Introduction
As a low-invasive tumour treatment method,
photodynamic therapy (PDT) can selectively accumulate
photosensitizers at the tumour site and generate a large number of
reactive oxygen species under the irradiation of a specific
wavelength of visible light. Subsequently, it can directly destroy
tumour cells, block the microvessels of tumour tissue and activate
the inflammatory and immune responses of the tumour site, which
aids in tumour treatment. PDT has various advantages, such as
specific targeting and high repeatability, and is a key supplement
to surgical resection, chemotherapy and radiotherapy (1). It has also been shown to lead to a
good response in certain tumour types, including oesophageal
(2), lung (3), breast (4) and skin (5) cancer, and its use has been approved
in numerous countries (1,6). However, the treatment response to
certain tumour types is poor, and tumour regrowth occurs following
PDT (7). In the clinical process
of PDT for tumours, the low concentration of photosensitizers is
used due to the particularity of tumour blood vessels. Furthermore,
due to the occurrence of the physical characteristics of light, its
light intensity decreases with the depth of the tumour; thus, the
efficacy of PDT decreases with the increasing depth of the tumour
and does not reach the lethal dose (8), that is, low-dose PDT.
As a critical part of the tumour microenvironment,
tumour blood vessels are not only the channels for tumour nutrition
delivery and tumour cell escape, but also play a crucial role in
tumour cell invasion and metastasis, thus also greatly affecting
the tolerance to treatment (1,9). In
PDT for tumours, there are factors that can induce angiogenesis and
reduce the therapeutic effect (1).
To reduce angiogenesis induced by PDT for tumours and enhance the
therapeutic effects of PDT, Ferrario et al (10) developed a model of PDT combined
with anti-angiogenic drugs to treat tumours. They used PDT combined
with anti-VEGF drugs for treatment, which effectively reduced the
blood vessel density of breast cancer in mice and improved the cure
rate of breast cancer from 39% with PDT alone to 80-90% with
combination treatment (10).
Furthermore, Peng et al (11) reported that the combination of PDT
and anti-VEGF drugs effectively inhibited tumour angiogenesis and
growth.
Previous studies have indicated that the combination
of PDT with anti-angiogenic drugs provides a novel anticancer
treatment strategy (12,13). Moreover, it has been hypothesized
that this combination has great prospects for the development of
anti-angiogenic drugs that target key molecules in the tumour
angiogenesis signalling pathway. However, other studies have found
that PDT combined with anti-VEGF drugs cannot effectively inhibit
tumour angiogenesis for a long period of time (6,14).
Drug resistance has been reported to occur at ~6 months following
the use of anti-VEGF drugs, and the long-term survival rate of
patients has not found to be significantly prolonged following
combination treatment (6,14). Therefore, it is of utmost
importance to identify key molecules in other anti-angiogenesis
signalling pathways that can serve as targets in PDT combined with
other therapies.
Activin receptor-like-kinase 1 (ALK1) is a type I
receptor of the TGF-β family, specifically a serine/threonine
kinase receptor. It is a homodimeric transmembrane glycoprotein
integrated into the cell membrane, which is mainly expressed in
vascular endothelial cells and other vascular-rich tissues. As a
signalling pathway that differs from the VEGF signalling pathway
(15), ALK1 regulates endothelial
cell proliferation, migration and lumen formation, and plays a
decisive role in regulating vascular development and pathological
angiogenesis (16,17).
Notably, ALK1 has been widely reported in tumour
blood vessels, and the expression of ALK1 is positively associated
with tumour vascular density (16,18).
Cunha et al (19) found
that reducing the expression level of ALK1 via genetic or
pharmacological approaches inhibited tumour growth and
angiogenesis. Hawinkels et al (20) confirmed through in vivo and
in vitro experiments on three types of tumour cells that the
use of drugs targeting ALK1 effectively inhibited tumour
angiogenesis and enhanced the chemotherapeutic effects. Clinical
studies have found that the use of drugs targeting ALK1 can reduce
the density of capillaries in tumours, inhibit various tumour
growths and inhibit the metastasis of breast cancer (21,22).
There is evidence to indicate that the abnormal
activation of the ALK1 signalling pathway plays a key role in the
occurrence and development of tumour angiogenesis; thus, the
inhibition of ALK1 may be a key strategy with which to prevent
tumour angiogenesis. In order to clarify the role of ALK1 in
PDT-induced tumour angiogenesis, the present study used human
umbilical vein endothelial cells (HUVECs) co-cultured with breast
cancer cell MDA-MB-231 (termed HU-231 cells) as the research object
to better simulate the tumour intravascular environment in
vivo. The present study aimed to reveal the role of the
anti-angiogenesis target, ALK1, in PDT-induced tumour angiogenesis,
thereby providing a theoretical basis for PDT combined with
anti-ALK1 in the treatment of tumours.
Materials and methods
Cells and cell culture
The MDA-MB-231 human breast cancer cells (cat. no.
SCSP-5043) were purchased from The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences and HUVECs (cat. no.
C0035C) were purchased from Thermo Fisher Scientific, Inc. (the
cells were frozen and stored at the Chongqing Key Laboratory of
Translational Medicine in Major Metabolic Diseases, Chongqing,
China). The cells were routinely and individually cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin and streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2. The HUVECs were co-cultured with the
MDA-MB-231 breast cancer cells and termed HU-231 cells, which were
established as experimental subjects in the present study to
simulate vascular endothelial cells in the intra-tumour
environment. All cell cultures were mycoplasma species-negative.
