Introduction
Pancreatic cancer is one of the most intractable
human malignancies and the seventh leading cause of cancer-related
death worldwide (1-3). The 5-year survival rate of
pancreatic ductal adenocarcinoma, which is a major histological
subtype of pancreatic tumors, remains poor (8% in the USA in 2017,
and 15-20% in Japan in 2018), even after surgery, due to its
strongly invasive and metastatic characteristics (1,4).
Chemotherapy, including gemcitabine, S-1 and 5-fluorouracil (5-FU),
and radiation treatment are the current options for patients with
unresectable, recurrent or metastatic cancer (1-4).
Although potential drug combinations have been tested in clinical
trials, their efficacy is limited due to the drug resistance of
pancreatic cancer (5-7). A detailed understanding of the
molecular mechanisms regulating the tumorigenesis and progression
of pancreatic cancer is needed for the development of more
effective treatments.
Accumulating evidence indicates the significance of
cancer stem cells (CSCs), which are resistant to current anticancer
medicines and radiation, in tumor initiation, progression,
recurrence, metastasis and, ultimately, patient death (8-10).
Therefore, specific and effective therapies against CSCs are
needed, and previous studies have reported the potential of drugs
that target CSC markers or signaling pathways (11-15). The targeting of membrane
transporters that are specifically upregulated in CSCs represents
one of the key novel strategies in cancer treatment.
Membrane transporters and transporters/ion channels
have been demonstrated to be involved in the biological processes
of cancer cells, and a cellular physiological approach exhibits
potential as a potential strategy in specific cancer therapies
(16-18). Our previous studies reported that
a number of ion channels, including transient receptor potential
vanilloid 2 (TRPV2), were highly expressed in squamous cell
carcinoma (ESCC) CSCs (19,20). The cytotoxic concentration of
tranilast, an analog of a tryptophan metabolite that specifically
inhibits TRPV2, needed to suppress cell proliferation was lower for
CSCs compared with non-CSCs. Tranilast is typically used in the
treatment of inflammatory diseases, such as allergic
conjunctivitis, asthma, dermatitis, keloids and hypertrophic scars
(21). The results of our
aforementioned studies suggested that TRPV2 may serve a role in the
maintenance and homeostasis of CSCs and may act as a targeted
therapeutic agent for ESCC (20).
However, transporter/ion channel expression profiles and their
oncogenic functions in pancreatic CSCs (PCSCs) have not been
examined in detail to date.
Therefore, the aims of the present study were to
determine the expression levels and function of transporters/ion
channels in PCSCs obtained from pancreatic carcinoma cell lines.
The results demonstrated that various genes related to ion
channels, including the voltage-gated potassium channel (Kv), were
upregulated in PCSCs compared with non-PCSCs. The study also
examined whether 4-aminopyridine (4-AP), a Kv inhibitor widely used
in the treatment of multiple sclerosis (MS) and Charcot-Marie-Tooth
disease, exerted specific inhibitory effects on PCSCs in
vitro and in vivo.
Materials and methods
Cell lines, culture and materials
PK59, PANC1, PK1, PK45H, KP4-1, AsPC1 and SUIT-2
cells were obtained from the Cell Engineering Division in RIKEN
BioResource Center and cultured as previously described (20). PK59, PANC1, PK1, PK45H, AsPC1 and
SUIT-2 cells were maintained in rPMi-1640 medium (Nacalai Tesque,
Inc.) supplemented with 100 u/ml penicillin, 100 µg/ml
streptomycin and 10% FBS (Nacalai Tesque, Inc.). KP4-1 cells were
cultured in DMEM plus HamF12 medium (Nacalai Tesque, Inc.)
supplemented with 100 u/ml penicillin, 100 µg/ml
streptomycin and 10% FbS. Cells were cultured in flasks or dishes
in a humidified incubator at 37°C with 5% Co2 in air.
4-AP was purchased from Nacalai Tesque, Inc. 5-Fu was purchased
from FUJIFILM Wako Pure Chemical Corporation.
Detection of CSCs using Aldefluor
fluorescence and cell sorting
The expression of ALDH1A1 in PK59 cells was
confirmed using an Aldefluor kit according to the manufacturer's
instructions (Stemcell Technologies, Inc.) (20,22). PK59 cells were centrifuged (150 ×
g; 23°C; 5 min) and resuspended in Aldefluor buffer and 3 ml of the
resulting cell suspension (1.0×106 cells/ml) was mixed
with 45 µl activated Aldefluor substrate. The mixture with
added ALDH1A1 inhibitor dieth-ylaminobenzaldehyde (DEAB) was used
as a negative control. Following incubation at 37°C for 45 min away
from light, the cells were centrifuged (150 × g; 23°C; 5 min),
resuspended (1.0×106 cells/ml) in Aldefluor buffer and
maintained on ice for 1 h. The cells were subsequently isolated by
flow cytometry using the Cell Sorter SH800 (Sony Corporation) and
categorized into two subgroups based on fluorescence and cell
scattering. Cells expressing ALDH1A1 treated with or without 4-AP
were analyzed using the BD Accuri C6 flow cytometer and the
associated software (BD Biosciences).
CSC culture
Cells expressing high levels of ALDH1A1 were
separated from PK59 cells using FACS and cultured in tumorsphere
medium containing rPMi-1640 medium with 100 u/ml penicillin, 100
µg/ml streptomycin, 2% b27 supplement (gibco; Thermo Fisher
Scientific, inc.), 10 ng/ml epidermal growth factor and 10 ng/ml
fibroblast growth factor (both Invitrogen; Thermo Fisher
Scientific, Inc.) for 7 days in ultra-low attachment 6-well plates
(Corning, Inc.) (20). The
presence of tumorspheres in plates was detected under an inverted
light microscope (magnification, ×40). Tumorspheres were recovered
by centrifugation (300 × g; 23°C; 10 min), counted, and dissociated
into single cells by processing with trypsin-EDTA and gentle
mechanical crushing using a glass pipette (20). The obtained single cells were
re-plated to allow the reformation of spheres. The spheres were
passaged every 4-7 days when their diameter reached ~100 µm
(20).
Drug sensitivity test
PCSCs separated from adherent and spheroid PK59
cells were seeded in 96-well microplates at a concentration of
2,000 cells/well. The cells were treated with increasing
concentrations of 5-FU or 4-AP for 72 h (5-FU, 0.16-80 µM;
4-AP, 0.08-40 mM). WST-8 assay (Cell Count Reagent SF; Nacalai
Tesque, Inc.) was performed to evaluate the viability of cells
treated with 5-FU or 4-AP. A total of 10 µl of this reagent
was added per well and the cells were incubated for 90 min at 23°C.
The number of viable cells was determined by measuring absorbance
at 490 nm using microplate reader.
Reverse transcription-quantitative
(RT-qPCR)
Total RNA was extracted from PK59 cells and PK59
CSCs using an RNeasy kit (Qiagen, Inc.); the concentration of the
cells before RNA extraction was not measured. The concentration of
RNA was measured after extraction, and the RNA concentration was
adjusted to 50 ng/µl. Reverse transcription was performed
using a High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, inc.) as follows: 25°C for 10
min, 37°C for 120 min and 85°C for 5 min). The 7300 real-Time PCr
System (Applied biosystems; Thermo Fisher Scientific, inc.) with
TaqMan gene expression Assays (Applied biosystems; Thermo Fisher
Scientific, inc.) were used according to the manufacturer's
instructions. The PCR thermocycling conditions were as follows:
initial denaturation at 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and at 60°C for 1 min. The expression levels of the
following mRNAs were determined: ALDH1A1 (cat. no. HS00946916_m1),
potassium voltage-gated channel (KCN) subfamily B member 1 (KCNB1;
cat. no. Hg00270657_m1), KCNC1 (cat. no. Hg00428197_m1), KCND1
(cat. no. Hg01085825_m1), SOX 2 (cat. no. Hs 010530 49_ s1), CD 4 4
(cat. no. Hs00153304_m1), CD133 (cat. no. Hs01009259_m1), and CXCR4
(cat. no. Hs00607978) (all Applied Biosystems; Thermo Fisher
Scientific, inc.). The expression levels were normalized to those
of the housekeeping gene β-actin (cat. no. Hs01060665_g1; Applied
Biosystems; Thermo Fisher Scientific, Inc.). Assays were performed
in triplicate.
Small interfering RNA (siRNA)
transfection
PK59 cells were seeded at a density of
1.0×105 cells/well on 6-well plates and were transfected
with 20 nM Aldh1A1 or KCNB1 siRNA (Stealth RNAi™; cat. nos.
