Introduction
Endometrial cancer is the most common gynecological
malignancy in developed countries and is increasing in both
incidence and associated mortality (1-3).
Most patients with endometrial cancer are diagnosed in the early
stages and exhibit a favorable 5-year relative survival rate (95%),
if the appropriate surgical procedure is provided (4). On the other hand, patients with
regional spread and distant metastasis beyond the uterus have a
poor 5-year relative survival rate (69 and 17%, respectively)
(4). Although radiation therapy,
hormonal therapy and chemotherapy with cytotoxic agents have been
used for advanced and recurrent endometrial cancer, the
effectiveness of these therapies is limited. It is reported that
the efficacy of the first-line chemotherapy is 30-57% and that the
median progression-free survival is <1 year (5,6). Of
note, the effectiveness of second-line chemotherapy is more limited
(6). Therefore, novel biomarkers
are required to select patients with endometrial cancer with poor
prognosis at the time of biopsy or initial surgery.
Claudins (CLDNs) are tetraspan proteins with a short
cytoplasmic N-terminus, two extracellular loops and a C-terminal
cytoplasmic domain (7). CLDNs form
tight junctions and are composed of >20 subtypes in humans. They
function as a physical barrier or gate of small molecules (8-11)
and as signaling platforms to coordinate diverse cellular behaviors
(10-12). CLDNs are expressed in distinct
patterns in different tissues and cells. Furthermore, CLDNs are
useful cancer biomarkers, as they are frequently upregulated and
are associated with malignant traits of cancers, such as invasion,
migration, metastasis and chemoresistance (13,14).
Recent studies by our group demonstrated that
aberrant CLDN6 expression is a biomarker for poor prognosis in
endometrial cancer, and that abnormal CLDN6 signaling enhances
malignant behaviors by AKT-dependent phosphorylation of estrogen
receptor-α (ERα) through the Src-family kinases (SFK)/PI3K/AKT
signaling pathway (15-17). Among CLDN subtypes, CLDN9 is the
closest member to CLDN6 (18) and
their genes are located adjacent to each other on the human genome.
While several experimental studies determined that CLDN9
overexpression promotes cancer malignancy (19,20),
endogenous expression of CLDN9 in cancer tissues has not been
indicated at the protein level and its clinicopathological
significance remains obscure due to the unavailability of selective
antibodies.
In the present study, it was demonstrated that high
CLDN9 expression predicts poor prognosis in patients with
endometrial cancer using a newly established specific monoclonal
antibody (mAb) (21). In addition,
it was indicated that the combination of CLDN9 and CLDN6 is
beneficial for predicting poor outcome in endometrial cancer.
Materials and methods
Cell culture, expression vectors, and
transfection
293T and Ishikawa cells were gifted by Professor
Suzuki, Fukushima Medical University (Fukushima, Japan) and
Professor Yamada, Wakayama Medical University (Wakayama, Japan),
respectively. They were grown in Dulbecco's Modified Eagle Medium
(Sigma-Aldrich; Merck KGaA) with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA) and 1%
penicillin-streptomycin-amphotericin B suspension (cat. no.
161-23181; Fujifilm).
The protein-coding regions of the human
CLDN1, CLDN4, CLDN5, CLDN6 and
CLDN9 genes were amplified from complementary or genomic DNA
extracted from 293T cells with the PrimeSTAR GXL (cat. no. R050A;
Clontech) PCR kit following the manufacturer's protocol. They were
cloned into the NotI/BamHI site of the
CSII-EF-MCS-IRES2-Venus plasmid (cat. no. RDB04384; RIKEN
BioResource Center). For transient expression of the target genes
(CLDN1, CLDN4, CLDN5, CLDN6 and
CLDN9), 5×106 293T cells were transfected with 10
µg of the indicated vectors using 30 µg of
Polyethyleneimine Max (PEI Max; Cosmo Bio Co., Ltd.) eight hours
after passage. The pTagRFP-laminB1 vector (cat. no. FP370; Evrogen)
was co-transfected with the CLDN9-expression vector to
visualize the nuclei in successfully transfected cells. The
transfection efficiency was evaluated by Venus expression with a
fluorescence microscope (IX71; Olympus Corporation).
Antibodies
Rat monoclonal antibodies (mAbs) against the
cytoplasmic tail of human CLDN6 and CLDN9 were generated using an
iliac lymph node method, as previously described (16,21).
Clone #15 for CLDN6 and clone 1D1 for CLDN9 were used in the
present study. A mouse anti-p53 mAb (cat. no. OP43; clone Ab-6;
Calbiochem; Merck KGaA) was used for evaluation of p53
expression.
Immunoblotting
Total cell lysates were prepared using CelLytic™ MT
Cell Lysis Reagent (cat. no. C3228; Sigma-Aldrich; Merck KGaA). The
protein concentration of the total cell lysates was measured by
Pierce™ BCA Protein Assay Kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.) and 0.5 µg was loaded per lane for
one-dimensional SDS-PAGE (12.5%). Subsequently, the protein bands
were electrophoretically transferred onto an Immobilon membrane
(MilliporeSigma). The membrane was blocked with PBS containing 4%
skimmed milk (Morinaga) for 30 min and treated with the supernatant
of the rat anti-CLDN9 hybridomaprima for 60 min at room
temperature. After washing with PBS three times, the membrane was
incubated with 1:2,000-fold diluted HRP-conjugated anti-rat IgG
(cat. no. NA935V; GE Healthcare). The signals were detected by
chemiluminescence (cat. no. WSE-7110EzWestLumi One; ATTO).