The experimental groups were as follows: The ALK1 inhibitor groups
(control, PDT, K02288 and PDT + K02288 group; K02288 was obtained
from Sigma-Aldrich, Merck KGaA), adenovirus transfection groups
(control, negative control, PDT, Ad-ALK1 and PDT + Ad-ALK1 group)
and PI3K inhibitor groups (control, PDT, LY294002 and PDT +
LY294002 group; LY294002 was obtained from Sigma-Aldrich, Merck
KGaA). The concentrations of K02288 and LY294002 used were 5 and 10
µmol/l, respectively. Both inhibitors were used to treat the
cells for 24 h.
PDT
The HU-231 cells were treated with the
photosensitizer, pyropheophorbide-a methyl ester (MPPa; cat. no.
P3787; Sigma-Aldrich, Merck KGaA) at the concentration of 10
µmol/l for 4 h and then washed twice with PBS. Following the
addition of 2 ml DMEM (Gibco; Thermo Fisher Scientific, Inc.), the
cells were irradiated for 5 sec with a wavelength of 630 nm and an
energy intensity of 10 mW/cm2 using a LED laser
(Chongqing Jinyu Laser Technology Co., Ltd.).
Adenovirus transfection
The HUVECs were inoculated into six-well plates at a
density of 6×104 (care was taken to avoid clustering)
and after 12 h, adenovirus AD-ALK1-RNAi (cat. no. 112177-1;
Shanghai Genechem Co., Ltd.; https://www.genechem.com.cn/; AD-ALK1-RNAi
(112177-1)-a, CCG GUC CAG AGA AGC CUA AAG UGA UCU CGA GAU CAC UUU
AGG CUU CUC UGG AUU UUU G; AD-ALK1-RNAi (112177-1)-b, AAU UCA AAA
AUCCAGAGA AGCCUA AAGUGAUCUCGA GAU CAC UUU AGG CUU CUC UGG A) and
negative control virus CON098 (Shanghai Genechem Co., Ltd.) were
added at a multiplicity of infection (MOI) of 1,000. After 12 h,
the solution was changed and included MDA-MB-231 cell chambers for
co-culture and subsequent experiments. The transfection efficiency
was determined by measuring the fluorescence intensity and
RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
The MDA-MB-231 and HUVECs were co-cultured at
5×104 cells/well in small chambers (BD Biosciences) and
24-well plates to investigate the appropriate drug concentrations
and light intensities, as well as the cellular activity of the four
groups of inhibitors. After 24 h, the medium of each well was
discarded and 700 µl CCK-8 solution (cat. no. C0037;
Beyotime Institute of Biotechnology) mixed with serum-free DMEM was
added to each well and the cells were further incubated for 1 h at
37°C in 5% CO2 for a further 1 h. The optical densities
(ODs) were measured using a microplate reader (Thermo Fisher
Scientific, Inc.). Cell survival was calculated as follows: Cell
survival (%)=(average OD of experimental group-average OD of blank
group)/(average OD of control group-average OD of blank group)
×100%. The wells in the blank group contained DMEM in CCK-8
solution only.
Colony formation assay
The MDA-MB-231 and HUVECs were co-cultured in small
chambers and six-well plates and treated with ALK1 inhibitor
(K02288) and transfected with adenovirus, respectively. After 24 h,
the HU-231 cells were counted at 800 cells/well and inoculated into
12-well plates. The culture was terminated when the appearance of
colonies visible to the naked eye was observed. The colonies were
fixed with 4% paraformaldehyde (cat. no. P0099; Beyotime Institute
of Biotechnology) for 30 min at room temperature and stained with
crystal violet solution (cat. no. C0121; Beyotime Institute of
Biotechnology) for 30 min at room temperature. The colonies were
photographed using an Android smartphone (Vivo X7 plus; Vivo Mobile
Communication Co., Ltd.) and counted using ImageJ software (ImageJ
1.53k; National Institutes of Health).
Wound healing assay
The MDA-MB-231 cells and HUVECs were co-cultured in
small chambers and six-well plates with serum-free DMEM and treated
with ALK1 inhibitor (K02288; 3×105 cells/well) and
transfected with adenovirus, respectively. Immediately following
PDT, the cells in all groups were scored with a gun tip (10
µl). DiI cell membrane fluorescent probe orange-red (cat.
no. MB4240; Meilunbio Co., Ltd.) was then added for 10 min and the
cells were washed in PBS. Images were obtained immediately at 0, 24
and 48 h later using a fluorescence microscope (Carl Zeiss AG). The
scratch areas were analysed using ImageJ software (ImageJ 1.53k;
National Institutes of Health).
Migration and invasion assays
The HUVECs and MDA-MB-231 cells were co-cultured at
5×104 cells/well in small chambers and 24-well plates
and 60 µl Matrigel gel (Corning, Inc.) diluted at 1:8 was
added to the small chambers prior to cell inoculation (for the
invasion assay). The cells were treated with ALK1 inhibitor
(K02288) and PDT. Following 24 h of co-culture (MDA-MB-231 and
HUVECs) in the adenovirus transfection group, the cells were
removed from each well and inoculated into 24-well Transwell
chambers at 5×104 cells/well for incubation at 37°C.
After 24 h, the cells that had not invaded the surface of the
Transwell chambers in each group were cleared using cotton swabs,
and the invading cells in the chambers were fixed with 4%
paraformaldehyde for 30 min at room temperature, washed with PBS
and stained with crystal violet for 30 min at room temperature. The
migration and invasion of the cells in the Transwells were observed
using a Zeiss light microscope (Carl Zeiss AG). Cell counts were
analysed using ImageJ software (ImageJ 1.53k; National Institutes
of Health).