HSS100366 and hSS180043; Invitrogen; Thermo Fisher Scientific,
Inc.) using the Lipofectamine® RNAiMAX reagent
(Invitrogen; Thermo Fisher Scientific, inc.) according to the
manufacturer's instructions. The cells were incubated with the
transfection mixture at 37°C for 24 h, following which the medium
was replaced. The Stealth RNAi™ siRNA negative control (cat. no.
12935112; invitrogen; Thermo Fisher Scientific, inc.) was used as
the negative control. The siRNA sequences were as follows: ALDH1A1
siRNA sense, 5′-CAG GAA CAG UGU GGG UGA AUU GCU A-3′ and antisense,
5′-UAG CAA UUC ACC CAC ACU GUU CCU G-3′; and KCNB1 siRNA sense,
5′-CCU AAG UUC UUA AGG CAG AAC UGU A-3′ and antisense, 5′-UAC AGU
UCU GCC UUA AGA ACU UAG G-3′.
Assessment of overexpression
Unsorted PK59 cells were transfected with the
control HaloTag plasmid (cat. no. G6591), ALDH1A1 HaloTag plasmid
(cat. no. FHC09770) or KCNB1 HaloTag plasmid (cat. no. FXC27060)
using FuGENE HD transfection reagents (cat. no. E2311) (all Promega
Corporation) according to the manufacturer's instructions. Vector
transfection was confirmed by fluorescence microscopy to detect the
haloTag fusion protein stained by the
tetramethylrhodamine-conjugated HaloTag ligand (cat. no. G8252;
Promega Corporation) according to the manufacturer's protocol. A
cell proliferation assay was subsequently conducted using
ALDH1A1-expressing cells. Briefly, PK59 cells were seeded in 6-well
plates at a density of 0.75×105 cells/well and incubated
at 37°C with 5% Co2 for 24 h. Subsequently, the cells
were transfected with plasmid as aforementioned, detached from the
flasks with trypsin-EDTA at 48 and 72 h post-transfection and
counted with a hemocytometer.
Cell counting
Unsorted PK59 cells were seeded in 6-well plates at
a density of 0.75×105 cells/well and incubated at 37°C
with 5% CO2 for 24 h. Subsequently, the cells were
transfected with siRNA as aforementioned, detached from the flasks
with trypsin-EDTA at 72 h post-transfection and counted with a
hemocytometer.
Sample preparation and hybridization to
microarrays
Total RNA (50 ng/µl) was extracted from Pk59
cells and Pk59 CSCs using an RNeasy kit (Qiagen, inc.),
aforementioned. Microarray analyses were performed by Takara Bio,
Inc. Using the Agilent SurePrint G3 Human Gene Expression 8×60 K
microarray (Agilent Technologies, Inc.). RNA quality was assessed
using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.).
Total RNA was labeled with Cyanine-3 (Cy3) using a Low Input Quick
Amp Labeling kit (Agilent Technologies, Inc.). Samples were
purified on RNeasy columns (Qiagen, inc.). The Cy3-labeled cRNA was
fragmented and subsequently hybridized for 17 h. Following
hybridization, the slides were washed and immediately scanned with
an Agilent DNA Microarray Scanner (cat. no. G2565CA; Agilent
Technologies, Inc.) using the one-color setting for 8×60K array
slides.
Microarray data processing
Scanned images of the microarray slides were
examined using Feature Extraction Software 10.10 (Agilent
Technologies, Inc.) with default parameters to obtain signal
intensities with background subtraction and spatial detrending.
Gene expression profiles and functions were assessed with Ingenuity
Pathway Analysis (IPA) software (Ingenuity Systems, Inc.) as
previously described (17,19,20).
The obtained datasets have been submitted to the public curated
database Gene Expression Omnibus (GSE172185) (https://www.ncbi.nlm.nih.gov/geo).
Formation of tumorspheres
PCSCs were suspended in medium with or without 5 mM
of 4-AP, seeded at 100 or 200 cells/well in 96-well plates and
incubated at 37°C with 5% CO2 in air for one week. To
exclude any potential disturbances to the formation of
tumorspheres, no changes were made to the culture medium. The
formed spheres were counted using a phase-contrast microscope under
×40 magnification as previously described (20,23).
Measurement of the intracellular
concentration of chloride [(Cl−)]i
[Cl−]i was assessed
using the MQAE reagent (dojindo laboratories, inc.), a
chloride-sensitive fluorescent probe (24). PK59 cells were seeded in 24-well
plates at a density of 4×104 cells/well and incubated at
37°C with 5% Co2 for 48 h. Subsequently, MQAE reagent
dissolved in complete RPMI-1640 medium was applied, and the plates
were incubated at 37°C in a Co2 incubator for 12 h. The
plates were washed five times with PBS, and the fluorescence
intensity of MQAE was measured by fluorescence microscopy (BZ-X810;
Keyence Corporation); three fields of view were analyzed per sample
at ×100 magnification. Quantification was performed using a BZ-X810
analyzer and accompanying software (v.1.1.1.8; Keyence
Corporation).
Animal experimental protocol
All animal protocols were approved by the by the
Institutional Review Board of the Kyoto Prefectural university of
Medicine (Kyoto, Japan; approval no. M2019-267), and all
experiments were strictly conducted in accordance with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals. A total of 27 female BALB/c nude mice (age, 4 weeks) were
obtained from Shimizu Laboratory Supplies Co., Ltd. and maintained
under pathogen-free barrier conditions. The mice were provided with
ad libitum access to sterile food and water and housed with
a 12-h light-dark cycle at 24°C and 40-70% humidity. Suspensions of
5×105 Pk59 cells in 50 µl rPMi-1640 and 50
µl Matrigel matrix (Corning, Inc.) incubated with 5 mM 4-AP
for 48 h in vitro were subcutaneously injected into the
right side of the lower flanks of 4-week-old female nude mice, and
suspensions containing the same number of PK59 cells incubated
without 4-AP were injected into the left side (n=3). To investigate
the influence that the expression of Aldh1A1 has on tumor growth,
suspensions of 5×105 PK59 cells transfected with control
siRNA, ALDH1A1 siRNA, control plasmid or ALDH1A1 plasmid in
vitro were injected subcutaneously into the lower flanks of
4-week-old female nude mice (n=3 mice/group). For the assessment of
drug combination treatment, suspensions of 5×105 PK59
cells incubated with 4-AP, 5-FU or 5-FU combined with 4-AP for 48 h
in vitro were injected subcutaneously into the lower flanks
of 4-week-old female nude mice (n=3 mice). All 27 mice were
sacrificed using pentobarbital (120 mg/kg) 28 days or 35 days after
the injection, and the volumes of resected tumors were measured.
The volumes of tumors were calculated according to the following
formula (25): Tumor volume
(mm3)=1/2 × length × width2.
Humane endpoints were reached when the xenograft
tumor reached >10% of the animal body weight, the tumor diameter
was >20 mm, tumors metastasized or grew such that it led to
rapid body weight loss (>20%), or signs of immobility, a huddled
posture, the inability to eat, ruffled fur, self-mutilation,
ulceration, infection or necrosis were observed. All 27 animals
reached the study endpoints (on day 28 or 35) and were euthanized
by cervical dislocation under anesthesia by intraperitoneal
injection of pentobarbital (70 mg/kg). Death was verified by the
cessation of a heartbeat and dilated pupils.
Immunohistochemistry
Xenograft samples were embedded in paraffin
following 12-h formalin fixation at 4°C. Immunohistochemistry for
the ALDH1A1 protein was performed on 4-µm-thick paraffin
sections of tumor tissues using the avidin-biotin-peroxidase
method. After dewaxing paraffin sections with xylene and hydration
in a graded ethanol series (99.5, 90, 70 and 50%), endogenous
peroxidases were blocked by an incubation in 0.3%
H2O2 for 30 min at 23°C. An Avidin/biotin
blocking kit (vector laboratories, Inc.) was used to block
endogenous biotin, biotin receptors and avidin-binding sites. The
sections were subsequently treated with a protein blocker and
incubated at 4°C overnight with an anti-ALDH1A1 antibody (1:300;
cat. no. 611194; BD Pharmingen; BD Biosciences), followed by
visualization of the avidin-biotin-peroxidase complex using the
Vectastain ABC Elite kit (Vector Laboratories, Inc.) with
diaminobenzidine tetrahydrochloride. The sections were
counterstained with hematoxylin for 4 min at 23°C and subjected to
dehydration in a graded ethanol series (50, 70, 90 and 99.5%),
cleared in xylene and mounted in Entellan new (Sigma-Aldrich,
Inc.). Quantification of staining intensity was performed using
bz-x800 All-in-one fluorescence microscope and analyzer software
(magnification, ×400; n=3 mice/group).
