Introduction
Breast cancer is the most common type of cancer and
the main cause of cancer-related mortality among women (1). It has previously been reported that
breast cancer accounts for ~11.7% of all global cancer cases and
15.5% of all female cancer-related deaths (2). Breast cancer is divided into the
following four subtypes according to its molecular phenotypes: i)
Luminal A; ii) Luminal B; iii) human epidermal growth factor
receptor-2; and iv) triple-negative breast cancer (TNBC) (3). TNBC accounts for ~15% of all breast
cancer cases (4) and is
characterized by an obvious heterogeneity, an early age of onset, a
high risk of visceral metastasis, high tumor invasiveness and a
high histological grade (5). Due
to the high tumor heterogeneity of TNBC and the lack of a clear
therapeutic target, effective treatments are still lacking.
The tumor microenvironment (TME) plays a key role in
the occurrence and development of cancers (6). Tumor cells promote the evolution of
cancer by altering the TME to create favorable conditions for their
own survival (7). Tumor-associated
macrophages (TAMs) are the main immune cells in the TME and account
for 50-80% of mesenchymal cells (8). TAMs have a strong plasticity and
polarize into different phenotypes under the stimulation of the TME
and cytokines. Moreover, TAMs can be divided into macrophages
activated by the classical pathway, TAM/M1, or the alternative
pathway, TAM/M2, according to their different polarization modes.
TAM/M1 mainly contributes towards tumor inhibition, whereas TAM/M2
has more tumor promoting properties (9). Moreover, ~90% of breast
cancer-related deaths are caused by metastasis (10). Furthermore, cytokines in the TME
interact with tumor cells and thereby play a crucial role in the
process of tumor metastasis (11).
TAM/M2 are associated with a poor prognosis of breast, colorectal
and gastric cancer (12-14). Furthermore, epithelial-mesenchymal
transition (EMT) is associated with tumor metastasis and a poor
prognosis (15). A previous study
reported that TAMs enhanced the metastasis of colorectal cancer
cells by inducing the EMT process (16). Cancer stem cells (CSCs) are
self-regenerative and have a high carcinogenic potential; they are
thus considered to be related to chemotherapeutic resistance,
metastasis and recurrence (17).
TAM-derived cytokines enhance the stemness of cancer via the EMT
process (18). TAM/M2 induce
angiogenesis and secrete pro-angiogenic factors, such as vascular
endothelial growth factor (VEGF), degrade the tumor extracellular
matrix and help tumor cells to evade the immune system. These
factors help promote tumor metastasis, immunosuppression and drug
resistance, and provide nutrition and metastasis pathways that
contributes towards tumor growth (19). Therefore, reversing the
polarization of TAM/M2, reducing their recruitment and blocking the
tumor-promoting function of TAM/M2 may prove to be a potential
novel antitumor therapeutic strategy.
Programmed death receptor-1 (PD-1) is an inhibitory
co-receptor that binds to programmed death ligand-1 (PD-L1) to
effectively inhibit T-cell activity, thereby reducing the T-cell
recognition of tumor cells and enabling tumor cells to evade immune
supervision (20). The expression
of PD-1 and PD-L1 in patients with TNBC are higher compared with
other molecular types of breast cancer (21). PD-L1 inhibitors have a long-lasting
effect on advanced TNBC (22). A
previous study reported that tumor drug resistance significantly
restricted the later efficacy of PD-1/PD-L1 inhibitors (23). PD-L1 expression in the TME of tumor
cells and host immune cells assists tumor cell immune evasion.
Therefore, monitoring PD-L1 expression levels in tumor tissues is
more important than monitoring PD-L1 expression levels in tumor
cells (24). The expression of
PD-L1 plays a decisive role in host immune cells, rather than tumor
cells (25). Therefore, the
expression of PD-L1 in TAMs plays a key role in tumor progression.
It can thus be hypothesized that TNBC may induce TAM/M2
polarization and promote tumor evolution. However, whether αPD-L1
inhibits TNBC malignant progression by reversing TAM/M2
polarization has not yet been reported, at least to the best of our
knowledge. Therefore, the present study aimed to investigate the
role of αPD-L1 in the regulation of TAM polarization in the TME of
TNBC, to reveal the molecular role of αPD-L1 in reversing TAM
polarization towards the M2 phenotype, and to provide a novel
therapeutic strategy for refractory TNBC.
Materials and methods
Cells and cell culture
The Yanbian University Cancer Research Center
(Yanji, China) supplied the human TNBC (MDA-MB-231 and Hs578T) cell
lines, the mouse 4T1 cell line, immortalized HUVECs, the human
monocyte THP-1 cell line, and the mouse macrophage RAW264.7 cell
line. All cell lines were cultured in RPMI-DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% streptomycin-penicillin (100 U/ml). THP-1
cells were differentiated into macrophages using 100 ng/ml
phorbol-12-myristate 13-acetate (PMA; Sigma-Aldrich; Merck KGaA)
for 24 h. The cells were cultured at 37°C in an atmosphere of 5%
CO2.
Flow cytometry
The RAW264.7 cells (5×105) were washed
twice with cell staining buffer (Biolegend, Inc.) and were
subsequently fixed using fixation buffer (Biolegend, Inc.). The
cell membranes were broken using permeabilization buffer
(Biolegend, Inc.). The cells were then stained with CD68-FITC
(1:100; cat. no. 137006; Biolegend, Inc.), CD206-phycoerythrin
(1:100; cat. no. 141706; Biolegend, Inc.) and CD86-allophycocyanin
(1:100; cat. no. 105011; Biolegend, Inc.) antibodies at 4°C for 2 h
(1:100; Biolegend, Inc.). The cells were subsequently suspended in
500 µl cell staining buffer and examined using a BD Accuri
C6 flow cytometer (BD Biosciences). FlowJo V10.5.3 (TreeStar, Inc.)
software was employed for analysis.
Western blot analysis
The MDA-MB-231, Hs578T, RAW264.7 and THP-1 cells
were collected and the total protein was extracted using RIPA
lysate (RIPA lysis buffer: PMSF, 100:1). Nuclear and cytoplasmic
proteins was isolated using a Nuclear and Cytoplasmic Protein
Extraction kit (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. The protein concentration was
determined and quantified using a BCA kit (Roche Diagnostics).
Proteins (30 µg per lane) were separated using SDS-PAGE (8
and 10%) and the separated proteins were then transferred a to PVDF
membrane (MilliporeSigma). The membrane was placed in 5% non-fat
milk (BD Biosciences) and blocked for 2 h at room temperature to
remove non-specific binding sites. The membranes were incubated
with the primary antibodies at 4°C overnight and subsequently with
the secondary antibodies at room temperature for 1 h. Primary
antibodies specific for the following proteins were used: PD-L1
(1:1,000; cat. no. 60475 and 85164; CST Biological Reagents Co.,
Ltd.), CD86 (1:1,000; cat. no. sc-28347; Santa Cruz Biotechnology,
Inc.), CD206 (1:1,000; cat. no. 24595; CST Biological Reagents Co.,
Ltd.), VEGF (1:1,000; cat. no. sc-7269; Santa Cruz Biotechnology,
Inc.), MMP2 (1:1,000; cat. no. sc-13594; Santa Cruz Biotechnology,
Inc.), MMP9 (1:1,000; cat. no. sc-393859; Santa Cruz Biotechnology,
Inc.), E-cadherin (1:1,000; cat. no. 14472; CST Biological Reagents
Co., Ltd.), zonula occludens-1 (ZO-1; 1:1,000; cat. no. 8193; CST
Biological Reagents Co., Ltd.), N-cadherin (1:1,000; cat. no.
sc-8424; Santa Cruz Biotechnology, Inc.), vimentin (1:1,000; cat.
