Introduction
Pancreatic cancers ranked seventh in terms of the
number of cancer-related mortality in men and women worldwide in
2012, where the most common subtype is pancreatic ductal
adenocarcinoma (PDAC) (1). In
2017, an estimated 53,670 individuals were diagnosed with
pancreatic cancer and there were 43,090 pancreatic
cancer-associated deaths in the United States (2). The mortality rate of pancreatic
cancer has continued to increase, particularly in men, where the
relative 5-year survival rate is only 8% at present (2). The poor prognosis of patients with
PDAC is primarily due to the high degree of invasiveness,
difficulties for early diagnosis and high erroneous diagnostic
rates in addition to intrinsic resistance to chemotherapy,
radiotherapy and immunotherapy (3). Moreover, the curative efficacy of
specific immunotherapy regimens and molecularly targeted therapies
for the management of advanced stage PDAC is poor (4,5).
Therefore, there is an urgent need for the development of effective
treatments for the management of PDAC.
Interleukin (IL)-28 receptor α subunit(IL-28RA) is a
transmembrane protein that serves as a subunit of the type II
cytokine receptor and binds to type III interferons (IFNs) or λ
IFNs, IL-28A and IL-28B when combined with another IL receptor
subunit, such as IL-10 receptor β (6). IL-28RA binds most readily to λ
interferon, followed by IL-28A and IL-28B (7). The signaling pathways activated by
the type III IFNs exhibit notable similarities with those activated
by type I IFNs (8), and exhibit
antiviral activity via the Janus kinase (JAK)-STAT pathway
(9), especially against hepatitis
B and influenza virus (10-13). Similar growth inhibitory functions
of IFN-λ and type I IFNs have been reported in several types of
cancer cells, including in pancreatic neuroendocrine BON1 tumor
cells (14-16), where similar signal transduction
pathways are activated by IFN-λ and type I IFNs, although no
detectable homology was found in their receptor subunits, IL-28RA
and interferon α and β receptor subunit 1 (15). Both of these factors bind to their
respective receptor complexes and predominantly modulate STAT1 and
STAT2 phosphorylation, but can also regulate STAT3 and STAT5
phosphorylation to a lesser degree (14,17). In addition, signals activated by
IFN-λ receptor scan regulate the expression of several established
type I IFN-responsive target genes, including interferon-stimulated
genes and suppressor of cytokine signaling 1 (9). Previous reports suggested that type
I IFNs showed antitumor ability against different types of
pancreatic cancer cell lines, including PANC-1, MiaPaCa-2 and
BxPc-3 cells (18,19). Overexpression of IL-28RA in
pancreatic cancer cells exerted inhibitory effects on proliferation
and invasion (18). Additionally,
IL-28RA increased the apoptotic rate of pancreatic cancer cell and
upregulated the expression of the pro-apoptotic protein BAX, whilst
decreasing the expression of the anti-apoptotic protein Bcl-2; in a
manner that may be associated with inhibition of the JAK2 and STAT3
signaling pathway (18). It was
also found that IL-28RAwas also involved in breast cancer and colon
cancer development, where IL-28RA was positively associated with
the prognosis of patients (20,21).
Immunofluorescence and western blotting analysis
were used to detect IL-28RA expression levels in PDAC tissues and
adjacent normal pancreatic tissues from human samples. To determine
the effect of IL-28RA on the development and progression of PDAC
with specific focus on the possible molecular mechanisms, stable
IL-28RA knockdown and IL-28RA overexpression were achieved using
the PDAC cell line PANC-1, in the present study. The effect of
IL-28RA on cell proliferation, cell cycle and migration ability
in vitro and the possible molecular mechanisms were
investigated. In addition, xenograft nude mouse models induced by
PANC-1 cells with stable IL-28RA overexpression and IL-28RA
knockdown were used to detect the effect of IL-28RA on
tumorigenesis and progression in vivo.
Materials and methods
Clinical specimens
The present study was approved by the Ethics
Committee of Anhui Medical University (Hefei, China). A total of
eight patients (sex, five males and three females; average age,
56.32±8.12 years) with stage I-II PDAC based on pathological
analysis according to Guideline for the diagnosis and treatment of
pancreatic adenocarcinoma (2014 edition) (22), who were diagnosed with PDAC with
no portal vein obstruction, intraperitoneal dissemination or
colonic ileus and treated with surgical resection at The First
Affiliated Hospital of Anhui Medical University between September
2015 and November 2016, were included in the present study. The
exclusion criterion was stage IV PDAC according to TNM
classification (22) with
complications, such as with indication for urgent surgery for
symptoms, including acute abdomen. The protocols used were
performed in accordance with the ethical guidelines described in
the Declaration of Helsinki and written informed consent was
obtained from each of the patients diagnosed in the present study
prior to surgery. PDAC and paired para-cancerous normal pancreatic
tissues were obtained from each individual for immunofluorescence
analysis and western blotting.
Cytokines, experimental materials and
reagents
Recombinant human IL-29 was purchased from Pepro
Tech, Inc. Theanti body against IL-28RA (cat. no. ab83865; 1:500)
was purchased from Abcam. A JAK inhibitor (JAKI, cat. no.
sc-204021) and antibodies againstSTAT1 (cat. no. sc-464; 1:500),
STAT3 (cat. no. sc-8019; 1:500), STAT5 (cat. no. sc-74442; 1:500),
AKT (cat. no. sc-5298; 1:500), phosphorylated (p)STAT1 (cat. no.
sc-8394; 1:500), pSTAT3 (cat. no. sc-8059; 1:500), pSTAT5 (cat. no.
sc-81524; 1:500), p AKT (cat. no. sc-514032; 1:500), CyclinB1 (cat.
no. sc-245; 1:500) and β-actin (cat. no. sc-47778; 1:1,000) were
acquired from Santa Cruz Biotechnology, Inc. LY294002, a PI3K
inhibitor, was purchased from Sigma-Aldrich; Merck KGaA. IL-28RA
recombinant plasmid (cat. no. RC221831) constructed in the
pCMV6-Entry plasmid carrying Kanamycin resistance in E. coli
selection and neomycin resistance for cell selection, control
plasmidp CMV6-Entry (cat. no. PS100001), IL-28RA short hairpin (sh)
RNA (cat. no. TR303938) constructed in p RS retroviral plasmids
with ampicillin and puromycin resistance markers and scrambled
shRNA plasmid (cat. no. TR20003) were purchased from Ori Gene
Technologies, Inc.
Cell culture
The human pancreatic tumor cell linePANC-1 was
obtained American Type Culture Collection and cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
Penicillin-Streptomycin at 37°C in a humidified incubator with 5%
CO2.