Correlation analysis in databases
Correlation analysis was performed using cBioportal
(www.cbioportal.org). Using the human
genome assembly hg19/grCh37, the gene expression at the mRNA level
of each case in databases was investigated. After selection of the
database, TCGA, PanCancer Atlas, the gene name 'ALDH1A1' was
entered and referred to the co-expression. This co-expression
function enables the investigation the relationships of gene
expression between ALDH1A1 and other genes. Spearman correlation
between the expression levels of ALDH1A1 and Kv in primary tumor
samples of human pancreatic cancer was performed using
cBioPortal.
Statistical analysis
All statistical analyses were conducted using the
statistical software JMP (version 12; SAS Institute, Inc.).
Statistical analysis was performed using the Mann-whitney u test
for two-group comparisons. One-way ANOVA was used to compare the
differences among multiple groups, followed by Tukey's multiple
comparisons post-hoc test. In the drug sensitivity assay,
IC50 values were calculated based on a non-linear
regression. Data are presented in the graphs as the mean ± SEM.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Acquisition of CSCs
The Aldefluor assay was performed using PK59 cells
to isolate cells that expressed high levels of ALDH1A1 by FACS
(Fig. 1A). The proportion of CSCs
in the Pk59 cell line was 3.9%. To confirm the characteristics of
CSCs, tumorsphere formation assay was performed, and the formation
of tumorspheres was observed under a microscope (Fig. 1B). Total RNA was extracted from
tumorspheres, and RT-qPCR was used to compare ALDH1A1 mRNA levels
in adherent PK59 cells (non-CSCs) and spheres (CSCs). Among PK59
cells, CSCs exhibited higher ALDH1A1 mRNA levels compared with
those in non-CSCs (Fig. 1C). The
Aldefluor assay was also performed using other pancreatic cell
lines, including PANC1, PK1, PK45H, KP4-1, AsPC1 and SUIT-2
(Fig. S1). Cells that strongly
expressed ALDH1A1 were only isolated from three cell lines, namely
PANK1, PK4-1 and SUIT-2 (Fig.
S1B). Among them, only cells isolated from SUIT-2 proliferated.
However, SUIT-2 cells expressing high levels of ALDH1A1 did not
form fine spheres in the sphere formation assay (Fig. S1C). Thus, only CSCs from the PK59
cell line were examined in the present study.
 | Figure 1Acquisition of CSCs in PK59 cells.
(A) Aldefluor assay in PK59 cells. Cells strongly expressing
ALDH1A1 were isolated using FACS. A specific inhibitor of ALDH,
DEAB, was used to control for background fluorescence. (B) Sorted
PK59 cells strongly expressing ALDH1A1 formed spheres in the sphere
formation assay. Magnification, ×40. Scale bar, 500 µm. (C)
Aldh1A1 mRNA expression levels were increased in CSCs compared with
those in non-CSC. Data are presented as the mean ± SEM. n=3.
*P<0.05 vs. non-CSCs. (D) The obtained CSCs exhibited
the ability to redifferentiate. CSCs cultured on normal plates
acquired adhesive properties and a typical morphology.
Magnification, ×40. Scale bar, 500 µm. (E) Anticancer drug
sensitivity of CSCs. 5-FU was more cytotoxic at a lower
concentration in non-CSCs compared with in CSCs in PK59 cells. n=4.
CSC, cancer stem cell; ALDH1A1, aldehyde dehydrogenase 1 family
member A1; ALDH, aldehyde dehydrogenase; 5-FU, 5-fluorouracil;
DEAB, diethylaminobenzaldehyde. |
To clarify whether established CSCs were capable of
redifferentiation, CSCs were seeded on normal plates in culture
medium. Three days later, these cells were observed to possess
adhesive properties and typical morphologies (Fig. 1D). The CSCs and non-CSCs of PK59
cells were treated with 5-FU to determine their resistance to
anticancer drugs. The IC50 values were ~3.47 and 6.07
µM in non-CSCs and CSCs, respectively (Fig. 1E). These results suggested that
the cytotoxicity of 5-FU was greater at lower concentrations in
non-CSCs compared with that in CSCs, which reflected the properties
of CSCs. The properties of CSCs were further assessed using
comparative analyses between sorted PK59 cells and unsorted PK59
cells of the expression profiles of other previously reported PCSC
markers, such as CD44, CD133 and SOX2 (4,26,27). In addition, ALDH1A1 overexpression
and knockdown experiments were performed to investigate its effects
on cell proliferation. Aldh1A1 mRNA levels were significantly
increased by the overexpression of ALDH1A1 in PK59 cells compared
with those in the control group (Fig.
2A), and CD44 and CD133 mRNA levels were also significantly
elevated (Fig. 2b). The number of
ALDH1A1 plasmid-transfected PK59 cells at 72 h post-transfection
was significantly higher compared with that of the control cells
(Fig. 2C). ALDH1A1 and SOX2 mRNA
levels were markedly decreased by the transfection of ALDH1A1 siRNA
into PK59 cells (Fig. 3A and B).
Tumorigenicity following transfection was slightly weaker in nude
mice inoculated with ALDH1A1 siRNA-transfected PK59 cells compared
with those injected with the control cells (Fig. 3C). By contrast, tumorigenicity was
slightly stronger in nude mice inoculated with ALDH1A1
plasmid-transfected PK59 cells compared with that in mice injected
with control cells (Fig. 3D).
These results suggested a potential relationship between ALDH1A1
and tumor formation, although not statistically significant in the
three mice, which supported the rationale for PK59 cells strongly
expressing ALDH1A1 to be defined as PCSCs.
Gene expression in PK59 CSCs
Gene expression data from PK59 CSCs and non-CSCs
were obtained using microarray and bioinformatics analyses. The
results of the microarray analysis revealed that the expression
levels of 4,870 genes in PK59 CSCs exhibited fold-changes >3.0
compared with those in non-CSCs. Among these, the expression levels
of 2,739 genes were upregulated, whereas the levels of 2,131 genes
were downregulated in PK59 CSCs. A list of 50 genes with the
greatest increases and decreases in expression levels in PK59 CSCs
is presented in Table SI. These
results revealed the upregulated expression levels of CSC markers,
such as SOX2, ALDH1A1 and CXCR4, in PK59 CSCs compared with those
in non-CSCs (Table SII). The
expression levels of ion channel-related genes in PK59 CSCs were
further analyzed using IPA software; the expression levels of 57
genes associated with ion channels were upregulated in PK59 CSCs
compared with those in non-CSCs (Table I).
 | Table IIon channel-related genes with high
expression levels in cancer stem cells isolated from PK59
cells. |
Table I
Ion channel-related genes with high
expression levels in cancer stem cells isolated from PK59
cells.