no. sc-6260; Santa Cruz Biotechnology, Inc.), Slug (1:1,000; cat.
no. sc-166476; Santa Cruz Biotechnology, Inc.), Twist (1:1,000;
cat. no. sc-81417; Santa Cruz Biotechnology, Inc.), CD44 (1:1,000;
cat. no. 3570; CST Biological Reagents Co., Ltd.), octamer-binding
transcription factor 4 (Oct4; 1:1,000; cat. no. 75643; CST
Biological Reagents Co., Ltd.), Nanog (1:1,000; cat. no. sc-374103;
Santa Cruz Biotechnology, Inc.), Bmi1 (1:1,000; cat. no. sc-390443;
Santa Cruz Biotechnology, Inc.), Sox2 (1:1,000; cat. no. sc-365823;
Santa Cruz Biotechnology, Inc.), CCAAT/enhancer-binding protein β
(CEBPβ; 1:1,000; cat. no. sc-7962; Santa Cruz Biotechnology, Inc.),
phosphorylated (p-)ERK (1:1,000; cat. no. 4695; CST Biological
Reagents Co., Ltd.), ERK (1:1,000; cat. no. 48303; CST Biological
Reagents Co., Ltd.), p-STAT6 (1:1,000; cat. no. 56554; CST
Biological Reagents Co., Ltd.), STAT6 (1:1,000; cat. no. sc-374021;
Santa Cruz Biotechnology, Inc.), p-STAT3 (1:1,000; cat. no. 9145;
CST Biological Reagents Co., Ltd.), STAT3 (1:1,000; cat. no. 9139;
CST Biological Reagents Co., Ltd.), β-actin (1:1,000; cat. no.
CW0096; CWBio Technology Co., Ltd.), GAPDH (1:1,000; cat. no.
CW0100M; CWBio Technology Co., Ltd.) and H3 (1:1,000; cat. no.
60932; CST Biological Reagents Co., Ltd.). Three different loading
controls were used (β-actin, GAPDH and H3) in this experiment.
Horseradish peroxidase-conjugated secondary antibodies, including
goat anti-mouse (1:3,000; cat. no. ZB-2305; ZSGB-BIO Technology
Co., Ltd.) and goat anti-rabbit (1:3,000; cat. no. ZB-2301;
ZSGB-BIO Technology Co., Ltd.) antibodies were used. Enhanced
chemiluminescence kit (CWBio Technology Co., Ltd.) was used to
detect antibody signals and images were collected. ImageJ (v. 1.48;
National Institutes of Health) software was employed for
analysis.
Immunofluorescence (IF) staining
MDA-MB-231, Hs578T and RAW264.7 cells, with a 30%
fusion rate, were seeded into six-well plates, fixed with anhydrous
methanol at room temperature for 15 min and permeated with 0.5%
Triton X-100 (CWBio Technology Co., Ltd.) at room temperature for
10 min. The cells were then blocked with 3% BSA (Beijing Solarbio
Science & Technology) at room temperature for 2 h.
Subsequently, the cells were incubated with primary antibody in 3%
BSA at 4°C overnight. Primary antibodies specific for the following
proteins were used: CD206 (1:100; cat. no. 24595; CST Biological
Reagents Co., Ltd.), E-cadherin (1:100; cat. no. 14472; CST
Biological Reagents Co., Ltd.), Vimentin (1:100; cat. no. sc-6260;
Santa Cruz Biotechnology, Inc.), CD44 (1:100; cat. no. 3570; CST
Biological Reagents Co., Ltd.), p-STAT3 (1:100; cat. no. 9145; CST
Biological Reagents Co., Ltd.) and STAT3 (1:100; cat. no. sc-8059;
Santa Cruz Biotechnology, Inc.). Following incubation with the
primary antibodies, the cells were then incubated with Alexa Fluor
488 goat anti-rabbit IgG (1:400; cat. no. A11008; Invitrogen;
Thermo Fisher Scientific, Inc.) or Alexa Fluor 568 goat anti-mouse
IgG (1:400; cat. no. A11004; Invitrogen; Thermo Fisher Scientific,
Inc.) at room temperature for 1 h. The cells were counterstained
with DAPI and imaged using a Leica SP5II confocal microscope (Leica
Microsystems GmbH).
ELISA
ELISA was performed according to the manufacturer's
instructions. The concentrations of interleukin (IL)-10 (Mlbio;
cat. no. ml037873; Shanghai Enzyme-linked Biotechnology Co., Ltd.)
and IL-12 (Mlbio; cat. no. ml037868; Shanghai Enzyme-linked
Biotechnology Co., Ltd.) in the RAW264.7 cell culture supernatants
were determined using the corresponding kits.
MTT assay
The MDA-MB-231, Hs578T and RAW264.7 cells
(5×103)/well were seeded and incubated in 96-well plates
at 37°C for 24 h. Conditioned medium (CM) or drugs (IL-13, 30
ng/ml; and/or αPD-L1, 15 µg/ml) were added and the cells
were further incubated at 37°C for 0, 24, 48 and 72 h.
Subsequently, 100 µl MTT reagent (1 mg/ml; Beyotime
Institute of Biotechnology) were added to each well and the cells
were incubated at 37°C for 4 h under the same conditions.
Subsequently, 100 µl DMSO (Beijing Solarbio Science &
Technology) were added to each well and the optical density at 490
nm was assessed using a full-wavelength multifunctional microplate
reader (Tecan, Inc.). At least five wells/group were analyzed and
the experiment was repeated three times.
Apoptosis assay
The MDA-MB-231, Hs578T and RAW264.7 cells
(1×106) were washed twice with binding buffer and were
then stained using the Annexin V-FITC Apoptosis Detection kit (BD
Biosciences) according to the manufacturer's instructions. The
cells were analyzed using a BD Accuri C6 flow cytometer (BD
Biosciences). FlowJo V10.5.3 (TreeStar, Inc.) software was employed
for analysis.
Cell morphology
RAW264.7 cells, with a 30% fusion rate, were
incubated in 6-well plates at 37°C for 12 h and were then treated
with the TAM/M2-inducing factor, IL-13 (10, 20 and 30 ng/ml;
Biolegend, Inc.) and/or αPD-L1 (15 µg/ml; 10F.9G2 monoclonal
antibody; cat no. BE0101; Bio X Cell) at 37°C for 48 h.
Morphological cell changes were observed using a microscope and
images were obtained (IX73, Olympus Corporation).
CM preparation
RAW264.7 and THP-1 (PMA) cells were treated with
IL-13 and/or αPD-L1 at 37°C for 48 h. The cells were then cultured
in serum-free medium at 37°C for 24 h, and three types of CM
(control, IL-13 and IL-13 + αPD-L1) were prepared. The CM was
directly used in the assays or stored at −80°C. The CM was filtered
and 2% FBS was added.
Transwell assay
The MDA-MB-231 and Hs578T cells (5×104)
in 100 µl serum-free RPMI-DMEM were seeded into the upper
chamber. The bottom chamber was filled with RPMI-DMEM with 10% FBS.
The cells were incubated at 37°C for 6 h. The upper chambers were
replaced with different types of CM. Cells passing through the
subsurface of the filtration membrane were fixed with 4%
paraformaldehyde at room temperature for 20 min and were then
stained with 0.1% crystal violet at room temperature for 20 min. In
total, three fields (magnification, ×200) were randomly selected
for imaging using a microscope (IX73; Olympus Corporation). Image-J
software (v. 1.46; National Institutes of Health) was used to
quantify the number of cells in each field.
Wound healing assay
Cells, with a 80% fusion rate, were incubated in
six-well plates at 37°C for 24 h. The wound was scratched
vertically using a 200 µl pipette tip and the plates were
washed three times with PBS to remove dead cells in the well. The
cells with serum-free CM (containing IL-13 and/or αPD-L1) were
imaged using a microscope (IX73; Olympus Corporation) at 0, 12 and
24 h.
