Introduction
Ovarian cancer (OC) is a deadly malignancy of the
female reproductive system responsible for a high number of deaths
each year. It is estimated that by the end of 2021 approximately
21,410 will be diagnosed with ovarian cancer, while 13,770 women
will die from this disease in the US alone (1). Epithelial ovarian cancers (EOCs) are
the most common subtype of ovarian tumor and may arise from
epithelial cells lining the ovaries, peritoneum and fallopian tubes
(2). Frontline treatment for EOCs
involve tumor debulking surgery followed by platinum-based
chemotherapy (3). Patients with a
large tumor burden may also undergo neoadjuvant chemotherapy
treatment in an effort to reduce the tumor volume prior to surgery
(3). After frontline treatment,
patients with advanced OC often receive maintenance therapy to
prolong their progression-free survival (PFS) (4). The National Comprehensive Cancer
Network (NCCN) guidelines recommend several maintenance therapies,
including anti-angiogenic bevacizumab and poly(ADP-ribose)
polymerase (PARP) inhibitors for patients with advanced disease
(4).
Despite these therapeutic advancements, most
patients diagnosed with metastatic OC experience disease relapse.
Repeated tumor recurrences result in the emergence of chemotherapy
resistance, particularly to platinum-based drugs, and patients
eventually succumb to platinum-resistant disease (5). Effective treatment of patients with
chemo-resistant disease remains a major clinical challenge and is
the focus of the present study.
Response to platinum-based chemotherapy is dependent
on multiple parameters including tumor biology and histology,
molecular alterations, and tumor microenvironment (6,7).
Patient sensitivity to platinum drugs is determined based on the
progression-free interval after completing multiple cycles of
chemotherapy infusion. Patients are deemed platinum-sensitive if
disease relapse occurs at more than 6 months after administration
of chemotherapy while those with tumor recurrence less than 6
months after chemotherapy are classified as platinum-resistant
(6). For treatment of
platinum-resistant patients, NCCN guidelines recommend use of
single nonplatinum-based chemotherapy drugs such as docetaxel,
etoposide, gemcitabine, liposomal doxorubicin, and topotecan
(4). These guidelines also
recommend enrollment of patients with platinum-resistant OC in
clinical trials testing new therapies. Overall, treatment of
chemoresistant disease is focused on relieving patients of
disease-related symptoms, controlling adverse reactions to drugs,
and maintaining quality of life. Hence, there is a critical need
for novel therapeutic interventions that can effectively reduce the
mortality rate and prolong the overall survival of patients with
platinum-resistant OC.
Several molecular factors may contribute to the
development of therapy resistance in OC such as reduced cellular
accumulation of platinum due to altered expression of membrane
transporter proteins, increased drug efflux, upregulated DNA
repair, reduced apoptosis, and increased autophagy (8). In the present study, we exploit
defects in apoptotic processes as a possible therapeutic target for
treating platinum-resistant OC. Cancer cells are known to evade
apoptosis by overexpression of a highly conserved class of
anti-apoptotic proteins called inhibitor of apoptosis (IAP)
proteins (9). IAP proteins exert
their anti-apoptotic activity either through direct inhibition of
caspases or through indirect ubiquitination of caspase proteins
(9). Eight IAP proteins have been
identified in humans [reviewed in Finlay et al (10)]. Out of these IAP proteins, some of
the most extensively studied proteins include cellular IAP proteins
(cIAP1 and cIAP2) and XIAP (9).
Deregulated cIAP and XIAP levels have been correlated with response
to therapy or disease progression in different types of cancer
including ovarian cancer (11-14). Survivin, another important member
of the IAP family has also been investigated in different cancer
types and has been linked to tumor progression and metastasis
(15,16), therapy-resistance (17,18) and poor prognosis (19,20).
Second mitochondrial activator of caspase (SMAC)
mimetics are small molecules designed to mimic endogenous SMAC
proteins released by mitochondria (21). SMAC mimetic molecules predispose
cells to apoptosis by inhibiting IAP proteins (21). Multiple clinical trials are
exploring the potential of such IAP inhibitors administered as a
single agent and in combination with chemotherapeutics for
treatment of various types of cancer [reviewed in Morrish et
al (21)]. We hypothesize
that combining SMAC mimetics with carboplatin, the standard
chemotherapy drug used in the treatment of OC, may enhance
carboplatin-induced cell death. To test this hypothesis, we have
utilized birinapant, a highly potent SMAC mimetic known to target
cIAP1/2 and XIAP in combination with carboplatin to target
platinum-resistant ovarian cancer cells. Birinapant is a promising
IAP protein antagonist that has demonstrated antitumor activity in
preclinical models of head and neck squamous cell carcinoma (HNSCC)
(22), and other solid tumors
including triple-negative breast cancer (23,24), non-small cell lung cancer
(25), and ovarian cancer
(26). The outcome of a clinical
trial testing birinapant as a monotherapy in advanced ovarian,
fallopian tube, and peritoneal cancers (NCT01681368) has
demonstrated its tolerability in a dose-dependent manner but was
not found efficacious as a single agent (27).
In the present study, the efficacy of birinapant and
carboplatin combination therapy was evaluated using in vitro
and in vivo models of platinum-resistant EOCs. A 3D organoid
bioassay was utilized to test the in vitro therapeutic
efficacy of co-therapy across a panel of ovarian cancer cell lines
and platinum-resistant primary patient tumor samples. The results
demonstrated that birinapant with carboplatin was effective in
targeting a subset of ovarian cancer cell lines and
platinum-resistant patient ovarian tumors.
Materials and methods
Cell lines and primary patient tumor
samples
Ovarian cancer cell lines including SKOV3, OVCAR3,
CaOV3, Kuramochi and OAW28 were purchased from the American Type
Culture Collection (ATCC). OVCAR4 and OVCAR8 cell lines were
obtained from the National Cancer Institute Division of Cancer
Treatment and Diagnosis (NCI/DCTD) repository through Material
Transfer Agreement (MTA). Cell lines were grown in recommended
media (RPMI supplemented with 10% FBS or DMEM supplemented with 10%
FBS) in a 5% CO2 humidified incubator at 37°C and used
within 10 passages. All cell lines used in this study were
frequently authenticated by STR analysis. Primary patient tumor
samples were obtained from patients after providing informed
consent through protocols approved by the UCLA Office of the Human
Research Protection Program (IRB# 10-000727). Patients ages ranged
from 43 to 75 years. Tumor samples were mechanically and
enzymatically dissociated with 1 mg/ml collagenase and dispase
solution (Gibco; Thermo Fisher Scientific, Inc.) and cryopreserved
in buffer (90% FBS, 10% DMSO) for experimental use.