Immunofluorescence
Ishikawa cells (5.0×105) were grown on
coverslips coated with Cellmatrix Type I-A (Nitta Gelatin). After
48 h, the samples were fixed in ice-cold ethanol for 10 min. After
washing with PBS, they were preincubated in PBS containing 5%
skimmed milk (Morinaga) at room temperature. They were subsequently
incubated overnight at 4°C with the supernatant of anti-CLDN9
hybridoma, followed by washing with PBS three times and incubation
with the secondary antibody, 1:400 diluted Alexa Fluor 488
AffiniPure Donkey Anti-Rat IgG antibody (cat. no. 712-545-150;
Jackson ImmunoResearch) for 60 min at room temperature. After
washing with PBS, the specimens were mounted with Fluoro-Gel II
with DAPI (cat. no. 17985-51; Electron Microscopy Sciences). The
samples were observed and images were acquired with a fluorescent
microscope (IX71; Olympus Corporation).
Tissue collection, immunostaining and
histological analysis
Formalin-fixed paraffin-embedded (FFPE) tissue
sections were obtained from 248 patients with endometrial cancer
[age, mean ± standard deviation (SD) of 58.2±11.5 years; range,
30-83 years] who underwent hysterectomy and
bilateral-salpingo-oophorectomy and/or retroperitoneal
lymphadenectomy between January 2003 and March 2015 at Fukushima
Medical University Hospital (FMUH; Fukushima, Japan). The subjects
were limited to patients who were confirmed to have at least 5-year
outcomes and those who had died due to endometrial cancer and
metastasis. Detailed information, including postoperative pathology
diagnosis reports, age, stage [International Federation of
Gynecology and Obstetrics (FIGO) 2008] (22), histological type, histological
grade, Bokhman subtype (23),
lymphovascular space involvement (LVSI), lymph node metastasis,
distant metastasis, recurrence status, disease-specific survival
(DSS) and disease-free survival (DFS), was obtained. The staging of
patients encountered between January 2003 and December 2007 was
modified in accordance with the FIGO 2008 system. Distant
metastasis was judged by diagnostic imaging. Normal adult tissues,
including the pituitary gland, cerebrum, liver, lung, and kidney,
were collected from autopsy specimens dissected at FMUH between
January 2013 and December 2014. Three to four specimens among six
cases (a 29-year-old male, 42-year-old female, 51-year-old female,
57-year-old male, 65-year-old male and a 71-year-old female) were
examined and a representative image was presented for each
organ.
For immunostaining, the FFPE tissue blocks were
sliced into 5-µm-thick sections, deparaffinized with xylene
and rehydrated using a graded series of ethanol. The sections were
then immersed in 0.3% hydrogen peroxide in methanol for 20 min at
room temperature to block endogenous peroxidase activity. Antigen
retrieval was performed by incubating the sections in boiling 10 mM
citric acid buffer (pH 5.0) using a microwave for 10 min. After
blocking with 5% skimmed milk (Morinaga) at room temperature for 30
min, the sections were incubated overnight at 4°C with supernatants
of the rat anti-CLDN6 or CLDN9 hybridoma. After washing with PBS, a
secondary antibody reaction was performed by using the Histofine
Simple Stain mouse MAX-PO kit (cat. no. 414311; Nichirei
Biosciences, Inc.) for 3′,3′-diaminobenzidine as a chromogen
according to the manufacturer's instructions. Immunostaining for
CLDN6 was performed as previously described (16). p53 was stained following the
manufacturer's protocol.
Immunostaining results were interpreted by two
independent pathologists and one gynecologist using a
semiquantitative scoring system. The immunostaining reactions were
evaluated according to signal intensity (SI; 0, negative; 1, weak;
2, moderate; 3, strong). The receiver operating characteristic
(ROC) curve was plotted and analyzed (Fig. S1) to determine the optimal cut-off
values of the SI for CLDN9 expression. CLDN6 expression was
assessed by the immunoreactive score (IRS) as described previously
(16). p53 mutation was assessed
following the most accepted criteria (24).
Statistical analysis
The χ2 test was used to evaluate the
relationship between CLDN9 expression and clinicopathological
parameters such as age, stage, histological type, histological
grade, Bokhman subtype, LVSI, lymph node metastasis, distant
metastasis, DSS and DFS. Survival analysis was performed using the
Kaplan-Meier method and differences between groups were analyzed
using the log-rank test. Cox regression according to the univariate
and multivariate model was used to identify predictors of survival.
In addition, the expression of CLDN9 and CLDN6 was compared and
statistical analysis was performed in a similar way. Two-tailed
P-values of <0.05 were considered to indicate a statistically
significant result. When comparing the disease-specific and
disease-free survival among four groups, P<0.125 was used as the
threshold for a statistically significant result to counteract the
multiple comparisons problem by applying Bonferroni correction. All
statistical analyses were performed using SPSS version 24.0
software (IBM Corporation).