Soft-agar colony-forming assay
In 96-well plates 1% agarose (Beijing Solarbio
Science & Technology) in complete medium was applied and was
solidified at 37°C for 30 min. The MDA-MB-231 and Hs578T cells
(1×103) were mixed with 0.3% agarose in CM and were
plated on top of the bottom layer of 1% agarose. Subsequently,
complete medium was applied on top of the cell layer. The number
and size of the colonies were observed using a microscope (citation
5; BioTek Instruments, Inc.) after 14 days.
Endothelial tube formation assay
Matrigel (BD Biosciences) and RPMI-DMEM were diluted
1:1 in 96-well plates and solidified at 37°C for 4 h. HUVECs
(2×104) were incubated in 2:1 diluted CM and culture
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
streptomycin-penicillin (100 U/ml) at 37°C for 4 h. The capillary
structure was imaged using a microscope (IX73; Olympus
Corporation).
Cell co-culture system
RAW264.7 cells (5×104) were added to 600
µl RPMI-DMEM with 10% FBS, which was added to the bottom
chamber. The MDA-MB-231 or Hs578T cells (1×105) were
added to 100 µl RPMI-DMEM with 10% FBS, which was added to
the upper chamber. The cells were co-cultured at 37°C for 6 h and
subsequently αPD-L1 (15 µg/ml) was added at 37°C for 48 h.
The supernatant was harvested for use in ELISA, and the RAW264.7
cells were harvested for use in western blot analysis and flow
cytometry.
The RAW264.7 cells (5×104) in 100
µl RPMI-DMEM with 10% FBS were seeded into the upper
chamber. The MDA-MB-231 or Hs578T cells (1×105) in 100
µl RPMI-DMEM with 10% FBS were seeded into the bottom
chamber. The cells were co-cultured at 37°C for 6 h and αPD-L1
subsequently incubated at 37°C for 48 h. The MDA-MB-231 or Hs578T
cells were then collected for use in western blot analysis, wound
healing and Transwell assays.
The following six groups were established: i)
Negative control group, RAW264.7 cells were cultured separately;
ii) positive control group, RAW264.7 cells were treated with IL-13
to produce TAM/M2; iii) co-culture group 1, RAW264.7 cells were
co-cultured with MDA-MB-231 cells; iv) co-culture treated group 1,
RAW264.7 cells were co-cultured with MDA-MB-231 cells with αPD-L1
treatment; v) co-culture group 2, RAW264.7 cells were co-cultured
with Hs578T cells; vi) co-culture treated group 2, RAW264.7 cells
were co-cultured with Hs578T cells with αPD-L1 treatment.
Animal experiments
The animal experiments were conducted between
November, 2021 to December, 2021. A total of 20 female BALB/c mice
(age, 5 weeks, weighing ~20 g) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. All mice were housed
under specific-pathogen-free conditions (temperature, 22°C;
humidity, 50%; light/dark cycle, 12/12 h), the animals were also
provided with free access to food and water, and the padding was
replaced twice a week. Animal health and behavior were monitored
every day. The 4T1 cells (5×105/100 µl) were
subcutaneously injected into the right flanks of the mice to
establish a tumor model. After the tumors were palpable, the mice
were randomly divided into the negative control (n=5) and αPD-L1
group (n=5). αPD-L1 (200 µg) was administered
intraperitoneally to the mice once a day every 3 days (26). Tumor size was measured every 3 days
and tumor volume was calculated using the following formula: Length
× width2 ×0.5. The animals were sacrificed 25 days after
the αPD-L1 treatment. For detecting lung metastases, 4T1 cells
(1×105/100 µl) were injected into the tail vein
of the mice. After 7 days, the mice were randomly divided into the
negative control (n=5) and αPD-L1 group (n=5). αPD-L1 (200
µg) was administered intraperitoneally to the mice once a
day every 3 days. The animals were sacrificed at 45 days after the
αPD-L1 treatment. The humane endpoints of the experiment were as
follows: i) Tumor burden, ≥10% body weight; ii) tumor volume,
>2,000 mm3; iii) weight loss, ≥20% body weight; iv)
ulceration or infection on tumor; v) no movement for >24 h; and
vi) no eating or drinking. In this experiment, no mice were found
dead. All mice were sacrificed by cervical dislocation following an
intraperitoneal injection of sodium pentobarbital (30 mg/kg) and
sacrifice was confirmed when the mice had stopped breathing and did
not respond to stimulation. The lungs were collected and the
surface nodules were quantified. The tumor and lung tissues were
fixed with 10% formalin at 4°C for 24 h. The paraffin-embedded
tumor tissues were sliced into 4-µm-thick sections. The
expression of the markers was confirmed using immunohistochemical
staining. Animal euthanasia was performed via cervical dislocation
under 2% isoflurane anesthesia. The present study was approved by
the Animal Ethics Committee of Yanbian University (approval no.
YD20220916004), and was performed according to the guidelines of
the Committee on Animal Research and Ethics.
Hematoxylin and eosin (H&E)
Tumor and lung tissue sections were dewaxed and
rehydrated, stained with H&E (ZSGB-BIO Technology Co., Ltd.) at
room temperature for 5 min and the stained tissue was evaluated
under a microscope (IX73; Olympus Corporation).
Immunohistochemistry (IHC)
Tumor and lung tissue sections were dewaxed and
dehydrated. Subsequently, antigen retrieval was performed using
microwave heating in 10 mM citrate buffer (pH 7.0) at 80°C for 20
min. Endogenous peroxidase was blocked with 3%
H2O2 (ZSGB-BIO Technology Co., Ltd.) at room
temperature for 30 min. The tissue sections were incubated with
primary antibodies at 4°C overnight. Primary antibodies specific
for the following proteins were used: CD206 (1:100; cat. no. 24595;
CST Biological Reagents Co., Ltd.), E-cadherin (1:100; cat. no.
14472; CST Biological Reagents Co., Ltd.), vimentin (1:100; cat.
no. sc-6260; Santa Cruz Biotechnology, Inc.), CD44 (1:100; cat. no.
3570; CST Biological Reagents Co., Ltd.), VEGF (1:100; cat. no.
sc-7269; Santa Cruz Biotechnology, Inc.). Following incubation with
the primary antibodies, the samples were incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. PV9005; ZSGB-BIO
Technology Co., Ltd.) at room temperature for 1 h. The samples were
stained using DAB (ZSGB-BIO Technology Co., Ltd.) at room
temperature for 5 min and counterstained with hematoxylin at room
temperature for 1 min. The images were obtained using a microscope
(IX73; Olympus Corporation).
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used for statistical analysis. A two-tailed unpaired
Student's t-test was used to compare the mean values of two groups.
One-way ANOVA with Tukey's post hoc test were used to compare the
mean values of multiple groups. All experiments were repeated in
triplicate and their mean values are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
aPD-L1 reverses TAM/M2 polarization
RAW264.7 cells were treated with continuous
concentrations of the TAM/M2-inducing factor, IL-13 (0, 10, 20 and
30 ng/ml) for 48 h. IF staining, western blot analysis and ELISA
demonstrated that IL-13 promoted the levels of the TAM/M2 marker,
CD206, in the RAW264.7 cell cytoplasm and the secretion of IL-10 in
a concentration-dependent manner. IL-13 at 30 ng/ml had the most
pronounced effect and this concentration was selected for use in
further experiments (Fig. 1A-D).
The RAW264.7 cells were treated with continuous concentrations of
αPD-L1 for 24, 48 and 72 h. The results of MTT assay demonstrated
that αPD-L1 at concentrations ≤15 µg/ml did not affect the
proliferation of the RAW264.7 cells. αPD-L1 at a concentration of
15 µg/ml was thus selected for use in further experiments
(Fig. 1E).
 | Figure 1αPD-L1 reverses TAM/M2 polarization.