In vitro 3D organoid bioassay
As previously described (28,29), in this bioassay, 5,000 cells/well
were suspended in Matrigel matrix (Corning Inc.) and MammoCult
growth medium (STEMCELL Technologies) and plated around the rim of
the wells of a 96-well plate. Organoids were allowed to grow for
1-2 days followed by drug treatment in a dose-dependent manner for
3 consecutive days. After drug treatment, cell viability was
determined using an ATP luminescence assay (CellTiter-Glo 3D
viability Assay kit; Promega Corp.). For testing platinum
sensitivity, each cell line was treated with carboplatin at
increasing concentrations of 0, 10, 25, 50, 100, 150, 200 and 250
µM. For co-therapy testing, cells were treated with a range
of doses of carboplatin (0-50 µM) and birinapant (0-50
nM).
As a measure for quality control, cryopreserved
primary tumor samples with cell viability less than 60% after
thawing were excluded from the study. Only those primary samples
that formed visible organoids in the 3D organoid bioassay were
included in this study, confirmed by examination under a
microscope. Assay results were rejected if the luminescence value
in vehicle-treated wells were found to be similar to the blank
control [Matrigel and growth media]. Staurosporine-treated cells
were used as a positive control for cell death in each
experiment.
Drug synergy analysis
Synergy between birinapant and carboplatin was
quantified using the Loewe additivity index model available in a
web-based package SynergyFinder 2.0 (30). Synergy scores were calculated
across all tested drug concentrations and combinations and
visualized as a two-dimensional interaction surface over the dose
matrix (Loewe synergy plots). Based on the Loewe additivity model,
a summary synergy score was generated. Drug interactions were
classified as likely synergistic (score >10), antagonistic
(score <-10), or additive (score -10 to +10). Synergy scores
shown in this study were obtained from the SynergyFinder 2.0 tool
as of 10/04/2021.
TCGA data analysis
Information regarding the genetic alterations in
cIAP1, cIAP2 and XIAP genes were obtained from
the Cancer Genome Atlas (TCGA) database through cBioPortal
(http://www.cbioportal.org). Data shown
in this study were extracted from PanCancer datasets as of
10/04/2021. We focused on TCGA data for 8 cancer types known to be
treated with platinum-based chemotherapy (31-34).
Western blot analysis
For assessing the endogenous levels of IAP proteins
in 7 EOC cell lines, growing cells were harvested, lysed using RIPA
lysis buffer supplemented with protease inhibitor cocktail (Thermo
Fisher Scientific, Inc.). Cell lysates were centrifuged at 12,000 ×
g for 10 min at 4°C. The supernatant was collected, and protein
concentration was measured using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts (40 µg) of protein
were loaded in each lane and resolved on NuPAGE 4-12% Bis-Tris gels
(Thermo Fisher Scientific, Inc.). Resolved proteins were
transferred to nitrocellulose membranes (Millipore), probed with
appropriate antibodies and detected by chemiluminescent reagent
(Millipore, WBKLS0500). Protein bands were visualized using a
Bio-Rad ChemiDoc Imaging system (Bio-Rad Laboratories).
For measuring the IAP protein levels in SKOV3 and
OVCAR8 cells after co-therapy treatment, both cell lines were
treated in 2D cell culture with carboplatin alone, birinapant
alone, or the combination of the two drugs at a concentration
corresponding to the 48 h half maximal inhibitory concentration
(IC50) of each drug. After 24 h of drug treatment, cells
were lysed and processed the same way as mentioned above. For
measuring IAP protein levels in PDX subcuticular tumors, an
additional step of sonication (Thermo Fisher Scientific sonicator)
was performed after tumors were lysed in RIPA lysis buffer.
Antibodies
Antibodies used in this study included: cIAP1 [Cell
Signaling Technology, Inc. (CST), cat. no. 7065], cIAP2 (CST, cat.
no. 3130), XIAP (CST, cat. no. 14334), GAPDH (CST, cat. no. 5174),
Ki67 (Agilent Technologies, cat. no. M724001-2), and Pax8
(Proteintech, cat. no. 10336-1-AP). For western blot experiments,
cIAP1, cIAP2, XIAP and GAPDH antibodies were used at a dilution of
1:1,000. For IHC experiments Ki67 and Pax8 antibodies were used at
a dilution of 1:100 and 1:500 respectively.
Apoptosis analysis by flow cytometry
Apoptosis was detected by Annexin V and PI staining
using BD Pharmingen Apoptosis Detection Kit (cat. no. 556547).
Cells (0.5 million/well) were seeded in 6-well plates. Cells were
treated with various concentration of carboplatin, birinapant, or
the co-therapy for 72 h. Cells were then harvested and stained with
Annexin V/PI following the manufacturer's protocol. Cell death was
analyzed using a BD FACS ARIA flow cytometer and FlowJo software
(version 10.8.0; BD Biosciences). Cell death was represented as the
percentage of Annexin V+ cell population after drug
treatment. For each cell line, three independent experiments were
performed.
Tumor necrosis factor (TNF)α ELISA
Cells were seeded at a density of 0.5 million
cells/well in a 6-well plate and allowed to grow until
approximately 80% confluency was reached. Cells were then washed
with 1X PBS, incubated with fresh media, and treated with
carboplatin, birinapant, or co-therapy at a concentration
corresponding to the 48 h IC50 of each drug. After 72 h
of drug treatment, 100 µl of culture supernatant was
collected from each treatment group for TNFα measurement by ELISA
kit (Thermo Fisher Scientific, Inc., cat. no. BMS223HS).
Recombinant TNFα protein was used as a positive control in these
experiments. Two independent experiments were performed with
duplicate wells for each condition.
Neutralization of TNFα molecules
Cells were seeded at a density of 5,000 cells/well
in 96-well plate. Cells were then pre-treated with 10 µg/ml
of anti-TNFα antibody (R&D Systems, cat. no. MAB610-100) for 2
h followed by addition of carboplatin, birinapant, or combination
of two drugs at the 48 h IC50 of each drug. After 72 h
of drug treatment, cell viability was measured using an ATP
luminescence assay (CellTiter-Glo 3D viability Assay kit, Promega
Corp.). IgG antibody was used as a non-specific antibody control.
Two independent experiments were performed with triplicate wells
for each condition in each cell line tested.
Establishment of a platinum-resistant
patient-derived xenograft model
PDX models were established from a primary pleural
effusion sample, EOC2, clinically classified as recurrent,
platinum-resistant high-grade serous ovarian cancer. For PDX
generation, 1 million cryopreserved cells were injected as a cell
suspension in the intraperitoneal space of a female NSG mouse. The
cells were re-passaged in mice twice. At the end of the second
passage, PDX cells were harvested and evaluated by histological and
genomic analyses. Short tandem repeat (STR) analyses performed
using extracted genomic DNA from PDX cells demonstrated that this
PDX retained greater than 90% identity to its parental tumor.
Single nucleotide polymorphism analysis was also performed,
confirming relatedness of PDX cells and parental tumor cells.
Immunohistochemistry (IHC) staining
Histologic slides containing PDX subcuticular tumor
fragments from each cohort after drug treatment were reviewed by a
gynecology pathologist NAM. PAX8 immunostaining was evaluated for
nuclear expression to confirm Mullerian origin of the tumors. Ki67
was also evaluated for nuclear staining for proliferation index.
