolis
N, Opalka B, Boes T, Seeber S, Moritz T and Flasshove M:
Differential expression of drug-resistance-related genes between
sensitive and resistant blasts in acute myeloid leukemia. Acta
Haematol. 117:8–15. 2007. View Article : Google Scholar
|
|
50
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu R, Qiu X, Hong S, Meng L, Hong X, Qiu
J, Yang J, Zhuang G and Liu Z: Clinical significance of TIPE
expression in gastric carcinoma. Onco Targets Ther. 9:4473–4481.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Monteith JA, Mellert H, Sammons MA,
Kuswanto LA, Sykes SM, Resnick-Silverman L, Manfredi JJ, Berger SL
and McMahon SB: A rare DNA contact mutation in cancer confers p53
gain-of-function and tumor cell survival via TNFAIP8 induction. Mol
Oncol. 10:1207–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Laliberté B, Wilson AM, Nafisi H, Mao H,
Zhou YY, Daigle M and Albert PR: TNFAIP8: A new effector for
Galpha(i) coupling to reduce cell death and induce cell
transformation. J Cell Physiol. 225:865–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han Y, Tang Z, Zhao Y, Li Q and Wang E:
TNFAIP8 regulates Hippo pathway through interacting with LATS1 to
promote cell proliferation and invasion in lung cancer. Mol
Carcinog. 57:159–166. 2018. View Article : Google Scholar
|
|
55
|
Xie Y, Zhou F and Zhao X: TNFAIP8 promotes
cell growth by regulating the Hippo pathway in epithelial ovarian
cancer. Exp Ther Med. 16:4975–4982. 2018.PubMed/NCBI
|
|
56
|
Yang C, Xu W, Meng X, Zhou S, Zhang M and
Cui D: SCC-S2 facilitates tumor proliferation and invasion via
activating wnt signaling and depressing hippo signaling in
colorectal cancer cells and predicts poor prognosis of patients. J
Histochem Cytochem. 67:65–75. 2019. View Article : Google Scholar :
|
|
57
|
Liu T, Xia B, Lu Y, Xu Y and Lou G:
TNFAIP8 overexpression is associated with platinum resistance in
epithelial ovarian cancers with optimal cytoreduction. Hum Pathol.
45:1251–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang DL and Yang N: TNFAIP8 regulates
cisplatin resistance through TAF-Iα and promotes malignant
progression of esophageal cancer. Eur Rev Med Pharmacol Sci.
24:4775–4784. 2020.PubMed/NCBI
|
|
59
|
Xing Y, Liu Y, Liu T, Meng Q, Lu H, Liu W,
Hu J, Li C, Cao M, Yan S, et al: TNFAIP8 promotes the proliferation
and cisplatin chemoresistance of non-small cell lung cancer through
MDM2/p53 pathway. Cell Commun Signal. 16:432018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu S, Li W, Wu Z, Cheng T, Wang P, Li N,
Liang X, Chi M, Zhang S, Ma Y, et al: TNFAIP8 promotes cisplatin
resistance in cervical carcinoma cells by inhibiting cellular
apoptosis. Oncol Lett. 17:4667–4674. 2019.PubMed/NCBI
|
|
61
|
Liu Y, Ni XY, Chen RL, Li J and Gao FG:
TIPE attenuates the apoptotic effect of radiation and cisplatin and
promotes tumor growth via JNK and p38 activation in Raw264.7 and
EL4 cells. Oncol Rep. 39:2688–2694. 2018.PubMed/NCBI
|
|
62
|
Wang J, Gao H, Liu G, Gu L, Yang C, Zhang
F and Liu T: Tumor necrosis factor α-induced protein 8 expression
as a predictor of prognosis and resistance in patients with
advanced ovarian cancer treated with neoadjuvant chemotherapy. Hum
Pathol. 82:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Niture S, Ramalinga M, Kedir H, Patacsil
D, Niture SS, Li J, Mani H, Suy S, Collins S and Kumar D: TNFAIP8
promotes prostate cancer cell survival by inducing autophagy.
Oncotarget. 9:26884–26899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Niture S, Gyamfi MA, Lin M, Chimeh U, Dong
X, Zheng W, Moore J and Kumar D: TNFAIP8 regulates autophagy, cell
steatosis, and promotes hepatocellular carcinoma cell
proliferation. Cell Death Dis. 11:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rastgoo N, Wu J, Liu M, Pourabdollah M,
Atenafu EG, Reece D, Chen W and Chang H: Targeting CD47/TNFAIP8 by
miR-155 overcomes drug resistance and inhibits tumor growth through
induction of phagocytosis and apoptosis in multiple myeloma.