Establishment of stably transfected
PANC-1 cells
PANC-1 cells were seeded ata density of
5×104 cells/well in 24-well plates. PANC-1 cells in the
24-well plates were grown to 80-90% confluence and transfected with
the IL-28RA recombinant plasmid (IL-28RA constructed in pCMV6-entry
mammalian expression vector), control pCMV6-entrymammalian
expression plasmid, shIL-28RApRS plasmid and shRNA pRS scrambled
shRNA Vector using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, DNA-lipid complex was prepared by mixing 0.2 µg
plasmid with Lipofectamine® 3000 and was added to cells
after 15 min incubation at room temperature. IL-28RA recombinant
plasmid- and control plasmid pCMV6-entry-transfected cells were
selected at 37°C for 2 weeks using G418 (800 µg/ml; Gibco;
Thermo Fisher Scientific, Inc.) 24 h after infection, whilst cells
transfected with plasmids encoding shIL-28RA and scrambled shRNA
were selected at 37°C for 2 weeks using puromycin (1 µg/ml;
cat. no. P9620; Sigma-Aldrich; Merck KGaA). Stably transfected
cells resistant to G418 or puromycin were grown, following which
the expression levels of IL-28RA were screened using western
blotting to confirm transfection efficiency.
Hematoxylin and eosin (H&E) and
tissue immunofluorescence staining
Formalin-fixed paraffin-embedded sections of PDAC
and paired paracancerous normal tissues were used for H&E
staining and immunofluorescence analysis to investigate IL28RA
expression levels. Briefly, PDAC tissues and paired paracancerous
normal tissues were collected, immediately fixed in 10% formalinat
room temperature for 24 h. The tissues were dehydrated in an
ascending gradient of ethanol followed by xylene and embedded in
paraffin. The embedded tissues were sectioned at 4-µm
thickness and dewaxed using xylene and hydrated in a descending
series of ethanol solutions.
For H&E staining, the sections were stained in
hematoxylin for 5 min at room temperature and eosin for 10 sec at
room temperature. The stained sections were observed and pictured
at ×200 magnification using a Nikon light microscope (Nikon TS2-FL;
Nikon Corporation) after sealing the slides with neutral
balsam.
For immunofluorescence staining, citrate buffer (pH
6.0) was used to heat the sections for antigen recovery at 95°C for
6 min followed by 30 min treatment in H2O2 at
room temperature after cooling down passively. Following
non-specific antigen blocking in 3% BSA (Beyotime Institute of
Biotechnology) with 0.2 Triton X-100/PBS at room temperature for 20
min, the specimens were stained with the IL-28RA-specific antibody
(1:100) overnight at 4°C. Tissues were then extensively washed and
the sections were incubated with goat anti-rabbit IgG secondary
antibody with FITC-conjugation (1:100; cat. no. ZF-0311; ZSGB-BIO,
Ori Gene Technologies, Inc.) at room temperature for 2 h in a dark
box. DAPI working solution at 10 µg/ml (cat. no. C1005;
Beyotime Institute of Biotechnology) was used to stain the nuclei
for 10 min at room temperature and the sections were mounted using
colorless mounting solution. The fluorescence images of the
specific stained sections were imaged using a fluorescence
microscope (magnification, ×200; Olympus Corporation).
Western blotting assay
PDAC and paired paracancerous normal tissues from
patients were washed using RIPA buffer (Sigma-Aldrich; Merck KGaA)
on ice to obtain the total proteins. PANC-1 cells were cultured,
transfected as aforementioned, stimulated with IL-29 (200 ng/ml),
JAKI (800 nM) or LY294002 (50 µM) at 37°C for 72 hand
harvested. After measuring the total protein concentration using
the bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology), equal amounts of total protein (30 µg/lane)
lysate from tissues or cells were resolved using 12% SDS-PAGE and
transferred onto PVDF membranes. Appropriate dilutions of specific
primary antibodies against IL-28RA, cyclinB1, pSTAT1, pSTAT3,
pSTAT5, STAT1, STAT3, STAT5, pAKT, AKT (all at 1:500) and β-actin
(1:1,000) were used to incubate the membranes at 4°C overnight
followed by 2 h incubation at room temperature with the
corresponding horseradish peroxidase-conjugated goat anti-rabbit
IgG (cat. no. AP132P; 1:10,000) and goat anti-mouse IgG (cat. no.
AP130; 1:10,000) secondary antibodies (Sigma Aldrich, Merck KGaA)
diluted in TBS solution. Signals were visualized using a Super
Signal West Femto Trial kit (Thermo Fisher Scientific, Inc.), and
images of the bands were taken using a Chemi Scope (Clinx Science
Instrument Co. Ltd.). The bands on western blots were quantified
using the Quantity One software (v4.6.6; Bio-Rad Laboratories,
Inc.).
Cell viability assay
A total of 3×103 stably transfected or
control PANC-1 cells in 100 µl culture medium were seeded
into 96-well plates, in triplicate and treated with IL-29 (200, 300
and 500 ng/ml), JAKI (800 nM) or LY294002 (50 µM) at 37°C
for 48 h. An MTS salt assay (Promega Corporation) was performed to
measure cell growth. Briefly, 20 µl MTS reagent was added to
each well at the end of cell treatment, followed by 4 h incubation
at 37°C. The absorbance value at a wavelength of 490 nm was
measured using a PerkinElmer Wallac Victor 2 1420 Multi label
Counter (PerkinElmer, Inc.) following color development. The growth
inhibition rate was calculated by the following formula: Inhibition
rate (%)=[1−(mean OD treated groups−mean OD blank
controls)/(OD control groups−OD blank
controls).
Cell cycle analysis
Stably transfected cells, at a density of
1×105 cells/well, were seeded into six-well plates and
routinely cultured to 80-90% confluence, followed by treatment with
IL-29 (200 ng/ml), JAKI (800 nM) or LY294002 (50 µM) at 37°C
for 72 h. Cell cycle distribution was analyzed using a BD FACSV
flow cytometer (BD Biosciences) using a Cell Cycle Analysis kit
(cat. no. C1052; Beyotime Institute of Biotechnology), according to
the manufacturer's protocol. Briefly, the cells were dissociated
and resuspended at a density of 5×105 cells/ml, fixed in
cold 70% ethanol for 30 min at 4°C and treated with propidium
iodide at 50 µg/ml staining buffer (Beijing Biosea
Biotechnology Co., Ltd.) in the dark at 4°C for 30 min. The flow
cytometry data were analyzed using the Mod Fit program version 3.1
(Verity Software House, Inc.).