Scoring was based on the estimation of the percentage of tumor
cells stained by the antibody visualized by light microscopy. The
mitotic figures were counted on hematoxylin and eosin stained
slides on 10 high power fields (40× objective). Mean number of
mitosis between the treatment groups was compared by ANOVA with
non-constant variance allowed.
Animals
All animal experiments were approved by the UCLA
Animal Research Committee (protocol 2008-153) and conducted under
the supervision of the UCLA Division of Laboratory Animal Medicine.
Six- to eight-week-old female NSG mice
(NOD.Cg-Prkdscid//2rgtm1Wjl/SzJ) were
purchased from Jackson Laboratories for all in vivo
experiments. All mice were housed in specific pathogen-free (SPF)
facilities. They were kept in autoclaved cages with sterile bedding
and food. Mice were anesthetized by isoflurane gas in oxygen.
Following the Institutional Animal Care and Use Committee (IACUC)
guidelines, 3-5% isoflurane was used for initial induction and 1-3%
was used for maintenance. For euthanization, mice were exposed to
gradually increasing concentrations of CO2 at a flow
rate ranging between 30-70% chamber volume/min as per the American
Veterinary Medical Association (AVMA) guidelines. Cervical
dislocation was used as a confirmatory method of euthanasia after
CO2 exposure. At the time of euthanization, tumor volume
of each mouse was <1,000 mm3 consistent with (IACUC)
guidelines.
Statistical methods
Results are reported as means ± standard error. For
normally distributed data, the P-values for comparing means were
calculated using one-way analysis of variance (ANOVA), two-way
ANOVA or two way (mixed) repeated measure ANOVA (tumor mean
comparison) when animals were measured repeatedly over time. When
data were not normal as indicated by the Shapiro-Wilks test,
P-values were computed with non-parametric Kruskal-Wallis test.
Calculations were performed using R version 4.0.5 (R Foundation for
Statistical Computing, Vienna, Austria).
Tumor volumes were calculated using the modified
ellipsoid formula: 1/2 × length × (width)2. The baseline
volume on day 0 (start of drug treatment) was subtracted from all
subsequent tumor volumes of the same animal since day 0 baseline
was not the same for all animals. After this standardization, the
relation between tumor volume and time (day) was approximately
linear in each animal. Thus, the growth rate in mm3 per
day for each animal was computed via linear regression on each
animal and the mean rates were compared among the four groups using
a one-way ANOVA where the variances were allowed to be
heterogeneous.
Results
Utilization of a 3D in vitro bioassay for
testing the platinum sensitivity of human ovarian cancer cell
lines
One of the challenges in the treatment of OC is the
absence of biomarkers that could prospectively predict patients'
response to standard platinum-based chemotherapy. OC tumors are
characterized as platinum-sensitive or platinum-resistant only
after chemotherapy drugs are administered. Hence, in vitro
drug response assays that are designed to test and predict the
sensitivity of patient tumor cells to existing chemotherapies may
provide a useful tool for treatment of OC patients. Although these
assays hold great promise in the field of precision medicine, they
have not yet proven efficacious for clinical application and
therefore, no such assay has been FDA approved for clinical use at
this time (35). In the present
study, a 3D organoid-based drug testing platform (28) was utilized to assess the response
of OC cell lines to carboplatin. Platinum sensitivity of the OC
cell lines measured in this bioassay were correlated with reported
response to platinum drugs for each cell line (36,37).
In this bioassay (28,29), OC cells were grown as 3D organoids
embedded within an extracellular matrix hydrogel called Matrigel
(Fig. 1A). On the first day of
this bioassay, a mixture of cells and Matrigel were plated around
the rims of wells in a 96-well plate and overlaid with growth
media. Cells were allowed to generate organoids for 24 h. Growing
organoids were treated with increasing concentrations of
carboplatin (0-250 µM) for three consecutive days, with
daily drug replenishment. After drug treatment, the viability of
the cells was measured using an ATP-based luminescence assay
(CellTiter Glo, Promega Corp.). Sensitivity to carboplatin was
determined using an IC50 value, defined as the drug
concentration causing 50% inhibition. IC50 values for
each cell line were obtained from carboplatin dose response curves
generated using the ATP-based viability assay.
The viability plots for each cell line demonstrated
a dose-dependent decrease in cell viability. Differential
sensitivity to carboplatin was noted in all cell lines tested
(Fig. 1B). Based on the
IC50 of carboplatin, OVCAR3 cells were found to be
carboplatin sensitive (IC50 <40 µM), Kuramochi
and OVCAR8 cells were highly carboplatin resistant (IC50
>85 µM). The remaining cell lines exhibited intermediate
carboplatin resistance (Fig. 1C).
Platinum sensitivity measured with this bioassay correlated with
platinum sensitivity of each cell line reported by others (36,37).
This 3D organoid bioassay was capable of classifying
cell lines as carboplatin-sensitive vs. carboplatin-resistant.
Since organoids are known to better recapitulate in vivo
tumor architecture (38), this
bioassay may provide a tool for testing single drug or combination
therapies in ovarian cancer. Therefore, next this bioassay was
utilized to test the efficacy of a combination therapy in targeting
platinum resistant ovarian cancer cells.
A subset of human platinum-resistant
ovarian cancer cell lines was targeted with a combination of
carboplatin and birinapant in vitro
Cancer cells are known to overexpress highly
conserved anti-apoptotic proteins called inhibitor of apoptosis
(IAP) proteins (9). These
proteins are predominantly known for inhibiting apoptotic cell
death via regulation of caspases and are reported to be involved in
tumor cell survival, chemo-resistance, disease progression, and
poor prognosis [reviewed in LaCasse et al (39)]. Data from The Cancer Genome Atlas
(TCGA) database also demonstrated that gene amplification is a
common alteration in key IAPs, namely cIAP1, cIAP2, and XIAP in
ovarian cancer and other cancers treated with platinum-based
chemotherapy drugs (31-34) (Fig.
S1A). Taken together, this evidence provides a rationale to
activate apoptosis in platinum-resistant ovarian cancer cells by
targeting IAP proteins. We combined birinapant, a small molecule
that mimics natural IAP protein antagonist SMAC, with carboplatin
to target platinum-resistant OC cell lines in vitro. Due to
its pro-apoptotic activity and tolerability, birinapant and several
other SMAC mimetic molecules have been evaluated in early clinical
trials as a monotherapy or in combination with other
chemotherapeutic drugs to target different types of cancers
[reviewed in Morrish et al (21)]. The combination of SMAC mimetics,
including birinapant and carboplatin, has also been previously
explored and demonstrated efficacy in targeting OC cell lines using
preclinical models (26,40). In this study, we expanded on these
previous investigations by testing the in vitro efficacy of
birinapant and carboplatin combination across a panel of 7 EOC cell
lines using the 3D organoid bioassay. This 3D organoid bioassay
offers several advantages. First, as a preclinical model for drug
testing, it retains complex tumor architecture similar to in
vivo tumors (28). Second, it
is compatible with automation and high throughput drug screening
allowing testing of combination therapies in a scalable and
reproducible way (28).
