Introduction
Gastric cancer (GC) is one of the most common
malignant tumors worldwide (1).
Although the current mortality rate of GC has decreased,
unresectable GC remains intractable (2). Guidelines for GC indicate that the
primary treatment option for advanced-stage GC is palliative
chemotherapy (3,4); however, the majority of patients
develop resistance, as the mean survival rate for these patients is
~1 year. Several agents targeting the molecules expressed in GC
cells have been used to enhance the therapeutic efficacy of
chemotherapy in GC. However, apart from HER2 or VEGFR2 inhibitors,
the majority of drugs did do not lead to an improved survival.
Comprehensive molecular analysis has revealed that a high
proportion of non-cancerous stroma within the primary tumor of GC
leads to a poor prognosis and an unsatisfactory response to
chemotherapy (5,6).
Cancer-associated fibroblasts (CAFs) are one of the
major components of GC stromal areas. Previous studies have
demonstrated that CAFs enhance the resistance of GC to chemotherapy
(7,8). However, the exact mechanisms
involved in CAF-induced resistance to therapy have not yet been
described, and inhibitors to suppress the effect of CAF have not
yet been applied in clinical settings, at least to the best of our
knowledge. CAFs can produce various cytokines and growth factors,
which can activate intracellular signaling pathways in cancer cells
(9,10). It has been described that the
Janus-activated kinase (JAK)/signal transducer and activator of
transcription 3 (STAT3) axis may be the primary signal transduction
pathway activated by CAF-produced cytokines and chemokines
(11). Moreover, it plays a
crucial role in enhancing resistance to cancer therapy (12). Taken together, the JAK/STAT3
pathway may be considered a promising target which may be used to
combat the CAF-induced resistance of GC to chemotherapy.
The JAK/STAT3 signaling pathway is an attractive
target for cancer treatment as it can promote the proliferation,
survival and migration of cancer cells (13). Although the US Food and Drug
Administration (FDA) approved the JAK1/2 inhibitor, ruxolitinib,
for hematopoietic neoplasms, the benefit of JAK- or STAT3-specific
inhibitors has not been reported in clinical trials for solid
malignancies (14). Moreover, the
JAK/SATA3 pathway has a variety of biological functions in normal
cells and tissues; therefore, blocking this pathway requires a
proper appreciation of the possible side-effects associated with
its inhibition (12,15,16).
Recent studies have reported that phytochemicals,
such as flavonoids or polyphenols in medicinal and edible plants
have antioxidant, anti-inflammatory and anticancer properties.
Among the numerous phytochemicals, curcumin (diferuloylmethane), a
polyphenol compound derived from the roots of Curcuma longa,
is a biologically active compound of the Indian spice, turmeric.
This compound has been shown to possess biological activities, such
as antioxidant, antimicrobial, anti-inflammatory and anticancer
activities (17-20). In a previous study, curcumin was
found to exert a potent anti-tumor effect in human uterine
leiomyosarcoma SKN cells via the inhibition of the activity of the
AKT/mTOR pathway (21).
Furthermore, another study reported that curcumin blocked the
initial carcinogenic process in colorectal cancer animal models
induced by azoxymethane (22).
In a previous study, curcumin was shown to inhibit
the STAT3 signaling pathway by binding to the JAK activation loop
in a time-and concentration-dependent manner (23). However, the suppressive effects of
curcumin on the development of chemotherapeutic resistance in GC
have not yet been evaluated, at least to the best of our knowledge.
Thus, the present study aimed to determine whether curcumin can
effectively suppress the CAF-mediated resistance to 5-fluorouracil
(5-FU) in GC cells.
Materials and methods
Cells and cell culture
The GC cell lines, MKN1 (KCLB no. 80101), MKN74
(KCLB no. 80104) and SNU668, (KCLB no. 00668) were purchased from
the Korean Cell Line Bank. The cells were cultured in RPMI-1640
medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(FBS; HyClone; Cytiva), 1% penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and 1% amphotericin B (SEARCH BIO Inc.).
The cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2. CAFs were isolated from fresh GC
patient specimens as described in a previous study by the authors
(24). For co-cultures, CAFs were
seeded into the upper chambers of 6-well Transwells and GC cell
lines were seeded into the bottom of six-well tissue culture
dishes. DMEM/high glucose medium (HyClone; Cytiva; supplemented
with 5% FBS) was added to both the upper and bottom chambers,
allowing interaction between the two cell types.
Reagents
The STAT3 inhibitor (WP1066; cat. no. 573097) and
5-FU (cat. no. F6627) were purchased from MilliporeSigma.
Recombinant human C-C motif chemokine ligand 2 (CCL2; cat. no.
279-MC-050), C-X-C motif chemokine ligand (CXCL)1 (cat. no.
275-GR-010) and CXCL8 (cat. no. 208-IL-050) were purchased from
R&D Systems, Inc., and recombinant human interleukin (IL)-6
(cat. no. PHC0064) was purchased from Gibco; Thermo Fisher
Scientific, Inc.
Western blot analysis
Cells were washed with phosphate-buffered saline
(PBS) and lysed in boiling sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) sample buffer [62.5 mM Tris (pH
6.8), 1% SDS, 10% glycerol and 5% β-mercaptoethanol]. The lysates
were scraped on a plate with a pipette and boiled for 5 min at
100°C. Protein concentrations were determined using the reducing
agent compatible/detergent compatible RC/DC protein assay (Bio-Rad
Laboratories, Inc.). Equal amounts (30 µg) of protein from
each sample were resolved by SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (MilliporeSigma). The
immunoblots were blocked by incubation in 5% skim milk, 25 mM Tris
(hydroxymethyl) aminomethane-HCl (pH 8.0), 150 mM NaCl, and 0.1%
Tween®-20 for 1 h at 25°C. The membranes were incubated
overnight at 4°C with the following primary antibodies: Anti-STAT3
(1:1,000 dilution; cat. no. 9139), anti-phosphorylated (p-) STAT3
(1:1,000; cat. no. 9145), anti-AKT (1:1,000; cat. no. 9272),
anti-p-AKT (1:1,000; cat. no. 4060), anti-mTOR (1:1,000; cat. no.
2972), anti-p-mTOR (1:1,000; cat. no. 2971S), anti-cleaved PARP
(1:1,000; cat. no. 9542; Cell Signaling Technology), anti-cleaved
caspase-3 (1:1,000; cat. no. 9664) (all from Cell Signaling
Technology, Inc.), anti-Bcl-2 (1:1,000; cat. no. sc-509; Santa Cruz
Biotechnology, Inc.), anti-survivin (1:500; cat. no. ADI-AAP-275;
Enzo Life Sciences, Inc.) anti-β-actin (1:5,000; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.). The membranes were then washed
three times with Tris-buffered saline with Tween-20 (TBST),
followed by their corresponding horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. no. 115-545-146; Jackson
ImmunoResearch Laboratories, Inc.) for 1 h at room temperature.