Results
Verification of anti-CLDN9 mAb
First, the reactivity and specificity of the
anti-CLDN9 mAb were tested against the C-terminal cytoplasmic
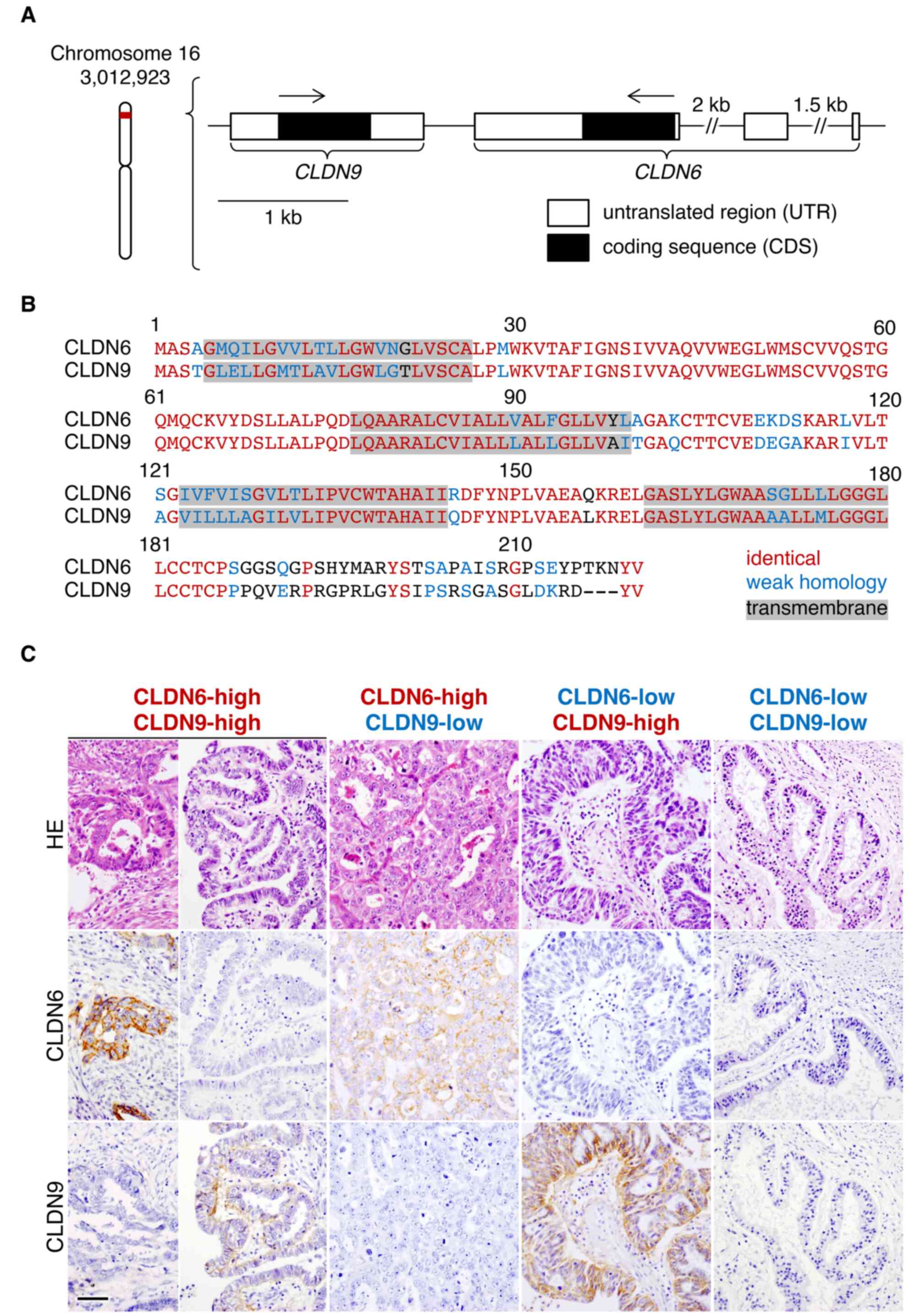
region of human CLDN9 (Fig. 1A),
which was recently established by our group (21). To this end, 293T cells were
transiently transfected with individual CLDN expression vectors,
followed by western blot and immunohistochemistry using whole-cell
extracts and cell blocks, respectively. As presented in Fig. 1B and C, the anti-CLDN9 mAb appeared
to specifically recognize CLDN9 but not CLDN1, CLDN4, CLDN5 or
CLDN6, which are the four closest members to CLDN9 among the CLDN
family. In addition, immunofluorescence staining using the
anti-CLDN9 mAb revealed positive signals along cell-cell borders in
the endometrial cancer cell line Ishikawa overexpressing CLDN9
(Fig. 1D). Furthermore, CLDN9
expression was detected in normal human pituitary gland (Fig. 1E), but not in cerebral, liver, lung
or kidney tissue (Fig. S2).
Differential expression of CLDN9 among
endometrial cancer subjects
Next, the expression of CLDN9 was evaluated by
immunohistochemistry in endometrial cancer tissues resected from
248 patients, whose demographic and clinicopathological
characteristics are presented in Table
I. CLDN9 was mainly distributed along the cell membranes of
endometrial carcinoma cells, but it was diffusely localized in
certain cases (Fig. 2). The
expression of CLDN9 was semi-quantified by determining the SI, as
the percentage of positive cells was not >10% in most cases
(mean ± SD, 6.30±6.84%). The SI varied among the subjects, which
was 3 in 18 subjects (7.3%), 2 in 25 (10.1%), 1 in 46 (18.5%) and 0
in 159 (64.1%) (Fig. S3). Based
on the ROC analysis, the samples were divided into two groups: Low
CLDN9 expression (SI <2) and high CLDN9 expression (SI ≥2;
Fig. S1).
 | Table IClinicopathological characteristics
of patients with endometrial cancer (n=248). |
Table I
Clinicopathological characteristics
of patients with endometrial cancer (n=248).
| Parameter | Value |
|---|
| Age, years | 58.1±11.5
(30-83) |
| Stage | |
| I | 182 (73.4) |
| II | 6 (2.4) |
| III | 40 (16.1) |
| IV | 20 (8.1) |
| Histological
type | |
| Endometrioid
carcinoma | 226 (91.1) |
| Grade 1 | 145 (58.5) |
| Grade 2 | 40 (16.1) |
| Grade 3 | 41 (16.5) |
| Serous
carcinoma | 7 (2.8) |
| Clear
carcinoma | 6 (2.4) |
| Mucinous
carcinoma | 2 (0.8) |
| Other | 7 (2.8) |
High CLDN9 expression is an independent
poor prognostic marker for endometrial cancer
Kaplan-Meier plots revealed significantly shorter
DSS and DFS in the high CLDN9 expression group than in the low
expression group (Fig. 3A and B).
The 5-year DSS rates in the low and high CLDN9 expression groups
were 87.8 and 62.8%, and the DFS rates were 84.9 and 62.8%,
respectively.