Tube formation assay
The MDA-MB-231 cells and HUVECs were co-cultured in
small chambers and six-well plates and treated with ALK1 inhibitor
(K02288) and transfected with adenovirus were incubated at 37°C and
5% CO2 for 24 h. Following incubation, cells were
inoculated on confocal dishes (BD Biosciences) with plates coated
with 200 µl Matrigel at a concentration of 5×105
cells per well. Tubes were incubated at 37°C and 5% CO2
for 9 h and then fixed with 4% paraformaldehyde for 30 min at room
temperature, lysed with Triton-X (cat. no. P0096; Beyotime
Institute of Biotechnology) for 15 min at room temperature, stained
with phallacidin (cat. no. C2205S; Beyotime Institute of
Biotechnology) for 30 min at room temperature, and then stained
with DAPI (cat. no. C1002; Beyotime Institute of Biotechnology) for
20 min at room temperature. Tubes were photographed in five random
microscope fields using a fluorescence microscope (Leica
Microsystems GmbH). ImageJ Angiogenesis Analyzer software (ImageJ
1.53k; National Institutes of Health) was then used to analyse the
raw images. In this process, a map of each imaging tube was
generated and the number of nodes, junctions, branches and branch
lengths were calculated using ImageJ software (ImageJ 1.53k;
National Institutes of Health).
5-Ethynyl-2'-deoxyuridine (EdU)
assay
The MDA-MB-231 cells and HUVECs were co-cultured in
small chambers and six-well plates and treated with ALK1 inhibitor
(K02288) and transfected with adenovirus. After 24 h, the HU-231
cells were counted as 2×104 cells/well respectively and
inoculated into confocal dishes. EdU assay was then performed using
an EdU kit (cat. no. C0078S; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The nuclei were stained
with Hoechst 33342 for 10 min at room temperature. The cells were
counted under a fluorescence microscope (Carl Zeiss AG).
Immunofluorescence staining
The HUVECs co-cultured with the MDA-MB-231 cells
were treated with ALK1 inhibitor (K02288) and transfected with
adenovirus. After 24 h, the HU-231 cells were counted as
2×104 cells/well respectively and inoculated into
confocal dishes. After 24 h, the cells were fixed in 4%
paraformaldehyde at room temperature for 15 min. The samples were
then blocked with Fast Blocking Solution (cat. no. p30500; New Cell
& Molecular Biotech Co., Ltd.) for 30 min at room temperature.
The cell were then incubated with primary antibody ALK1 (1:150
dilution; cat. no. ab68703; Abcam) overnight at 4°C, followed by
incubation with fluorescent secondary antibody goat anti-rabbit
(1:500 dilution; cat. no. SA00003; Proteintech Group, lnc.) for 1 h
at room temperature. Subsequently, the cells were incubated with
DAPI for 10 min at room temperature. Finally, fluorescence was
detected using a fluorescence microscope (Leica Microsystems
GmbH).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the SteadyPure
Universal RNA Extraction kit according to the manufacturer's
protocol [cat. no. AG21017; Accurate Biotechnology (Hunan) Co.,
Ltd.] and quantified spectrophotometrically using a NanoDrop
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.). The
Evo M-MLV Mix kit with gDNA Clean for qPCR (cat. no. AG11728;
Accurate Biotechnology (Hunan) Co., Ltd.) was used for reverse
transcription(37°C for 15 min and 85°C for 5 sec). The primers used
were the following: GAPDH forward, GCA CCG TCA AGG CTG AGA
AC and reverse, TGG TGA AGA CGC CAG TGG A; ALK1 forward, CAG
CCC ACG AAT CAT CTC CC and reverse, GTC CCC TGT CAC TCC ACT TC;
inhibitor of DNA binding 1 (ID1) forward, ATC GCA TCT TGT
GTC GCT GAA and reverse, ATT CCT CTT GCC CCC TGG AT; AKT
forward, CTG TTC TTC CAC CTG TCC CG and reverse, TAA TGT GCC CGT
CCT TGT CC. The SYBR-Green Premix Pro Taq HS qPCR kit according to
the manufacturer's instructions (cat. no. AG11701; Accurate
Biotechnology (Hunan) Co., Ltd.) and the CFX96™ real-time PCR
detection system (Bio-Rad Laboratories, Inc.) were employed to
perform qPCR under the following conditions: Initial hold at 95°C
for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec
and annealing/extension at 60°C for 30 sec. The 2−ΔΔCq
method (23) was used to analyse
the relative expression levels.
Western blot analysis
Cellular proteins were collected following 24 h of
treatment in each group and all cells were lysed using RIPA lysis
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
containing protease inhibitor and phosphatase inhibitor for 15 min
on ice. The protein content was determined using a BCA protein
assay kit (cat. no. P0012; Beyotime Institute of Biotechnology).
Equal amounts of protein (20 µg) were subjected to SDS-PAGE
(12.5% gel) and then transferred onto a nitrocellulose membrane
(Invitrogen; Thermo Fisher Scientific, Inc.) using the wet
electroblotting system (Bio-Rad Laboratories, Inc.). Subsequently,
the membrane was blocked with 5% non-fat milk (cat. no. P0216;
Beyotime Institute of Biotechnology) for 2 h at room temperature,
followed by overnight incubation at 4°C with the respective primary
antibodies: Rabbit polyclonal antibody (pAb) to ALK1 (1:1,000
dilution; cat. no. ab68703; Abcam), rabbit monoclonal antibody
(mAb) to phosphorylated (p-)Smad1/5 (1:1,000 dilution; cat. no.