Protein detection was performed using an enhanced chemiluminescence
kit (AbClon, Inc.). The pixel volumes were evaluated using ImageJ
software (version 1.52; National Institutes of Health), and the
results were normalized to the corresponding blots.
Cell viability assay
The cells were seeded at 1×104 cells per
well in 96-well plates, and cell proliferation was measured using a
highly sensitive water-soluble tetrazolium salt-based viability
assay kit (DoGen Bio). After seeding the cells, 10 µl
EZ-Cytox solution were added per well, and the cells were incubated
for 1 h at 37°C. The absorbance was measured at 450 nm using a
Tecan microplate reader (Infinite F50; Tecan Group, Ltd.). All
experiments were performed in triplicate. To evaluate the effects
of curcumin on cell viability, the cells were treated with various
concentrations (0.2, 1.0, 5.0, 25, 125, 625 and 3,125 µM) of
5-FU for 72 h. The To IC50 values were calculated as
follows: The inhibitor concentration against the percent activity
is plotted [(I)-activity % graph]. Using the linear (y=mx + n) or
parabolic (y=ax2 + bx + c) equation on this graph for y=50 value x
point yields the IC50 value.
Secretome and transcriptome analysis
Secretome analysis was performed using the Proteome
Profiler Human Cytokine Array kit (R&D Systems Inc.) to
identify upregulated secretory factors in the culture supernatants
of MKN1, MKN74 and SNU668 cells co-cultured with CAFs as compared
with MKN1, MKN74, and SNU668 cells cultured without CAFs.
Conditioned medium (CM) from the MKN1, MKN74, and SNU668 cell
cultures was collected after 48 h in serum-free medium and
incubated with arrays containing 36 human cytokine-specific
antibodies at 4°C for 24 h. After the membranes were treated with a
luminescence reagent, X-ray films were developed to identify the
pixel volume of the spots related to the quantity of each secreted
protein. The pixel volumes were evaluated using ImageJ software
(version 1.52; National Institutes of Health), and the results were
normalized to the corresponding spots. Using RNeasy (Qiagen, Inc.),
RNA was extracted from the MKN1, MKN74 and SNU668 cells cultured
alone or together with CAFs. The RNA quality was assessed using a
2100 Bioanalyzer (Agilent Technologies, Inc.) and cDNA was
synthesized using the GeneChip WT (Whole Transcript) Amplification
kit (Thermo Fisher Scientific, Inc.). Labeled target DNA (~5.5
µg) was hybridized to Affymetrix GeneChip Human Gene 2.0 ST
Arrays (Thermo Fisher Scientific, Inc.). Array data processing and
analysis were performed using Affymetrix GeneChip Command Console
Software (6.0+ version; Thermo Fisher Scientific, Inc.). Gene set
enrichment analysis (GSEA) was used to identify upregulated
pathways in MKN74 and SNU668 cells co-cultured with CAFs relative
to MKN74 and SNU668 cells alone. Pathway categories were obtained
from the Molecular Signatures Database (MSigDB, https://software.broadinstitute.org). The cut-off
value for statistical significance used in GSEA was a false
discovery rate (FDR) of 25%. To reduce the likelihood of
false-positive results, the present study used an FDR value of 5%
as the cut-off level for enriched gene sets.
Patient samples
Of the patients diagnosed with GC who were treated
with neoadjuvant chemotherapy/chemoradiotherapy following surgery
at the Shanghai Cancer Center, Shanghai, China from July, 2019 to
October, 2020, surgically resected paraffin-embedded tissues from
50 patients were collected. Normal tissue collected from adjacent
tumor area in patients. The use of these specimens was approved by
the Institutional Review Board of Shanghai Cancer Center (IRB no.
050432-4-1911D). Informed consent was obtained from all the
participants. The combined regimens based on 5-FU were administered
for neoadjuvant chemotherapy from two to six cycles, and 7 patients
were additionally treated with radiation at 4,500 cGy. Following
chemotherapy or chemoradiation therapy, all patients were
curatively resected by total or subtotal gastrectomy with proper
lymph node dissection.
All hematoxylin and eosin (H&E)-stained sections
were reviewed by an experienced pathologist (LW) to confirm the GC
cancer proportion and to be assigned a grade of responsiveness to
chemotherapy in the primary GC tissues according to the grading
system suggested by the national comprehensive cancer network
(25).
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded xenograft and
primary GC tumors were sectioned, affixed onto microscopic slides,
deparaffinized with xylene, hydrated using a diluted alcohol
series, and immersed in 0.3% H2O2 in methanol
to quench endogenous peroxidase activity. The sections were treated
with citrate buffer (10 mM, pH 6.0) for antigen retrieval. To
reduce non-specific staining, each section was treated with 20%
aqua block (Abcam) in TBST for 30 min. The sections were incubated
overnight at 4°C with the primary rabbit monoclonal anti-cleaved
caspase-3 antibody (1:100; cat. no. 9664, Cell Signaling
Technology, Inc.) for xenograft tumors and the primary rabbit
monoclonal anti-p-STAT3 antibody (1:100; cat. no. 9145, Cell
Signaling Technology, Inc.) for human GC primary tumors in antibody
diluent solution (GBI Labs). The following day, the sections were
incubated with polyclonal anti-rabbit secondary antibody (1:100;
cat. no. AbC-5003; AbClon, Inc.) for 60 min at room temperature.
The chromogen used was 3,3′-diaminobenzidine (Thermo Fisher
Scientific, Inc.), and the sections were counterstained with Harris
hematoxylin (cat. no. S3309, Dako; Agilent Technologies, Inc.) at
room temperature for 5 min. For cleaved caspase-3
immunohistochemical analysis of the harvested tumor tissue, the
images were captured using an Olympus BX53 microscope with an
Olympus DP73 camera (Olympus Corporation) at ×200 magnification. In
the images, the areas of entire tumors and brown-stained lesions
were quantified by densitometry using ImageJ software (version
1.52; National Institutes of Health). The percentage of apoptotic
cells was calculated as the ratio of stained area to the total
area. Differences in apoptotic marker expression among the
treatment groups were analyzed using the Mann-Whitney U test.
Analysis of gene expression dataset for
GC
To investigate the association of STAT3-related
genes and the tumor stroma with outcomes of patients with GC, GC
mRNA profile data were downloaded from the publicly available GEO
database (GSE62254) with 300 GC patient samples and clinical
information and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analyzed using GSEA methods. The GSE62254 dataset was
generated based on the GPL570 platform (Affymetrix Human Genome
U133 Plus 2.0). To infer the proportion of stromal fibroblasts in
GC tissues, the Estimation of Stromal and Immune cells in
MAliganant tumor tissues (ESTIMATE) algorithm on the transcriptome
data was used. In addition, to annotate gene sets related to the
STAT3 signaling pathway, MSigDB we used, and the STAT3 pathway was
one of the C2 gene sets representing canonical pathways from
pathway resources. A total of eight genes (MAPK3,
TYK2, JAK1, MTOR, MAP1, STAT3,
JAK3 and JAK2) were included in the STAT3 pathway,
and patients were classified into the high and low STAT3 pathway
expression groups using the median expression of the eight genes as
the cut-off value. The correlations of the STAT3 pathway gene set
with the stromal score and several cytokine- or chemokine-related
genes were evaluated using Pearson's correlation analysis.