| Gene symbol | UniGene ID | Gene name | Fold-change |
|---|
| GJC1 | Hs.712052 | Gap junction
protein γ1 | 629.762 |
| CACNA2D1 | Hs.282151 | Calcium
voltage-gated channel auxiliary subunit α2δ1 | 303.458 |
| TMEM63C | Hs.22452 | Transmembrane
protein 63C | 234.969 |
| KCTD4 | Hs.23406 | Potassium channel
tetramerization domain-containing 4 | 217.796 |
| KCNB1 | Hs.84244 | Potassium
voltage-gated channel subfamily B member 1 | 191.214 |
| HCN1 | Hs.353176 |
Hyperpolarization-activated cyclic
nucleotide-gated potassium channel 1 | 179.023 |
| ANXA6 | Hs.412117 | Annexin A6 | 135.343 |
| KCNG3 | Hs.352633 | Potassium
voltage-gated channel modifier subfamily G member 3 | 104.575 |
| SCN9A | Hs.439145 | Sodium
voltage-gated channel α subunit 9 | 83.685 |
| MCOLN2 | Hs.591446 | Mucolipin 2 | 71.243 |
| KCNF1 | Hs.23735 | Potassium
voltage-gated channel modifier subfamily F member 1 | 67.786 |
| KCNG1 | Hs.118695 | Potassium
voltage-gated channel modifier subfamily G member 1 | 60.852 |
| TRPM8 | Hs.366053 | Transient receptor
potential cation channel subfamily M member 8 | 60.393 |
| TRPC1 | Hs.250687 | Transient receptor
potential cation channel subfamily C member 1 | 55.342 |
| SLC9A1 | Hs.469116 | Solute carrier
family 9 member A1 | 39.349 |
| GRIN2A | Hs.411472 | glutamate
ionotropic receptor nMdA type subunit 2A | 37.432 |
| TMEM150C | Hs.507676 | Transmembrane
protein 150C | 37.276 |
| MCOLN3 | Hs.535239 | Mucolipin 3 | 32.26 |
| GABRD | Hs.113882 | γ-aminobutyric acid
type A receptor δ subunit | 31.534 |
| KCND1 | Hs.55276 | Potassium
voltage-gated channel subfamily D member 1 | 28.252 |
| GRIK2 | Hs.98262 | Glutamate
ionotropic receptor kainate type subunit 2 | 24.759 |
| ZACN | Hs.714919 | Zinc-activated ion
channel | 22.824 |
| SCN8A | Hs.710638 | Sodium
voltage-gated channel α subunit 8 | 21.842 |
| LRRC8C | Hs.412836 | Leucine-rich
repeat-containing 8 VRAC subunit C | 15.768 |
| KCNC1 | Hs.552896 | Potassium
voltage-gated channel subfamily C member 1 | 15.316 |
| CACNA1G | Hs.591169 | Calcium
voltage-gated channel subunit α1 G | 14.324 |
| KCNH1 | Hs.553187 | Potassium
voltage-gated channel subfamily H member 1 | 10.901 |
| CACNA1H | Hs.459642 | Calcium
voltage-gated channel subunit α1 h | 10.788 |
| GRIN3B | Hs.660378 | glutamate
ionotropic receptor nMdA type subunit 3B | 10.067 |
| CACNA1I | Hs.125116 | Calcium
voltage-gated channel subunit α1 i | 9.816 |
| TTYH2 | Hs.27935 | Tweety family
member 2 | 9.742 |
| KCNMA1 | Hs.144795 | Potassium
calcium-activated channel subfamily M α 1 | 9.038 |
| KCNK13 | Hs.510191 | Potassium two pore
domain channel subfamily K member 13 | 8.942 |
| CACNG4 | Hs.514423 | Calcium
voltage-gated channel auxiliary subunit γ4 | 8.593 |
| KCNE2 | Hs.551521 | Potassium
voltage-gated channel subfamily E regulatory subunit 2 | 8.265 |
| KCNMB3 | Hs.591285 | Potassium
calcium-activated channel subfamily M regulatory β subunit 3 | 8.138 |
| HCN4 | Hs.86941 |
Hyperpolarization-activated cyclic
nucleotide-gated potassium channel 4 | 7.770 |
| CLCN5 | Hs.166486 | Chloride
voltage-gated channel 5 | 7.703 |
| CACNA1A | Hs.501632 | Calcium
voltage-gated channel subunit α1 A | 7.116 |
| ABCC9 | Hs.732701 | ATP-binding
cassette subfamily C member 9 | 7.108 |
| GPM6A | Hs.75819 | Glycoprotein
M6A | 6.524 |
| KCTD13 | Hs.534590 | Potassium channel
tetramerization domain-containing 13 | 6.269 |
| ASIC1 | Hs.274361 | Acid-sensing ion
channel subunit 1 | 5.888 |
| CACNA2D2 | Hs.476273 | Calcium
voltage-gated channel auxiliary subunit α2δ2 | 5.886 |
| KCNQ2 | Hs.161851 | Potassium
voltage-gated channel subfamily Q member 2 | 5.540 |
| KCNT1 | Hs.104950 | Potassium
sodium-activated channel subfamily T member 1 | 5.086 |
| PKDREJ | Hs.241383 | Polycystin family
receptor for egg jelly | 5.076 |
| KCNK12 | Hs.591586 | Potassium two pore
domain channel subfamily K member 12 | 4.632 |
| ITPR1 | Hs.567295 | Inositol
1,4,5-trisphosphate receptor type 1 | 4.594 |
| CACNB4 | Hs.120725 | Calcium
voltage-gated channel auxiliary subunit β4 | 4.450 |
| CNGA4 | Hs.434618 | Cyclic
nucleotide-gated channel α 4 | 4.342 |
| CLCA4 | Hs.567422 | Chloride channel
accessory 4 | 4.080 |
| KCNQ1 | Hs.95162 | Potassium
voltage-gated channel subfamily Q member 1 | 3.529 |
| TRPV2 | Hs.279746 | Transient receptor
potential cation channel subfamily V member 2 | 3.415 |
| SCN3B | Hs.4865 | Sodium
voltage-gated channel β subunit 3 | 3.412 |
| GRINA | Hs.594634 | Glutamate
ionotropic receptor NMDA type subunit-associated protein 1 | 3.196 |
| CLCN3 | Hs.481186 | Chloride
voltage-gated channel 3 | 3.168 |
The top-ranking genes related to potassium
voltage-gated channels, based on fold change expression in the
microarray results, were selected for further analysis. RT-qPCR was
conducted to validate the results obtained in the microarray
analysis. In PK59 cells, KCNB1, KCNC1 and KCND1 mRNA expression
levels were significantly higher in CSCs compared with those in
non-CSCs (Fig. 4A). These results
demonstrated that ion channels, which are involved in maintaining
CSCs, exhibited high expression levels. Among these ion channels,
KCNB1 was selected, and it was found that blocking this channel
with 4-AP inhibited the proliferation of PK59 cells, which was
suggested that inhibition of KCNB1 could be a potential therapeutic
target for pancreatic cancer. Even after five passages, the high
mRNA expression levels of Kv and CSC markers were maintained in
PK59 CSCs (Fig. 5A and B). These
results demonstrated the constant high expression of Kv in CSCs,
which reinforced the possibility that Kv may be a therapeutic
target for pancreatic cancer.
 | Figure 4Verification of gene expression and
drug sensitivity of non-CSCs or CSCs. (A) verification of gene
expression by RT-qPCR. The expression levels of three selected
Kv-related genes, KCNB1, KCNC1 and KCND1, in CSCs were compared
with those in the non-CSCs of PK59 cells using RT-qPCR. n=3.
*P<0.05 vs. non-CSCs. (B) drug sensitivity of CSCs to
the Kv inhibitor 4-AP. 4-AP was more cytotoxic at a lower
concentration in CSCs compared with non-CSCs isolated from PK59
cells. n=4. (C) Representative images of cultured non-CSCs or CSCs
treated with various concentrations of 4-AP. Magnification, ×40.