(A) Flow cytometry was used to determine the percentage of
CD206+/CD68+ RAW264.7 cells (Q2 region)
treated with IL-13 (0, 10, 20 and 30 ng/ml). (B and C)
Immunofluorescence staining and western blot analysis were
performed to determine the protein expression levels of CD206 in
RAW264.7 cells treated with IL-13 (magnification, ×400). The
markers were all expressed in the cytoplasm. (D) ELISA was
performed to determine the IL-10 levels in RAW264.7 cells treated
with IL-13. (E) RAW264.7 cell viability was determined using MTT
assay when the cells were treated with αPD-L1 (0, 5, 10 and 15
µg/ml). (F) Western blot analysis was used to determine the
protein expression levels of PD-L1 in RAW264.7 cells treated with
IL-13 (30 ng/ml) and/or αPD-L1 (15 µg/ml). (G) RAW264.7 cell
proliferation was analyzed using MTT assay when the cells were
treated with IL-13 and/or αPD-L1. (H) RAW264.7 cell apoptosis was
analyzed using flow cytometry when the cells were treated with
IL-13 and/or αPD-L1. (I and J) CD86 and CD206 expression levels in
the RAW264.7 cells treated with IL-13 and/or αPD-L1 were determined
using flow cytometry and western blot analysis. (K) IL-12 and IL-10
levels in RAW264.7 cells treated with IL-13 and/or αPD-L1 were
assessed using ELISA. (L) RAW264.7 cell morphology was imaged using
a microscope when the cells were treated with IL-13 and/or αPD-L1.
*P<0.05, **P<0.01 and
***P<0.001, vs. control. ns, not significant; αPD-L1,
programmed death-ligand 1 inhibitor; TAM/M2, tumor-associated
macrophages/M2-type. |
To examine the potential effects of macrophages on
TNBC progression, 100 ng/ml PMA were used to induce the
differentiation of THP-1 cells into macrophages, which were
characterized by the expression of the recognized macrophage
marker, CD68 (Fig. S1A). To
investigate the functions of αPD-L1 in TAM/M2 cells, the protein
expression levels of PD-L1 were examined in TAM/M2 cells using
western blot analysis. The results demonstrated that the PD-L1
protein expression levels were upregulated in the TAM/M2 cells and
that αPD-L1 downregulated the expression of PD-L1 (Figs. 1F and S1B). Subsequently, it was demonstrated
that IL-13 (30 ng/ml) and/or αPD-L1 (15 µg/ml) had no
notable effect on the proliferation and apoptosis of RAW264.7
cells, as shown by MTT and flow cytometric assays (Fig. 1G and H). The results of flow
cytometry, western blot analysis and ELISA demonstrated that αPD-L1
inhibited the TAM/M2 marker, CD206, and the IL-10 levels induced by
IL-13. However, the expression of the TAM/M1 marker, CD86, and the
IL-12 levels were not markedly affected by IL-13 and/or αPD-L1
(Figs. 1I-K and S1B). To further investigate the effects
of αPD-L1 on TAM/M2 polarization, changes in cell morphology were
observed using a microscope. IL-13 stimulated the RAW264.7 cells
and changed the morphology from a round or oval shape to a long
spindle shape, whereas αPD-L1 inhibited cell extension (Fig. 1L). These data thus suggested that
αPD-L1 inhibited TAM/M2 polarization.
aPD-L1 inhibits the TAM/M2-induced
migration and angiogenesis of TNBC cells
The RAW264.7 and THP-1 cells were treated with IL-13
and/or αPD-L1 for 48 h and supernatant was replaced with serum-free
medium for 24 h and then collected as CM (Fig. 2A). To examine the effects of αPD-L1
on the interaction between TAM/M2 and TNBC cells, it was determined
that CM had no notable effect on the proliferation and apoptosis of
TNBC cells, as shown by MTT assay and flow cytometry (Fig. 2B and C). Subsequently, wound
healing and Transwell assays confirmed that the MDA-MB-231 and
Hs578T cells treated with the CM of IL-13/RAW264.7 or IL-13/THP-1
cells exhibited an increased cell migration, whereas the CM of
IL-13 + αPD-L1/RAW264.7 or IL-13 + αPD-L1/THP-1 cells reversed this
phenomenon (Figs. 2D and E, and
S1C and D).
Neovascularization in tumor tissues is an important
condition for malignant tumor metastasis that is regulated via
various chemokines in the TME (27). In the present study, to investigate
the effects of αPD-L1 on angiogenesis via TAM/M2 polarization in
vitro, an endothelial tube formation assay was performed. The
results demonstrated that HUVECs treated with the CM of
IL-13/RAW264.7 cells exhibited increased microtubule formation,
whereas the CM of IL-13 + αPD-L1/RAW264.7 cells reversed this
phenomenon (Fig. 2F).
Subsequently, western blot analysis was performed and the results
demonstrated that the MDA-MB-231 and Hs578T cells treated with the
CM of IL-13/RAW264.7 or IL-13/THP-1 cells exhibited upregulated
protein expression levels of VEGF, MMP2 and MMP9. However, the CM
of IL-13 + αPD-L1/RAW264.7 or IL-13 + αPD-L1/THP-1 cells reversed
this phenomenon (Figs. 2G and
S1E). These data thus suggested
that αPD-L1 inhibited TNBC cell migration and angiogenesis that was
induced by TAM/M2 polarization.
aPD-L1 inhibits the TAM/M2-induced EMT
process and the stemness of TNBC cells
To investigate whether αPD-L1 inhibits the EMT
process of TNBC cells via the regulation of TAM polarization,
western blot analysis and IF staining were performed. The results
demonstrated that the MDA-MB-231 and Hs578T cells treated with the
CM of IL-13/RAW264.7 or IL-13/THP-1 cells exhibited upregulated
protein expression levels of the mesenchymal markers, N-cadherin,
vimentin, Slug and Twist. Furthermore, the protein expression
levels of the epithelial markers, E-cadherin and ZO-1 were
downregulated. However, the MDA-MB-231 and Hs578T cells treated
with the CM of IL-13 + αPD-L1/RAW264.7 or IL-13 + αPD-L1/THP-1
cells exhibited a reversal of this phenomenon (Figs. 3A and B, and S1E).