Kaplan-Meier analysis and log-rank tests were used to investigate
the survival difference between the high and low mean expression of
actin alpha 2 (ACTA2), a marker of activated fibroblasts,
and STAT3 genes.
Flow cytometric analysis
The MKN1, MKN74 and SNU668 cells were seeded in a
six-well plate and treated with 5-FU (100 µM), CAF
co-culture and curcumin (20 µM) for 72 h. The cells were
stained with fluorescein isothiocyanate (FITC)-conjugated Annexin V
and propidium iodide (PI). FITC Annexin V and PI double-positive
cells were detected using a FACSCanto II flow cytometer (BD
Biosciences).
Animal model experiment
Animal care and handling procedures were performed
in accordance with the Ajou University School of Medicine
Institutional Animal Care and Use Committee guidelines, and all
animal experiments were approved by the Animal Research Committee
of the institution. The animal model was established using six- to
eight-week-old male athymic nude mice (Orient Bio, Inc.) weighing
16-18 g. For the experiments, four 4 groups of mice were used with
6 mice in each group. The mice in all groups were intraperitoneally
injected with 1×106 SNU668 cells with 1×106
CAFs. The groups were as follows: Group 1, untreated mice; group 2,
mice treated with curcumin alone; group 3, mice treated with 5-FU
alone; and group 4, mice treated 5-FU with curcumin. To establish
xenograft tumors, the cells were suspended in 50 µl Matrigel
mixed with PBS. In total, 1×106 tumor cells (SNU668)
with 5×105 fibroblasts were subcutaneously implanted
into the flanks of BALB/c-nu nude mice (Orient Bio, Inc.). To
investigate the effects of 5-FU and curcumin on tumor formation in
the mice, at 7 or 10 days following cell transplantation, the mice
were treated intraperitoneally with 5-FU (25 µg/g body
weight) and curcumin (100 µg/g body weight) three times a
week for 3 weeks. Tumor volume and body weight were monitored
throughout the study period. In all experiments, tumor dimensions
were measured using calipers, and the tumor volume was calculated
using the following formula: Tumor volume (mm3)=(a ×
b2)/2, where a=length in mm, and b=width in mm. The
method of euthanasia used for the mice was CO2
asphyxiation followed by cervical dislocation (CO2 was
introduced into the chamber at a rate of 30-70% of the chamber
volume per min to minimize distress). After euthanizing the mice,
all tumors were harvested for tumor weight measurement and IHC
staining.
Statistical analysis
All experiments were performed independently in
triplicate. The results are presented as the mean ± standard error
(SE). To compare means of continuous variables between the two
groups, datasets were analyzed using an unpaired or paired t-test
for normally distributed data or a Mann-Whitney U test or Wilcoxon
test for non-normal data. A one-way analysis of variance (ANOVA),
followed by Tukey's post hoc test, was used to compare means across
three or more groups. The Chi-squared test was used to determine
whether there was an association between two or more categorical
variables. Statistical analyses were performed using IBM SPSS
statistics (version 21 for Mac OS X; IBM, Inc.) and GraphPad Prism
(version 6.0, for Mac OS X; GraphPad Software, Inc.) software. The
bioinformatics data using the public dataset were analyzed using
the R package. Values of P<0.05, P<0.01 or P<0.001 were
considered to indicate statistically significant or highly
statistically significant differences, respectively.
Results
CAFs induce chemoresistance to 5-FU in GC
cell lines
To initially evaluate the paracrine effects of CAFs
on the resistance of GC cell lines to chemotherapy, first, CM was
isolated from CAFs (CAF-CM), which was added to the MKN1, MKN74 and
SNU668 cells treated with 5-FU. The MKN1, MKN74 and SNU668 cells
stimulated with CAF-CM exhibited a greater resistance (higher
IC50) to 5-FU than those cultured in normal medium, as
revealed by a cell viability test (Fig. 1A). In addition, a Transwell
co-culture system was used to investigate the CAF-induced changes
in apoptotic markers, including the expression of PARP and
caspase-3. It was found that co-culture with CAFs markedly
decreased the expression of cleaved PARP and caspase-3 in the
5-FU-treated MKN1, MKN74 and SNU668 cells (Fig. 1B). In addition, it was found that
the 5-FU-induced apoptotic morphologies, including cellular
shrinkage and the formation of apoptotic bodies, as well as the
reduction in live (attached) cell numbers were markedly attenuated
in the cancer cells co-cultured with CAFs. When treated with 5-FU,
while the majority of the GC cells were killed or scattered, the
CAF-co-cultured GC cells displayed a normal morphology (Fig. 1C). These results suggest that CAFs
confer the resistance to 5-FU of GC cell lines through the
suppression of apoptosis.
CAFs activate the JAK2/STAT3 signal
transduction pathway in GC cell lines
To identify CAF-specific secreted molecules and
CAF-induced gene expression in GC cells, secretome analysis for CM
and transcriptome analysis of GC cells co-cultured with CAFs were
performed. It was hypothesized that increased proteins in the CM of
GC cells co-cultured with CAFs, compared to the CM of GC cells
cultured without CAFs, would be the main ligands to stimulate GC
cells. Thus, the levels of secreted proteins in CM between GC and
GC co-culture with CAFs were compared (Fig. 2A). In most pairs, the intensity of
CCL2, CXCL1, IL-6 and IL-8 in the CM from GC cells co-cultured with
CAFs was higher than that in the GC cells not cultured with CAFs
(Fig. 2A). Subsequently, under
the same conditions as the secretome analysis, transcriptome
analysis was performed using mRNA extracted from the harvested GC
cells. The results revealed that mRNA extracted through GSEA was
positively associated with IL-6/JAK/STAT3 signaling (Fig. 2A; MKN-74 cell enrichment score,
1.83; nominal P<0.001, FDR Q= 0.001, FWER P= 0.006; SNU668 cell
enrichment score, 1.45; nominal P<0.01, FDR Q=0.01, FWER
P=0.145).