In vitro wound healing assay
Stably transfected cells were plated at a density of
4×105 cells/ml in six-well plates following
trypsinization and resuspension. Cells were incubated to yield a
confluent monolayer before creating the wound. Wounds were created
using P1000 pipette tips and incubated with IL-29 (200 ng/ml), JAKI
(800 nM) or LY294002 (50 µM) in DMEM supplemented with 2%
heat-inactivated FBS at 37°C (23). Images were taken immediately after
wounding (0 h), after 24 and 48 hunder Nikon light microscope
(magnification, ×100; Nikon TS2-FL; Nikon Corporation). Quantity on
e4.6.6 software (Bio-Rad Laboratories, Inc.) was used to measure
the distance migrated by cells after 24 and 48 h. Results are
presented as the percentage residual of wound area, and the
percentage of the distance after treatment relative to the distance
before treatment. The percentage of residual wound area (%) was
calculated by the width of the wound at 24 or 48 h divided by the
width of the wound at their corresponding 0 h.
Animal experiments
For in vivo oncogenicity experiments, a total
12 male BALB/c nude mice, aged 4 weeks (weight, 16.32±86 g), were
obtained from Beijing Vital River Laboratory Animal Technology Co.,
Ltd. and adaptively fed for 1 week before experiment. Only males
were used in the present study because female mice have estrous
cycles which would introduce another variable to negatively
influence data analysis (24).
The animal experiments were approved by the Anhui Medical
University Institutional Animal Care and Treatment Committee
(approval no. 20140257) and the mice were raised in the
specific-pathogen-free laboratory animal room under human
conditions at 22±2°C with 55±5% humidity under a 12-h light/dark
cycle with food and water provided ad libitum according to
guidelines described by the Anhui Medical University Institutional
Animal Care and Treatment Committee (25). Animal health and behavior were
monitored twice daily by food and water intake, general assessment
of animal activity and fur condition. The animal should be excluded
from the study and euthanized before the predetermined time point
if the size of a subcutaneous tumor was observed to be >2000
mm3 or with decreased food or water intake. No animal
reached any of the humane endpoints in the present study. Animal
death was verified by the absence of breathing and heartbeat.
PANC-1 Cells stably transfected with pCMV6-IL-28RA,
pCMV6,shIL-28RA or scrambled shRNA were inoculated subcutaneously
into male BALB/c nude mice 2 weeks after transfection [n=6 for each
group, 1×107 cells injection (26) in 0.2 ml PBS] in the hips
(pCMV6-IL-28RA and pCMV6 were transfected in the same mice at right
and left hips respectively, shIL-28RA and scrambled shRNA were
transfected in the same mice at right and left hips respectively).
When the tumors reached 0.4-0.5 cm in size (~2 weeks later), PBS or
IL-29 (16 mg/kg/50 µl)was injected intratumorally into the
mice, two times a week for 3 weeks and tumor growth was monitored
in detail. The tumors were allowed to grow for 24 days and the
tumor volumes were monitored every 3 days. On day 24 of PBS or
IL-29 injection, the mice with administered with pentobarbital (50
mg/kg) by intraperitoneal injection 1 h before surgery and
euthanasia by cervical dislocation, where the tumors were carefully
resected and weighed. The tumor size was calculated as (a x
b2)/2, where a is the length and b is the width of the
resected tumors.
Statistical analysis
Data are presented as the mean ± standard error of
experimental repeats (n=3 for in vitro assays; n=6 mice per
groups for in vivo assays). Data were analyzed using SPSS
version 12.0 (SPSS, Inc.). Differences between groups were compared
using Tukey test. P<0.05 was considered to indicate a
statistically significant difference.
Results
IL-28RA expression is downregulated in
PDAC tissues
IL-28RA expression in PDAC tissues and paired
adjacent normal pancreatic tissues from eight patients with PDAC
was assessed using immunofluorescence staining. Strong IL-28RA
immunoreactivity was observed in the normal pancreatic tissues with
little expression in the nucleus, whereas its expression in the
tumor tissues was markedly lower (Fig. 1A). H&E staining of PDAC tissue
slides showed the ductal glandular structure formed by tumor cells
with abundant fibrous stroma (Fig.
1B). Western blotting also showed that expression of IL-28RA in
PDAC tissues was lower compared with that in the corresponding
paired adjacent normal pancreatic tissue (Fig. 1C and D), confirming the results of
the immunofluorescence staining.
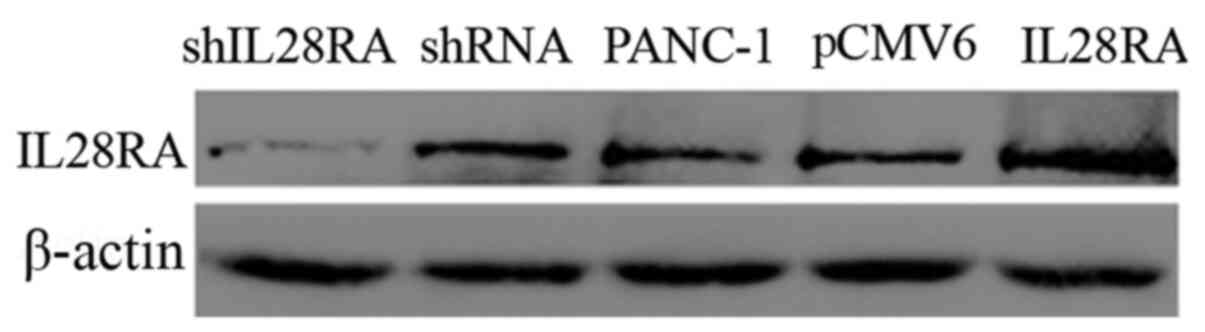
Construction of stably transfected cell
lines
To elucidate the roles of IL-28RA in PDAC
development, stably transfected PANC-1 cell lines in which IL-28RA
was overexpressed or downregulated were established. The levels of
IL-28RA expression in the transfected PANC-1 cells were determined
using western blot analysis. As shown in Fig. 2, IL-28RA expression was markedly
increased in the IL-28RA recombinant plasmid-transfected cells
compared with that in cells transfected with the empty plasmid
pCMV6-entry. By contrast, expression of IL-28RA was markedly
decreased in cells transfected withshIL-28RA compared with that in
cells transfected with the scrambled shRNA.
IL-29 exerts a cytostatic effect on the
PANC-1cells
Type III IFN interaction with IL-28R has been
reported to induce anti-proliferative responses in several types of
cancers (23-25). MTS assays were therefore performed
to determine the effect of IL-28RA on PANC-1 cell viability.
Stimulation of PANC-1 cells with IL-29 (500 ng/ml) for 48 h reduced
cell viability by 40.37% (Fig.