Among the clinicopathological factors, high CLDN9
expression was significantly associated with non-endometrioid
histology (P=0.021) and lymph node metastasis (P=0.012; Table II). By contrast, younger age
(P=0.370), stage III/IV (P=0.072), histological grade 3 (P=0.431),
histological type II (which includes endometrioid carcinoma grade
3, serous carcinoma and clear cell carcinoma; P=0.101),
lymphovascular space involvement (LVSI; P=0.070) and distant
metastasis (P=0.414) were not related to high CLDN9 expression.
 | Table IIRelationship between CLDN9 expression
and clinicopathological factors in patients with endometrial cancer
(n=248). |
Table II
Relationship between CLDN9 expression
and clinicopathological factors in patients with endometrial cancer
(n=248).
| Parameter | Total | CLDN9-high
(n=43) | CLDN9-low
(n=205) | P-value |
|---|
| Age, years | | | | 0.370 |
| <50 | 53 (21.4) | 7 (16.3) | 46 (22.4) | |
| ≥50 | 195 (78.6) | 36 (83.7) | 159 (77.6) | |
| Stage | | | | 0.072 |
| I/II | 188 (75.8) | 28 (65.1) | 160 (78.0) | |
| III/IV | 60 (24.2) | 15 (34.9) | 45 (22.0) | |
| Histological
type | | | | 0.021a |
| Endometrioid | 226 (91.1) | 35 (81.4) | 191 (93.1) | |
| Serous | 7 (2.8) | 1 (2.3) | 6 (2.9) | |
| Clear | 6 (2.4) | 4 (9.3) | 2 (1.0) | |
| Mucinous | 2 (0.8) | 0 (0.0) | 2 (1.0) | |
| Others | 7 (2.8) | 3 (7.0) | 4 (2.0) | |
| Histological
grade | | | | 0.431 |
| 1/2 | 185 (74.6) | 27 (62.8) | 158 (77.1) | |
| 3 | 41 (16.5) | 8 (18.6) | 33 (16.1) | |
| Other | 22 (8.9) | 8 (18.6) | 14 (6.8) | |
| Histological
classification | | | | 0.101 |
| Type I | 185 (74.6) | 27 (62.8) | 158 (77.1) | |
| Type II | 54 (21.8) | 13 (30.2) | 41 (20.0) | |
| Other | 9 (3.6) | 3 (7.0) | 6 (2.9) | |
| LVSI | | | | 0.070 |
| (−) | 178 (71.8) | 26 (60.5) | 152 (74.1) | |
| (+) | 70 (28.2) | 17 (39.5) | 53 (25.9) | |
| Nodal stage | | | | 0.012 |
| N0 | 203 (81.9) | 30 (69.7) | 173 (84.4) | |
| N1 | 34 (13.7) | 11 (25.6) | 23 (11.2) | |
| Unknown | 11 (4.4) | 2 (4.7) | 9 (4.4) | |
| Metastasis
stage | | | | 0.414 |
| M0 | 229 (92.3) | 41 (95.3) | 188 (91.7) | |
| M1 | 19 (7.7) | 2 (4.6) | 17 (8.3) | |
In the univariate analysis, stage III/IV [hazard
ratio (HR)=15.69, 95% confidence interval (CI) 7.47-32.96,
P<0.001], endometrioid type [HR=0.35 (95% CI, 0.16-0.76),
P=0.008], histological grade 3 [HR=4.02 (95% CI, 2.01-8.02),
P<0.001], histological type II [HR=3.85 (95% CI, 2.02-7.35),
P<0.001], LVSI [HR=8.99 (95% CI, 4.50-17.97), P<0.001], lymph
node metastasis [HR=12.70 (95% CI, 6.37-25.32), P<0.001],
distant metastasis [HR=14.25 (95% CI, 7.45-27.26), P<0.001] and
high CLDN9 expression [HR=3.64 (95% CI, 1.94-6.81), P<0.001]
were significant prognostic factors for DSS of patients with
endometrial cancer (Table III).
In addition, p53 abnormality was not associated with CLDN9 in
histological type II cases (Table
SI).
 | Table IIIUnivariate analysis of
disease-specific survival in patients with endometrial cancer. |
Table III
Univariate analysis of
disease-specific survival in patients with endometrial cancer.
| Variable | HR | 95% CI | P-value |
|---|
| Age ≥50 years | 2.64 | 0.94-7.39 | 0.066 |
| Stage III/IV | 15.69 | 7.47-32.96 | <0.001 |
| Endometrioid
type | 0.35 | 0.16-0.76 | 0.008 |
| Histological grade
3 | 4.02 | 2.01-8.02 | <0.001 |
| Type II | 3.85 | 2.02-7.35 | <0.001 |
| LVSI (+) | 8.99 | 4.50-17.97 | <0.001 |
| N1 | 12.70 | 6.37-25.32 | <0.001 |
| M1 | 14.25 | 7.45-27.26 | <0.001 |
| CLDN9-high | 3.64 | 1.94-6.81 | <0.001 |
Subsequently, Cox multivariate analysis was
performed to determine the independent predictors of survival.
Among the variables analyzed, stage III/IV [HR=6.00 (95% CI,
1.94-18.56), P=0.002], LVSI [HR=3.34 (95% CI, 1.21-9.25), P=0.020],
distant metastasis [HR=6.74 (95% CI, 2.32-19.57), P<0.001] and
high CLDN9 expression [HR=4.99 (95% CI, 1.96-12.70), P<0.001]
were independent prognostic factors for DSS of patients with
endometrial cancer (Table IV). By
contrast, older age, endometrioid type, histological grade 3,
histological type II and lymph node metastasis were no independent
prognostic variables for them.
 | Table IVMultivariate analysis of
disease-specific survival in patients with endometrial cancer. |
Table IV
Multivariate analysis of
disease-specific survival in patients with endometrial cancer.
| Variable | HR | 95% CI | P-value |
|---|
| Stage III/IV | 6.00 | 1.94-18.56 | 0.002 |
| LVSI (+) | 3.34 | 1.21-9.25 | 0.020 |
| M1 | 6.74 | 2.32-19.57 | <0.001 |
| CLDN9-high | 4.99 | 1.96-12.70 | <0.001 |
CLDN9 expression correlates with CLDN6
expression in endometrial cancer
CLDN9 largely shares its alignment with CLDN6
(18) and its genetic locus is
adjacent to CLDN6 (Fig. 4A and B).