 | Figure 2Identification of the JAK2/STAT3 axis
as a specific communicator between CAFs and GC cells. (A) Schematic
diagram of secretome analysis of conditioned media and
transcriptome and western blot analysis for MKN1 cells, MKN74 cells
and SNU668 cells. IL-6, IL-8 and CCL2 were
secreted at higher levels in co-cultured with CAFs relative to
MKN74 cells and SNU668 cells cultured alone. These factors were all
associated with the JAK/STAT3 signal transduction pathway. Gene set
enrichment analysis revealed that the IL-6/JAK/STAT3
signaling-related genes were enriched in MKN74 cells and SNU668
cells co-cultured with CAFs (MKN-74 cell enrichment score, 1.83,
nominal P<0.001, FDR Q=0.001, FWER P=0.006; SNU668 cell
enrichment score, 1.456; nominal P<0.01, FDR Q=0.01, FWER
P=0.145). (B) KEGG pathway analysis expressed increased genes
between no co-culture and co-culture with CAFs in MKN1 cells, MKN74
cells and SNU668 cells. (C) Western blot analysis showing the
expression levels of the indicated proteins after co-culture with
CAFs in MKN1 cells, MKN74 cells and SNU668 cells. (D) Western blot
analysis showing the expression levels of the indicated proteins
following 5-FU treatment with or without co-culture with CAFs in
MKN1 cells, MKN74 cells and SNU668 cells. 5-FU, 5-fluorouracil;
CAF, cancer-associated fibroblast; GC, gastric cancer; CCL2, C-C
motif chemokine ligand 2; KEGG, Kyoto Encyclopedia of Gene and
Genomes; IL-6, interleukin-6; IL-8, interleukin-8; JAK,
Janus-activated kinase; STAT3, signal transducer and activator of
transcription 3. |
The genes encoding the secreted proteins were
functionally annotated according to the KEGG pathway map
(https://www.genome.jp/kegg/).
CCL2, CXCL1, IL-6 and CXCL8 are
involved in cytokine-chemokine receptor interactions and chemokine
signaling pathways, including the JAK/STAT3 signaling pathway
(Fig. 2B).
In addition, using western blot analysis, it was
confirmed that JAK2, STAT3, Bcl-2 and survivin were associated with
the JAK/STAT3 signal transduction pathway. Likewise, the GC cell
lines co-cultured with CAFs exhibited increased phosphorylation
levels of JAK2 (STAT3 upstream protein), Bcl-2 and survivin (STAT3
downstream proteins) (Fig. 2C).
To confirm the CAF-induced chemoresistance of GC cell lines treated
with 5-FU, western blot analysis was performed for the GC cell
lines co-cultured with CAFs. The GC cell lines co-cultured with
co-culture CAFs exhibited increased p-STAT3 levels when treated
with 5-FU. However, another pathway was not altered when the cells
were co-cultured with CAFs (Fig.
2D). Through this process, it was expected that the co-culture
of GC cells and CAFs would affect the intercellular signaling
pathway in GC cells, particularly the JAK2/STAT3 signaling
pathway.
Upregulation of p-STAT3 is associated
with the non-responsiveness of human primary GC tumors treated with
neoadjuvant therapy
Primary GC tumors, which were harvested from the
Shanghai Cancer Center cohort treated with neoadjuvant
chemotherapy, were divided into the response (grade I/II) and
non-response (grade III/IV) groups. These groups were selected
according to the pathological responsiveness of the surgical
specimens following neoadjuvant chemotherapy (Fig. 3A), and IHC for p-STAT3 was
measured from 0 to 80% according to the proportion of cells with
positive staining (Fig. 3B,
images). The mean proportion of p-STAT3 expression did not markedly
differ between the two groups. However, when the expression of
p-STAT3 was determined to be positive when the proportion of
positive cells was ≥3%, the positive expression of p-STAT3 was
significantly associated with a high proportion of poor
responsiveness (P=0.048; Fig. 3B,
table).
The high expression of the STAT3 pathway
gene set is significantly associated with a high stromal gene score
and a poor prognosis of patients with GC
A cohort containing 299 GC patients with available
transcriptome data (GSE62254) and clinical information from a
previously published report was analyzed (26). All patients included in this
dataset were diagnosed with advanced GC with T2 and greater depth
of invasion, and 90.3% were stage II or higher (Fig. 3C). According to the ESTIMATE
algorithm, the infiltration of stromal cells into tumor tissue was
estimated. A 'stromal score' was generated to reflect the presence
of fibrotic stromal cells in tumor tissues using the 141 genes
proposed (27). In the same
dataset, a 'STAT3_PATHWAY_GENES' was calculated using eight genes
generated from the MSigDB database. To investigate the correlation
between the 'stromal score' and 'STAT3_PATHWAY_GENES', Pearson's
correlation analysis was used and a positive correlation was
revealed (r=0.29, P<0.001). In addition, the correlation between
the genes encoding the suggested proteins and 'STAT3_PATHWAY_GENES'
was evaluated. The expression of CCL2, CXCL1,
IL-6 and CXCL8 positively correlated with
'STAT3_PATHWAY_GENES' in the GSE62254 dataset (Fig. 3D).
In the same cohort, the present study also aimed to
identify the prognostic role of the simultaneous expression of
ACTA2, a marker of fibroblasts, and STAT3 genes in
the outcomes of GC patients using the Kaplan-Meier curve with the
log-rank test. Patients with GC with high mean expression of
ACTA2 and STAT3 exhibited a significantly worse
prognosis compared to patients with a low expression (P<0.0001)
and a worse disease-free survival (P<0.0001) (Fig. 3E). These human GC data may suggest
that the accumulation of a fibrotic stroma, including CAFs is
significantly associated with STAT3 activation, and CAF-secreted
proteins, such as CCL2 and CXCL1 enhance the STAT3 signaling
pathway in GC tissues. The activation of STAT3 in the GC stroma
leads to resistance to treatments, and thus this may be a good
target for GC treatment.
Curcumin inactivates the CAF-induced
activation of the JAK2/STAT3 signal transduction pathway in GC cell
lines
It was hypothesized that CAFs may participate in the
development of drug resistance through the activation of the
JAK2/STAT3 pathway. Thus, the effects of curcumin on the tendency
of CAFs to lead to chemoresistance and the phosphorylation of
JAK2/STAT3 in GC cell lines were investigated. First, the toxicity
of curcumin on cancer cells was evaluated, and the results revealed
that the effects of curcumin at ≤20 µM on cell viability of
GC cell lines were minimal (Fig.
S1). Moreover, curcumin at <20 µM significantly
decreased the CAF-induced phosphorylation of JAK/STAT3 in GC cells
in a concentration-dependent manner (Fig. 4A). Curcumin also affected the
expression of Bcl-2 and survivin, which are downstream of the STAT3
pathway in GC cell lines (Fig.
4A). However, curcumin treatment did not decrease the
phosphorylation of mTOR and AKT (Fig. S2). Following treatment with 5-FU,
the CAF-induced phosphorylation of JAK2/STAT3 was compromised by
curcumin treatment in GC cell lines (Fig. 4B).