Therapeutic target proteins of birinapant (cIAP1/2
and XIAP) were evaluated in these 7 cell lines using western blot
analysis (Fig. S1B). For
co-therapy testing, cell line-derived organoids were treated with
increasing concentrations of carboplatin (0-50 µm) and
birinapant (0-50 nM) in the 3D-organoid bioassay (Fig. 2A). After 3 days of drug treatment,
cell viability was measured using an ATP-based assay (Fig. S1C). Drug synergy was scored using
the Loewe additivity model available in the online package
SynergyFinder 2.0 (30).
Four out of the 7 cell lines (OVCAR8, SKOV3, Kuramochi, and OAW28)
demonstrated a positive synergy score that indicates increased cell
death upon co-treatment with birinapant and carboplatin compared to
the sum of the single agents (Fig. 2B
and C). The combined effects were found to be likely additive
in the OVCAR4 cell line while the effects of these two drugs were
found to be likely antagonistic in OVCAR3 and CaOV3 cell lines
(Fig. 2B and C). Overall, these
in vitro results demonstrated that addition of birinapant to
carboplatin treatment enhanced cell death in a subset of OC cell
lines.
Combination treatment induces apoptosis
in platinum-resistant ovarian cancer cell lines
Both birinapant and carboplatin are known to induce
cell death. Birinapant-mediated cell death occurs by inhibition of
IAP proteins (21) while
carboplatin forms DNA lesions that block DNA synthesis and results
in cell death (41). Therefore,
the combined effect of carboplatin and birinapant on cellular
apoptosis was investigated by flow cytometry. We selected a highly
platinum-resistant cell line, OVCAR8, and a moderately platinum
resistant cell line, SKOV3, as these two cell lines demonstrated
Loewe synergy scores greater than 10 when treated with carboplatin
and birinapant combination in vitro (Fig. 2C).
Quantification of drug-induced cell death was
facilitated by Annexin V/PI staining using flow cytometry. Both
SKOV3 and OVCAR8 cells were treated with carboplatin, birinapant,
or the combination of both drugs in 2D cell culture at
concentrations corresponding to IC50 values of each
drug. After 72 h of drug treatment, cells were harvested, stained
with Annexin V/PI and analyzed by flow cytometry (Fig. 3A). The percentage of dead cells
(Annexin V+) was significantly increased when either
cell line was treated with the co-therapy (CP+BP) compared to
single agents (CP or BP) (Fig. 3B and
C). Degradation of cIAP1 was detected in both cell lines by
birinapant alone, or in combination with carboplatin (Fig. S2). However, birinapant had no
effect on XIAP expression levels in these cell lines.
 | Figure 3Birinapant and carboplatin
combination induces apoptosis in SKOV3 and OVCAR8 cell lines. (A)
Experimental schema for measuring drug-induced apoptosis in OVCAR8
and SKOV3 cells using flow cytometry. Cells were treated with
carboplatin (CP), birinapant (BP), or co-therapy (CP+BP) for 72 h,
stained with FITC-Annexin V and PI, then analyzed by flow
cytometry. (B and C) Representative FACS plots showing early
apoptotic cells (Q3), late apoptotic cells (Q2), in SKOV3 and
OVCAR8 cells (left). Quantification of cell death (right) is
measured as % Annexin V+ cell population (Q3+Q2) (mean ±
SEM, n=3, one way-ANOVA, *P=0.0248,
**P=0.0004, ***P=0.0001 and
****P<0.0001). (D) Workflow for measurement of
secreted TNFα using ELISA. SKOV3 and OVCAR8 cells were treated with
vehicle (veh), carboplatin (CP), birinapant (BP), or the
combination (CP+BP) for 72 h (left). Quantification of TNFα protein
concentration in pg/ml for each cell line (right) is shown (mean ±
SEM, n=2, one-way ANOVA, **P=0.0032,
****P<0.0001). (E) Workflow to measure cell death
after neutralization with the anti-TNFα antibody. OVCAR8 and SKOV3
cells were pre-treated with anti-TNFα antibody for 2 h followed by
drug treatment for 72 h (left). Cell viability as measured using
the ATP-based luminescence assay is shown (right, mean ± SEM, n=2,
two-way ANOVA, **P=0.0024, ****P<0.0001).
For all experiments, SKOV3 and OVCAR8 cells were treated with drugs
at a concentration corresponding to half maximal inhibitory
concentration (IC50) values [For SKOV3, (CP)=40
µm, (BP)=30 nM; for OVCAR8, (CP)=100 µM, (BP)=100
nM]. |
Several studies have reported that SMAC mimetics
including birinapant stimulate cells to release cytokine TNFα which
binds to membrane-bound TNF receptors and triggers an
apoptosis-signaling pathway (42,43). The role of TNFα in co-therapy
targeted SKOV3 and OVCAR8 cells was examined by measuring the
release of TNFα molecules using ELISA. In this experiment, both
cell lines were treated with carboplatin (CP), birinapant (BP), or
the combination of the two agents (CP+BP) in 2D cell culture at
concentrations corresponding to the IC50 of each drug
(Fig. 3D). After 72 h of drug
treatment, 100 µl of culture supernatant was collected from
each treatment group for TNFα measurement by ELISA. Results
demonstrated that co-therapy treated OVCAR8 and SKOV3 cells
secreted significantly more TNFα compared to carboplatin alone
(Fig. 3D, right panel). This
suggests that TNFα signaling may also contribute to overall drug
synergy observed in these cell lines. To further confirm the role
of TNFα in mediating pro-apoptotic signaling, anti-TNFα
neutralizing antibody was used to block the interaction between
TNFα and its membrane bound TNF receptor. In this experiment, both
cell lines were pre-treated with anti-TNFα antibody for 2 h
followed by addition of carboplatin (CP), birinapant (BP), or the
combination of the two agents (CP+BP) for 72 h (Fig. 3E). After drug treatment, cell
viability was measured using an ATP-based luminescence assay.
Neutralization of TNFα molecules with anti-TNFα antibody was found
to partially reverse co-therapy-induced cytotoxicity. An increase
in cell viability was observed in SKOV3 and OVCAR8 cells treated
with the co-therapy following pre-treatment with the anti-TNFα
antibody (Fig. 3E, right
panel).
Overall, the findings demonstrated that apoptosis
was induced in the co-therapy-treated platinum-resistant OVCAR8 and
SKOV3 cells. It also suggests that mechanisms of drug synergy may
be TNFα-dependent in these cell lines.