3A) when compared with that in control cells (0 ng/ml). Due to
almost the same inhibitory effect conferred between the 200 and 300
ng/ml concentrations, whilst cytotoxicity occurred at 500 ng/ml,
IL-29 at 200 ng/ml was chosen to treat the cells in the following
experiments. In certain types of cells, including the monkey kidney
cell line COS-1 and the human colon cancer cell line HT-29, IFN-λ
has been reported to induce STAT phosphorylation (17). Consequently, western blotting was
used to investigate whether IL-29 treatment induced the
phosphorylation of AKT, STAT1, STAT3 and STAT5 in PANC-1 cells. The
results showed that IL-29 activated STAT1 when cells were treated
from 1 to 24 h, whilst also markedly increasing STAT3
phosphorylation with a peak at 1 h. However, IL-29 conferred no
notable effects on STAT5 phosphorylation (Fig. 3B). IL-29 only slightly increased
the phosphorylation levels of AKT after 1 h before the levels were
lower compared with that at baseline from 6 h onwards (Fig. 3B).
IL-28RA affects PANC-1 cell growth in
vitro via regulation of the STAT1 and AKT phosphorylation
levels
To investigate the importance of IL-28RA on cell
growth, shIL-28RA-transfected and IL-28RA-overexpressing PANC-1
cells were used. The effect of IL-29 (200 ng/ml) on the viability
of the transfected PANC-1 cells was detected using an MTS assay.
The results showed that viability was significantly reduced in the
IL-28RA-overexpressing cells compared with that in cells
transfected with the pCMV6 vector control (Fig. 4A). By contrast, shIL-28RA
transfection significantly increased cell viability compared with
that in cells transfected with the scrambled shRNA (Fig. 4A). Furthermore, the results of
western blotting revealed that STAT1 phosphorylation was markedly
increased in the PANC-1 cells overexpressing IL-28RAwhen compared
with that transfected with the pCMV6 control plasmid vector, but no
obvious change was observed in the IL-28RA-knockdown cells compared
with that in the scrambled shRNA control group. The levels of p AKT
were upregulated in the IL28RA-knockdown cells and decreased in the
IL-28RA-overexpressing cells compared with those in cells
transfected with scrambled shRNA and pCMV6 vector control,
respectively (Fig. 4C), following
treatment with IL-29.
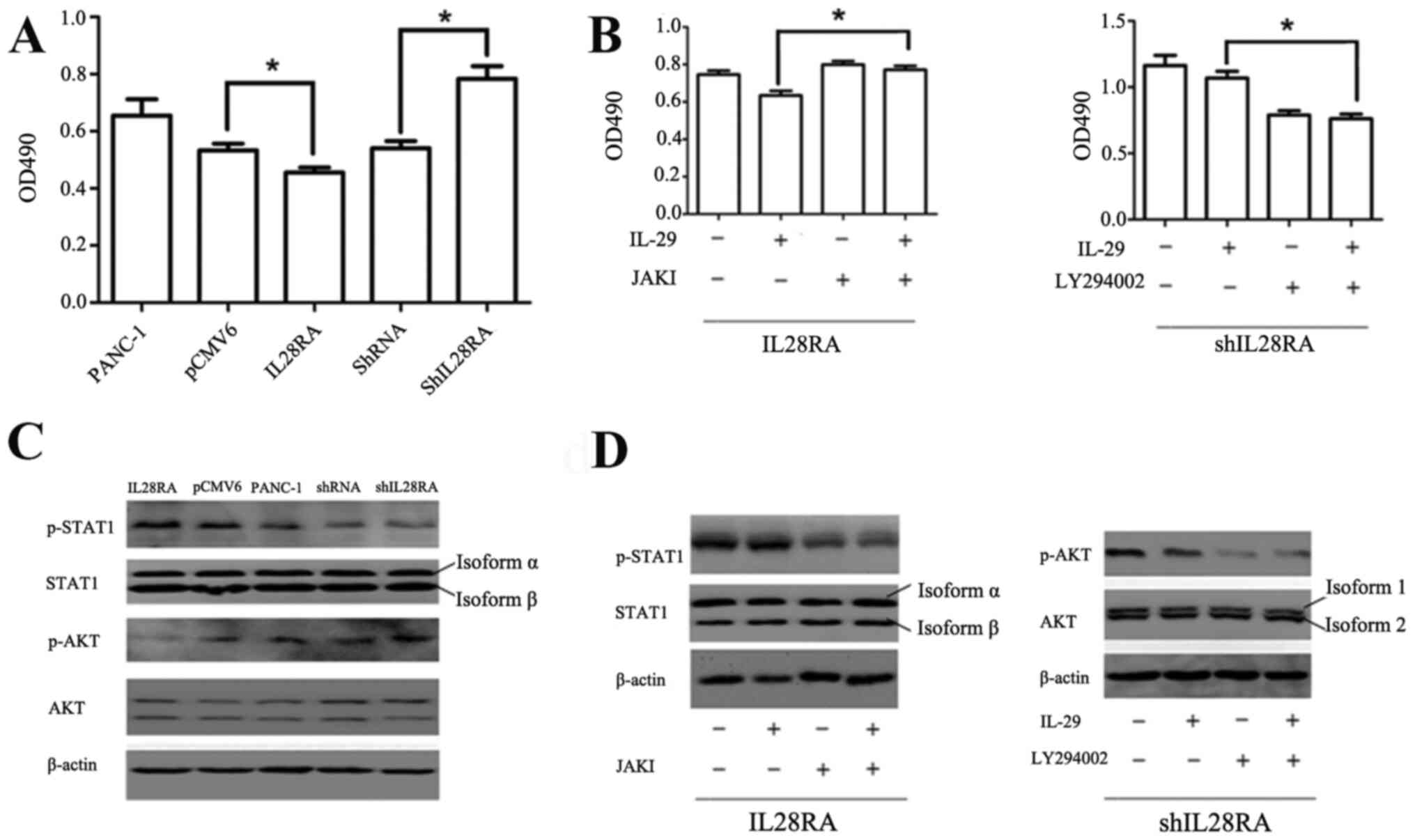 | Figure 4Effects of IL-28RA overexpression or
knockdown on PANC-1 cell viability in the presence of JAKI or
LY294002, respectively. (A) MTS analysis of the effects of IL-28RA
overexpression or IL-28RA knockdown on PANC-1 cell viability.
*P<0.05. (B) MTS analysis of the effects of JAKI or
LY294002 on cell viability in IL28RA-overexpressing cells or
IL-28RA-knockdown cells, respectively, in the presence of IL-29.