It was demonstrated that the four types of cytokines
(CCL2, CXCL1, IL-6 and IL-8) from the KEGG pathway analysis
affected the JAK2/STAT3 signaling pathway in GC cells. Western blot
analysis revealed that the levels of p-JAK2/STAT3 increased
following treatment with the four cytokines in GC cell lines;
however, the expression of mTOR/AKT was not altered. Curcumin
decreased the JAK2/STAT3 phosphorylation levels, similar to
treatment following co-culture with CAFs (Fig. S3A). These results indicate that
CAF-derived cytokines (CCL2, CXCL1, IL-6 and IL-8) can stimulate
the activation of the JAK2/STAT3 signaling pathway in GC cell lines
and may cause the resistance of GC cell lines to 5-FU. To confirm
the primary role of CAF-induced STAT3 activation on
chemoresistance, CAF-stimulated GC cells were treated with STAT3
specific inhibitor. The STAT3 inhibitor downregulated the
CAF-induced phosphorylation of STAT3 in a concentration-dependent
manner (Fig. S3B). The cell
viability assay revealed that the resistance to 5-FU increased by
CAF-CM was reduced by the STAT3 inhibitor (Fig S3C). Those findings indicated that
the curcumin-induced inhibition of phosphorylation of STAT3 could
cause the synergistic effect of 5-FU in CAF-stimulated GC
cells.
To determine the effects of curcumin on the
CAF-induced chemoresistance of GC cell lines, the present study
then whether curcumin added to the CAF-CM decreased resistance
compared to the cells cultured with CAF-CM and treated only with
5-FU using cell viability assay. The CM from CAF cultures was added
to the MKN1, MKN74, and SNU668 cells treated with 5-FU. The cell
viability assay revealed that co-treatment with CAF-CM decreased
the cytotoxicity of 5-FU. However, treatment with curcumin
significantly decreased the viability of the GC cell lines
(Fig. 4C). These data strongly
suggest that curcumin treatment increased the sensitivity of CAFs
to 5-FU in GC cell lines. Moreover, to determine the mechanisms
through which curcumin affects the resistance of GC cells treated
with 5-FU to apoptosis, the GC cells co-cultured with CAFs were
treated with curcumin and 5-FU. The induction of apoptosis was
confirmed through cleaved PARP and caspase-3 expression in the GC
cell lines. The results revealed that the increased levels of
cleaved PARP and caspase-3 upon 5-FU treatment were suppressed by
CAF co-culture, and then restored by curcumin treatment (Fig. 4D). Furthermore, under the same
conditions, the changes in the proportion of Annexin V and PI
double-positive MKN74 and SNU668 cells was monitored using FACS
analysis (P<0.05; Fig. 4E).
These results suggest that CAFs confer the resistance of GC cell
lines to 5-FU through the inhibition of apoptosis, and that
curcumin attenuates the chemoresistance of GC cell lines through
the JAK2/STAT3 signaling pathway.
Combined treatment with curcumin and 5-FU
inhibits CAF-induced tumor growth in the xenograft model of human
GC
To investigate whether curcumin contributes to
suppressed tumorigenesis in a mouse model, a GC cell line and
patient-derived primary CAFs were established using a previously
described method (23). In
previous research, the authors investigated the in vivo
effect of fibroblasts on resistance to 5-FU in GC cell line
xenografts (8), which were
established using only GC cell lines (1×106 cells each)
or mixed with CAFs (1×105). Tumor volume in xenografts
mixed with CAFs suppressed the effects of 5-FU. Therefore, the
present study investigated whether combination treatment (curcumin
and 5-FU) suppressed the growth of GC cell line-derived xenografts
(1×106 cells each) mixed with CAFs (5×105).
Six mice were used in each group. The results demonstrated that
combined treatment suppressed the tumor volume of the xenografts
mixed with CAFs (Fig. 5A). The
mean weight of the extracted tumors from the mice injected with GC
cells mixed with CAFs and subjected to combination treatment
(curcumin and 5-FU) was significantly lower than that of the single
treatment and control (DMSO). By contrast, the combination
treatment was not significantly different from that of the 5-FU
treatment alone (Fig. 5B). The
diameter of the tumor was 10.75 by 10.47 and the maximum tumor
volume in a single treatment group was 589.21 mm3
according to the calculation method. IHC revealed that the addition
of curcumin to the 5-FU treatment of CAF-mixed tumors upregulated
the expression of cleaved caspase-3 compared to the vehicle
treatment group (Fig. 5C). During
treatment, all mice survived and there was no difference in mouse
body weight among the treatment groups (Fig. S4). Overall, the in vivo
experiments revealed that curcumin treatment increased the
sensitivity of xenograft tumors containing CAFs to 5-FU through
increased apoptosis without any other side-effects observed in the
mice.
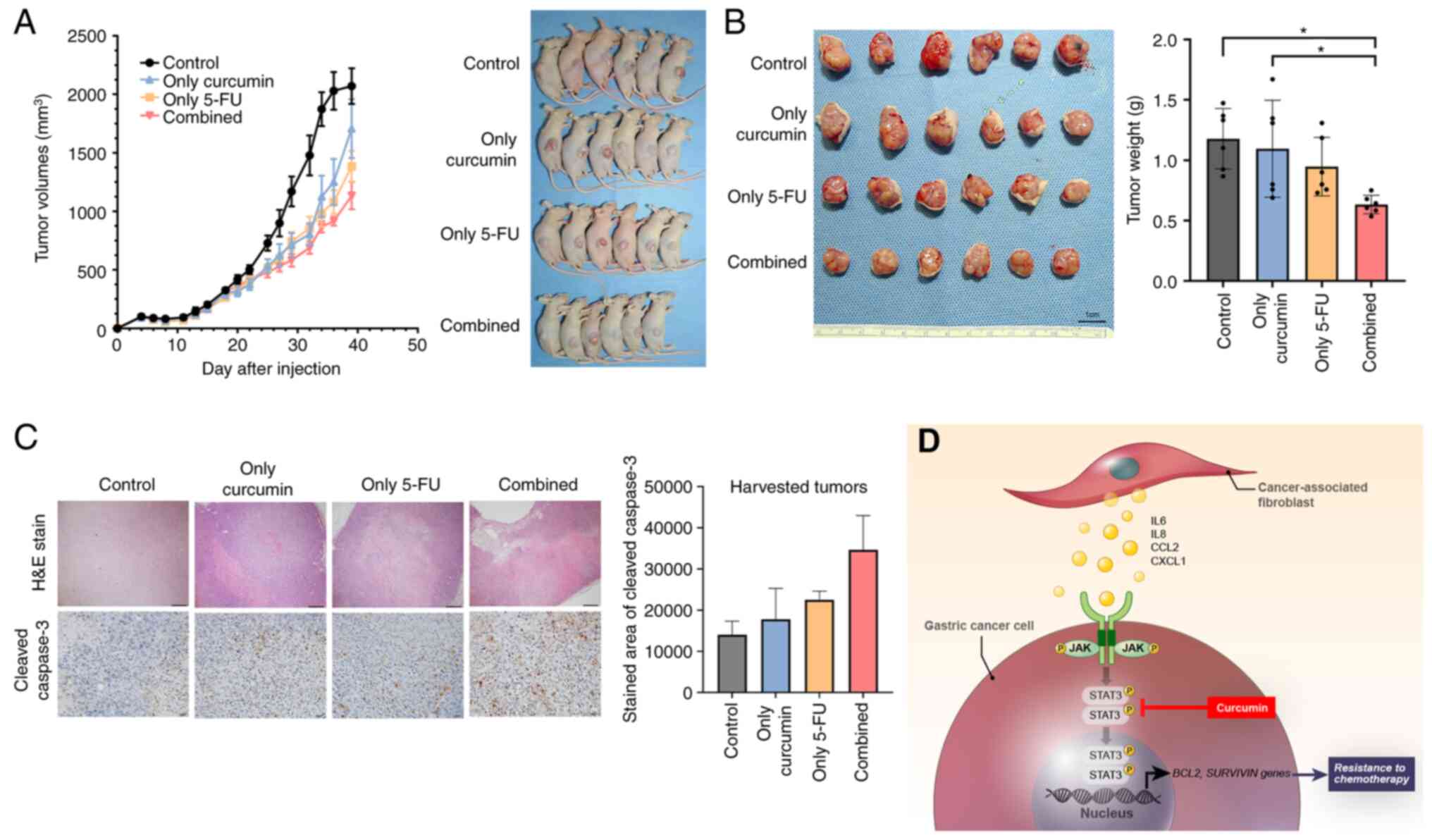 | Figure 5GC xenograft tumor growth inhibition
by combination treatment with Curcumin and 5-FU. (A) Mice growing
subcutaneous tumors were randomized to receive 21 intraperitoneal
injections three times a week of either DMSO or 5-FU or curcumin or