9516T; Cell Signaling Technology, Inc.), rabbit mAb to Smad1
(1:1,000 dilution; cat. no. 6944T; Cell Signaling Technology,
Inc.), rabbit pAb to ID1 (1:1,000 dilution; cat. no. ab230679;
Abcam), rabbit mAb to PI3K (1:1,000 dilution; cat. no. 3011T; Cell
Signaling Technology, Inc.), rabbit pAb to p-PI3K (1:1,000
dilution; cat. no. bs-6417R; Bioss), rabbit mAb to AKT (1:1,000
dilution; cat. no. 4685S; Cell Signaling Technology, Inc.), rabbit
mAb to p-AKT (1:1,000 dilution; cat. no. 4060S; Cell Signaling
Technology, Inc.) and rabbit mAb to β-actin (1:2,000 dilution; cat.
no. AC028; ABclonal, Inc.), followed by incubation with goat
anti-rabbit IgG secondary antibody (1:10,000 dilution; cat. no.
ZB-2301; Zsbio) for 1 h at room temperature. The immunoreactive
bands were then visualised using the Fusion FX.EDGE (Vilber Bio
Imaging). Visualisation was carried out using the new Super ECL
assay (Thermo Fisher Scientific, Inc.), which was then exposed to
film and analysed using ImageJ software (ImageJ 1.53k; National
Institutes of Health).
Statistical analysis
Data are represented as the mean ± SD. Statistical
analyses were performed using GraphPad Prism9 software (GraphPad
Software Inc.). The differences between two groups were analysed
using a Student's t-test. Multiplegroup statistical comparisons
were performed using oneway ANOVA with Tukey's multiplecomparisons
test. The quantification of cell numbers, colony numbers, wound gap
closure and western blot band integrated density were performed
using ImageJ software (ImageJ 1.53k; National Institutes of
Health). All experiments were performed in triplicate. P<0.05
was considered to indicate a statistically significant
difference.
Results
Low-dose PDT induces tumour angiogenesis
and ALK1 inhibitor reverses this effect
To establish a model of tumour angiogenesis induced
by low-dose PDT, CCK-8 assay was used to detect the appropriate
MPPa drug concentration and light intensity in the co-culture
system. As illustrated in Fig. 1A,
the 10 µmol/l MPPa concentration had no significant effect
on cell viability, while 15 and 20 µmol/l MPPa significantly
inhibited cell viability; therefore, the 10 µmol/l MPPa
concentration was used in subsequent PDT experiments. Moreover, the
cell viability was related to the light intensity, which was 10
MPPa µmol/l. Furthermore, when the light energy density was
0.04 J/cm2, the proliferative activity of the HU-231
cells was the strongest (Fig. 1B);
thus, this light intensity was used in subsequent experiments.
Additionally, when the concentration was 5 µmol/l, K02288
(ALK1 inhibitor) had no significant effect on cell viability.
However, at 10, 20 and 40 µmol/l, K02288 decreased cell
viability (Fig. 1C) in a
concentration-dependent manner. Therefore, in the subsequent
experiments, 5 µmol/l K02288 was used. The results of CCK-8
assay for the four groups of cells (the control, PDT, K02288 and
PDT + K02288 group) revealed that PDT combined with K02288
significantly inhibited HU-231 cell viability compared with the
control and PDT groups (Fig.
1D).
 | Figure 1Effect of PDT on the viability and
proliferation of HU-231 cells. (A) Effects of various
photosensitizer (MPPa) concentrations (5, 10, 15 and 20
µmol/l) on the viability of HU-231 cells. (B) Effects of
different activin receptor-like kinase-1 inhibitor (K02288)
concentrations (2.5, 5, 10, 20 and 40 µmol/l) on HU-231 cell
viability. (C) Cell viability under different light intensities
(1.6, 3.2, 4, 8, 16 and 24 ×10−2 J/cm2). (D)
Cell viability of the control group, PDT group, K02288 group and
PDT + K02288 group. (E and F) Cell proliferation of the control,
PDT, K02288 and PDT + K02288 groups (scale bars, 200 µm). (G
and H): Proliferative ability of the cells in the four groups. The
red colour represents the proliferating cells stained with EdU, and
the blue colour represents all cells stained with Hoechst 33342
(scale bars, 20 µm). *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. PDT, photodynamic therapy; MPPa,
pyropheophorbide-a methyl ester. |
Furthermore, colony forming and EdU assays were used
to detect the proliferative ability of the HU-231 cells, which
revealed that the colony number and proliferation rate of the
low-dose PDT group were significantly higher than those of the
other groups. These findings indicated that low-dose PDT enhanced
the proliferative ability of the HU-231 cells. There was no
significant difference between the K02288 and the control group.
However, the colony number and proliferation rate of the PDT +
K02288 group were significantly reduced compared with the control
group and PDT group, indicating that PDT combined with K02288
weakened the proliferative ability of the cells and reversed tumour
angiogenesis induced by low-dose PDT (Fig. 1E-H).
The horizontal and vertical migratory abilities of
the HU-231 cells were determined using wound healing and Transwell
migration assays, respectively. The results of the wound healing
assay (Fig. 2A and B) revealed
that the wound healing rate of the low-dose PDT group at 24 and 48
h was significantly higher than that of the control group,
indicating that low-dose PDT enhanced the horizontal migratory
ability of the HU-231 cells. However, the migration area was
significantly reduced following combination treatment with K02288,
which weakened the horizontal migratory ability of the cells. The
Transwell migration assay (Fig. 2C and
D) revealed that the number of migrating cells in the low-dose
PDT group was significantly higher than that in the other groups,
indicating that low-dose PDT enhanced the vertical migratory
ability of the HUVECs co-cultured with MDA-MB-231 cells. However,
following combination treatment with K02288, the number of
migrating cells was significantly reduced, which weakened the
vertical migratory ability of the cells.
 | Figure 2Effects of low-dose PDT on the
migration, invasion and tubular formation of HU-231 cells. (A and
B) Horizontal migratory ability of the cells in the control, PDT,
K02288 and PDT + K02288 groups at 0, 24 and 48 h (scale bars, 200
µm). (C and D) Vertical migratory ability of the cells in
the control, PDT, K02288 and PDT + K02288 groups (scale bars, 200
µm). (E and F) Invasive ability of the cells in the control,
PDT, K02288 and PDT + K02288 groups (scale bars, 200 µm).