Scale bar, 250 µm. Data are presented as the mean ± SEM. Kv,
voltage-gated potassium channel; 4-AP, 4-aminopyridine; RT-qPCR,
reverse transcription-quantitative PCR; KCN, potassium
voltage-gated channel; KCNB1, KCN subfamily B member 1; KCNC1, KCN
subfamily C member 1; KCND1, KCN subfamily D member 1; CSCs, cancer
stem cells; ACTB, β-actin. |
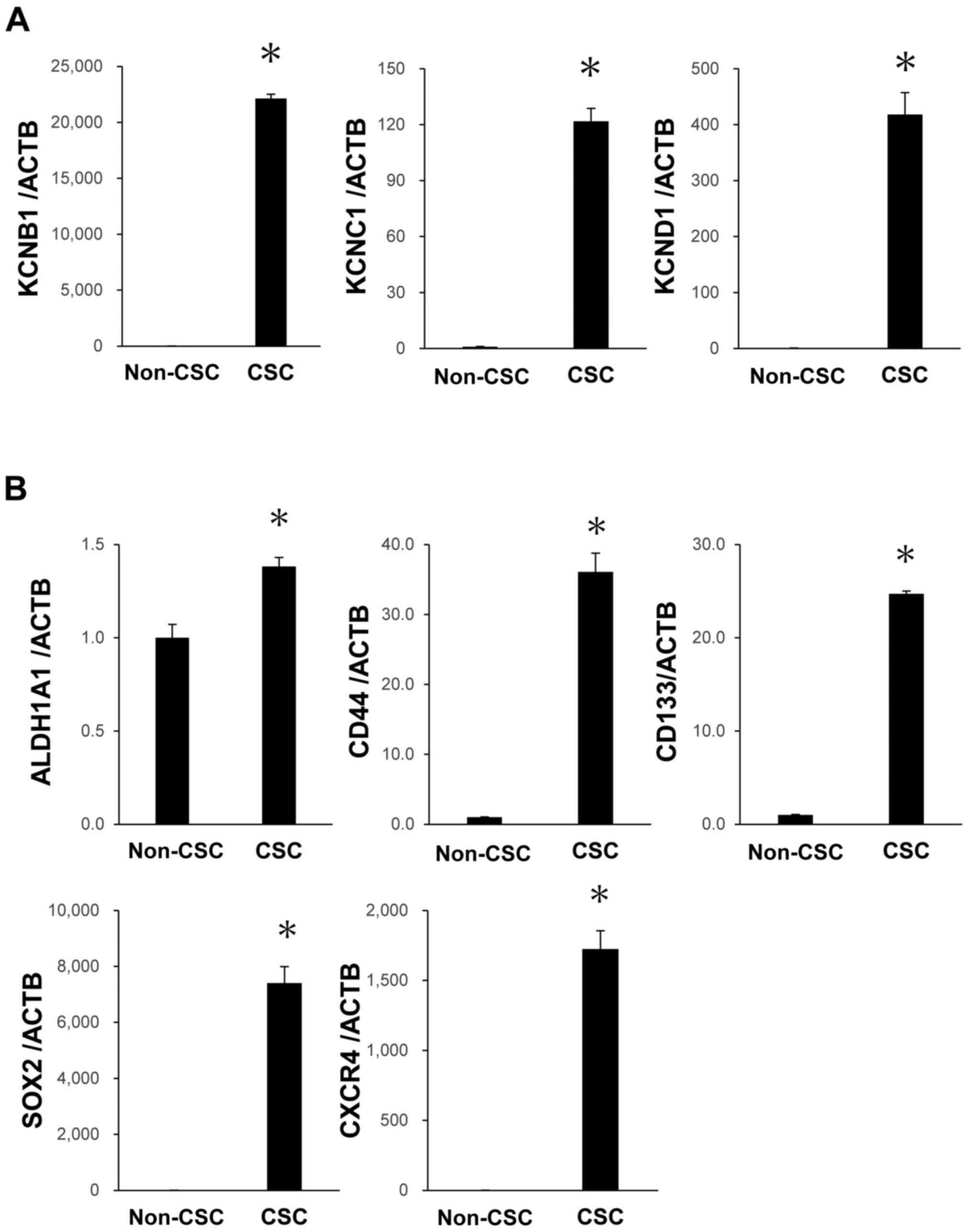 | Figure 5Expression levels of Kv and CSC
markers in PK59 CSCs after five passages. (A) high mRNA expression
levels of KCNB1, KCNC1 and KCND1 were maintained in PK59 CSCs. (B)
High mRNA expression levels of ALDH1A1, CD44, CD133, SOX2 and CXCR4
were maintained in PK59 CSCs. mRNA expression levels were analyzed
using reverse transcription-quantitative PCR. n=3. Data are
presented as the mean ± SEM. *P<0.05 vs. non-CSCs.
Kv, voltage-gated potassium channel; KCN, potassium voltage-gated
channel; KCNB1, KCN subfamily B member 1; KCNC1, KCN subfamily C
member 1; KCND1, KCN subfamily D member 1; CSC, cancer stem cell;
ACTB, β-actin. |
Effects of 4-AP on CSCs
The present study subsequently analyzed the
overexpression of Kv in PK59 CSCs. To clarify the effects of the
inhibition of Kv, PK59 non-CSCs and CSCs cells were both treated
with 4-AP. Among PK59 cells, IC50 values were
approximately 5.65 and 0.64 mM in non-CSCs and CSCs, respectively
(Fig. 4B and C). Therefore, the
cytotoxicity of 4-AP appeared to be greater in CSCs compared with
that in non-CSCs. A sphere formation assay was further performed
using PK59 CSCs treated with or without 4-AP. The number of spheres
formed by the Pk59 CSCs was significantly lower in cells treated
with 4-AP (Fig. 6). When non-CSCs
(PK59 cell line) were treated with 4-AP, the population of cells
strongly expressing Aldh1A1 significantly reduced compared with
that in the control group (Fig.
7). Therefore, 4-AP specifically inhibited the activity of CSCs
strongly expressing Kv. Overexpression experiments on PK59 cells
using a KCNB1 plasmid were conducted in order to investigate its
effects on cell proliferation and the function of Kv in PCSCs.
KCNB1 mRNA levels in PK59 cells were markedly increased by KCNB1
plasmid transfection compared with those in cell transfected with
the control plasmid (Fig. 8A).
The number of KCNB1 plasmid-transfected PK59 cells was
significantly higher compared with that of the control cells at 72
h post-transfection (Fig. 8B).
The effects of 4-AP or 5-FU on cell proliferation were then
assessed, and the results demonstrated that the number of 4-AP- or
5-FU-treated cells was significantly lower compared with that of
untreated control cells (Fig.
S2). Co-treatment with 4-AP and 5-FU revealed that 4-AP
enhanced the inhibitory effects of 5-FU (Fig. S2).
Regarding the molecular mechanisms by which Kv
maintains stemness and cell proliferation, the present study
focused on changes in the intracellular ion environment. Our
previous studies reported the roles of intracellular Cl−
in cancer cell proliferation (28-31); thus, we hypothesized that the
inhibition of K+ channels by siRNA or 4-AP may affect
the movement of Cl−, which is the counter ion of
K+ (24). This
hypothesis was tested by measuring the fluorescence intensity of
MQAE, a Cl−-sensitive fluorescent probe, to evaluate the
intracellular ion concentration (Fig.
9). The results revealed that the fluorescence intensity of
MQAE was increased by the knockdown of KCNB1 or the treatment with
4-AP in PK59 cells compared with that in the corresponding control
groups (Fig. 9B and C). These
results suggested that the change in intracellular Cl−
induced by Kv may serve a key role in the molecular mechanisms
underlying the regulation of stemness and cell proliferation.
The present study also examined the effects of the
inhibition of Kv on tumor growth in vivo. PK59 cells
incubated with or without 4-AP for 48 h were subcutaneously
injected into nude mice, and the growth of tumor nodules was
assessed (Fig. 10). Tumor
volumes were significantly lower in the sites injected with PK59
cells treated with 4-AP compared with those formed by untreated
cells (Fig. 10). The levels of
CSC markers in tumor tissues were further analyzed. The number of
ALDH1A1-stained cells in tumors formed by 4-AP-treated PK59 cells
was markedly lower compared with those formed by the untreated
cells (Fig. 11A). Furthermore,
ALDH1A1 mRNA levels were significantly lower in tumors treated with
4-AP compared with those formed by the untreated cells (Fig. 11B). Tumor weights were also
significantly lower in mice injected with PK59 cells treated with
4-AP combined with 5-FU compared with those in mice inoculated with
cells treated with 5-FU alone (Fig.
12). These results suggested that the inhibition of Kv with
4-AP strongly suppressed the development of pancreatic tumors in
vivo.
Correlation analysis between ALDH1A1 and
KCNB1 in databases
Analysis using the cBioPortal database revealed that
the expression levels of ALDH1A1 and KCNB1 are positively
correlated in primary tumor samples of human pancreatic cancer
(Fig. 13).
Discussion
Previous studies have demonstrated that several
CSC-specific markers are present in pancreatic cancer, including
ALDH1, CD133, CD24, CD44, CXCR4, EPCAM, ABCG2, c-Met and nestin
(4,26,27). In addition, the high expression
level of ALDH1 is associated with tumorigenic cells in pancreatic
ductal adenocarcinoma (32-35). Aldefluor has been successfully
applied to detect enhanced ALDH1 activity and isolate CSCs from
pancreatic cancer cells (33,35). In the present study, the Aldefluor
assay was used to isolate and obtain PCSCs following tumorsphere
formation. The obtained results demonstrated that PCSCs strongly
expressed ALDH1A1, exhibited the capacity to redifferentiate and
were resistant to chemotherapy. Gene expression data confirmed the
upregulated expression of 57 genes in ion channels in PK59 CSCs,
indicating the potential efficacy of selective ion channel
inhibitors as a targeted treatment against CSCs. Among these genes,
the role and function of K+ channels in CSCs were
subjected to further analysis. The Aldefluor assay was performed
using various cell lines, including PANC1, PK1, PK45H, PK59, KP4-1,
AsPC1 and SUIT-2. Cells that strongly expressed ALDH1A1 with FACS
were isolated from four cell lines, including PK59, PANK1, PK4-1
and SUIT-2. However, tumorspheres were only obtained from cells
isolated from PK59, and thus, CSCs from one cell line were examined
in the present study. Our previous study demonstrated that several
K+ channels were upregulated in CSCs from esophageal
squamous cell carcinoma (20),
suggesting that similar transporters may be expressed in other
PCSCs.