combined treatment (5-FU + curcumin). The tumor volume was measured
using calipers (n=6 mice in each group) (B) The tumor weight was
measured using a precision electronic balance. Representative
images of excised tumors in SNU668 models. Scale bar, 1 cm. (C)
Representative micrographs showing H&E staining and
immunohistochemical staining for cleaved caspase-3 in harvested
xenograft tumors derived from SNU668 cells mixed with CAFs after
treatment. Scale bar, 100 µm. (D) Schematic diagram of the
findings of the present study. CAF-induced cytokines activate the
JAK2/STAT3 pathway in gastric cancer cells via paracrine signaling,
which allows tumor cells to increasingly oppose apoptosis and
increase their survival and resistance to chemotherapy. Curcumin,
inhibitor for STAT3, that is a nature product, inhibits the
CAF-induced activation of the JAK2/STAT3 signaling pathway in GC
cells and consequently enhance the efficacy of chemotherapeutic
drugs. 5-FU, 5-fluorouracil; GC, gastric cancer; CCL2, C-C motif
chemokine ligand 2; CAF, cancer-associated fibroblast; H&E,
hematoxylin and eosin; IL-6, interleukin-6; IL-8, interleukin-8;
JAK, Janus-activated kinase; STAT3, signal transducer and activator
of transcription 3. *P<0.05. |
Discussion
In the present study, it was found that CAF-produced
cytokines, such as IL-6 and IL-8, and chemokines, such as CCL2
enhanced resistance to chemotherapy through the activation of the
JAK2/STAT3 axis in GC cells. It was also confirmed that the
CAF-activated JAK2/STAT3 axis was efficiently inhibited by the
natural product, curcumin. Finally, the present study examined
whether curcumin could restore resistance to chemotherapy in the
in vitro and in vivo experimental models (Fig. 5D).
In a previous study by the authors, it was
demonstrated that IL-6 usually originates from CAFs in GC tissues
and is consequently involved in resistance to chemotherapy through
the activation of the JAK/STAT3 signaling pathway in GC cells
(8). In addition, it was found
that the IL-6 receptor inhibitor exerted a negative effect on
CAF-induced resistance in GC experimental models (8). However, CAFs may be a source of
other secreted proteins, such as IL-8 or CCL2, which can enhance
the JAK/STAT3 pathway (28,29). Thus, the inhibition of the common
JAK/STAT3 pathway would be more ideal for reducing the effect of
CAFs on GC cells than the IL-6R inhibitor. In the present study,
secretome analysis revealed that the levels of IL-6, IL-8 and CCL2
were increased in CAF-co-cultured CM compared to the CM of cancer
cells cultured alone. In addition, transcriptome analysis with gene
function analysis revealed that the upregulated genes in CAFs
co-cultured with GC cells positively correlated with the JAK/STAT3
subset, and this result was validated using western blot analysis.
It was hypothesized that CAF-derived molecules may be candidates
for activating the JAK/STAT3 signal transduction pathway in GC
cells involved in resistance to chemotherapy.
STAT proteins are involved in the regulation of
essential signal transduction pathways that control proliferation,
differentiation and cell homeostasis (13,30,31). The receptors activated by
ligand-binding can phosphorylate specific tyrosine residues of STAT
proteins through activation of members of the Janus family of
protein tyrosine kinases (JAKs). Of the seven STAT subtypes, STAT3
has been suggested as a critical mediator in maintaining the
aggressive phenotype in many cancer cells through its retained
activity (32). Moreover,
previous studies have reported that the increased phosphorylation
of STAT3 is associated with a poor response to chemoradiation, and
the inhibition of STAT3 sensitizes cancer cells to 5-FU treatment
through the downregulation of STAT3-dependent proteins, such as
cyclin D or survivin (33-36).
These previous results support the findings of the present study,
demonstrating that CAF-induced STAT3 activation promotes resistance
to chemotherapy in GCs. Therefore, the suppression of JAK/STAT3
signaling activated by CAFs may be an effective therapeutic
strategy to overcome fibrotic stroma-induced resistance to
chemotherapy.
Over the past several decades, various strategies
have been tested to target STAT3 for the development of potential
anticancer drugs. These include small molecules that target
different domains of STAT3 and decoy oligonucleotides. The
structural analysis of STAT3 has generated several chemicals, such
as OPB-31121 or C188-9, targeting the SH2 domain of STAT3, and
these have yielded promising results in experimental studies
(37,38). However, OPB-31121 in phase I
clinical trials for hepatocellular carcinoma only yielded a minimal
response, while poor pharmacokinetic properties and severe
peripheral nerve toxicity were observed (39). In addition, a number of other
chemical drugs targeting STAT3 often exert adverse effects, such as
fatigue, infection and diarrhea, which may be related to the
physiological function of STAT3 in non-cancerous tissues; thus, few
drugs targeting STAT3 have currently been approved for use in
clinical settings (40).