(G-J) Ability of the cells to form tubes in the control, PDT,
K02288 and PDT + K02288 groups (scale bars, 200 µm).
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. PDT,
photodynamic therapy. |
Transwell invasion assay (Fig. 2E and F) also revealed that the
number of invasive cells in the low-dose PDT group was
significantly higher than that in the other groups, indicating that
low-dose PDT enhanced the invasive ability of the HU-231 cells.
However, following combination treatment with K02288, the number of
invasive cells was significantly reduced, which weakened the
invasive ability of cells and reversed the promoting effects of
low-dose PDT on tumour vascular invasion.
Furthermore, the tube formation assay (Fig. 2G-J) revealed that the tube-forming
length, tube-forming node and tube-forming ring number of the
low-dose PDT group were significantly higher than those of the
other groups, indicating that low-dose PDT enhanced the
tube-forming ability of the HU-231 cells. However, following
combination treatment with K02288, the tube-forming length,
tube-forming node and tube-forming ring number were significantly
reduced, which weakened the tube-forming ability of the cells. It
also reversed the effects induced by low-dose PDT, wherein the
tube-forming ability of the tumour blood vessels was enhanced.
Low-dose PDT induces tumour angiogenesis
and ALK1 knockdown reverses this effect
Colony formation and EdU assays were used to detect
the proliferative ability of the HU-231 cells. The colony number
and proliferation rate of the low-dose PDT group were significantly
higher than those of the other groups. Moreover, a significant
difference between the Ad-ALK1 group and the control group was
observed, indicating that the knockdown of the ALK1 gene
inhibited cell proliferation. After PDT was combined with Ad-ALK1,
the colony number and proliferation rate significantly differed
from those of the control group and PDT group, which severely
weakened the cell proliferative ability and reversed the promoting
effects on tumour angiogenesis induced by low-dose PDT (Fig. 3).
 | Figure 3Effects of low-dose PDT on the
viability and proliferation of HU-231 cells. (A and B) Cell
proliferative ability of the cells in the control group, NC group,
PDT group, Ad-ALK1 group and PDT + Ad-ALK1 group. (C and D)
Proliferative ability of the cells in the five groups. The red
colour represents the proliferating cells stained with EdU, and the
blue colour represents all cells stained with Hoechst 33342 (scale
bars, 30 µm. *P<0.05, **P<0.01
and ***P<0.001. NC, negative control; Ad, adenovirus;
PDT, photodynamic therapy; ALK1, activin receptor-like
kinase-1. |
The horizontal and vertical migratory abilities of
the HU-231 cells were analysed using wound healing and Transwell
migration assays, respectively. The wound healing assay (Fig. 4A and B) revealed that the wound
healing rate of the low-dose PDT group at 24 and 48 h was
significantly higher than that of the control group. There was no
significant difference between the Ad-ALK1 group and the control
group. However, the migration area of the PDT + Ad-ALK1 group was
significantly reduced compared with that of the control and PDT
groups, indicating the severely weakened horizontal migratory
ability of the cells. Moreover, the effects induced by low-dose PDT
were reversed, wherein the horizontal migratory ability of new
tumour blood vessels was enhanced. The Transwell migration assay
(Fig. 4C and D) also revealed that
the number of migrating cells in the low-dose PDT group was
significantly higher than that in the other groups. However,
compared with the and PDT groups, the number of migrating cells in
the PDT + Ad-ALK1 group was significantly reduced, which weakened
the vertical migratory ability of the cells. It further reversed
the effects of low-dose PDT, wherein the vertical migratory ability
of new tumour vessels was enhanced.
 | Figure 4Effects of low-dose PDT on the
migration, invasion and tubular formation of HU-231 cells. (A and
B) Horizontal migratory ability of the cells in the control group,
NC group, PDT group, Ad-ALK1 group and PDT + Ad-ALK1 group at 0, 24
and 48 h (scale bars, 200 µm). (C and D) Vertical migration
of the cells in the control, NC, PDT, Ad-ALK1 and PDT + Ad-ALK1
groups (scale bars, 200 µm). (E and F): Vertical invasion of
the cells in the control, NC, PDT, Ad-ALK1 and PDT + Ad-ALK1 groups
(scale bars, 200 µm). (G-J) Ability of the cells to form
tubes in the control, NC, PDT, Ad-ALK1 and PDT + Ad-ALK1 groups
(scale bars, 200 µm). *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. NC, negative control; Ad, adenovirus;
PDT, photodynamic therapy; ALK1, activin receptor-like
kinase-1. |
Transwell invasion assay (Fig. 4E and F) revealed that the number of
invasive cells in the low-dose PDT group was significantly higher
than that in other groups. However, compared with the control and
PDT groups, the number of invasive cells in the PDT + Ad-ALK1 group
was significantly reduced, which weakened the invasive ability of
cells and reversed the effect induced by low-dose PDT, wherein the
invasive ability of new tumour blood vessels was enhanced.