K+ channels serve a key role in multiple
cellular functions, such as the regulation of cell volume,
differentiation, proliferation, migration and apoptosis (36-38). K+ channels may be
classified according to several criteria, including the stimulus to
which they respond and their biophysical and structural properties,
into the following four main families: Kv, calcium-activated
K+ channels, inward-rectifier k+ channels and
two-pore-domain K+ channels (36,38). Kv are selectively permeable to
K+ ions and comprise a large family of heterogeneous
groups of ion channels forming 12 subfamilies (Kv1-Kv12) (36-38). Kv are widely distributed in a
number of cancer cell types, and their oncogenic potential has been
documented (36,38). Kv are also potential molecular
targets for anticancer therapies, and their blockers and antibodies
have been investigated and used in previous studies (38). In pancreatic cancer cells,
epigenetic mechanisms, such as DNA methylation, have been
implicated in the altered expression of Kv1.3 (39). The targeting of Kv1.3 selectively
reduces tumor progression in mouse models of pancreatic ductal
adenocarcinoma (40-42). Clofazimine promotes neoplastic
B-cell death by inhibiting Kv1.3 in chronic lymphocytic leukemia
(43). Kv1.1 blockers, such as
KAaH1 and KAaH2, inhibit cell migration and adhesion in colon
adenocarcinoma, breast cancer and glioblastoma (44). The tricyclic antidepressant
imipramine, an antidepressant Kv10.1 antagonist, increases survival
rates in patients with moderate Kv10.1 expression in brain cancers
(45). The expression of Kv11.1
has been detected in pancreatic ductal adenocarcinoma (46). Furthermore, Kv11.1 has been
demonstrated to participate in the P13k/Akt-dependent pathway, and
its blockade inhibits tumor growth, angiogenesis, and metastasis
(47).
Previous studies have analyzed the roles of Kv in
various types of stem cells. For example, Wang et al
(48) have reported that rat
mesenchymal stem cells (MSCs) heterogeneously express distinct
types of the K+ channel, and that Kv channel activity
modulates the cell cycle progression, affecting the proliferation
of MSCs. Zhang et al (49)
have reported that Kv10.1 regulates cell proliferation and
differentiation in human bone marrow-derived MSCs. Morokuma et
al (50) have demonstrated
that the modulation of Kv7.1 confers a hyperproliferative invasive
phenotype on embryonic stem cells. Bai et al (51) have described the various types of
Kv expressed in human adipose tissue-derived stem cells. However,
further studies are needed to clarify the expression, specific
roles and functions of kv in CSCs. To the best of our knowledge,
the present study is the first to investigate the expression of Kv
in PCSCs and the inhibitory effects of 4-AP, a potent Kv inhibitor,
on their proliferation.
4-AP has been used as a medical agent to regulate
the symptoms of MS (52,53) and has demonstrated effectiveness
as a symptomatic treatment for decreased walking capacity in
patients with MS (52). Phase iii
trials reported an ~25% increase in walking speed in 40% of
patients and improved muscle strength in the lower extremities
(54,55), and 4-AP was approved as a compound
by the U.S. Food & Drug Administration in 2010. Furthermore,
4-AP exhibits antitumor activities against various types of cancer
cells, such as neuroblastoma (56), Schwann cells cultured from tumors
that arise in neurofibromatosis type 1 (57), melanoma (58), prostate cancer (59), malignant astrocytoma (60), hepatoblastoma (61), acute myeloid leukemia and glioma
(62,63) cells. Ru et al (64) have reported that 4-AP induces
glioma cell apoptosis by reducing the expression of
microRNA-10b-5p. These anticancer activities of 4-AP are mostly
attributed to its inhibition of Kv. However, the effects of 4-AP on
CSCs remain unknown. The present study elucidated the mechanisms by
which 4-AP may suppress the proliferation of CSCs through its
effects as an inhibitor of Kv.
Luo et al (65) have recently demonstrated that 4-AP
inhibits cell proliferation, induces apoptosis and enhances the
sensitivity of a cisplatin (CDDP)-resistant lung cancer cell line
to CDDP by upregulating phosphatase and tensin homolog, suggesting
the potential of 4-AP as a therapeutic agent for patients with
resistance to anticancer agents. Although advances have been
achieved in chemotherapy for patients with pancreatic cancer
through the application of various key agents, such as 5-FU, S-1,
CDDP and gemcitabine, the prognosis of this cancer remains poor as
recurrence is common in patients with advanced disease (1-7).
In the present study, 4-AP inhibited tumorsphere formation in PCSCs
and tumor growth in vivo compared with those in the
corresponding control groups. Furthermore, 4-AP decreased the
population of cells strongly expressing ALDH1A1 among PK59 cells.
These results suggested the potential of 4-AP as a candidate drug
in combination with anticancer agents for treatment-resistant
pancreatic cancer.
Our previous studies demonstrated that
[Cl−]i controlled by Cl− channels
may be an important messenger (28-31), and that a change in
[Cl−]i induced cell cycle arrest at the
G0/G1 phase via mitogen-activated protein
kinases in cancer cells (28,29). Since Cl− is the counter
ion of K+, the inhibition of K+ channels may
affect its movement (24). In the
present study, the depletion of kCnb1 or treatment with 4-AP
altered the fluorescence intensity of MQAE. These results suggested
that Kv may regulate the stemness and cell proliferation by
controlling [Cl−]i in pancreatic cancer.
Pancreatic cancer is a desmoplastic tumor with
fibroblasts, and a number of factors render the tumor environment a
hostile milieu for antitumor immune cells, such as hypoxia,
hypoglycemia and lactic acidosis (3-7).
Therefore, a xenograft model does not simulate a real tumor. On the
other hand, analysis of the cBioPortal database in the present
study revealed that the expression levels of ALDH1A1 and KCNB1
positively correlated in primary tumor samples of human pancreatic
cancer, suggesting that similar results to our in vitro
analyses are obtainable under in vivo conditions.
In conclusion, the results of the present study
demonstrated that several ion channels, including Kv, were strongly
expressed in PCSCs. The cytotoxicity of 4-AP against Kv was greater
at a lower level in CSCs compared with that in non-CSCs. Although
further studies are needed on the role and function of Kv in CSCs,
its inhibitor 4-AP has potential as a novel therapeutic target
against pancreatic cancer.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AS, TomK, TosK, MK, KK, HirI, KS, TA, HK, RM, SK,
HisI, AT, TK, HF, KO and EO designed the research. AS, TomK and EO
wrote the paper. AS, TomK, TosK, MK, KK and HirI performed cell
culture, molecular biology and animal experiments. AS, TomK, KK and
HirI confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental methods were performed in
accordance with relevant guidelines and regulations. The animal
protocol was approved by the Institutional Animal Care and Use
Committee of Kyoto Prefectural university of Medicine (Kyoto,
Japan), and all experiments were performed in accordance with the
National Institutes of Health Guide for Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
This study was supported by Grants-in-Aid for
Scientific Research (C) (grant nos. 17K10602, 17K10710, 18K08628,
18K08689, 19K09202 and 19K09182) and a Grant-in-Aid for Young
Scientists from the Japan Society for the Promotion of Science
(grant no. 19K18160).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe Estimates
for 40 countries in 2012. Eur J Cancer. 49:1374–1403. 2013.
View Article : Google Scholar
|
|
3
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar
|
|
4
|
Ishiwata T, Matsuda Y, Yoshimura H, Sasaki
N, Ishiwata S, Ishikawa N, Takubo K, Arai T and Aida J: Pancreatic
cancer stem cells: Features and detection methods. Pathol Oncol
Res. 2:797–805. 2018. View Article : Google Scholar
|
|
5
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
national cancer institute of Canada clinical trials group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
6
|
Louvet C, Labianca R, Hammel P, Lledo G,
Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et
al: Gemcitabine in combination with oxaliplatin compared with
gemcitabine alone in locally advanced or metastatic pancreatic
cancer: Results of a GERCOR and GISCAD phase III trial. J Clin
Oncol. 23:3509–3516. 2005. View Article : Google Scholar
|
|
7
|
Hu G, Li F, Ouyang K, Xie F, Tang X, Wang
K, Han S, Jiang Z, Zhu M, Wen D, et al: Intrinsic gemcitabine
resistance in a novel pancreatic cancer cell line is associated
with cancer stem cell-like phenotype. Int J Oncol. 40:798–806.
2012.
|
|
8
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
9
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar
|
|
10
|
Toh TB, Lim JJ and Chow EK: Epigenetics in
cancer stem cells. Mol Cancer. 16:292017. View Article : Google Scholar
|
|
11
|
Long A, Giroux V, Whelan K A, Hamilton KE,
Tetreault MP, Tanaka K, Lee JS, Klein-Szanto AJ, Nakagawa H and
Rustgi AK: WNT10A promotes an invasive and self-renewing phenotype
in esophageal squamous cell carcinoma. Carcinogenesis. 36:598–606.