Curcumin, a natural product derived from turmeric used as a dietary
spice and coloring agent, has a negative effect on the binding of
JAK protein to cytokine receptors, thereby blocking the
phosphorylation and activation of STAT3 (22). It was first tested in an
experimental model of lung cancer, demonstrating an inhibitory
effect on STAT3 phosphorylation in a time-and
concentration-dependent manner (22). In addition, curcumin inhibits IL-6
induced STAT3 phosphorylation in myeloma cells (41). While the drug metabolites
generated from synthetic drug sources can create a variety of
adverse side-effects, drugs formed from natural sources, such as
curcumin, may have limited side-effects. In the present study, low
concentrations of curcumin, which was not toxic to GC cells,
effectively suppressed CAF-induced JAK/STAT3 activation, as well as
STAT3-dependent proteins, such as Bcl-2 and survivin. Moreover,
additional low-dose curcumin had a positive effect on
chemotherapy-induced apoptosis in experimental models. These
results suggest that low-dose curcumin treatment or a
turmeric-based diet during chemotherapy in GC patients may be an
effective strategy which may be used to improve the response.
In the present study, in the animal experiments,
although additional curcumin treatment significantly reduced tumor
growth compared to the control, it did not exhibit a significant
difference from the 5-FU treatment alone. In fact, the limitation
of curcumin is its low bioavailability and short time for
metabolization as a natural product. Therefore, different curcumin
analogs have been synthesized to increase their stability. FLLL32
has two hydrogens of curcumin replaced with a spiro-cyclohexyl ring
and methyl groups, which can improve its stability (42). Several previous reports have
described that FLLL32 potently reduced tumor growth of xenografts
in bone and breast cancers (43,44). Therefore, curcumin analogs more
effectively improve the response to chemotherapy in GC models,
including CAFs, compared to curcumin, in future studies.
In conclusion, the present study demonstrated that
CAFs enhanced resistance to chemotherapy in GC through the
activation of the JAK2/STAT3 signaling pathway. Curcumin, a natural
product derived from turmeric, effectively reduced CAF-induced
STAT3 activation in GC cells and subsequently enhanced the effects
of chemotherapy in GC models. The combination of curcumin and
conventional chemotherapy may thus be a promising strategy with
which to overcome resistance to chemotherapy in GC with profuse
fibroblasts.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IHH and HH performed the majority of the
experiments, analyzed the data and managed the clinical
information, and were also involved in the conception and design of
the study. LW and HH provided the resources. DL, KSC, HJO and THK
were involved in data curation. LW, HYJ, and TMK were involved in
formal analysis. JW and IHH were involved in the investigative
aspects of the study (e.g., data collection). JW, IHH and HH
drafted the manuscript. IHH, HH and HJO provided administrative,
technical, or material support. IHH, KSC and HH supervised the
study. IHH and HH confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai Cancer Center (IRB No. 050432-4-1911D).
Informed consent was obtained from all the participants, and
procedures were conducted according to the Declaration of Helsinki.
Animal care and handling procedures were performed in accordance
with the Ajou University School of Medicine Institution Animal Care
and Use Committee guidelines, and all animal experiments were
approved by the Animal Research Committee of the institution.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Research Foundation of Korea (NRF) funded by the Ministry of
Education (no. 2020R1A6A1A03043539 and 2020R1I1A1A01070961 and a
NRF grant funded by the Korean government, the Ministry of Science
and ICT (no. 2020R1A2C1006273).
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
CAF
|
cancer-associated fibroblast
|
|
CCL2
|
C-C motif chemokine ligand 2
|
|
CM
|
conditioned medium
|
|
GC
|
gastric cancer
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
IL-6
|
interleukin-6
|
|
IL-8
|
interleukin-8
|
|
JAK
|
Janus-activated kinase
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar
|
|
3
|
Guideline Committee of the Korean Gastric
Cancer Association (KGCA), Development Working Group & Review
Panel: Korean practice guideline for gastric cancer 2018: An
evidence-based, multi-disciplinary approach. J Gastric Cancer.
19:1–48. 2019. View Article : Google Scholar
|
|
4
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2018 (5th edition).
Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar
|
|
5
|
Wu Y, Grabsch H, Ivanova T, Tan IB, Murray
J, Ooi CH, Wright AI, West NP, Hutchins GG, Wu J, et al:
Comprehensive genomic meta-analysis identifies intra-tumoural
stroma as a predictor of survival in patients with gastric cancer.
Gut. 62:1100–1111. 2013. View Article : Google Scholar
|
|
6
|
Lei Z, Tan IB, Das K, Deng N, Zouridis H,
Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, et al: Identification
of molecular subtypes of gastric cancer with different responses to
PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology.
145:554–565. 2013. View Article : Google Scholar
|
|
7
|
Ma J, Song X, Xu X and Mou Y:
Cancer-associated fibroblasts promote the chemo-resistance in
gastric cancer through secreting IL-11 targeting JAK/STAT3/Bcl2
pathway. Cancer Res Treat. 51:194–210. 2019. View Article : Google Scholar
|
|
8
|
Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM,
Choi KS, Son SY, Han SU, Brekken RA, Lee D and Hur H: Targeting
interleukin-6 as a strategy to overcome stroma-induced resistance
to chemotherapy in gastric cancer. Mol Cancer. 18:682019.
View Article : Google Scholar
|
|
9
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar
|
|
10
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
11
|
Kuzet SE and Gaggioli C: Fibroblast
activation in cancer: When seed fertilizes soil. Cell Tissue Res.
365:607–619. 2016. View Article : Google Scholar
|
|
12
|
Resemann HK, Watson CJ and Lloyd-Lewis B:
The Stat3 paradox: A killer and an oncogene. Mol Cell Endocrinol.
382:603–611. 2014. View Article : Google Scholar
|
|
13
|
Aaronson DS and Horvath CM: A road map for
those who don't know JAK-STAT. Science. 296:1653–1655. 2002.
View Article : Google Scholar
|
|
14
|
Sweet K, Hazlehurst L, Sahakian E, Powers
J, Nodzon L, Kayali F, Hyland K, Nelson A and Pinilla-Ibarz J: A
phase I clinical trial of ruxolitinib in combination with nilotinib
in chronic myeloid leukemia patients with molecular evidence of
disease. Leuk Res. 74:89–96. 2018. View Article : Google Scholar
|
|
15
|
McLornan DP, Khan AA and Harrison CN:
Immunological consequences of JAK inhibition: Friend or foe? Curr
Hematol Malig Rep. 10:370–379. 2015. View Article : Google Scholar
|
|
16
|
Wake MS and Watson CJ: STAT3 the
oncogene-still eluding therapy? FEBS J. 282:2600–2611. 2015.
View Article : Google Scholar
|
|
17
|
Gupta SC, Patchva S, Koh W and Aggarwal
BB: Discovery of curcumin, a component of golden spice, and its
miraculous biological activities. Clin Exp Pharmacol Physiol.
39:283–299. 2012. View Article : Google Scholar
|
|
18
|
Lee WH, Bebawy M, Loo CY, Luk F, Mason RS
and Rohanizadeh R: Fabrication of curcumin micellar nanoparticles
with enhanced anti-cancer activity. J Biomed Nanotechnol.
11:1093–1105. 2015. View Article : Google Scholar
|
|
19
|
Lee WH, Loo CY, Young PM, Rohanizadeh R
and Traini D: Curcumin nanoparticles attenuate production of
pro-inflammatory markers in lipopolysaccharide-induced macrophages.