Birinapant in combination with
carboplatin demonstrates efficacy in a subset of primary human
ovarian cancers
Molecular alterations that drive OC progression and
chemotherapy resistance are highly variable between individual
patients. The heterogenous nature of OC tumors poses a challenge in
selecting the most effective treatment for individual patients
based on their unique tumor traits. This challenge has steered the
field of cancer therapeutics towards precision medicine approaches
whereby the selection of therapy is tailored to each individual
patient's tumor. Exploring the potential of this approach, we
utilized the 3D organoid bioassay as a precision medicine tool to
test the therapeutic efficacy of carboplatin and birinapant
combination in a panel of platinum-resistant primary OC tumor
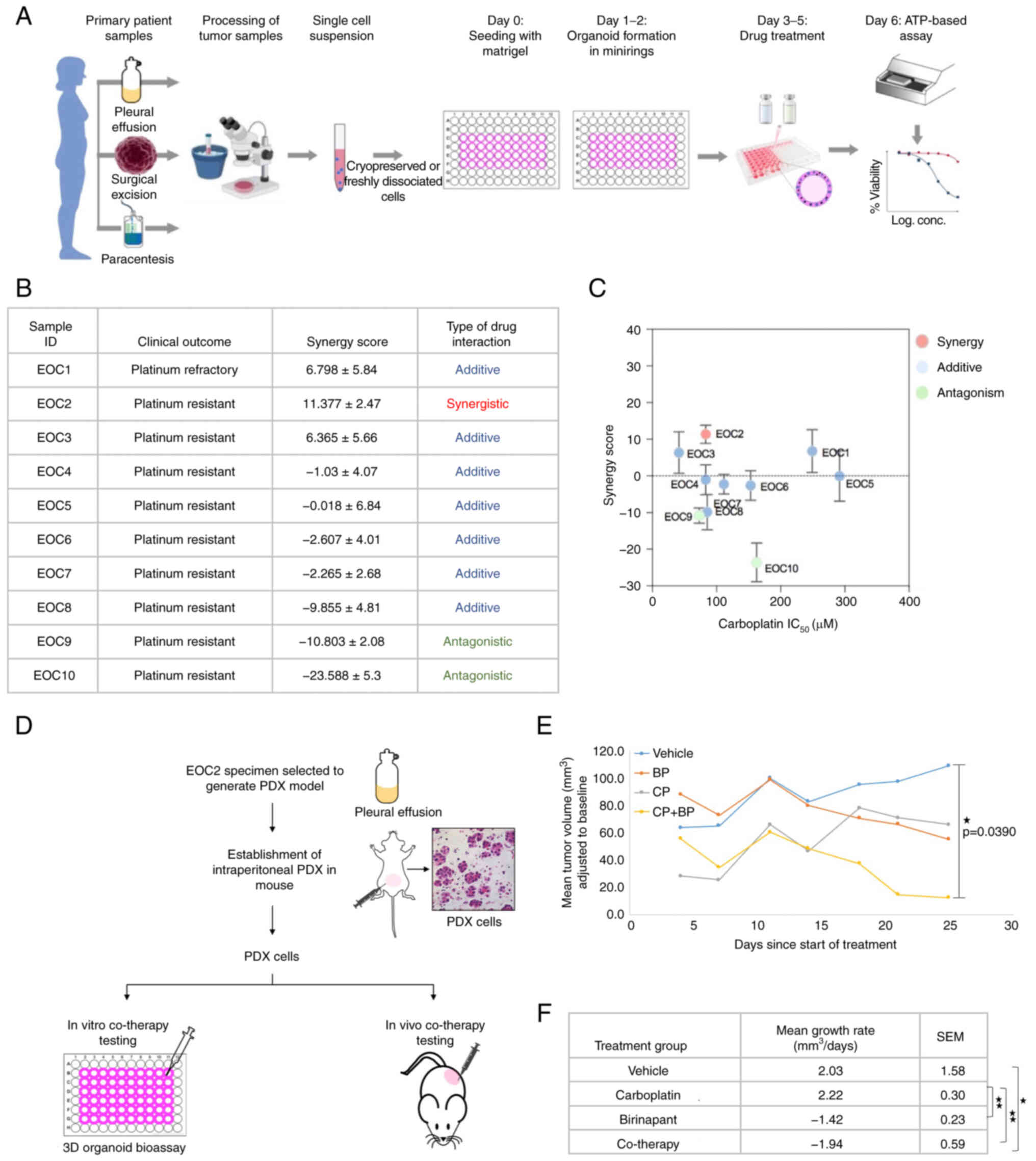
samples (Fig. 4A). A total of 10
platinum-resistant EOC specimens were tested in this study
(Fig. 4B).
In this bioassay, dissociated tumor cells either
freshly processed or cryopreserved were mixed with Matrigel and
plated in tissue culture plates similar to experiments performed
using the OC cell lines. Patient-derived organoids were then
treated with carboplatin (0-50 µM), birinapant (0-50 nM), or
co-therapy for 3 consecutive days followed by assessment of cell
viability and synergy score calculation (Fig. S3A). Loewe synergy scores
calculated by the SynergyFinder tool demonstrated that
co-therapy treatment was likely synergistic in targeting 1/10,
additive in 7/10, and antagonistic in 2/10 platinum resistant
primary tumor samples (Fig. 4B and
C). The IC50 of carboplatin and birinapant was also
measured for each sample (Fig.
S3B). Clinically classified platinum-resistant tumor samples
tested in this bioassay demonstrated high IC50 values of
carboplatin (above 40 µM).
To examine the therapeutic efficacy of the
co-therapy in vivo, one primary tumor sample that
demonstrated maximum Loewe synergy score in vitro (EOC2) was
used to generate a platinum-resistant patient-derived xenograft
(PDX) model in immunocompromised mice (Figs. 4D and S4A). The efficacy of the co-therapy was
confirmed ex vivo in the 3D organoid bioassay. Similar to
its parental patient tumor sample (EOC2), PDX cells were found
sensitive to co-therapy (Fig.
S4B). Levels of IAP proteins in PDX cells were also measured
using western blot analysis (Fig.
S4C).
To assess the efficacy of birinapant, carboplatin,
and the co-therapy in vivo, PDXs were generated in n=17
female NSG mice by subcuticular injection of one million PDX
cells/mouse (Fig. S4D). One
mouse was randomly selected and euthanized before treatment to
confirm establishment of subcuticular tumors (Fig. S4E). The remaining mice were then
randomized into treatment groups (n=4/group). Treatment was
initiated when the average tumor volume of all PDX-bearing mice
reached approximately 125 mm3. Mice were treated with
either vehicle, carboplatin (50 mg/kg i.p. 1×/week), birinapant (30
mg/kg i.p. 2×/week), or the combination of both drugs for 4 weeks.
In the co-therapy-treated mice, birinapant was administered 4 h
before carboplatin. During the treatment period, the mouse body
weight and size of tumors were recorded twice a week. After 4 weeks
of drug treatment, mice were euthanized, and tumors were
harvested.