The tube formation assay (Fig. 4G-J) demonstrated that the tube
length, tube node and tube ring number of the low-dose PDT group
were significantly higher than those of the other groups,
indicating that low-dose PDT enhanced the tube-forming ability of
the HU-231 cells. However, the tube length, tube node and tube ring
number of the PDT + Ad-ALK1 group were significantly reduced
compared with the control and PDT groups, indicating the suppressed
tube-forming ability of the cells. It also reversed the effect
induced by low-dose PDT, wherein tumour angiogenesis was
enhanced.
Involvement of the ALK1-Smad1/5-ID1
pathway in low-dose PDT-induced tumour angiogenesis
The aforementioned experiments verified that ALK1
was involved in the enhancement of HU-231 cell activity,
proliferation, migration, invasion and tube formation abilities of
the tumours induced by low-dose PDT. Furthermore, to elucidate the
role of ALK1 in the enhancement of HU-231 cell activity and the
ability of proliferation, migration, invasion and tube formation
induced by low-dose PDT, the gene level changes (Fig. 5A and C) of ALK1 and ID1 in the
control, low-dose PDT, K02288 and PDT + K02288 groups at 0, 2, 3,
6, 10 and 24 h following treatment were detected using RT-qPCR. In
the low-dose PDT group, ALK1 expression was significantly increased
(Fig. 5A) compared to that in the
control group at 2 h following treatment. No significant
differences were observed at the other time points. Furthermore,
ID1 expression significantly decreased in the K02288 group and PDT
+ K02288 group, apart from the time point of 0 h (Fig. 5C). In addition, the gene level
changes of ALK1 and ID1 were also detected at 2 h following
adenovirus transfection and ALK1 knockdown. The results
revealed that both ALK1 and ID1 expression levels were
significantly elevated in the PDT group compared with the control
group, while the decrease in ALK1 and ID1 expression levels in the
Ad-ALK1 and PDT + Ad-ALK1 groups were significant compared to the
control group (Fig. 5B and D).
 | Figure 5Expression of ALK1 and ID1 at
different time points in each group. (A) Expression of ALK1 at
different time points (0, 2, 3, 6, 10 and 24 h) in the control,
PDT, K02288 and PDT + K02288 groups. (B) Expression of ALK1 at 2 h
in the control, NC, PDT, Ad-ALK1 and PDT + Ad-ALK1 groups. (C)
Expression of ID1 at different time points (0, 2, 3, 6, 10 and 24
h) in the control, PDT, K02288 and PDT + K02288 groups. (D)
Expression of ID1 at 2 h in the control, NC, PDT, Ad-ALK1 and
Ad-ALK1 + PDT groups. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. NC, negative control; Ad, adenovirus;
PDT, photodynamic therapy; ALK1, activin receptor-like kinase-1;
ID1, inhibitor of DNA binding 1. |
These findings indicated that during PDT-induced
tumour angiogenesis, the expression of ALK1 was significantly
increased at 2 h, and that of its downstream molecule, ID1, was
significantly decreased following treatment with ALK1 inhibitor (at
2, 3, 6, 10 and 24 h) or ALK1 knockdown (2 h).
Subsequently, using immunofluorescence to detect the
content of ALK1 in HU-231 cells, the fluorescence intensity of the
PDT group was observed to be significantly stronger than that of
the control group, while the fluorescence intensity significantly
decreased following combination treatment with PDT with K02288
(Fig. 6A and B).
 | Figure 6The association between low-dose
PDT-induced tumour angiogenesis and the ALK1-Smad1/5-ID1 pathway.
(A and B) Expression of ALK1 in the control, PDT, K02288 and PDT +
K02288 groups (scale bars, 50 µm). (C-F) Expression of ALK1,
Smad 1, p-Smad1/5 and ID1 proteins in the control, PDT, K02288 and
PDT + K02288 groups. (G-J) Expression of ALK1, Smad 1, p-Smad1/5
and ID1 proteins in the control, NC, PDT, Ad-ALK1 and PDT + Ad-ALK1
groups. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. NC,
negative control; Ad, adenovirus; p-, phosphorylated; PDT,
photodynamic therapy; ALK1, activin receptor-like kinase-1; ID1,
inhibitor of DNA binding 1. |
Western blot analyses also revealed that the
expression of ALK1, p-Smad1/5 and ID1 was upregulated in the
low-dose PDT group, whereas it was inhibited following combination
treatment with PDT with K02288. Following the adenovirus knockdown
of ALK1, the levels of ALK1, p-Smad1/5 and ID1 were also
significantly decreased compared with the control, negative control
and PDT groups (Fig. 5C-J).
Furthermore, the tumour angiogenesis induced by
low-dose PDT may have been attributed to the significant increase
in the expression of ALK1, p-Smad1/5 and ID1. After PDT was
combined with inhibitor drugs or adenovirus, the expression of the
aforementioned molecules was significantly decreased, confirming
that tumour angiogenesis induced by low-dose PDT may be related to
this pathway.