*P<0.05. (C) Western blotting of STAT1 and AKT
phosphorylation levels in the transfected PANC-1 cell lines. (D)
Western blot analysis of STAT1 phosphorylation levels in
IL-28RA-overexpressing cells in the absence or presence of JAKI in
the presence of IL-29, or the phosphorylation levels of AKT in
shIL-28RA-cells with or without LY294002 treatment, in the presence
of IL-29. The specific antibody against STAT1 recognizes both
isoform α and isoform β with different molecule weights of 91 and
84 kDa, respectively. The specific antibody against AKT recognizes
both isoform 1 and isoform 2 with different molecule weights of 56
and 48 kDa, respectively. shRNA, short hairpin RNA; p-,
phosphorylated; OD, optical density; IL, interleukin; IL-28RA,
interleukin-28 receptor α subunit; JAKI, Janus kinase
inhibitor. |
Next, JAKI (800 nM) or LY294002 (50
µM)combined with 200 ng/ml IL-29 were used to treat cells to
determine which component of the JAK-STAT and PI3K-AKT signaling
pathways was involved in mediating the effects of IL-29 and
IL-28RAin stably transfected PANC-1 cells. Compared with cells
treated with IL-29 alone, JAKI reversed the IL-29/IL28R2A-induced
increases in pSTAT1 levels, whereas LY294002 reversed the
shIL28RA-induced increases in p-AKT (Fig. 4D). JAKI significantly reversed the
inhibitory effects of IL-29 on the viability of
IL-28RA-overexpressingcells, whilstblockingp AKT using LY294002
significantly reduced cell viability in cells transfected with
shIL-28RA (Fig. 4B).
IL-28RA regulates cell cycle progression
via regulation of cyclin B1 expression
Cell cycle distribution of the transfected PANC-1
cells was next evaluated following treatment with IL-29 (200 ng/ml)
for 72 h. The proportion of cells in the G2/M phases was
significantly increased in IL-28RA-knockdown cells when compared
with that in the scrambled shRNA control cell group, whilst
IL-28RAoverexpression resulted in a significant reduction in the
proportion of cells in the G2/M phase compared with
cells transfected with the control pCMV6 plasmid (Fig. 5A and B). In addition, compared
with their corresponding transfection controls (scrambled shRNA and
pCMV6), western blotting showed that cyclin B1 expression was
downregulated by the overexpression of IL-28RA,but was upregulated
by IL-28RA knockdown (Fig.
5C).
Additionally, the effect of the JAKI and LY294002
treatment on IL-28RA-overexpressing and IL-28RA-knockdown cells,
respectively, on cell cycle distribution was investigated. As shown
in Fig. 5D, JAKI significantly
increased the proportion of cells in the G2/M phase
cells and significant decrease the proportion of cells in the S
phase compared with that in untreated JAKI IL28RA-overexpressing
cells. Compared with those in untreated IL-28RA-knockdown cells,
significantly decreased proportions of cells in the G2/M
phase were observed in LY294002-treated cells. Western blotting
showed that, when compared to the transfected cells or the
transfected cells treated with IL-29, JAKI upregulated cyclin B1
expression in IL-28RA-overexpressing cells, whilst LY294002
downregulated cyclin B1 expression in IL-28RA-knockdown cells, but
with no obvious changes were observed in cells treated with only
IL-29 (Fig. 5E).
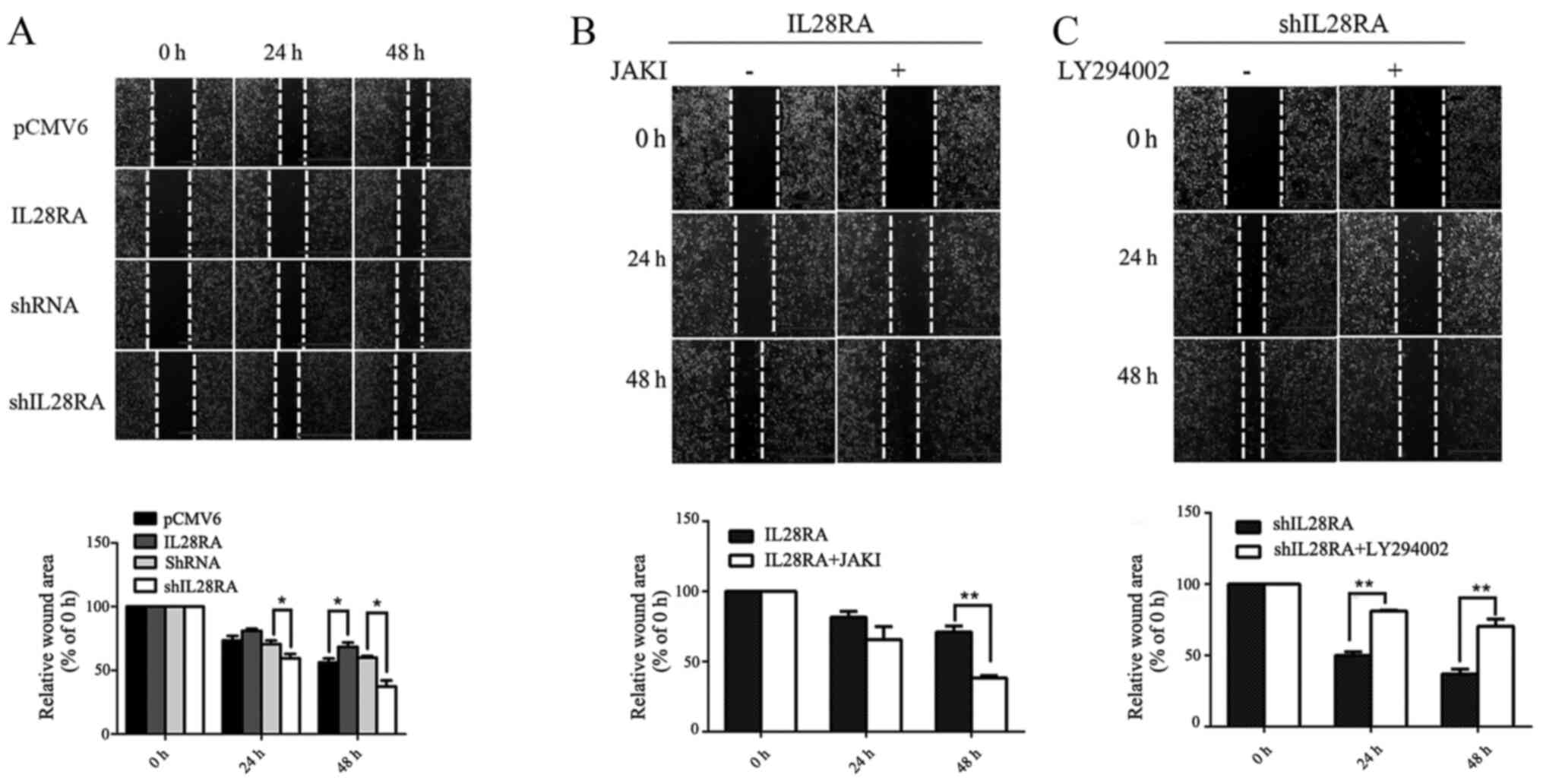
Overexpression of IL-28RA expression
results in impaired cell motility
In vitro wound healing assays were performed
to assess the effects of IL-28RA on PANC-1 cell migration following
treatment with IL-29 for every condition. Overexpression of IL-28RA
significantly reduced PANC-1 cell migration at 48 h compared with
that in cells transfected with the PCMV6 control plasmid (Fig. 6A), as shown by the increased wound
area. Opposite trend was observed in IL-28RA-knockdown cells, which
exhibited significant increases in migration at both 24 and 48 h,
with reduced residual wound areas when compared with those in cells
transfected with the non-targeting control shRNA (Fig. 6A). Cell migration in
IL-28RA-overexpressing and IL-28RA-knockdownPANC-1 cells was
investigated following treatment with JAKI and LY294002,
respectively (Fig. 6B and C). The
results showed a reduction in wound area at both 24 and 48 h in the
JAKI-treated, IL-28RA-overexpressing cells compared with that in
untreated IL-28RA-overexpressing cells, suggesting increased
migration in the former. By contrast, there was a significant
increase in wound area at 24 and 48 h in LY294002-treated,
shIL-28RA-transfected cells compared with that the untreated
IL-28RA-silenced cells, suggesting that LY294002 reduced cell
migration in this case. These results suggest that IL-28RA may
inhibit the migratory potential of PANC-1 in vitro. JAKI and
LY294002 partially reversed the inhibitory or enhancing effects on
PANC-1 cell migration by IL-28RA-overexpression or IL-28RA
knockdown, respectively (Fig. 6B and
C).