Tumor volume of each mouse was adjusted by
subtracting the baseline tumor volume as measured at the start of
treatment (day 0). At the end of treatment (day 25), the smallest
mean tumor volume (adjusted to baseline) was observed in the
co-therapy-treated mice compared to vehicle, carboplatin-treated or
birinapant-treated group, with mean difference statistically
significant with the vehicle-treated mice only (P=0.0390) (Fig. 4E).
Comparison of the mean tumor growth rates between
treatment groups demonstrated that the mean tumor volume in the
vehicle and carboplatin-treated groups increased with time at the
rate of (2.03±1.58 mm3/day) and (2.22±0.30
mm3/day) respectively whereas it decreased in the
birinapant-treated and co-therapy-treated mice at the rate of
(-1.42±0.23 mm3/day) and (-1.94±0.59 mm3/day)
respectively (Fig. 4F). Tumors
harvested after treatment were also weighed and histologically
analyzed (Fig. S4F and S4G). IHC
staining for the cell proliferation biomarker Ki-67 in these tumor
fragments demonstrated no significant differences in the percentage
of Ki67-positive cells across the 4 treatment groups (Fig. S4H). The mean number of mitosis
(per high power field) was found lower in the birinapant (0.6377)
and co-therapy (0.720)-treated groups compared to the vehicle
(1.533) and carboplatin (1.388) groups, although it did not reach
statistical significance (Fig.
S4I). No signs of drug toxicity as measured by mouse body
weight were observed (Fig.
S4J).
Overall, the results highlight consistency between
in vitro and in vivo therapeutic responses of cancer
cells to combination therapy as measured by the 3D organoid
bioassay. This suggests that the 3D organoid bioassay may be used
as valuable preclinical research tool in the field of cancer
therapeutics to evaluate the efficacy of targeted therapies.
Additionally, the results indicate the efficacy of carboplatin and
birinapant combination in targeting a subset of platinum-resistant
primary human ovarian cancers.
Discussion
In the present study, we utilized a 3D-organoid
bioassay (28,29) as an in vitro platform to
measure platinum sensitivity of ovarian cancer cell lines.
Assay-predicted results were found to be correlated with reported
platinum sensitivities for each cell line. The bioassay was also
used to test platinum sensitivity of 10 primary patient tumor
samples. We observed that clinically classified platinum-resistant
tumor samples demonstrated high IC50 values for
carboplatin that ranged from 40 to 291.8 µM. These results
suggest that the 3D-organoid bioassay may be utilized as a
potential companion diagnostic (CDx) for predicting ovarian cancer
patients' response to platinum-based chemotherapy. As a potential
precision medicine tool, this bioassay offers several advantages
(28,29). First, as demonstrated in this
study, the bioassay allows formation of epithelial ovarian cancer
organoids from fluid samples (ascites, pleural effusions) as well
as from dissociated tumor cells obtained from surgical specimens.
Although no biopsy samples were tested in this study, the low
cellular requirement would make this assay compatible for testing
small biopsies as well. Second, assay results were obtained within
one week, as previously reported (28), making it suitable for
time-sensitive therapeutic decision making. However, for clinical
utility, the bioassay needs to be validated prospectively with a
large cohort of ovarian cancer tumor samples in a clinical
trial.
Another major goal of this study was to target
platinum-resistant epithelial ovarian cancer cells. Chemo-resistant
cancer cells are known to evade therapy-induced apoptosis. As a
promising therapeutic strategy for activating apoptosis in ovarian
cancer cells, we utilized a small molecule inhibitor called
birinapant designed to inhibit anti-apoptotic IAP proteins in
combination with carboplatin. The in vitro efficacy of the
co-therapy was tested across a panel of epithelial ovarian cancer
cell lines in the 3D organoid bioassay. Results from these studies
demonstrated enhanced cell death for a subset of platinum-resistant
cell lines treated with the co-therapy compared to single agents.
Similarly, the combination of birinapant and carboplatin was found
effective in targeting a subset of platinum-resistant primary
patient tumor samples in vitro. The combination therapy also
demonstrated some efficacy in targeting a platinum-resistant PDX
model. The correlation between in vitro and in vivo
therapeutic response of these PDX cells and the parental human
tumor demonstrates the potential of the 3D organoid bioassay as a
high throughput drug testing platform.
Our findings in the present study are consistent
with published data demonstrating that combining SMAC mimetics with
carboplatin could target ovarian cancers using in vitro and
in vivo preclinical models (26,40). Several studies have explored
birinapant activity in combination with different anticancer
agents. The combination of birinapant with several chemotherapies
including carboplatin/paclitaxel, docetaxel, irinotecan,
gemcitabine or liposomal doxorubicin have also been evaluated in a
Phase I/II clinical trial for treatment of patients with advanced
or metastatic solid tumors (NCT01188499). The results from this
clinical trial demonstrated that birinapant could be well-tolerated
when combined with multiple chemotherapies (44). Birinapant has also been tested in
combination with gemcitabine, oxaliplatin/5-fluorouracil, TNFα,
TRAIL and docetaxel for targeting preclinical models of breast
cancer (24), colorectal cancer
(45), and head and neck squamous
cell carcinomas (HNSCC) respectively (46). Other than standard chemotherapy
drugs, birinapant has also shown synergy with CAR-T therapy and
radiotherapy in targeting colorectal cancer (47) and HNSCC (22), respectively. A recent study has
suggested the significance of sequential drug administration for
increased drug synergy between birinapant and carboplatin in
targeting OVCAR8 xenografts (26). Here, birinapant was injected after
carboplatin administration. In our study, we administered
birinapant 4 h prior to carboplatin injection in the PDX-bearing
mice. The rationale for this sequence of drug administration was to
activate birinapant-mediated apoptosis in the tumor cells to better
prime them for carboplatin-induced cytotoxicity. The optimal
sequence for administration of these two drugs may require more
investigation.
Although the development of birinapant as an
anticancer therapeutic is promising, there remain some challenges
to be addressed. One of the main challenges is to identify and
develop biomarkers of response to birinapant. Several studies have
assessed and demonstrated that IAP levels do not correlate with
sensitivity to birinapant (48,49). Zinngrebe et al demonstrated
that protein expression levels of cIAP1 and XIAP were similar in
SMAC mimetic-sensitive and SMAC mimetic-insensitive primary B-cell
acute lymphoblastic leukemia samples (48). McCann et al also reported
that expression of IAP proteins alone could not be correlated to
birinapant sensitivity in a panel of colorectal cancer cell lines
(49). Similarly, in our study,
we did not see a correlation between IAP protein levels and
response to birinapant in the ovarian cancer cell lines tested. In
an attempt to characterize predictive biomarkers of response to
birinapant, a recent study has identified a 12-protein signature
consisting of apoptotic proteins that could segregate responders
and non-responders to birinapant and chemotherapy combinations in
colorectal cancer (49). Another
study identified a biomarker set of 4 genes including
TNFRSF1A, TSPAN7, DIPK1C and MTX2 for
prediction of response to SMAC mimetics in pediatric precursor-cell
acute lymphoblastic leukemia (48). Although these studies have paved
the way for personalized treatment of cancer using SMAC mimetics,
their successful clinical implementation across different cancers
requires further evaluation in clinical trials. In the absence of
any reliable biomarkers, optimization of the 3D organoid bioassay
may potentially offer a precision medicine tool to predict
therapeutic responses ex vivo.