 | Figure 3αPD-L1 inhibits the EMT process and
the stemness of TNBC promoted by TAM/M2. (A) Protein expression
levels of EMT markers, E-Cadherin, ZO-1, N-Cadherin, Vimentin, Slug
and Twist, in MDA-MB-231 and Hs578T cells treated with CM, as
determined using western blot analysis. (B) E-cadherin and vimentin
protein expression levels in MDA-MB-231 and Hs578T cells treated
with CM were analyzed using IF staining. The markers were all
expressed in the cytoplasm (magnification, ×400). (C) Protein
expression levels of the stemness markers, CD44, Oct4, Nanog, Bmi1
and Sox2, in MDA-MB-231 and Hs578T cells treated with CM, as
determined using western blot analysis. (D) CD44 protein expression
levels in MDA-MB-231 and Hs578T cells treated with CM were assessed
using IF staining. The markers were all expressed in the cytoplasm
(magnification, ×400). *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. αPD-L1, programmed death-ligand 1
inhibitor; EMT, epithelial-mesenchymal transition; TAM/M2,
tumor-associated macrophages/M2-type; ZO-1, zonula occludens-1; CM,
conditional media; IF, immunofluorescence. |
Tumor cells express high levels of stem cell surface
markers following EMT progression, which indicates that these cells
have become stem cells (28). In
the present study, to investigate whether αPD-L1 inhibits the
stemness of TNBC cells via the regulation of TAM/M2 polarization,
western blot analysis and IF staining were performed. The results
demonstrated that the MDA-MB-231 and Hs578T cells treated with the
CM of IL-13/RAW264.7 or IL-13/THP-1 cells exhibited upregulated
protein expression levels of the stemness markers, CD44, Oct4,
Nanog, Bmi1 and Sox2. However, the MDA-MB-231 and Hs578T cells
treated with the CM of IL-13 + αPD-L1/RAW264.7 or IL-13 +
αPD-L1/THP-1 exhibited a reversal of this phenomenon (Figs. 3C and D, and S1F). The present study then investigated
whether αPD-L1 inhibits CSC properties without affecting cell
proliferation. There is increasing evidence to suggest that 3D cell
culture is a more accurate reflection of the TME 2D culture
(29). Therefore, the soft agar
colony formation assay, a technique widely used to assess CSC
proliferation, was used herein (30). The results demonstrated that TNBC
cells treated with the CM of IL-13/RAW264.7 cells exhibited an
increased colony number and size, whereas the CM of IL-13 +
αPD-L1/RAW264.7 reversed this phenomenon (Fig. S2). These data thus suggested that
αPD-L1 inhibited the EMT process and the stemness of TNBC cells by
reversing TAM/M2 polarization, which thereby inhibited TNBC
metastasis and angiogenesis.
aPD-L1 reverses TAM/M2 polarization in
the co-culture system
The effects of TAM/M2 polarization on TNBC cells
were identified and therefore a co-culture system of TAMs and TNBC
cells was established to simulate this interaction in the TME
(Fig. 4A). Flow cytometry and
western blot analysis confirmed that the percentage of
CD206+/CD68+ (TAM/M2) cells and the levels of
CD86 and CD206 in the RAW264.7 cells co-cultured with TNBC cells
were upregulated, whereas αPD-L1 downregulated the percentage of
CD206+/CD68+ cells. However, the percentages
of CD86+/CD68+ (TAM/M1) cells were not
significantly altered in the co-culture system with or without
αPD-L1 (Fig. 4B and C). To further
support these results, ELISA was performed and the results
demonstrated that the IL-10 levels in the positive control group,
co-culture group 1 and co-culture group 2 were increased, whereas
αPD-L1 reversed this phenomenon. However, the IL-12 levels were not
altered by the co-culture system with or without αPD-L1 (Fig. 4D). These data suggested that αPD-L1
regulated the interaction between TAMs and TNBC cells.
 | Figure 4αPD-L1 reverses TAM/M2 polarization
in the co-culture of TNBC cells and TAMs. (A) Schematic diagram of
TNBC cells and the TAMs co-culture system. (B) RAW264.7 cells were
co-cultured with MDA-MB-231 and Hs578T cells. The percentages of
CD86+/CD68+ and
CD206+/CD68+ treated with or without αPD-L1
in the co-culture system were determined using flow cytometry
assay. (C) CD86 and CD206 protein expression levels, when the cells
were treated with or without αPD-L1 in co-culture, were determined
using western blot analysis. (D) IL-12 and IL-10 levels, in cells
treated with or without αPD-L1 in co-culture, were assessed using
ELISA. *P<0.05, **P<0.01,
***P<0.001, and ****P<0.0001. ns, not
significant; αPD-L1, programmed death-ligand 1 inhibitor; TAM/M2,
tumor-associated macrophages/M2-type; TNBC, triple-negative breast
cancer. |
TNBC cells induce TAM/M2 polarization,
and positive feedback promotes the malignant evolution of TNBC
cells
To investigate whether TAM/M2 cells promote the
malignant progression of TNBC cells in a co-culture system, a
co-culture system was established and wound healing and Transwell
assays were performed. The results demonstrated that TAM/M2
promoted the migration of TNBC cells, whereas αPD-L1 reversed this
phenomenon (Fig. 5A and B). To
further investigate whether TAM/M2 directly induces the
angiogenesis of TNBC, western blot analysis was performed. The
results demonstrated that TAM/M2 upregulated the protein expression
levels of VEGF, MMP2 and MMP9 in the TNBC cells, whereas αPD-L1
reversed these effects (Fig. 5C).
Furthermore, TAM/M2 upregulated the protein expression levels of
mesenchymal and stemness markers in TNBC cells and downregulated
those of epithelial markers, whereas αPD-L1 reversed the EMT
process and the stemness of TNBC cells (Fig. 5D and E). On the whole, these data
suggested that αPD-L1 potentially inhibited the migration and
angiogenesis of TNBC cells via the regulation of the TAM
polarization-mediated EMT process and cancer stemness.
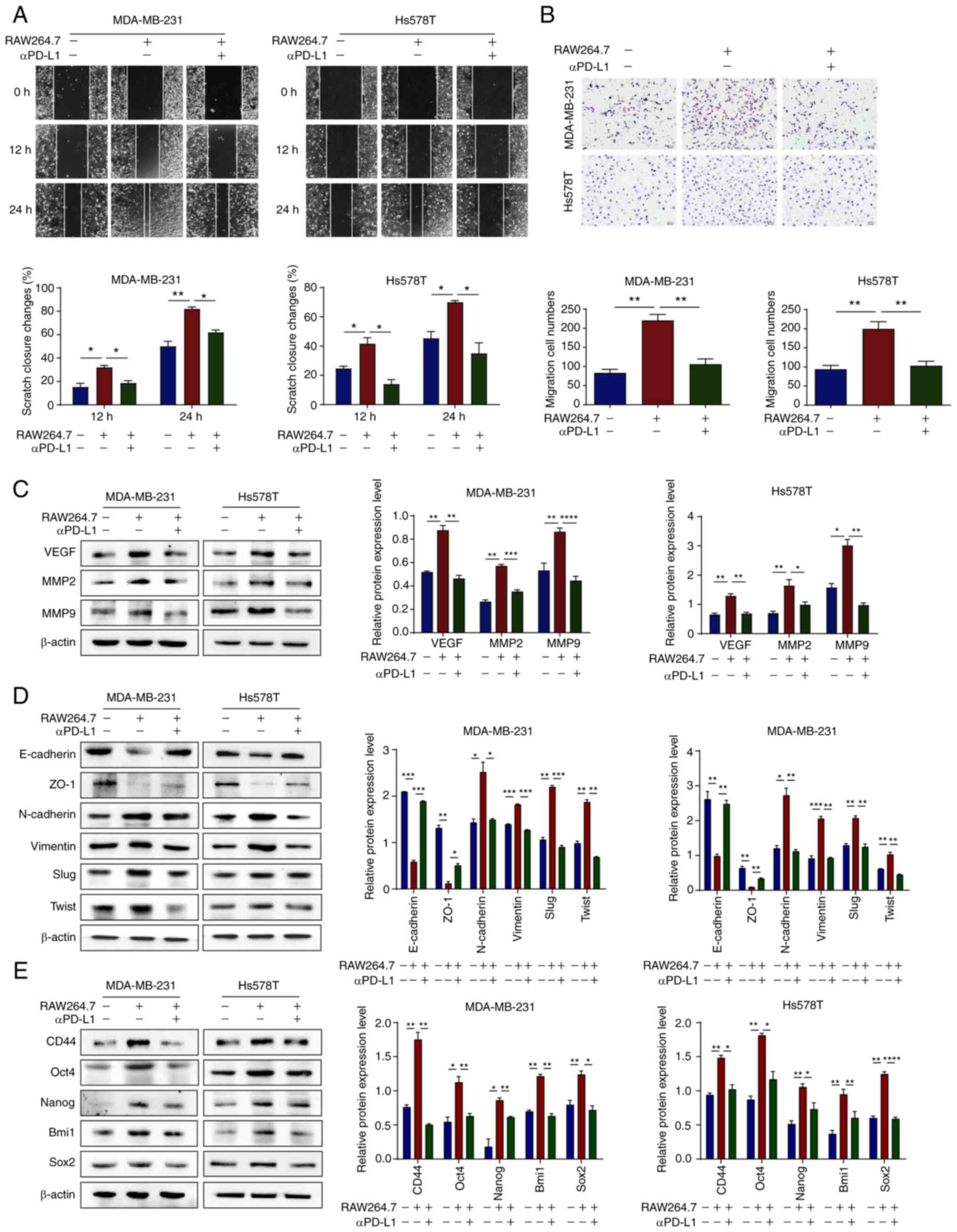 | Figure 5αPD-L1 reverses TNBC malignant
evolution in the co-culture of TNBC cells and TAMs. (A and B)
MDA-MB-231 and Hs578T cell migration capacity was assessed using
wound healing and Transwell assays when the cells were treated with
or without αPD-L1 in a co-culture system (magnification, ×200). (C)
Protein expression levels of VEGF, MMP2 and MMP9 in MDA-MB-231 and