Furthermore, none of the two genes are primarily expressed in adult
tissues, except for the inner ear, olfactory epithelium and
anterior pituitary glands (21,25),
suggesting that they are regulated by similar mechanisms. Thus, it
was hypothesized that upregulation of CLDN6 and CLDN9 may be
mutually correlated in endometrial cancer tissues. To compare the
expression of CLDN9 and CLDN6, the 248 patients with endometrial
cancer were classified into four groups according to the expression
levels of CLDN9 and CLDN6 (Fig.
4C). In the high CLDN6 expression group (18 cases), 10 (55.6%)
and 8 (44.4%) had high and low CLDN9 expression, respectively,
whereas in the low CLDN6 expression group (n=230), 33 (14.3%) and
197 (85.7%) cases had high and low CLDN9 expression, respectively.
It was determined that high expression of CLDN9 in endometrial
cancer cases was significantly associated with high CLDN6
expression (P<0.001; Table V),
although CLDN6 and CLDN9 were principally expressed in different
tumor cells within endometrial cancer tissues (Fig. 4C, panels of CLDN6-high/CLDN9-high).
Of note, unlike CLDN9, p53 abnormality was positively associated
with CLDN6 in histological type II cases (Table SII).
 | Table VAssociation between CLDN6 and CLDN9
expression in patients with endometrial cancer. |
Table V
Association between CLDN6 and CLDN9
expression in patients with endometrial cancer.
| CLDN6 | CLDN9
| P-value |
|---|
| Low | High |
|---|
| Low | 197 (79.4) | 33 (13.3) | <0.001 |
| High | 8 (3.2) | 10 (4.0) | |
Combination of CLDN6 and CLDN9 expression
is advantageous in identifying patients with endometrial cancer
with poor prognosis
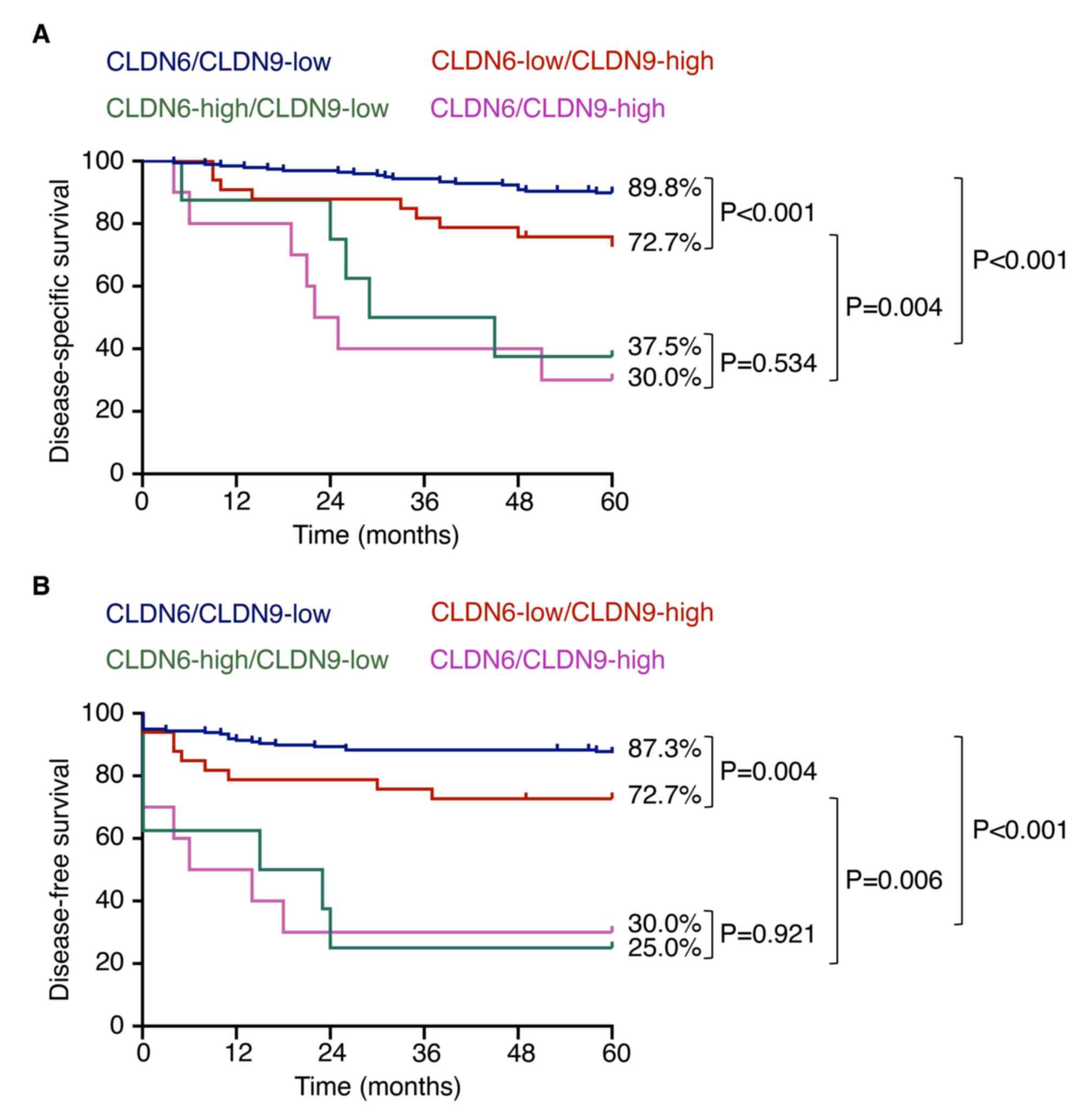
Kaplan-Meier plots revealed that DSS and DFS in the
high CLDN6 expression group were significantly lower than those in
the low CLDN6 expression group regardless of the CLDN9 expression
levels (Fig. 5). Furthermore,
there were significant differences in both DSS and DFS between the
subjects with CLDN6-low/CLDN9-low and those with
CLDN6-low/CLDN9-high. Their 5-year DSS rate was 89.8% in the former
and 72.7% in the latter group.