Despite these shortcomings, the antitumor activity
and safety profile of birinapant highlights its potential as an
effective anticancer drug when combined with other
chemotherapeutics. In summary, work by other investigators and
results from this study provide a rationale for evaluation of the
efficacy of birinapant in combination with carboplatin in clinical
trials for patients with platinum-resistant ovarian cancers.
Supplementary Data
Availability of data and materials
All data generated or analyzed during the current
study are included in this article. Data sharing is not applicable
to this article, as no datasets were generated or analyzed during
the current study.
Authors' contributions
Conception and design were carried out by TS and SM.
In vitro assays were conducted by TS and AN. Processing of
patient tumor samples was accomplished by AN. The animal study was
performed by TS, AN and NR. The IHC study was conducted by GD and
NAM. Writing, review of the manuscript was conducted by TS, AN, and
SM. Study supervision was carried out by TS and SM. All authors
read and approved the manuscript and confirmed the generated data
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All primary patient tumor samples tested in this
study were obtained from consented patients through protocols
approved by the UCLA Office of the Human Research Protection
Program (IRB# 10-000727). All animal experiments were approved by
the UCLA Animal Research Committee (protocol 2008-153) and
conducted under the supervision of the UCLA Division of Laboratory
Animal Medicine.
Patient consent for publication
Patients provided written informed consent for
publication of any associated data maintaining their identity
confidentiality.
Competing interests
Authors declare no competing interests.
Acknowledgments
We would like to thank Dr Jeffrey Gornbein
(biostatistician), the UCLA Translational Pathology Core Laboratory
(TPCL) and the Broad Stem Cell Research Center (BSCRC) Flow
Cytometry Core for their assistance. We also thank the patients and
their relatives without whom this study would not have been
possible.
Funding
AN and SM are partially supported by a Department of Veterans
Affairs Merit Award (grant no. I01BX004651).
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desai A, Xu J, Aysola K, Qin Y, Okoli C,
Hariprasad R, Chinemerem U, Gates C, Reddy A, Danner O, et al:
Epithelial ovarian cancer: An overview. World J Transl Med. 3:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–121. 2021. View Article : Google Scholar
|
|
4
|
Kaplan DA: Overview of the Updated NCCN
Guidelines on Ovarian Cancer. 6:2020.
|
|
5
|
Berek JS, Crum C and Friedlander M: Cancer
of the ovary, fallopian tube, and peritoneum. Int J Gynaecol
Obstet. 119(Suppl 2): S118–S129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baert T, Ferrero A, Sehouli J, O'Donnell
DM, González-Martín A, Joly F, van der Velden J, Blecharz P, Tan
DSP, Querleu D, et al: The systemic treatment of recurrent ovarian
cancer revisited. Ann Oncol. 32:710–725. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Yang Y, Yang J, Zhao X and Wei X:
Tumor microenvironment in ovarian cancer: Function and therapeutic
strategy. Front Cell Dev Biol. 8:7582020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Kang Y, Chen L, Wang H, Liu J,
Zeng S and Yu L: The drug-resistance mechanisms of five
platinum-based antitumor agents. Front Pharmacol. 11:3432020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubrez L, Berthelet J and Glorian V: IAP
proteins as targets for drug development in oncology. Onco Targets
Ther. 9:1285–1304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finlay D, Teriete P, Vamos M, Cosford NDP
and Vuori K: Inducing death in tumor cells: Roles of the inhibitor
of apoptosis proteins. F1000Res. 6:5872017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pluta P, Jeziorski A, Cebula-Obrzut AP,
Wierzbowska A, Piekarski J and Smolewski P: Expression of IAP
family proteins and its clinical importance in breast cancer
patients. Neoplasma. 62:666–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofmann HS, Simm A, Hammer A, Silber RE
and Bartling B: Expression of inhibitors of apoptosis (IAP)
proteins in non-small cell human lung cancer. J Cancer Res Clin
Oncol. 128:554–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imoto I, Tsuda H, Hirasawa A, Miura M,
Sakamoto M, Hirohashi S and Inazawa J: Expression of cIAP1, a
target for 11q22 amplification, correlates with resistance of
cervical cancers to radiotherapy. Cancer Res. 62:4860–4866.
2002.PubMed/NCBI
|
|
14
|
Miyamoto M, Takano M, Iwaya K, Shinomiya
N, Kato M, Aoyama T, Sasaki N, Goto T, Suzuki A, Hitrata J and
Furuya K: X-chromosome-linked inhibitor of apoptosis as a key
factor for chemoresistance in clear cell carcinoma of the ovary. Br
J Cancer. 110:2881–2886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Y, Ma W, Huang X, Cao L, Li H, Jiang
Y, Lu N and Yin Y: Effect of survivin on tumor growth of colorectal
cancer in vivo. Int J Clin Exp Pathol. 8:13267–13272. 2015.
|
|
16
|
Zhao G, Wang Q, Wu Z, Tian X, Yan H, Wang
B, Dong P, Watari H, Pfeffer LM, Guo Y, et al: Ovarian primary and
metastatic tumors suppressed by survivin knockout or a novel
survivin inhibitor. Mol Cancer Ther. 18:2233–2245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park E, Gang EJ, Hsieh YT, Schaefer P,
Chae S, Klemm L, Huantes S, Loh M, Conway EM, Kang ES, et al:
Targeting survivin overcomes drug resistance in acute lymphoblastic
leukemia. Blood. 118:2191–2199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moriai R, Tsuji N, Moriai M, Kobayashi D
and Watanabe N: Survivin plays as a resistant factor against
tamoxifen-induced apoptosis in human breast cancer cells. Breast
Cancer Res Treat. 117:261–271. 2009. View Article : Google Scholar
|
|
19
|
Span PN, Sweep FCGJ, Wiegerinck ET,
Tjan-Heijnen VC, Manders P, Beex LV and de Kok JB: Survivin is an
independent prognostic marker for risk stratification of breast
cancer patients. Clin Chem. 50:1986–1993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K and Tokuda M: Survivin expression and its correlation
with cell proliferation and prognosis in epithelial ovarian tumors.
Int J Oncol. 21:315–320. 2002.PubMed/NCBI
|
|
21
|
Morrish E, Brumatti G and Silke J: Future
therapeutic directions for Smac-Mimetics. Cells. 9:4062020.