2015. View Article : Google Scholar
|
|
12
|
Chen Q, Song S, Wei S, Liu B, Honjo S,
Scott A, Jin J, Ma L, Zhu H, Skinner HD, et al: ABT-263 induces
apoptosis and synergizes with chemotherapy by targeting stemness
pathways in esophageal cancer. Oncotarget. 6:25883–25896. 2015.
View Article : Google Scholar
|
|
13
|
Yue D, Zhang Z, Li J, Chen X, Ping Y, Liu
S, Shi X, Li L, Wang L, Huang L, et al: Transforming growth
factor-betal promotes the migration and invasion of sphere-forming
stem-like cell subpopulations in esophageal cancer. Exp Cell Res.
336:141–149. 2015. View Article : Google Scholar
|
|
14
|
Brungs D, Aghmesheh M, Vine KL, Becker TM,
Carolan MG and Ranson M: Gastric cancer stem cells: Evidence,
potential markers, and clinical implications. J Gastroenterol.
51:313–326. 2016. View Article : Google Scholar
|
|
15
|
Li Y, Rogoff HA, Keates S, Gao Y,
Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB and Li CJ:
Suppression of cancer relapse and metastasis by inhibiting cancer
stemness. Proc Natl Acad Sci USA. 112:1839–1844. 2015. View Article : Google Scholar
|
|
16
|
Shiozaki A, Ichikawa D, Otsuji E and
Marunaka Y: Cellular physiological approach for treatment of
gastric cancer. World J Gastroenterol. 20:11560–11566. 2014.
View Article : Google Scholar
|
|
17
|
Shiozaki A, Ariyoshi Y, Iitaka D, Kosuga
T, Shimizu H, Kudou M, Konishi T, Shoda K, Arita T, Konishi H, et
al: Functional analysis and clinical significance of sodium iodide
symporter expression in gastric cancer. Gastric Cancer. 22:473–485.
2019. View Article : Google Scholar
|
|
18
|
Kosuga T, Shiozaki A, Kudou M, Yamazato Y,
Ichikawa D, Komatsu S, Konishi H, Okamoto K, Shoda K, Arita T, et
al: Blockade of potassium ion transports enhances
hypotonicity-induced cytocidal effects in gastric cancer.
Oncotarget. 8:101394–101405. 2017. View Article : Google Scholar
|
|
19
|
Kudou M, Shiozaki A, Yamazato Y,
Katsurahara K, Kosuga T, Shoda K, Arita T, Konishi H, Komatsu S,
Kubota T, et al: The expression and role of TRPV2 in esophageal
squamous cell carcinoma. Sci Rep. 9:160552019. View Article : Google Scholar
|
|
20
|
Shiozaki A, Kudou M, Ichikawa D, Fujiwara
H, Shimizu H, Ishimoto T, Arita T, Kosuga T, Konishi H, Komatsu S,
et al: Esophageal cancer stem cells are suppressed by tranilast, a
TRPV2 channel inhibitor. J Gastroenterol. 53:197–207. 2018.
View Article : Google Scholar
|
|
21
|
Darakhshan S and Pour AB: Tranilast: A
review of its therapeutic applications. Pharmacol Res. 91:15–28.
2015. View Article : Google Scholar
|
|
22
|
Almanaa TN, Geusz ME and Jamasbi RJ: A new
method for identifying stem-like cells in esophageal cancer cell
lines. J Cancer. 4:536–548. 2013. View Article : Google Scholar
|
|
23
|
Johnson S, Chen H and Lo PK: In vitro
tumorsphere formation assays. Bio Protoc. 3:e3252013. View Article : Google Scholar
|
|
24
|
Miyazaki H, Shiozaki A, Niisato N and
Marunaka Y: Physiological significance of hypotonicity-induced
regulatory volume decrease: Reduction in intracellular
Cl− concentration acting as an intracellular signaling.
Am J Physiol Renal Physiol. 292:F1411–F1417. 2007. View Article : Google Scholar
|
|
25
|
Fan P, Zhang Y, Liu L, Zhao Z, Yin Y, Xiao
X, Bauer N, Gladkich J, Mattern J, Gao C, et al: Continuous
exposure of pancreatic cancer cells to dietary bioactive agents
does not induce drug resistance unlike chemotherapy. Cell Death
Dis. 7:e22462016. View Article : Google Scholar
|
|
26
|
Rasheed ZA and Matsui W: Biological and
clinical relevance of stem cells in pancreatic adenocarcinoma. J
Gastroenterol Hepatol. 27(Suppl 2): S15–S18. 2012. View Article : Google Scholar
|
|
27
|
Matsuda Y, Kure S and Ishiwata T: Nestin
and other putative cancer stem cell markers in pancreatic cancer.
Med Mol Morphol. 45:59–65. 2012. View Article : Google Scholar
|
|
28
|
Miyazaki H, Shiozaki A, Niisato N, Ohsawa
R, Itoi H, Ueda Y, Otsuji E, Yamagishi H, Iwasaki Y, Nakano T, et
al: Chloride ions control the G1/S cell-cycle checkpoint by
regulating the expression of p21 through a p53-independent pathway
in human gastric cancer cells. Biochem Biophys Res Commun.
366:506–512. 2008. View Article : Google Scholar
|
|
29
|
Ohsawa R, Miyazaki H, Niisato N, Shiozaki
A, Iwasaki Y, Otsuji E and Marunaka Y: Intracellular chloride
regulates cell proliferation through the activation of
stress-activated protein kinases in MKN28 human gastric cancer
cells. J Cell Physiol. 223:764–770. 2010.
|
|
30
|
Shiozaki A, Otsuji E and Marunaka Y:
Intracellular chloride regulates the G(1)/S cell cycle progression
in gastric cancer cells. World J Gastrointest Oncol. 3:119–122.
2011. View Article : Google Scholar
|
|
31
|
Tanaka S, Miyazaki H, Shiozaki A, Ichikawa
D, Otsuji E and Marunaka Y: Cytosolic Cl− affects the
anticancer activity of paclitaxel in the gastric cancer cell line,
MKN28 cell. Cell Physiol Biochem. 42:68–80. 2017. View Article : Google Scholar
|
|
32
|
Rausch V, Liu L, Kallifatidis G, Baumann
B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zoller
M, et al: Synergistic activity of sorafenib and sulforaphane
abolishes pancreatic cancer stem cell characteristics. Cancer Res.
70:5004–5013. 2010. View Article : Google Scholar
|
|
33
|
Kim MP, Fleming JB, Wang H, Abbruzzese JL,
Choi W, Kopetz S, McConkey DJ, Evans DB and Gallick GE: ALDH
activity selectively defines an enhanced tumor-initiating cell
population relative to CD133 expression in human pancreatic
adenocarcinoma. PLoS One. 6:e206362011. View Article : Google Scholar
|
|
34
|
Rasheed ZA, Yang J, Wang Q, Kowalski J,
Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et
al: Prognostic significance of tumorigenic cells with mesenchymal
features in pancreatic adenocarcinoma. J Natl Cancer Ins.
102:340–351. 2010. View Article : Google Scholar
|
|
35
|
Wu HY, Yang MC, Ding LY, Chen CS and Chu
PC: p21-Activated kinase 3 promotes cancer stem cell phenotypes
through activating the Akt-GSK3ß-ß-catenin signaling pathway in
pancreatic cancer cells. Cancer Lett. 456:13–22. 2019. View Article : Google Scholar
|
|
36
|
Comes N, Serrano-Albarras A, Capera J,
Serrano-Novillo C, Condom E, Ramon Y, Cajal S, Ferreres JC and
Felipe A: Involvement of potassium channels in the progression of
cancer to a more malignant phenotype. Biochim Biophys Acta.
1848:2477–2492. 2015. View Article : Google Scholar
|
|
37
|
Rao VR, Perez-Neut M, Kaja S and Gentile
S: Voltage-gated ion channels in cancer cell proliferation. Cancers
(Basel). 7:849–875. 2015. View Article : Google Scholar
|
|
38
|
Serrano-Novillo C, Capera J,
Colomer-Molera M, Condom E, Ferreres JC and Felipe A: Implication
of voltage-gated potassium channels in neoplastic cell
proliferation. Cancers (Basel). 11:2872019. View Article : Google Scholar
|
|
39
|
Brevet M, Fucks D, Chatelain D, Regimbeau
JM, Delcenserie R, Sevestre H and Ouadid-Ahidouch H: Deregulation
of 2 potassium channels in pancreas adenocarcinomas: Implication of
KV1.3 gene promoter methylation. Pancreas. 38:649–654. 2009.