Knockdown of IL-28RA promotes development
of PDAC cells in vivo
To further examine the biological effects of IL-28RA
in the occurrence and progression of PDAC in vivo,
pancreatic cancer cells with IL-28RA expression enhanced or knocked
down were subcutaneously injected into mice to analyze
tumorigenesis in vivo. As shown in Fig. 7A-D, the volumes and weights of
tumors formed by IL-28RA-overexpressing PANC-1 cells were smaller
and lighter compared with those in mice injected with the cells
transfected with the pCMV6 control with significant differences
after 12 days growth. Conversely, in mice injected with the
shIL-28RA-transfectedPANC-1 cells, the tumors were larger and
heavier compared with those in mice injected with the scrambled
shRNA control PANC-1 cells, with significant differences after 6
days. The maximum tumor diameter and the maximum tumor volume
observed were 14 mm and 300 mm3, respectively. Together,
the in vitro and in vivo experiments showed that
IL-28RA exhibited a suppressive effect on tumor growth in PANC-1
cells.
Discussion
Pancreatic cancer frequently develops in an
insidious but rapid manner during the early stages, making early
diagnosis difficult (3). However,
at latter stages, it exhibits a high degree of invasiveness and
metastasis (3). The curative
effects of conventional radiotherapy and chemotherapy is
unsatisfactory, since the majority of patients are already
diagnosed with moderate to advanced stages pancreatic cancer at
presentation (27). To overcome
the difficulties of early diagnosis and poor treatment efficacy,
development of novel diagnostic and therapeutic methods for
pancreatic cancer are required.
The present study was performed to determine whether
IL-28RA served important roles in the occurrence and development of
PDAC, in addition to its potential as a target for the diagnosis
and treatment of PDAC. Immunofluorescence staining and western
blotting analysis showed that the IL-28RA expression levels in
human PDAC tissues were lower compared with that in matched
adjacent precancerous tissues. Stimulation of IL-28RA-mediated
signaling by IL-29 resulted in notable inhibition of cell
viability, suggesting that IL-28RA may be used as a marker for the
diagnosis of PDAC. IL-29/IL-28RA may exhibit anti-proliferative
effects and act as an inhibitory factor in PDAC. IL-28RA, the
receptor of type III IFNs, has been detected in the majority of
human tissues (6), such that
IL-28RA has been reported to be implicated in tumor cell
proliferation (16,28). Reduced IL-28RA expression in tumor
tissues was associated with accelerated tumor growth and low
survival rates of patients (29).
The anti-tumor activity of type III IFNs governed by IL-28RA has
been documented to involve the inhibition of cell proliferation and
mitosis, induction of cell cycle arrest and cell apoptosis
(30). These aforementioned roles
may be mediated by increased caspase activity coupled with the
induction of p21 and R b de phosphorylation (31).
To determine the specific role of IL-28RA and
underlying molecular mechanisms, stably transfected PANC-1 cells
overexpressing IL-28RA or with IL-28RA expression knocked down were
established together with the pCMV6 vector control and scrambled
shRNA control. Overexpression of IL-28RA in PANC-1 cells decreased
the proportion of G2/M phase cells and significantly
reduced the viability of pancreatic cells, in vitro and
in vivo. Additionally, reduced migration capacity was
observed in PANC-1 cells overexpressing IL-28RA. Conversely,
IL-28RA knockdown by shRNA resulted in increased cell viability and
migration compared with those in cells transfected with the
scrambled shRNA control. These results support the notion that
IL-28RA served as a suppressor of PDAC. Western blotting revealed
increased cyclin B1 expression in shIL-28RA PANC-1 cells, but
cyclin B1 protein expression levels in pCMV-IL-28RA PANC-1 cells
was decreased, suggesting that regulation of cyclin B1 expression
may be a mechanism by which IL-28RA inhibits PDAC progression.
In mouse embryonic fibroblasts and STAT1-deficient
human fibroblasts U3A, STAT is necessary for the inhibitory effects
of IFN-γ on cell proliferation (32). JAK-STAT signaling pathway is a key
pathway involved in the biological functions of IL-28RA,where STAT
downstream is a tumor suppressor protein, suggesting that the
JAK/STAT signaling pathway also serves an important role in
tumorigenesis in addition to regulating inflammatory and immune
responses (33). STAT can also be
suppressed by the expression of the proto-oncogene c-Myc to promote
cell cycle progression (34).
Furthermore, in a previous in vivo experiment, fibrosarcoma
growth and metastasis was reduced by STAT1-reconstitution in
STAT1-deficientfibrosarcoma RAD-105 cells when compared with those
in the mice injected with STAT1-deficientfibrosarcoma RAD-105 cells
(35).
In addition, previous studies have shown that ~50%
of pancreatic cancer types exhibit increased PI3K signaling
activity, which can be assessed based on the AKT phosphorylation
levels and has been frequently correlated with an undifferentiated
state and poor prognosis of the malignant tumor (36,37). It has also been found that the AKT
pathway is activated in early precancerous lesions and the
initiation of pancreatic lesions may be associated with KRAS
mutations or inflammation (38).