Hs578T cells, with or without αPD-L1 in a co-culture system, were
determined using western blot analysis. (D) Protein expression
levels of the EMT markers, E-cadherin, ZO-1, N-cadherin, vimentin,
Slug and Twist, in MDA-MB-231 and Hs578T cells, with or without
αPD-L1 in a co-culture system, were determined using western blot
analysis. (E) Protein expression levels of the stemness markers,
CD44, Oct4, Nanog, Bmi1 and Sox2 in MDA-MB-231 and Hs578T cells,
with or without αPD-L1 in a co-culture system were determined using
western blot analysis. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. αPD-L1, programmed death-ligand 1
inhibitor; TNBC, triple-negative breast cancer; TAM/M2,
tumor-associated macrophages/M2-type; VEGF, vascular endothelial
growth factor; EMT, epithelial-mesenchymal transition; ZO-1, zonula
occludens-1. |
aPD-L1 regulates TAM/M2 polarization by
downregulating STAT3 phosphorylation and preventing its entry into
the nucleus
To further investigate the molecular mechanisms
through which αPD-L1 regulates TAM polarization, several
immune-related signaling pathways were examined. The CEBPb, MAPK
and STAT signaling pathways are all involved in TAM/M2 polarization
(31,32). In the present study, western blot
analysis was performed and the results demonstrated that IL-13,
with or without αPD-L1, did not affect the protein expression
levels of CEBPβ. However, IL-13 upregulated the protein expression
levels of p-ERK, p-STAT6 and p-STAT3. Of note, the protein
expression levels of p-ERK and p-STAT6 exhibited no notable changes
following αPD-L1 treatment. However, the expression levels of
p-STAT3 were significantly downregulated following αPD-L1 treatment
(Figs. 6A and S1B). IL-13 binding with its receptor
promotes STAT3 phosphorylation to form a dimer in the nucleus
(33). The location of STAT3 and
p-STAT3 was subsequently determined using IF staining and
nucleoplasm separation assays. The results confirmed that IL-13 led
to the translocation of STAT3 from the cytoplasm to the nucleus,
whereas αPD-L1 led to the translocation of STAT3 from the nucleus
to the cytoplasm (Fig. 6B-D).
 | Figure 6αPD-L1 inhibits TAM/M2 polarization
via the prevention of STAT3 phosphorylation and nuclear
translocation. (A) The protein expression levels of CEBPβ, p-ERK,
ERK, p-STAT6, STAT6, p-STAT3 and STAT3 in RAW264.7 cells treated
with IL-13 and/or αPD-L1 were determined using western blot
analysis. (B and C) The protein expression levels and nuclear
translocation of STAT3 and p-STAT3 in RAW264.7 cells treated with
IL-13 and/or αPD-L1 were assessed using immunofluorescence staining
(magnification, ×400). (D) Cytosol and nuclear protein expression
levels of STAT3 and p-STAT3 in RAW264.7 cells treated with IL-13
and/or αPD-L1 were assessed using western blot analysis.
*P<0.05, **P<0.01 and
***P<0.001. ns, not significant; αPD-L1, programmed
death-ligand 1 inhibitor; TAM/M2, tumor-associated
macrophages/M2-type; CEBPβ, cAMP response element-binding
protein/CCAAT-enhancer-binding protein β; p-, phosphorylated. |
aPD-L1 prevents lung metastasis in vivo
by targeting TAM/M2
The effects of αPD-L1 on lung metastasis in
subcutaneous and intravenous models of TNBC were then investigated.
αPD-L1 was dissolved in PBS and administered to mice
intraperitoneally at a dose of 200 µg and the control group
was treated with PBS (Fig. 7A).
There were no significant differences in tumor growth and body
weight between the two groups in the TNBC subcutaneous models
(Fig. 7B and C and F). However,
αPD-L1 reduced the number of metastatic nodes on the lung tissue
surface. H&E staining of the lung tissues further confirmed the
presence of lung metastases (Fig. 7D
and E). Similar results were obtained in the TNBC intravenous
model (Fig. 7G-I). These data
suggested that αPD-L1 potentially prevented lung metastasis in both
subcutaneous and intravenous models of TNBC.
 | Figure 7αPD-L1 inhibits lung metastasis in
vivo. (A) Schematic diagram of the animal assays performed. (B
and C) Tumor volume in the subcutaneous cell-transplanted mice was
recorded over time throughout the animal assay. (D and H) The
number of lung metastatic nodules was determined. (E) Lung
metastases in mice that were subcutaneously injected with TNBC
cells. Lung tissue images, H&E scan images and H&E
magnification images (magnification, ×100) are shown, respectively.
(F and G) Body weight of subcutaneous and intravenous
cell-transplanted mice was recorded over time throughout the animal
assay. (I) Lung metastases in mice that were intravenously injected
with TNBC cells. Lung tissue images, H&E scan images and
H&E magnification images (magnification, ×100) are shown,
respectively. (J and K) CD206 protein expression levels in lung and
tumor tissues were assessed using immunohistochemistry
(magnification: upper panels, ×100, lower panels, ×200). (L-O)
Protein expression levels of E-cadherin, vimentin, VEGF and CD44 in
tumors tissues were determined using immunohistochemistry
(magnification: upper panels, ×100, lower panels, ×200).
*P<0.05. αPD-L1, programmed death-ligand 1 inhibitor;
TNBC, triple-negative breast cancer; VEGF, vascular endothelial
growth factor. |
Subsequently, IHC and IF staining demonstrated that
αPD-L1 reduced CD206 expression in the lung and tumor tissues,
which suggested that αPD-L1 potentially inhibited TAM/M2
polarization in vivo (Fig. 7J
and K). IHC staining further demonstrated that αPD-L1
upregulated the protein expression levels of E-cadherin, and
downregulated the protein expression levels of vimentin, VEGF and
CD44 (Fig. 7L-O). These data thus
suggested that αPD-L1 potentially played a significant role in the
evolution of TNBC (Fig. 8).
Discussion
The TME is complex and changeable and is closely
associated with the occurrence and metastasis of tumors. TAMs are a
key component of the TME (34).
Cytokines, such as IL-10 and TGF-β are secreted by TAM/M2 and can
inhibit the tumor immune response and promote tumor development
(35). Tumor cells can also
release biomolecules into the TME and promote TAM/M2 polarization.