The PI3K/AKT pathway is not involved in
low-dose PDT-induced tumour angiogenesis
To determine whether tumour angiogenesis induced by
low-dose PDT is related to the PI3K/AKT pathway in addition to the
classic Smad pathway, the gene level changes of AKT after the
combined application of ALK1 inhibitor (K02288) or the adenovirus
knockdown of ALK1 was detected using RT-qPCR. AKT expression
exhibited no significant change in each group at the different time
points (Fig. 7A and B).
 | Figure 7The association between tumour
angiogenesis induced by low-dose PDT and the PI3K/AKT pathway. (A)
The expression of AKT in the control, PDT, K02288 and PDT + K02288
groups at different time points (0, 2, 3, 6, 10 and 24 h). (B) The
expression of AKT at 2 h in the control, NC, PDT, Ad-ALK1 and PDT +
Ad-ALK1 groups. (C and D) The protein expression of AKT, p-AKT,
PI3K and p-PI3K in the control, PDT, K02288 and PDT + K02288 groups
after 24 h of treatment. (E-G) The protein expression of AKT,
p-AKT, PI3K and p-PI3K in the control, PDT, Ad-ALK1 and PDT +
Ad-ALK1 groups after 24 h of treatment. (H) Effects of various PI3K
inhibitor (LY294002) concentrations (5, 10, 20 and 40
µmol/l) on HU-231 cell viability. (I-K) Protein expression
of AKT, p-AKT, PI3K and p-PI3K in the control, PDT, LY294002 and
PDT + LY294002 groups after 24 h of treatment
*P<0.05, **P<0.01 and
***P<0.001. ns, not significant (P>0.05); NC,
negative control; Ad, adenovirus; p-, phosphorylated; PDT,
photodynamic therapy; ALK1, activin receptor-like kinase-1. |
Similarly, western blot analyses further revealed no
significant changes in the PI3K/AKT total protein and
phosphorylation levels (Fig. 7C and
D) when PDT was used at a low dose alone or in combination with
the ALK1 inhibitor, K02288. Moreover, following adenovirus
knockdown, AKT phosphorylation was enhanced, while the PI3K and
p-PI3K levels were not significantly altered, which was consistent
with the observations of the inhibitor group (Fig. 7E-G). Subsequently, the PI3K
inhibitor, LY294002, was used to verify whether low-dose
PDT-induced tumour vascular proliferation was associated with
PI3K/AKT signalling pathway. The appropriate drug concentration was
first screened using CCK-8 assay and no significant effect was
observed on cell viability at the concentration of 10 µmol/l
(Fig. 7H); thus, this
concentration was subsequently used for the subsequent western
blotting experiments. In the western blot analysis, the protein
expression of AKT, p-AKT, PI3K and p-PI3K was measured in four
groups (control, PDT, LY294002 and PDT + LY294002 group) (Fig. 7I-K). The expression of p-PI3K and
p-AKT in the LY294002 and PDT + LY294002 groups was significantly
decreased compared with the control group and there was no
significant difference between the two groups; similarly, there was
no significant difference in the expression of p-PI3K and p-AKT
between the PDT group and the control group. This suggests that the
PI3K/AKT pathway may not be involved in tumour angiogenesis induced
by low-dose PDT.
Discussion
PDT has unique advantages, such as minimal
invasiveness, low systemic toxicity and no drug resistance
(20). The main principle of PDT
in cancer treatment includes the systemic or local application of a
non-toxic photosensitive dye, a photosensitizer. Following the
selective aggregation of the photosensitizer in tumours, the
photosensitizer is stimulated by visible light at an appropriate
wavelength. The presence of oxygen in cells and tissues leads to
the production of cytotoxic substances and the activation of
signalling pathways, thereby causing cell death and tumour tissue
destruction (24). However, due to
the specificity of tumour blood vessels, the concentration of
photosensitizer accumulation in the tumour is low. Moreover, the
light intensity decreases with the depth of the tumour. In
addition, the tumour resides in a hypoxic condition, which causes
PDT to affect the deep part of the tumour in a low-dose state
(8). Subsequently, the induced
oxidative stress and cell damage activate the tumour cell
survival-promoting signalling pathway (25). This may be a potential reason for
the poor response of PDT to some tumours and tumour regrowth
following PDT treatment (26).
Previous research has reported that low-dose PDT not only affects
cell proliferation and differentiation, but also has a
tumour-protecting effect (27,28),
and improves the tumorigenic potential of cells (29). In vivo studies have also
found that low-dose PDT increases the proliferation of cerebral
vascular endothelial cells in nude mice (30). Therefore, the present study focused
on the effects of low-dose PDT on the tumour vascular
endothelium.
Herein, to better simulate the tumour intravascular
environment in vivo, HUVECs co-cultured with breast cancer
cell line MB-231 (HU-231 cells) were used as the research object.
The photosensitizer concentration (10 µmol/l), combined with
the light intensity (0.04 J/cm2) that can significantly
increase the cell activity, was further used to simulate the tumour
angiogenesis induced by low-dose PDT. Consistently, in the present
study, low-dose PDT did indeed induce the proliferation of tumour
vascular endothelial cells and enhanced their migration, invasion
and tube formation.
It has been demonstrated that ALK1 plays a key role
in the regulation of physiological and pathological angiogenesis
(31). In humans, ALK1 mutation
can cause type II hereditary haemorrhagic telangiectasia, which is
characterized by telangiectasia, arteriovenous malformations and
periodic bleeding (32). In a
genetic study on mice, ALK1 gene mutation was observed to induce
the death of mice at the embryonic stage, owing to vascular
malformations, such as excessive fusion of capillary plexus and
excessive expansion of large blood vessels (33). It has been demonstrated that the
deletion of ALK1 not only inhibits the proliferation and migration
of endothelial cells, but also the differentiation and maturation
of vascular smooth muscle cells and the recruitment to the original
vascular network formed by endothelial cells (34). Therefore, the role of ALK1 in
tumour angiogenesis induced by low-dose PDT was explored
herein.
The present study observed that in the co-culture of
MDA-MB-231 breast cancer cell and HUVECs, after low-dose PDT was
combined with ALK1 inhibitor (K02288) or the adenovirus knockdown
of ALK1, the significantly enhanced proliferation, migration,
invasion and tubulogenesis induced by low-dose PDT were suppressed.