View Article : Google Scholar
|
|
40
|
Leanza L, Zoratti M, Gulbins E and Szabo
I: Mitochondrial ion channels as oncological targets. Oncogene.
33:5569–5581. 2014. View Article : Google Scholar
|
|
41
|
Zaccagnino A, Manago A, Leanza L,
Gontarewitz A, Linder B, Azzolini M, Biasutto L, Zoratti M, Peruzzo
R, Legler K, et al: Tumor-reducing effect of the clinically used
drug clofazimine in a SCID mouse model of pancreatic ductal
adenocarcinoma. Oncotarget. 8:38276–38293. 2017. View Article : Google Scholar
|
|
42
|
Leanza L, Romio M, Becker KA, Azzolini M,
Trentin L, Manago A, Venturini E, Zaccagnino A, Mattarei A,
Carraretto L, et al: Direct pharmacological targeting of a
mitochondrial ion channel selectively kills tumor cells in vivo.
Cancer Cell. 31:516–531.e10. 2017. View Article : Google Scholar
|
|
43
|
Szabo I, Trentin L, Trimarco V, Semenzato
G and Leanza L: Biophysical characterization and expression
analysis of Kv1.3 potassium channel in primary human leukemic B
cells. Cell Physiol Biochem. 37:965–978. 2015. View Article : Google Scholar
|
|
44
|
Aissaoui D, Mlayah-Bellalouna S, Jebali J,
Abdelkafi-Koubaa Z, Souid S, Moslah W, Othman H, Luis J, ElAyeb M,
Marrakchi N, et al: Functional role of Kv1.1 and Kv1.3 channels in
the neoplastic progression steps of three cancer cell lines,
elucidated by scorpion peptides. Int J Biol Macromol.
111:1146–1155. 2018. View Article : Google Scholar
|
|
45
|
Martinez R, Stühmer W, Martin S, Schell J,
Reichmann A, Rohde V and Pardo L: Analysis of the expression of
Kv10.1 potassium channel in patients with brain metastases and
glioblastoma multiforme: Impact on survival. BMC Cancer.
15:8392015. View Article : Google Scholar
|
|
46
|
Sette A, Spadavecchia J, Landoulsi J,
Casale S, Haye B, Crociani O and Arcangeli A: Development of novel
anti-Kv 11.1 antibody-conjugated PEG-TiO2 nanoparticles
for targeting pancreatic ductal adenocarcinoma cells. J Nanopart
Res. 15:21112013. View Article : Google Scholar
|
|
47
|
Crociani O, Lastraioli E, Boni L, Pillozzi
S, Romoli MR, D'Amico M, Stefanini M, Crescioli S, Masi A, Taddei
A, et al: hERG1 channels regulate VEGF-A secretion in human gastric
cancer: Clinicopathological correlations and therapeutical
implications. Clin Cancer Res. 20:1502–1512. 2014. View Article : Google Scholar
|
|
48
|
Wang SP, Wang JA, Luo RH, Cui WY and Wang
H: Potassium channel currents in rat mesenchymal stem cells and
their possible roles in cell proliferation. Clin Exp Pharmacol
Physiol. 35:1077–1084. 2008. View Article : Google Scholar
|
|
49
|
Zhang YY, Yue J, Che H, Sun HY, Tse HF and
Li GR: BKCa and hEag1 channels regulate cell proliferation and
differentiation in human bone marrow-derived mesenchymal stem
cells. J Cell Physiol. 229:202–212. 2014. View Article : Google Scholar
|
|
50
|
Morokuma J, Blackiston D, Adams DS,
Seebohm G, Trimmer B and Levin M: Modulation of potassium channel
function confers a hyperproliferative invasive phenotype on
embryonic stem cells. Proc Natl Acad Sci USA. 105:16608–16613.
2008. View Article : Google Scholar
|
|
51
|
Bai X, Ma J, Pan Z, Song YH, Freyberg S,
Yan Y, Vykoukal D and Alt E: Electrophysiological properties of
human adipose tissue-derived stem cells. Am J Physiol Cell Physiol.
293:C1539–C1550. 2007. View Article : Google Scholar
|
|
52
|
Jensen HB, Ravnborg M, Dalgas U and
Stenager E: 4-Aminopyridine for symptomatic treatment of multiple
sclerosis: A systematic review. Ther Adv Neurol Disord. 7:97–113.
2014. View Article : Google Scholar
|
|
53
|
Solari A, Uitdehaag B, Giuliani G, Pucci E
and Taus C: Aminopyridines for symptomatic treatment in multiple
sclerosis. Cochrane Database Syst Rev. 2002:CD0013302002.
|
|
54
|
Goodman AD, Brown TR, Edwards KR, Krupp
LB, Schapiro RT, Cohen R, Marinucci LN and Blight AR; MSF204
Investigators: A phase 3 trial of extended release oral
dalfampridine in multiple sclerosis. Ann Neurol. 68:494–502. 2010.
View Article : Google Scholar
|
|
55
|
Goodman AD, Brown TR, Krupp LB, Schapiro
RT, Schwid SR, Cohen R, Marinucci LN and Blight AR; Fampridine
MS-F203 Investigators: Sustained-release oral fampridine in
multiple sclerosis: A randomised, double-blind, controlled trial.
Lancet. 373:732–738. 2009. View Article : Google Scholar
|
|
56
|
Rouzaire-Dubois B, Gerard V and Dubois JM:
Involvement of K+ channels in the quercetin-induced inhibition of
neuroblastoma cell growth. Pflugers Arch. 423:202–205. 1993.
View Article : Google Scholar
|
|
57
|
Fieber LA, Gonzâlez DM, Wallace MR and
Muir D: Delayed rectifier K currents in NF1 Schwann cells.
Pharmacological block inhibits proliferation. Neurobiol Dis.
13:136–146. 2003. View Article : Google Scholar
|
|
58
|
Nilius B and Wohlrab W: Potassium Channels
and Regulation of proliferation of human melanoma cells. J Physiol.
445:537–548. 1992. View Article : Google Scholar
|
|
59
|
Rybalchenko V, Prevarskaya N, Van
Coppenolle F, Legrand G, Lemonnier L, Le Bourhis X and Skryma R:
Verapamil inhibits proliferation of LNCaP human prostate cancer
cells influencing K+ channel gating. Mol Pharmacol. 59:1376–1387.
2001. View Article : Google Scholar
|
|
60
|
Chin LS, Park CC, Zitnay KM, Sinha M,
DiPatri AJ Jr, Perillan P and Simard JM: 4-Aminopyridine causes
apoptosis and blocks an outward rectifier K+ channel in malignant
astrocytoma cell lines. J Neurosci Res. 48:122–127. 1997.
View Article : Google Scholar
|
|
61
|
Kim JA, Kang YS, Jung MW, Kang GH, Lee SH
and Lee YS: Ca2+ influx mediates apoptosis induced by
4-aminopyridine, a K+ channel blocker, in HepG2 human
hepatoblastoma cells. Pharmacology. 60:74–81. 2000. View Article : Google Scholar
|
|
62
|
Wang W, Xiao J, Adachi M, Liu Z and Zhou
J: 4-aminopyridine induces apoptosis of human acute myeloid
leukemia cells via increasing [Ca2+]i through P2X7
receptor pathway. Cell Physiol Biochem. 28:199–208. 2011.
View Article : Google Scholar
|
|
63
|
Huang L, Li B, Li W, Guo H and Zou F:
ATP-sensitive potassium channels control glioma cells proliferation
by regulating ERK activity. Carcinogenesis. 30:737–744. 2009.
View Article : Google Scholar
|
|
64
|
Ru Q, Li WL, Xiong Q, Chen L, Tian X and
Li CY: Voltage-gated potassium channel blocker 4-aminopyridine
induces glioma cell apoptosis by reducing expression of
microRNA-10b-5p. Mol Biol Cell. 29:1125–1136. 2018. View Article : Google Scholar
|
|
65
|
Luo Z, Wang J, Li C, Qiu Y, Huang J, Huang
Y, Gu H, Wu B, Hu Z and Zhen Y: Upregulation of phosphatase and
tensin homolog is essential for the effect of 4-aminopyridine on
A549/CDDP cells. Mol Med Rep. 17:5996–6001. 2018.
|



