LY294002, a specific inhibitor of PI3K, can inhibit pancreatic
cancer cell proliferation and G1 phase progression
(39,40). Binding of IL-29 toIL-28RA
activates the JAK-STAT and PAKT intracellular signaling pathway and
results in anti-proliferative and pro apoptotic functions in human
melanoma cells (41).
In studying the signaling mechanism underlying the
IL-28RA mediated changes in PDAC cell growth and migration, it was
shown that although IL-29 treatment notably activated STAT1, but
the effects on phosphorylation levels of AKT were less clear, only
increasing slightly after 1 h before decreasing to levels lower
than the baseline after 6 h. pSTAT1 levels were also found to be
upregulated in the IL-28RA-overexpressing cells, whilst an increase
in p AKT levels was observed in the shIL-28RA-transfectedPANC-1
cells. Therefore, it was speculated that IL-28RA activated the
JAK-STAT1 pathway to mediate G1 phase arrest and
proliferation inhibition, but in IL-28RA knockdown cells the
PI3K-AKT signaling pathway was activated to stimulate cell
proliferation. To assess this hypothesis, the JAK inhibitor (JAKI)
or PI3K inhibitor (LY294002) were selected to treat the cells
overexpressing IL-28RA or with IL-28RA expression knocked down,
respectively. JAKI reversedIL-28RA-mediated reduction of cell
viability and migration, whilst LY294002 attenuated the increase in
cell viability and migration that was stimulated by IL-28RA
knockdown.
In summary, IL-28RA was shown to serve as a
tumor-inhibiting factor in PDAC both in vitro and in
vivo. ReducedIL-28RA expression was detected in PDAC tissues
compared with that in the paracancerous normal tissues.
Overexpression of IL-28RA inhibited PANC-1 cell viability and
migration and also induced G2/M phase arrest by
decreasing cyclin B1 expression. By contrast, IL-28RA knockdown
using shIL-28RA increased cell viability and cell migration by
increasing in cyclin B1 expression. JAKI blocked the increase in
the phosphorylation levels of STAT1 induced by IL-28RA
overexpression and reversed the reductions in cell viability and
migration induced by IL-28RA overexpression. In
shIL-28RA-transfected cells, AKT was activated and resulted in an
increase in cell viability and migration, which was reversed by
LY294002. These results suggest that the low expression of IL-28RA
in PDAC tissues may contribute to the pathogenesis of PDAC and
implicate an important role of IL-28RA in PDAC progression, where
sustained IL-29/IL-28RA signaling is partially JAK/STAT and
PI3K-AKT dependent. These results may provide insights into the
pathogenesis of PDAC and identify novel therapeutic methods for
PDAC. However, it should be noted that IL-28RA expression is lower
in cancer tissues. Therefore, a method for upregulating its
expression followed by examination of its downstream effects is
required.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ designed the study and revised the manuscript. LL
performed the majority of the experiments. DH performed the
histological examination. YQ and SZ analyzed and interpreted the
data, wrote and revised the manuscript. YX collected the specimen
from the patients with PDAC and revised the manuscript. ZH, YG, ML,
BJ and XL analyzed and interpreted the data. SZ and SZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Anhui Medical University (approval no. 20140896,
approved on 6 Nov. 2014). The study protocol conformed to the
ethical guidelines described in the 1975 Declaration of Helsinki
and written informed consent was obtained from each patient
included in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by funding from the General
Program of National Natural Science Foundation of China (grant no.
81271748) and the University Science Research Project of Anhui
Province (grant no. KJ2017A195).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arslan C and Yalcin S: Current and future
systemic treatment options in metastatic pancreatic cancer. J
Gastrointest Oncol. 5:280–295. 2014.PubMed/NCBI
|
|
4
|
Chen J, Xiao-Zhong G and Qi XS: Clinical
outcomes of specific immunotherapy in advanced pancreatic cancer: A
systematic review and meta-analysis. J Immunol Res.
2017:82823912017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez de Lapuente A, Alloza I, Goertsches
R, Zettl UK, Urcelay E, Arroyo R, Comabella M, Montalban X,
Antigüedad A and Vandenbroeck K: Analysis of the IL28RA locus as
genetic risk factor for multiple sclerosis. J Neuroimmunol.
245:98–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheppard P, Kindsvogel W, Xu W, Henderson
K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C,
Roraback J, et al: IL-28, IL-29 and their class II cytokine
receptor IL-28R. Nat Immunol. 4:63–68. 2003. View Article : Google Scholar
|
|
7
|
Syedbasha M, Linnik J, Santer D, O'Shea D,
Barakat K, Joyce M, Khanna N, Tyrrell DL, Houghton M and Egli A: An
ELISA based binding and competition method to rapidly determine
ligand-receptor interactions. J Vis Exp. 109:535752016.
|
|
8
|
Wack A, Terczyńska-Dyla E and Hartmann R:
Guarding the frontiers: The biology of type III interferons. Nat
Immunol. 16:802–809. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maher SG, Sheikh F, Scarzello AJ,
Romero-Weaver AL, Baker DP, Donnelly RP and Gamero AM: IFNalpha and
IFNlambda differ in their antiproliferative effects and duration of
JAK/STAT signaling activity. Cancer Biol Ther. 7:1109–1115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heidari Z, Moudi B, Mahmoudzadeh-Sagheb H
and Hashemi M: The correlation between interferon lambda 3 gene
polymorphisms and susceptibility to hepatitis B virus infection.
Hepat Mon. 16:e342662016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei H, Wang S, Chen Q, Chen Y, Chi X,
Zhang L, Huang S, Gao GF and Chen JL: Suppression of interferon
lambda signaling by SOCS-1 results in their excessive production
during influenza virus infection. PLoS Pathog. 10:e10038452014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lazear HM, Daniels BP, Pinto AK, Huang AC,
Vick SC, Doyle SE, Gale M Jr, Klein RS and Diamond MS: Interferon-λ
restricts west nile virus neuroinvasion by tightening the
blood-brain barrier. Sci Transl Med. 7:pp. 284ra592015, View Article : Google Scholar
|
|
13
|
Syedbasha M and Egli A: Interferon lambda:
Modulating immunity in infectious diseases. Front Immunol.
8:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zitzmann K, Brand S, Baehs S, Göke B,
Meinecke J, Spöttl G, Meyer H and Auernhammer CJ: Novel
interferon-lambdas induce antiproliferative effects in
neuroendocrine tumor cells. Biochem Biophys Res Commun.