The number of TAM/M2 cells in tumor tissues is related to the
malignant biological behavior and poor prognosis of various solid
tumors (36). Furthermore, the
activation of immune regulatory site PD-1/PD-L1 plays a critical
role in the process of immune cell recognition. Blocking the
expression of PD-L1 in host immune cells has more prominent
tumor-suppressive effects than inhibiting the expression of PD-L1
in tumor cells (24). Targeting
TAMs is an effective therapeutic approach with which to modulate
the activity of anti-PD-L1 agents in cancer treatment (37). This indicates that PD-L1 may play a
role by directly regulating macrophage activity. The results of the
present study suggested that αPD-L1 potentially reversed the
expression levels of PD-L1, the TAM/M2 marker, CD206, and the
levels of IL-10.
The receptor for advanced glycation end-products
regulates the TME by recruiting TAMs and therefore promotes the
progression and metastasis of TNBC (38). However, the effects of blocking
PD-1/PD-L1 on TAMs in the process of tumor metastasis remain to be
further elucidated. In the present study, the effects of αPD-L1 on
tumor cell migration via the regulation of TAM polarization in
vitro were investigated. The results indicated that αPD-L1
potentially reversed the migration of TNBC cells mediated by TAM/M2
polarization.
EMT contributes towards enhancing the capability of
cancer cells to migrate and invade, which is critical in tumor
metastasis. Numerous solid tumors have a large number of
macrophages in the TME, which can promote tumor metastasis
(39). Cancer stemness originates
from the fusion of TAM/M2 and breast cancer cells, and these hybrid
cells overexpress mesenchymal and cancer stemness markers (40). Therefore, tumor cells are likely to
become CSCs following the EMT of cancer cells (41). PD-1/PD-L1 induces immunosuppression
and promotes the EMT of cancer cells (42). The effects of αPD-L1 on the EMT and
the stemness of cancer cells were investigated in the present study
via the regulation of TAM polarization in vitro and in
vivo. The results demonstrated that αPD-L1 potentially reversed
the EMT process and the stemness of TNBC cells that were induced
via TAM/M2 polarization.
Neovascularization in tumor tissues is an important
condition for the metastasis of malignant tumors, and this process
is regulated by various chemokines in the TME (43). Macrophages promote the secretion of
VEGF by tumor cells to induce angiogenesis in local tumor tissues,
which provides nutrition for tumor growth and metastasis (44). 4-Hydroxphenyl retinamide inhibits
the TAM/M2 phenotype, which thereby inhibits the promotion of
angiogenesis (45). The expression
of VEGF can promote the metastasis of cancer cells (46). Furthermore, MMPs affect the
expression of tumor cell adhesion molecules and promote tumor cell
translocation beyond the basement membrane (47). The results of the present study
demonstrated that αPD-L1 downregulated VEGF, MMP2 and MMP9 protein
expression levels via the inhibition of TAM/M2 polarization. From
the results of the present study, it was suggested that αPD-L1
reversed the angiogenesis of TNBC induced by TAM/M2 polarization.
It can therefore be hypothesized that αPD-L1 can potentially
inhibit tumor metastasis and angiogenesis.
The conversion of the macrophage phenotype is
dependent on the activation of the signaling pathway response. The
transcription factor CEBPb regulates the expression of early growth
response protein 2 and participates in the polarization process of
macrophages (48). A previous
study demonstrated that tumor tissue promotes TAM/M2 polarization
and induces the metastasis of lung cancer via the upregulation ERK
phosphorylation (36). Moreover,
the STAT protein family regulates the biological behavior of tumor
cells and immune cells via inflammatory mediators. These proteins
play an important role in the process of inflammation-cancer
transformation. STAT3 signaling pathways serve an important role in
TAM/M2 polarization (49,50). IL-13 binds to the IL-13 receptor on
the surface of macrophages during TAM/M2 polarization and initiates
the intracellular tyrosine kinase phosphorylation cascade. STAT3 is
activated in the cytoplasm following phosphorylation at Y705 and
S727. Activated STAT3 can therefore be transferred to the nucleus
and promotes TAM/M2 polarization. The results of the present study
indicated that the phosphorylation and nuclear translocation of
STAT3 are potentially involved in the regulation of TAM/M2
polarization via αPD-L1.
In conclusion, the results of the present study
demonstrated that αPD-L1 potentially inhibited TAM/M2 polarization
in vitro and in vivo, which potentially contributed
to its anti-metastatic and anti-angiogenic function in TNBC.
Furthermore, STAT3 plays a crucial role in the effects of αPD-L1 on
TAM/M2 polarization. Therefore, PD-L1 may be a promising biomarker
for determining the prognosis of patients with TNBC and may provide
a potential therapeutic target for TAMs in anti-metastatic
treatment.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TJ and MZ designed the experiments. ZM, RZ and XW
performed the statistical analyses. ZM, RZ and XW performed the
experiments. ZM and RZ wrote the manuscript. TF and MZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Yanbian University (approval no. YD20220916004), and
was performed according to the guidelines of the Committee on
Animal Research and Ethics.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the National
Natural Science Foundation of China (no. 81960554).
References
|
1
|
Zuo TT, Zheng RS, Zeng HM, Zhang SW and
Chen WQ: Female breast cancer incidence and mortality in China,
2013. Thorac Cancer. 8:214–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaikh S and Rasheed A: Predicting
molecular subtypes of breast cancer with mammography and ultrasound
findings: Introduction of Sono-mammometry score. Radiol Res Pract.
2021:66919582021.PubMed/NCBI
|
|
4
|
Jiagge EM, Ulintz PJ, Wong S, McDermott
SP, Fossi SI, Suhan TK, Hoenerhoff MJ, Bensenhaver JM, Salem B,
Dziubinski M, et al: Multiethnic PDX models predict a possible
immune signature associated with TNBC of African ancestry. Breast
Cancer Res Treat. 186:391–401. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manjunath M and Choudhary B:
Triple-negative breast cancer: A run-through of features,
classification and current therapies. Oncol Lett. 22:5122021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Fan X and Houghton J: Tumor
microenvironment: The role of the tumor stroma in cancer. J Cell
Biochem. 101:805–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinez-Reyes I and Chandel NS: Cancer
metabolism: Looking forward. Nat Rev Cancer. 21:669–680. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emami F, Pathak S, Nguyen TT, Shrestha P,
Maharjan S, Kim JO, Jeong JH and Yook S: Photoimmunotherapy with
cetuximab-conjugated gold nanorods reduces drug resistance in
triple negative breast cancer spheroids with enhanced infiltration
of tumor-associated macrophages. J Control Release. 329:645–664.
2021. View Article : Google Scholar
|
|
9
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adorno-Cruz V, Hoffmann AD, Liu X,
Dashzeveg NK, Taftaf R, Wray B, Keri RA and Liu H: ITGA2 promotes
expression of ACLY and CCND1 in enhancing breast cancer stemness
and metastasis. Genes Dis. 8:493–508. 2021. View Article : Google Scholar :
|
|
11
|
Turajlic S and Swanton C: Metastasis as an
evolutionary process. Science. 352:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Hu JC, He SH, Lou B, Ding TB, Yang
JT, Mo MG, Ye DY, Zhou L, Jiang XC, et al: Sphingomyelin synthase 2
facilitates M2-like macrophage polarization and tumor progression
in a mouse model of triple-negative breast cancer. Acta Pharmacol
Sin. 42:149–159. 2021. View Article : Google Scholar :
|
|
13
|
Zhang XL, Hu LP, Yang Q, Qin WT, Wang X,
Xu CJ, Tian GA, Yang XM, Yao LL, Zhu L, et al: CTHRC1 promotes
liver metastasis by reshaping infiltrated macrophages through
physical interactions with TGF-β receptors in colorectal cancer.