In the univariate analysis specifically for patients
with endometrial cancer with low CLDN6 expression, stage III/IV
[HR=18.42 (95% CI, 7.49-45.38), P<0.001], endometrioid type
[HR=0.33 (95% CI, 0.13-0.86), P=0.023], histological grade 3
[HR=4.06 (95% CI, 1.80-9.14), P<0.001], histological type II
[HR=3.82 (95% CI, 1.77-8.26), P<0.001], LVSI [HR=9.70 (95% CI,
4.29-21.93), P<0.001], lymph node metastasis [HR=16.47 (95% CI,
7.08-38.29), P<0.001], distant metastasis [HR=16.51 (95% CI,
7.71-35.37), P<0.001] and high CLDN9 expression [HR=2.98 (95%
CI, 1.36-6.55), P=0.007] were significant prognostic variables for
DSS. However, older age was not a prognostic factor for endometrial
cancer with low CLDN6 expression (Table VI).
 | Table VICox univariate analysis of
disease-specific survival in patients with endometrial cancer with
low CLDN6 expression. |
Table VI
Cox univariate analysis of
disease-specific survival in patients with endometrial cancer with
low CLDN6 expression.
| Variable | HR | 95% CI | P-value |
|---|
| Age ≥50 years | 1.90 | 0.66-5.45 | 0.201 |
| Stage III/IV | 18.42 | 7.49-45.38 | <0.001 |
| Endometrioid
type | 0.33 | 0.13-0.86 | 0.023 |
| Histological grade
3 | 4.06 | 1.80-9.14 | <0.001 |
| Type II | 3.82 | 1.77-8.26 | <0.001 |
| LVSI (+) | 9.70 | 4.29-21.93 | <0.001 |
| N1 | 16.47 | 7.08-38.29 | <0.001 |
| M1 | 16.51 | 7.71-35.37 | <0.001 |
| CLDN9-high | 2.98 | 1.36-6.55 | 0.007 |
Discussion
The immunohistochemical analysis of the present
study using the specific anti-CLDN9 mAb revealed that CLDN9 is
differentially expressed in endometrial cancer tissues. For
instance, the SI varied among the subjects with endometrial cancer
and 43 in the 248 cases (17.3%) had high CLDN9 expression. In
addition, there was a variation in the percentage of CLDN9-positive
cells among the cases. Of note, similar heterogeneity was also
observed in CLDN6 expression in endometrial cancer tissues
(16).
Among the CLDN family, CLDN1 and CLDN2 are prone to
be expressed in histological type I and II endometrial cancers,
respectively, although the prognostic value remains to be
determined (26,27). The present study demonstrated that
high CLDN9 expression represents a poor prognostic marker for
endometrial cancer. This conclusion was drawn from the following
results: i) The DSS and DFS in the high CLDN9 expression group of
endometrial cancer subjects were significantly lower compared with
those in the low CLDN9 expression group; ii) high CLDN9 expression
was significantly associated with adverse clinicopathological
factors, including non-endometrioid type and lymph node metastasis;
iii) univariate analysis indicated that high CLDN9 expression was a
significant prognostic factor (HR=3.64); and iv) upon multivariate
analysis, high CLDN9 expression appeared to be an independent
prognostic factor for DSS of the endometrial cancer subjects
(HR=4.99). Thus, not only aberrant CLDN6 expression (16) but also high CLDN9 expression
predicts a poor outcome for endometrial cancer. The high CLDN9
expression significantly correlated with the high CLDN6 expression
in endometrial cancer, further supporting this conclusion.
Recently, endometrial cancer was classified into
four molecular groups based on genetic characteristics:
Ultramutated, hypermutated, copy-number low and copy-number high
(28,29). More recently, simplified
classifications have also been proposed for broader clinical
applications (30-32). However, these genetic
classifications are not applicable for all cases mainly due to
insufficient tumor cell content. In addition, protein expression
does not necessarily correlate with gene mutations or mRNA
expression (33-35). Therefore, a classification based on
protein signals in FFPE specimens, which directly reflects
molecular characteristics, is valuable. In the present study, it
was demonstrated that the protein expression of CLDN9 and CLDN6 in
FFPE specimens reflected the prognosis of patients with endometrial
cancer. In more detail, the CLDN6-high group of patients with
endometrial cancer exhibited markedly lower DSS and DFS
irrespective of the expression levels of CLDN9. In addition,
CLDN6-low/CLDN9-high cases of endometrial cancer had significantly
decreased DSS and DFS compared with CLDN6-low/CLDN9-low cases.
Furthermore, while the proportion of CLDN6-high cases was <10%,
which was also in line with a previous study (16), nearly a quarter of cases had high
expression of either CLDN6 or CLDN9. Thus, classification depending
on the expression of CLDN6 and CLDN9 (CLDN6-high,
CLDN6-low/CLDN9-high and CLDN6-low/CLDN9-low) would be beneficial
in the aspects of easy use and broad clinical applications. In
addition, abnormal expression of p53 was not associated with
CLDN9-high, indicating that CLDN9-high predicts poor prognosis
regardless of p53 mutation.
It remains elusive by which mechanisms high CLDN9
expression results in poor outcome for patients with endometrial
cancer. However, it was previously reported that aberrant CLDN6
expression promotes endometrial cancer progression in vitro
and in vivo via hijacking the CLDN6/SFK/PI3K/AKT/ERα pathway
(12,17). For instance, abnormal CLDN6
signaling stimulates not only cell proliferation but also
collective cell migration in the leading front of endometrial
cancer cells. Since CLDN6 recruits and activates SFKs in second
extracellular domain-dependent and Y196/200-dependent manners, both
of which are highly conserved in CLDN9 (12), CLDN9 may similarly act as a cancer
promoter. Alternatively, since CLDN2 activates Yes-associated
protein (YAP) and stimulates self-renewal of human colorectal
cancer stem-like cells (36),
CLDN9 may also promote YAP activity to advance endometrial cancer
progression.