Haematologica. 105:2813–2823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Woodward MJ, de Boer J, Heidorn S, Hubank
M, Kioussis D, Williams O and Brady HJ: Tnfaip8 is an essential
gene for the regulation of glucocorticoid-mediated apoptosis of
thymocytes. Cell Death Differ. 17:316–323. 2010. View Article : Google Scholar
|
|
67
|
Zhang C, Chakravarty D, Sakabe I, Mewani
RR, Boudreau HE, Kumar D, Ahmad I and Kasid UN: Role of SCC-S2 in
experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9
expression. Mol Ther. 13:947–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Day TF, Kallakury BVS, Ross JS, Voronel O,
Vaidya S, Sheehan CE and Kasid UN: Dual targeting of EGFR and IGF1R
in the TNFAIP8 knockdown non-small cell lung cancer cells. Mol
Cancer Res. 17:1207–1219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhong M, Li N, Qiu X, Ye Y, Chen H, Hua J,
Yin P and Zhuang G: TIPE regulates VEGFR2 expression and promotes
angiogenesis in colorectal cancer. Int J Biol Sci. 16:272–283.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ahn SH, Deshmukh H, Johnson N, Cowell LG,
Rude TH, Scott WK, Nelson CL, Zaas AK, Marchuk DA, Keum S, et al:
Two genes on A/J chromosome 18 are associated with susceptibility
to Staphylococcus aureus infection by combined microarray and QTL
analyses. PLoS Pathog. 6:e10010882010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Porturas TP, Sun H, Buchlis G, Lou Y,
Liang X, Cathopoulis T, Fayngerts S, Johnson DS, Wang Z and Chen
YH: Crucial roles of TNFAIP8 protein in regulating apoptosis and
Listeria infection. J Immunol. 194:5743–5750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sun H, Lou Y, Porturas T, Morrissey S, Luo
G, Qi J, Ruan Q, Shi S and Chen YH: Exacerbated experimental
colitis in TNFAIP8-deficient mice. J Immunol. 194:5736–5742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bordoloi D, Banik K, Shabnam B, Padmavathi
G, Monisha J, Arfuso F, Dharmarajan A, Mao X, Lim LHK, Wang L, et
al: TIPE family of proteins and its implications in different
chronic diseases. Int J Mol Sci. 19:29742018. View Article : Google Scholar :
|
|
74
|
Goldsmith JR, Spitofsky N, Zamani A, Hood
R, Boggs A, Li X, Li M, Reiner E, Ayyaz A, Etwebi Z, et al: TNFAIP8
controls murine intestinal stem cell homeostasis and regeneration
by regulating microbiome-induced Akt signaling. Nat Commun.
11:25912020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim SK, Ioannidis JPA, Ahmed MA, Avins AL,
Kleimeyer JP, Fredericson M and Dragoo JL: Two genetic variants
associated with plantar fascial disorders. Int J Sports Med.
39:314–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Govoni M, Bassi M, Vezzoli S, Lucci G,
Emirova A, Nandeuil MA, Petruzzelli S, Jellema GL, Afolabi EK,
Colgan B, et al: Sputum and blood transcriptomics characterisation
of the inhaled PDE4 inhibitor CHF6001 on top of triple therapy in
patients with chronic bronchitis. Respir Res. 21:722020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang HG, Hyde K, Page GP, Brand JP, Zhou
J, Yu S, Allison DB, Hsu HC and Mountz JD: Novel tumor necrosis
factor alpha-regulated genes in rheumatoid arthritis. Arthritis
Rheum. 50:420–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xue W, Tan W, Dong L, Tang Q, Yang F, Shi
X, Jiang D and Qian Y: TNFAIP8 influences the motor function in
mice after spinal cord injury (SCI) through meditating inflammation
dependent on AKT. Biochem Biophys Res Commun. 528:234–241. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Grigoryev DN, Dalal J, Becker ML and Ye
SQ: Combined meta-analysis of systemic effects of allogeneic stem
cell transplantation and systemic sclerosis. BMC Hematol. 14:72014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kumari R, Palaniyandi S, Strattan E, Huang
T, Kohler K, Jabbour N, Dalland J, Du J, Kesler MV, Chen YH and
Hildebrandt GC: TNFAIP8 deficiency exacerbates acute graft versus
host disease in a murine model of allogeneic hematopoietic cell
transplantation. Transplantation. 104:500–510. 2020. View Article : Google Scholar
|
|
81
|
Zhang X, Wang X, Khurm M, Zhan G, Zhang H,
Ito Y and Guo Z: Alterations of brain quantitative proteomics
profiling revealed the molecular mechanisms of diosgenin against
cerebral ischemia reperfusion effects. J Proteome Res.
19:1154–1168. 2020. View Article : Google Scholar : PubMed/NCBI
|