344:1334–1341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meager A, Visvalingam K, Dilger P, Bryan D
and Wadhwa M: Biological activity of interleukins-28 and -29:
Comparison with type I interferons. Cytokine. 31:109–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumoutier L, Tounsi A, Michiels T,
Sommereyns C, Kotenko SV and Renauld JC: Role of the interleukin
(IL)-28 receptor tyrosine residues for antiviral and
antiproliferative activity of IL-29/interferon-lambda 1:
Similarities with type I interferon signaling. J Biol Chem.
279:32269–32274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kotenko SV, Gallagher G, Baurin VV,
Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H
and Donnelly RP: IFN-lambdas mediate antiviral protection through a
distinct class II cytokine receptor complex. Nat Immunol. 4:69–77.
2003. View Article : Google Scholar
|
|
18
|
Yang L, Wei WC, Meng XN, Gao J, Guo N, Wu
FT and Zeng WW: Significance of IL28RA in diagnosis of early
pancreatic cancer and its regulation to pancreatic cancer cells by
JAK/STAT signaling pathway-effects of IL28RA on pancreatic cancer.
Eur Rev Med Pharmacol Sci. 23:9863–9870. 2019.PubMed/NCBI
|
|
19
|
Vitale G, van Eijck CH, van KoetsveldIng
PM, Erdmann JI, Speel EJ, van der WansemIng K, Mooij DM, Colao A,
Lombardi G, Croze E, et al: Type I interferons in the treatment of
pancreatic cancer: Mechanisms of action and role of related
receptors. Ann Surg. 246:259–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mucha J, Majchrzak K, Taciak B, Hellmén E
and Król M: MDSCs mediate angiogenesis and predispose canine
mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-λ)
signaling. PLoS One. 9:e1032492014. View Article : Google Scholar
|
|
21
|
Hui XW, Chen H, Zhang S, Ma X, Wang X and
Huang B: Antitumor activities of recombinant human interferon
(IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer.
Cancer Lett. 311:141–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pancreatic Surgery Group of Surgery Branch
of Chin: Guideline for the diagnosis and treatment of pancreatic
adenocarcinoma (2014 edition). Chin J Digest Surg. 11:831–837.
2014.
|
|
23
|
Ding Y, He J, Huang J, Yu T, Shi X, Zhang
T, Yan G, Chen S and Peng C: Harmine induces anticancer activity in
breast cancer cells via targeting TAZ. Int J Oncol. 54:1995–2004.
2019.PubMed/NCBI
|
|
24
|
Shansky RM: Are hormones a 'female
problem' for animal research? Science. 364:825–826. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
http://sydwzx.ahmu.edu.cn/2017/0114/c6570a79605/page.htm.
|
|
26
|
Bloomston M, Shafii A, Zervos EE and
Rosemurgy AS: TIMP-1 overexpression in pancreatic cancer attenuates
tumor growth, decreases implantation and metastasis, and inhibits
angiogenesis. J Surg Res. 102:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glassman DC, Palmaira RL, Covington CM,
Desai AM, Ku GY, Li J, Harding JJ, Varghese AM, O'Reilly EM and Yu
KH: Nanoliposomal irinotecan with fluorouracil for the treatment of
advanced pancreatic cancer, a single institution experience. BMC
Cancer. 18:6932018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Jin R, Zou BB, Li L, Cheng FW, Luo
X, Geng X and Zhang SQ: Activation of Toll-like receptor 7
regulates the expression of IFN-λ1, p53, PTEN, VEGF, TIMP-1 and
MMP-9 in pancreatic cancer cells. Mol Med Rep. 13:1807–1812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Wei J and He S: Integrative
genomic analyses on interferon-lambdas and their roles in cancer
prediction. Int J Mol Med. 25:299–304. 2010.PubMed/NCBI
|
|
30
|
Lasfar A, Gogas H, Zloza A, Kaufman HL and
Kirkwood JM: IFN-λ cancer immunotherapy: New kid on the block.
Immunotherapy. 8:877–888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato A, Ohtsuki M, Hata M, Kobayashi E and
Murakami T: Antitumor activity of IFN-lambda in murine tumor
models. J Immunol. 176:7686–7694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bromberg JF, Horvath CM, Wen Z, Schreiber
RD and Darnell JE Jr: Transcriptionally active Stat1 is required
for the antiproliferative effects of both interferon alpha and
interferon gamma. Proc Natl Acad Sci USA. 93:7673–7678. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quintás-Cardama A and Verstovsek S:
Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and
resistance. Clin Cancer Res. 19:1933–1940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramana CV, Grammatikakis N, Chernov M,
Nguyen H, Goh KC, Williams BR and Stark GR: Regulation of c-myc
expression by IFN-gamma through Stat1-dependent and -independent
pathways. EMBO J. 19:263–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang S, Bucana CD, Van Arsdall M and
Fidler IJ: Stat1 negatively regulates angiogenesis, tumorigenicity
and metastasis of tumor cells. Oncogene. 21:2504–2512. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaplan DH, Shankaran V, Dighe AS, Stockert
E, Aguet M, Old LJ and Schreiber RD: Demonstration of an interferon
gamma-dependent tumor surveillance system in immunocompetent mice.
Proc Natl Acad Sci USA. 95:7556–7561. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baer R, Cintas C, Therville N and
Guillermet-Guibert J: Implication of PI3K/Akt pathway in pancreatic
cancer: When PI3K isoforms matter? Adv Biol Regul. 59:19–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ebrahimi S, Hosseini M, Shahidsales S,
Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM and Avan A:
Targeting the Akt/PI3K signaling pathway as a potential therapeutic
strategy for the treatment of pancreatic cancer. Curr Med Chem.
24:1321–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu CY, Carpenter ES, Takeuchi KK, Halbrook
CJ, Peverley LV, Bien H, Hall JC, DelGiorno KE, Pal D, Song Y, et
al: PI3K regulation of RAC1 is required for KRAS-induced pancreatic
tumorigenesis in mice. Gastroenterology. 147:1405–1416.e7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reichert M, Saur D, Hamacher R, Schmid RM
and Schneider G: Phosphoinositide-3-kinase signaling controls
S-phase kinase-associated protein 2 transcription via E2F1 in
pancreatic ductal adenocarcinoma cells. Cancer Res. 67:4149–4156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guenterberg KD, Grignol VP, Raig ET,
Zimmerer JM, Chan AN, Blaskovits FM, Young GS, Nuovo GJ, Mundy BL,
Lesinski GB and Carson WE III: Interleukin-29 binds to melanoma
cells inducing Jak-STAT signal transduction and apoptosis. Mol
Cancer Ther. 9:510–520. 2010. View Article : Google Scholar : PubMed/NCBI
|