It is premature to discuss how the expression of
CLDN6 and CLDN9 is upregulated in endometrial cancer cells.
However, a previous study by our group demonstrated that the CLDN6
signaling ligand independently activates the expression of
endogenous CLDN6 gene in F9 embryonal carcinoma cells
(15). Therefore, the positive
loop of the CLDN6/SFK/AKT/RARγ cascade may contribute to inducing
and maintaining CLDN6 expression in endometrial cancer cells. As
the second extracellular domain and Y200 in CLDN6 are conserved in
CLDN9 as described above, the expression of CLDN9 may be
upregulated by a similar positive feedback mechanism.
As CLDNs are distributed on the cell surface, they
are good druggable targets of antibody therapy. Indeed, antibody
therapy against CLDN6, including CAR-T and antibody-drug
conjugates, exhibits efficiency in solid tumors (37-39).
Since CLDN9 has almost identical extracellular domains to those of
CLDN6, it appears feasible to obtain a bispecific antibody
targeting CLDN6 and CLDN9 together. Furthermore, CLDN9 and CLDN6
are rarely expressed in normal adult tissues, excluding the cochlea
(25), olfactory epithelia and
anterior pituitary glands (21),
implying that the antibody therapy may be less toxic to non-tumor
tissues. Therefore, taken together with the present findings that
CLDN6 and CLDN9 are poor prognostic factors, antibody therapy
targeting CLDN9 and CLDN6 would be promising for endometrial cancer
treatment.
In conclusion, the present study demonstrated that
upregulation of CLDN9 expression, determined using a selective mAb
against CLDN9, predicted poor prognosis for patients with
endometrial cancer. It is required to unveil the molecular
mechanisms of CLDN9 signals in endometrial cancer to develop a new
personalized medicine targeting CLDN9.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YE, KS, MakK, YK, ManK, AYH and TH performed
experiments. YE, KS and HC drafted the manuscript. KS and HC
conceived the study and supervised all experiments. KS, YE, YH and
HC reviewed the pathological diagnosis and/or analyzed
immunohistochemistry slides. SF, SS, TW and KF collected specimens
and assembled the database. YH, KF and HC helped with the writing
of the manuscript. YE, KS and HC confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent for
publication
The study was approved by the Ethics Committee of
Fukushima Medical University (Fukushima, Japan; approval code,
2019-311; approval date, Mar 18, 2020). The research was conducted
in accordance with the 1964 Helsinki Declaration or comparable
standards. This study was carried out by an opt-out method. Since
it was conducted as a retrospective study using cases with a
follow-up period of more than five years, the patients had already
died or stopped visiting the hospital. The experimental protocol
has been disclosed on the website and the patients or their
representatives were able to decline to participate in the survey
as they preferred.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Ms. Seiko Watanabe and Ms. Keiko
Watari, Fukushima Medical University for their technical assistance
and the Scientific English Editing Section of Fukushima Medical
University (Fukushima, Japan) for their help with the
manuscript.
Funding
This work was supported by JSPS KAKENHI (grant nos. 19K16615,
20K18224 and 20K16176) and by the Takeda Science Foundation.
Abbreviations:
|
CI
|
confidence interval
|
|
CLDN
|
claudin
|
|
DFS
|
disease-free survival
|
|
DSS
|
disease-specific survival
|
|
ERα
|
estrogen receptor α
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
HR
|
hazard ratio
|
|
IRS
|
immunoreactive score
|
|
LVSI
|
lymphovascular space involvement
|
|
mAb
|
monoclonal antibody
|
|
RARγ
|
retinoic acid receptor γ
|
|
SI
|
signal intensity
|
|
SFK
|
Src-family kinase
|
|
SD
|
standard deviation
|
|
YAP
|
Yes-associated protein
|
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henley SJ, Ward EM, Scott S, Ma J,
Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 126:2225–2249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu KH and Broaddus RR: Endometrial cancer.
N Engl J Med. 383:2053–2064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoki Y, Kanao H, Wang X, Yunokawa M,
Omatsu K, Fusegi A and Takeshima N: Adjuvant treatment of
endometrial cancer today. Jpn J Clin Oncol. 50:753–765. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bestvina CM and Fleming GF: Chemotherapy
for endometrial cancer in adjuvant and advanced disease settings.
Oncologist. 21:1250–1259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Furuse M, Fujita K, Hiiragi T, Fujimoto K
and Tsukita S: Claudin-1 and -2: Novel integral membrane proteins
localizing at tight junctions with no sequence similarity to
occludin. J Cell Biol. 141:1539–1550. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuse M and Tsukita S: Claudins in
occluding junctions of humans and flies. Trends Cell Biol.
16:181–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Itallie CM and Anderson JM: Claudins
and epithelial paracellular transport. Annu Rev Physiol.
68:403–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiba H, Osanai M, Murata M, Kojima T and
Sawada N: Transmembrane proteins of tight junctions. Biochim
Biophys Acta. 1778:588–600. 2008. View Article : Google Scholar
|
|
11
|
Zihni C, Mills C, Matter K and Balda MS:
Tight junctions: From simple barriers to multifunctional molecular
gates. Nat Rev Mol Cell Biol. 17:564–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugimoto K and Chiba H: The
claudin-transcription factor signaling pathway. Tissue Barriers.
9:19081092021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J: Context-dependent roles of claudins
in tumorigenesis. Front Oncol. 11:6767812021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabariès S and Siegel PM: The role of
claudins in cancer metastasis. Oncogene. 36:1176–1190. 2017.