View Article : Google Scholar :
|
|
22
|
Eytan DF, Snow GE, Carlson S, Derakhshan
A, Saleh A, Schiltz S, Cheng H, Mohan S, Cornelius S, Coupar J, et
al: SMAC mimetic birinapant plus radiation eradicates human head
and neck cancers with genomic amplifications of cell death genes
FADD and BIRC2. Cancer Res. 76:5442–5454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lalaoui N, Merino D, Giner G, Vaillant F,
Chau D, Liu L, Kratina T, Pal B, Whittle JR, Etemadi N, et al:
Targeting triple-negative breast cancers with the Smac-mimetic
birinapant. Cell Death Differ. 27:2768–2780. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie X, Lee J, Liu H, Pearson T, Lu AY,
Tripathy D, Devi GR, Bartholomeusz C and Ueno NT: Birinapant
enhances gemcitabine's antitumor efficacy in triple-negative breast
cancer by inducing intrinsic pathway-dependent apoptosis. Mol
Cancer Ther. 20:296–306. 2021. View Article : Google Scholar
|
|
25
|
Colombo M, Marabese M, Vargiu G, Broggini
M and Caiola E: Activity of birinapant, a SMAC mimetic compound,
alone or in combination in NSCLCs with different mutations. Front
Oncol. 10:5322922020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernandez LF, Dull AB, Korrapati S and
Annunziata CM: Smac-mimetic enhances antitumor effect of standard
chemotherapy in ovarian cancer models via Caspase 8-independent
mechanism. Cell Death Discov. 7:1342021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noonan AM, Bunch KP, Chen JQ, Herrmann MA,
Lee JM, Kohn EC, O'Sullivan CC, Jordan E, Houston N, Takebe N, et
al: Pharmacodynamic markers and clinical results from the phase II
Study of the SMAC-Mimetic birinapant in women with relapsed
platinum-resistant or refractory epithelial ovarian cancer. Cancer.
122:588–597. 2016. View Article : Google Scholar
|
|
28
|
Phan N, Hong JJ, Tofig B, Mapua M,
Elashoff D, Moatamed NA, Huang J, Memarzadeh S, Damoiseaux R and
Soragni A: A simple high-throughput approach identifies actionable
drug sensitivities in patient-derived tumor organoids. Commun Biol.
2:782019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen HTL and Soragni A: Patient-derived
tumor organoid rings for histologic characterization and
high-throughput screening. STAR Protoc. 1:1000562020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ianevski A, Giri AK and Aittokallio T:
SynergyFinder 2.0: Visual analytics of multi-drug combination
synergies. Nucleic Acids Res. 48(W1): W488–W493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Cancer Institute: Oxaliplatin.
Accessed September 15, 2021. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/oxaliplatin.
|
|
32
|
National Cancer Institute: Cisplatin.
Accessed September 15, 2021. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/cisplatin.
|
|
33
|
National Cancer Institute:
Discovery-Cisplatin and The Treatment of Testicular and Other
Cancers. Accessed September 15, 2021. Available from: https://www.cancer.gov/research/progress/discovery/cisplatin.
|
|
34
|
Decatris MP, Sundar S and O'Byrne KJ:
Platinum-based chemotherapy in metastatic breast cancer: Current
status. Cancer Treat Rev. 30:53–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burstein HJ, Mangu PB, Somerfield MR,
Schrag D, Samson D, Holt L, Zelman D and Ajani JA; American Society
of Clinical Oncology: American Society of Clinical Oncology
clinical practice guideline update on the use of chemotherapy
sensitivity and resistance assays. J Clin Oncol. 29:3328–3330.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haley J, Tomar S, Pulliam N, Xiong S,
Perkins SM, Karpf AR, Mitra S, Nephew KP and Mitra AK: Functional
characterization of a panel of high-grade serous ovarian cancer
cell lines as representative experimental models of the disease.
Oncotarget. 7:32810–32820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beaufort CM, Helmijr JC, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van
IJcken WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel
(OCCP): Clinical importance of in vitro morphological subtypes.
PLoS One. 9:e1039882014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kopper O, de Witte CJ, Lõhmussaar K,
Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost
N, Begthel H, et al: An organoid platform for ovarian cancer
captures intra- and interpatient heterogeneity. Nat Med.
25:838–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted therapies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thibault B, Genre L, Le Naour A, Broca C,
Mery E, Vuagniaux G, Delord JP, Wiedemann N and Couderc B: DEBIO
1143, an IAP inhibitor, reverses carboplatin resistance in ovarian
cancer cells and triggers apoptotic or necroptotic cell death. Sci
Rep. 8:178622018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar :
|
|
42
|
Vince JE, Wong WW, Khan N, Feltham R, Chau
D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et
al: IAP Antagonists Target cIAP1 to Induce TNFα-Dependent
Apoptosis. Cell. 131:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Probst BL, Liu L, Ramesh V, Li L, Sun H,
Minna JD and Wang L: Smac mimetics increase cancer cell response to
chemotherapeutics in a TNF-α-dependent manner. Cell Death Differ.
17:1645–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Amaravadi RK, Senzer NN, Martin LP,
Schilde RJ, LoRusso P, Papadopoulos KP, Weng DE, Graham M and Adjei
AA: A phase I study of birinapant (TL32711) combined with multiple
chemotherapies evaluating tolerability and clinical activity for
solid tumor patients. J Clin Oncol. 31(Suppl 15): S25042013.
View Article : Google Scholar
|
|
45
|
Fichtner M, Bozkurt E, Salvucci M, McCann
C, McAllister KA, Halang L, Düssmann H, Kinsella S, Crawford N,
Sessler T, et al: Molecular subtype-specific responses of colon
cancer cells to the SMAC mimetic Birinapant. Cell Death Dis.
11:10202020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eytan DF, Snow GE, Carlson SG, Schiltz S,
Chen Z and Van Waes C: Combination effects of SMAC mimetic
birinapant with TNFα, TRAIL, and docetaxel in preclinical models of
HNSCC. Laryngoscope. 125:E118–E124. 2015. View Article : Google Scholar
|
|
47
|
Michie J, Beavis PA, Freeman AJ, Vervoort
SJ, Ramsbottom KM, Narasimhan V, Lelliott EJ, Lalaoui N, Ramsay RG,
Johnstone RW, et al: Antagonism of IAPs Enhances CAR T-cell
Efficacy. Cancer Immunol Res. 7:183–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zinngrebe J, Schlichtig F, Kraus JM, Meyer
M, Boldrin E, Kestler HA, Meyer LH, Fischer-Posovszky P and Debatin
KM: Biomarker profile for prediction of response to SMAC mimetic
monotherapy in pediatric precursor B-cell acute lymphoblastic
leukemia. Int J Cancer. 146:3219–3231. 2020. View Article : Google Scholar
|
|
49
|
McCann C, Matveeva A, McAllister K, Van
Schaeybroeck S, Sessler T, Fichtner M, Carberry S, Rehm M, Prehn
JHM and Longley DB: Development of a protein signature to enable
clinical positioning of IAP inhibitors in colorectal cancer. FEBS
J. 288:5374–5388. 2021. View Article : Google Scholar : PubMed/NCBI
|