Pharm Res. 33:315–327. 2016. View Article : Google Scholar
|
|
20
|
Lee WH, Loo CY, Young PM, Traini D, Mason
RS and Rohanizadeh R: Recent advances in curcumin nanoformulation
for cancer therapy. Expert Opin Drug Deliv. 11:1183–1201. 2014.
View Article : Google Scholar
|
|
21
|
Wong TF, Takeda T, Li B, Tsuiji K,
Kitamura M, Kondo A and Yaegashi N: Curcumin disrupts uterine
leiomyosarcoma cells through AKT-mTOR pathway inhibition. Gynecol
Oncol. 122:141–148. 2011. View Article : Google Scholar
|
|
22
|
Byun SY, Kim DB and Kim E: Curcumin
ameliorates the tumor-enhancing effects of a high-protein diet in
an azoxymethane-induced mouse model of colon carcinogenesis. Nutr
Res. 35:726–735. 2015. View Article : Google Scholar
|
|
23
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar
|
|
24
|
Lee D, Ham IH, Son SY, Han SU, Kim YB and
Hur H: Intratumor stromal proportion predicts aggressive phenotype
of gastric signet ring cell carcinomas. Gastric Cancer. 20:591–601.
2017. View Article : Google Scholar
|
|
25
|
Ryan R, Gibbons D, Hyland JM, Treanor D,
White A, Mulcahy HE, O'Donoghue DP, Moriarty M, Fennelly D and
Sheahan K: Pathological response following long-course neoadjuvant
chemoradiotherapy for locally advanced rectal cancer.
Histopathology. 47:141–146. 2005. View Article : Google Scholar
|
|
26
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar
|
|
27
|
Wang H, Wu X and Chen Y: Stromal-immune
score-based gene signature: A prognosis stratification tool in
gastric cancer. Front Oncol. 9:12122019. View Article : Google Scholar
|
|
28
|
Tsuyada A, Chow A, Wu J, Somlo G, Chu P,
Loera S, Luu T, Li AX, Wu X, Ye W, et al: CCL2 mediates cross-talk
between cancer cells and stromal fibroblasts that regulates breast
cancer stem cells. Cancer Res. 72:2768–2779. 2012. View Article : Google Scholar
|
|
29
|
Wu J, Gao FX, Wang C, Qin M, Han F, Xu T,
Hu Z, Long Y, He XM, Deng X, et al: IL-6 and IL-8 secreted by
tumour cells impair the function of NK cells via the STAT3 pathway
in oesophageal squamous cell carcinoma. J Exp Clin Cancer Res.
38:3212019. View Article : Google Scholar
|
|
30
|
O'Shea JJ, Holland SM and Staudt LM: JAKs
and STATs in immunity, immunodeficiency, and cancer. N Engl J Med.
368:161–170. 2013. View Article : Google Scholar
|
|
31
|
Quintás-Cardama A and Verstovsek S:
Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and
resistance. Clin Cancer Res. 19:1933–1940. 2013. View Article : Google Scholar
|
|
32
|
Birner P, Toumangelova-Uzeir K, Natchev S
and Guentchev M: STAT3 tyrosine phosphorylation influences survival
in glioblastoma. J Neurooncol. 100:339–343. 2010. View Article : Google Scholar
|
|
33
|
Qin A, Yu Q, Gao Y, Tan J, Huang H, Qiao Z
and Qian W: Inhibition of STAT3/cyclinD1 pathway promotes
chemotherapeutic sensitivity of colorectal caner. Biochem Biophys
Res Commun. 457:681–687. 2015. View Article : Google Scholar
|
|
34
|
Spitzner M, Roesler B, Bielfeld C, Emons
G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz
J, et al: STAT3 inhibition sensitizes colorectal cancer to
chemoradiotherapy in vitro and in vivo. Int J Cancer. 134:997–1007.
2014. View Article : Google Scholar
|
|
35
|
Stella S, Tirrò E, Conte E, Stagno F, Di
Raimondo F, Manzella L and Vigneri P: Suppression of survivin
induced by a BCR-ABL/JAK2/STAT3 pathway sensitizes
imatinib-resistant CML cells to different cytotoxic drugs. Mol
Cancer Ther. 12:1085–1098. 2013. View Article : Google Scholar
|
|
36
|
Wen K, Fu Z, Wu X, Feng J, Chen W and Qian
J: Oct-4 is required for an antiapoptotic behavior of
chemoresistant colorectal cancer cells enriched for cancer stem
cells: Effects associated with STAT3/survivin. Cancer Lett.
333:56–65. 2013. View Article : Google Scholar
|
|
37
|
Brambilla L, Genini D, Laurini E, Merulla
J, Perez L, Fermeglia M, Carbone GM, Pricl S and Catapano CV:
Hitting the right spot: Mechanism of action of OPB-31121, a novel
and potent inhibitor of the signal transducer and activator of
transcription 3 (STAT3). Mol Oncol. 9:1194–1206. 2015. View Article : Google Scholar
|
|
38
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
Ligand-dependent and -independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar
|
|
39
|
Okusaka T, Ueno H, Ikeda M, Mitsunaga S,
Ozaka M, Ishii H, Yokosuka O, Ooka Y, Yoshimoto R, Yanagihara Y and
Okita K: Phase 1 and pharmacological trial of OPB-31121, a signal
transducer and activator of transcription-3 inhibitor, in patients
with advanced hepatocellular carcinoma. Hepatol Res. 45:1283–1291.
2015. View Article : Google Scholar
|
|
40
|
Beebe JD, Liu JY and Zhang JT: Two decades
of research in discovery of anticancer drugs targeting STAT3, how
close are we? Pharmacol Ther. 191:74–91. 2018. View Article : Google Scholar
|
|
41
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
42
|
Bill MA, Fuchs JR, Li C, Yui J, Bakan C,
Benson DM Jr, Schwartz EB, Abdelhamid D, Lin J, Hoyt DG, et al: The
small molecule curcumin analog FLLL32 induces apoptosis in melanoma
cells via STAT3 inhibition and retains the cellular response to
cytokines with anti-tumor activity. Mol Cancer. 9:1652010.
View Article : Google Scholar
|
|
43
|
Onimoe GI, Liu A, Lin L, Wei CC, Schwartz
EB, Bhasin D, Li C, Fuchs JR, Li PK, Houghton P, et al: Small
molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent
growth suppressive activity in osteosarcoma cells and tumor growth
in mice. Invest New Drugs. 30:916–926. 2012. View Article : Google Scholar
|
|
44
|
Yan J, Wang Q, Zou K, Wang L, Schwartz EB,
Fuchs JR, Zheng Z and Wu J: Inhibition of the JAK2/STAT3 signaling
pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep.
12:498–502. 2015. View Article : Google Scholar
|



