View Article : Google Scholar
|
|
15
|
Sugimoto K, Ichikawa-Tomikawa N, Kashiwagi
K, Endo C, Tanaka S, Sawada N, Watabe T, Higashi T and Chiba H:
Cell adhesion signals regulate the nuclear receptor activity. Proc
Natl Acad Sci USA. 116:24600–24609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kojima M, Sugimoto K, Tanaka M, Endo Y,
Kato H, Honda T, Furukawa S, Nishiyama H, Watanabe T, Soeda S, et
al: Prognostic significance of aberrant claudin-6 expression in
endometrial cancer. Cancers (Basel). 12:27482020. View Article : Google Scholar :
|
|
17
|
Kojima M, Sugimoto K, Kobayashi M,
Ichikawa-Tomikawa N, Kashiwagi K, Watanabe T, Soeda S, Fujimori K
and Chiba H: Aberrant claudin-6-adhesion signaling promotes
endometrial cancer progression via estrogen receptor α. Mol Cancer
Res. 19:1208–1220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lal-Nag M and Morin PJ: The claudins.
Genome Biol. 10:2352009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma RK, Chheda ZS, Das Purkayastha BP,
Gomez-Gutierrez JG, Jala VR and Haribabu B: A spontaneous
metastasis model reveals the significance of claudin-9
overexpression in lung cancer metastasis. Clin Exp Metastasis.
33:263–275. 2016. View Article : Google Scholar
|
|
20
|
Liu H, Wang M, Liang N and Guan L:
Claudin-9 enhances the metastatic potential of hepatocytes via
Tyk2/Stat3 signaling. Turk J Gastroenterol. 30:722–731. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higashi AY, Higashi T, Furuse K, Ozeki K,
Furuse M and Chiba H: Claudin-9 constitutes tight junctions of
folliculostellate cells in the anterior pituitary gland. Sci Rep.
11:216422021. View Article : Google Scholar
|
|
22
|
Mutch DG and Prat J: 2014 FIGO staging for
ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conlon N, Da Cruz Paula A, Ashley CW,
Segura S, De Brot L, da Silva EM, Soslow RA, Weigelt B and DeLair
DF: Endometrial carcinomas with a 'serous' component in young women
are enriched for DNA mismatch repair deficiency, lynch syndrome,
and POLE exonuclease domain mutations. Am J Surg Pathol.
44:641–648. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Köbel M, Ronnett BM, Singh N, Soslow RA,
Gilks CB and McCluggage WG: Interpretation of P53
immunohistochemistry in endometrial carcinomas: Toward increased
reproducibility. Int J Gynecol Pathol. 38(Suppl 1): S123–S131.
2019. View Article : Google Scholar
|
|
25
|
Nakano Y, Kim SH, Kim HM, Sanneman JD,
Zhang Y, Smith RJH, Marcus DC, Wangemann P, Nessler RA and Bánfi B:
A claudin-9-based ion permeability barrier is essential for
hearing. PLoS Genet. 5:e10006102009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sobel G, Németh J, Kiss A, Lotz G, Szabó
I, Udvarhelyi N, Schaff Z and Páska C: Claudin 1 differentiates
endometrioid and serous papillary endometrial adenocarcinoma.
Gynecol Oncol. 103:591–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szabó I, Kiss A, Schaff Z and Sobel G:
Claudins as diagnostic and prognostic markers in gynecological
cancer. Histol Histopathol. 24:1607–1615. 2009.PubMed/NCBI
|
|
28
|
Urick ME and Bell DW: Clinical
actionability of molecular targets in endometrial cancer. Nat Rev
Cancer. 19:510–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cancer Genome Atlas Research Network;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stelloo E, Nout RA, Osse EM,
Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, Nijman HW, Putter H, Bosse T, et al: Improved risk assessment
by integrating molecular and clinicopathological factors in
early-stage endometrial cancer-combined analysis of the PORTEC
cohorts. Clin Cancer Res. 22:4215–4224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kommoss S, McConechy MK, Kommoss F, Leung
S, Bunz A, Magrill J, Britton H, Kommoss F, Grevenkamp F, Karnezis
A, et al: Final validation of the ProMisE molecular classifier for
endometrial carcinoma in a large population-based case series. Ann
Oncol. 29:1180–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
León-Castillo A, de Boer SM, Powell ME,
Mileshkin LR, Mackay HJ, Leary A, Nijman HW, Singh N, Pollock PM,
Bessette P, et al: Molecular classification of the PORTEC-3 trial
for high-risk endometrial cancer: Impact on prognosis and benefit
from adjuvant therapy. J Clin Oncol. 38:3388–3397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Edfors F, Danielsson F, Hallström BM, Käll
L, Lundberg E, Pontén F, Forsström B and Uhlén M: Gene-specific
correlation of RNA and protein levels in human cells and tissues.
Mol Syst Biol. 12:8832016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fortelny N, Overall CM, Pavlidis P and
Freue GVC: Can we predict protein from mRNA levels? Nature.
547:E19–E20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paquet-Fifield S, Koh SL, Cheng L, Beyit
LM, Shembrey C, Mølck C, Behrenbruch C, Papin M, Gironella M,
Guelfi S, et al: Tight junction protein claudin-2 promotes
self-renewal of human colorectal cancer stem-like cells. Cancer
Res. 78:2925–2938. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reinhard K, Rengstl B, Oehm P, Michel K,
Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, et al: An
RNA vaccine drives expansion and efficacy of claudin-CAR-T cells
against solid tumors. Science. 367:446–453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong FE, Li GM, Tang YQ, Xi SY, Loong JHC,
Li MM, Li HL, Cheng W, Zhu WJ, Mo JQ, et al: Targeting tumor
lineage plasticity in hepatocellular carcinoma using an anti-CLDN6
antibody-drug conjugate. Sci Transl Med. 13:eabb62822021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuzaki J, Lele S, Odunsi K and Tsuji T:
Identification of Claudin 6-specific HLA class I- and HLA class
II-restricted T cell receptors for cellular immunotherapy in
ovarian cancer. Oncoimmunology. 11:20209832022. View Article : Google Scholar : PubMed/NCBI
|