Oncogene. 40:3959–3973. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang C, Lei X, Xiong L, Hu Z and Tang B:
HMGA1B/2 transcriptionally activated-POU1F1 facilitates gastric
carcinoma metastasis via CXCL12/CXCR4 axis-mediated macrophage
polarization. Cell Death Dis. 12:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar
|
|
16
|
Yang C, Dou R, Wei C, Liu K, Shi D, Zhang
C, Liu Q, Wang S and Xiong B: Tumor-derived exosomal
microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM
interaction to facilitate CRC metastasis. Mol Ther. 29:2088–2107.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Tan W and Wang C: Tumor-associated
macrophage-derived cytokines enhance cancer stem-like
characteristics through epithelial-mesenchymal transition. Onco
Targets Ther. 11:3817–3826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larionova I, Kazakova E, Gerashchenko T
and Kzhyshkowska J: New angiogenic regulators produced by TAMs:
Perspective for targeting tumor angiogenesis. Cancers (Basel).
13:32532021. View Article : Google Scholar
|
|
20
|
Long Y, Yu X, Chen R, Tong Y and Gong L:
Noncanonical PD-1/PD-L1 axis in relation to the efficacy of Anti-PD
therapy. Front Immunol. 13:9107042022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gatalica Z, Snyder C, Maney T, Ghazalpour
A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, et
al: Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common
cancers and their correlation with molecular cancer type. Cancer
Epidemiol Biomarkers Prev. 23:2965–2970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cimino-Mathews A, Foote JB and Emens LA:
Immune targeting in breast cancer. Oncology (Williston Park).
29:375–385. 2015.
|
|
23
|
Moser JC and Hu-Lieskovan S: Mechanisms of
resistance to PD-1 checkpoint blockade. Drugs. 80:459–465. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noguchi T, Ward JP, Gubin MM, Arthur CD,
Lee SH, Hundal J, Selby MJ, Graziano RF, Mardis ER, Korman AJ and
Schreiber RD: Temporally Distinct PD-L1 expression by tumor and
host cells contributes to immune escape. Cancer Immunol Res.
5:106–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juneja VR, McGuire KA, Manguso RT, LaFleur
MW, Collins N, Haining WN, Freeman GJ and Sharpe AH: PD-L1 on tumor
cells is sufficient for immune evasion in immunogenic tumors and
inhibits CD8 T cell cytotoxicity. J Exp Med. 214:895–904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY and
Mou XZ: The roles of tumor-associated macrophages in tumor
angiogenesis and metastasis. Cell Immunol. 353:1041192020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Zhao Y, Yao S, Cui X, Pan W, Huang
W, Gao J, Dong T and Zhang S: Nigericin inhibits epithelial ovarian
cancer metastasis by suppressing the cell cycle and
Epithelial-mesenchymal transition. Biochemistry (Mosc). 82:933–941.
2017. View Article : Google Scholar
|
|
29
|
Stankevicius V, Kunigenas L, Stankunas E,
Kuodyte K, Strainiene E, Cicenas J, Samalavicius NE and Suziedelis
K: The expression of cancer stem cell markers in human colorectal
carcinoma cells in a microenvironment dependent manner. Biochem
Biophys Res Commun. 484:726–733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wylie PG and Bowen WP: Determination of
cell colony formation in a high-content screening assay. Clin Lab
Med. 27:193–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neamatallah T: Mitogen-activated protein
kinase pathway: A critical regulator in Tumor-associated macrophage
polarization. J Microsc Ultrastruct. 7:53–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
LaPorte SL, Juo ZS, Vaclavikova J, Colf
LA, Qi X, Heller NM, Keegan AD and Garcia KC: Molecular and
structural basis of cytokine receptor pleiotropy in the
interleukin-4/13 system. Cell. 132:259–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boutilier AJ and Elsawa SF: Macrophage
polarization states in the tumor microenvironment. Int J Mol Sci.
22:69952021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma YY, He XJ, Wang HJ, Xia YJ, Wang SL, Ye
ZY and Tao HQ: Interaction of coagulation factors and
tumor-associated macrophages mediates migration and invasion of
gastric cancer. Cancer Sci. 102:336–342. 2011. View Article : Google Scholar
|
|
36
|
Sami E, Paul BT, Koziol JA and ElShamy WM:
The immunosuppressive microenvironment in BRCA1-IRIS-overexpressing
TNBC tumors is induced by bidirectional interaction with
tumor-associated macrophages. Cancer Res. 80:1102–1117. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santoni M, Romagnoli E, Saladino T,
Foghini L, Guarino S, Capponi M, Giannini M, Cognigni PD, Ferrara G
and Battelli N: Triple negative breast cancer: Key role of
Tumor-associated macrophages in regulating the activity of
anti-PD-1/PD-L1 agents. Biochim Biophys Acta Rev Cancer.
1869:78–84. 2018. View Article : Google Scholar
|
|
38
|
Nasser MW, Wani NA, Ahirwar DK, Powell CA,
Ravi J, Elbaz M, Zhao H, Padilla L, Zhang X, Shilo K, et al: RAGE
mediates S100A7-induced breast cancer growth and metastasis by
modulating the tumor microenvironment. Cancer Res. 75:974–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li B, Lu Y, Yu L, Han X, Wang H, Mao J,
Shen J, Wang B, Tang J, Li C and Song B: miR-221/222 Promote cancer
stem-like cell properties and tumor growth of breast cancer via
targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem Biol
Interact. 277:33–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Y, Chen D, Qi Y, Liu R, Li S, Zou H,
Lan J, Ju X, Jiang J, Liang W, et al: Evaluation of expression of
cancer stem cell markers and fusion gene in synovial sarcoma:
Insights into histogenesis and pathogenesis. Oncol Rep.
37:3351–3360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu T, Tang C, Tao R, Yong X, Jiang Q and
Feng C: PD-L1-mediated immunosuppression in oral squamous cell
carcinoma: Relationship with macrophage infiltration and epithelial
to mesenchymal transition markers. Front Immunol. 12:6938812021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Samples J, Willis M and Klauber-Demore N:
Targeting angiogenesis and the tumor microenvironment. Surg Oncol
Clin N Am. 22:629–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barnett FH, Rosenfeld M, Wood M, Kiosses
WB, Usui Y, Marchetti V, Aguilar E and Friedlander M: Macrophages
form functional vascular mimicry channels in vivo. Sci Rep.
6:366592016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong R, Gong Y, Meng W, Yuan M, Zhu H,
Ying M, He Q, Cao J and Yang B: The involvement of M2 macrophage
polarization inhibition in fenretinide-mediated chemopreventive
effects on colon cancer. Cancer Lett. 388:43–53. 2017. View Article : Google Scholar
|
|
46
|
Wang H, Yung MMH, Ngan HYS, Chan KKL and
Chan DW: The impact of the tumor microenvironment on macrophage
polarization in cancer metastatic progression. Int J Mol Sci.
22:65602021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Watroba S, Wisniowski T, Bryda J and
Kurzepa J: The role of matrix metalloproteinases in pathogenesis of
human bladder cancer. Acta Biochim Pol. 68:547–555. 2021.PubMed/NCBI
|
|
48
|
Veremeyko T, Yung AWY, Anthony DC,
Strekalova T and Ponomarev ED: Early growth response Gene-2 is
essential for M1 and M2 macrophage activation and plasticity by
modulation of the transcription factor CEBPβ. Front Immunol.
9:25152018. View Article : Google Scholar
|
|
49
|
Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu
W, Pan Z, Guo Q, Li B, Zhao S, et al: Hypoxic glioma-derived
exosomes promote M2-like macrophage polarization by enhancing
autophagy induction. Cell Death Dis. 12:3732021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qian M, Wang S, Guo X, Wang J, Zhang Z,
Qiu W, Gao X, Chen Z, Xu J, Zhao R, et al: Hypoxic glioma-derived
exosomes deliver microRNA-1246 to induce M2 macrophage polarization
by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene.
39:428–442. 2020. View Article : Google Scholar
|






