However, the ALK1 mRNA and protein expression levels increased
following low-dose PDT treatment, particularly at 2 h after
low-dose PDT treatment. These findings thus indicated that tumour
blood vessels were stimulated by stress after PDT treatment and the
expression of ALK1 in endothelial cells was upregulated, thereby
promoting the proliferation and migration of vascular endothelial
cells and participating in PDT-induced tumour angiogenesis.
Therefore, the inhibition of ALK1 could enhance the efficacy of PDT
in treating tumours. Based on pharmacological and genetic
approaches, PDT was combined with K02288 or ALK1 knockdown, which
revealed that the protein expression of ALK1 was significantly
downregulated compared with the control and low-dose PDT group.
This may be attributed to the fact that K02288 is a competitive
inhibitor. Furthermore, combining K02288 or ALK1 knockdown
significantly inhibited cell viability, proliferation, migration,
invasion and tube formation. Therefore, ALK1 can promote the
proliferation, migration and luminal formation of tumour vascular
endothelial cells induced by low-dose PDT; however, the inhibition
of related pathways can reduce the effects of low-dose PDT.
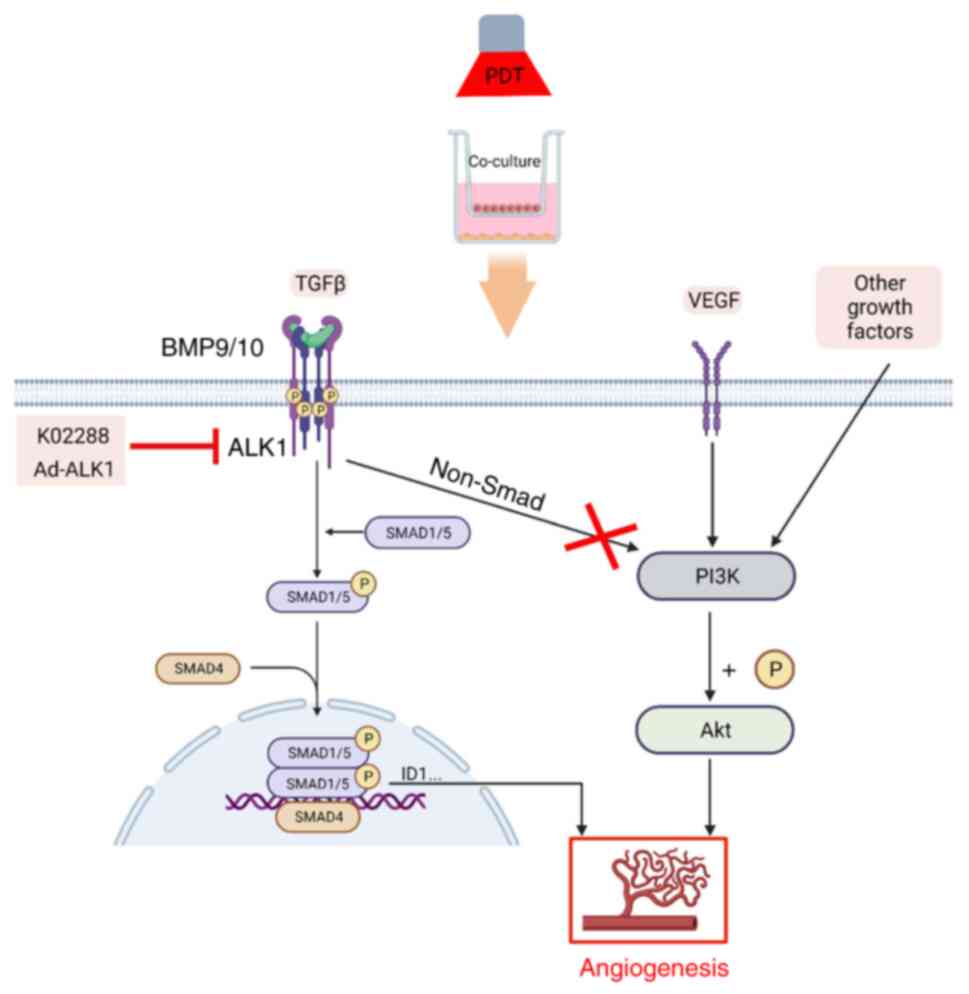
As a member of the TGF-β family, the classical
signalling pathway of ALK1 is the Smad pathway. The binding of ALK1
with the BMP9/10 receptor results in the sequential activation of
type II receptor BMPRII and type I receptor ALK1. This is then
followed by the phosphorylation of Smad1 and Smad5 on their
conservative C-terminal SXS motif through ALK1. Once
phosphorylated, Smad1, Smad5 and Smad4 form heterotrimeric
complexes and translocate to the nucleus (35), where they activate the
transcription of downstream target genes, such as ID1, ID3,
transmembrane protein 100 and other target genes. Subsequently,
downstream angiogenic factors are activated, thereby regulating
endothelial cell proliferation, migration and lumen formation, and
regulating angiogenesis (17,36,37).
The present study observed that following low-dose
PDT, the expression of ALK1, p-Smad1/5 and ID1 was upregulated.
After combining PDT with K02288 and the adenovirus knockdown of
ALK1, the expression of the aforementioned three genes was
significantly reduced. This finding confirms that ALK1-Smad1/5-ID1,
a classic SMAD pathway, may be involved in the low-dose PDT-induced
vascular proliferation.
In addition to regulating the Smad pathway, ALK1 can
also affect the function of the vascular endothelium through the
non-Smad pathway, such as the PI3K and AKT pathway. Moreover, in a
previous study, the knockout of BMP-9 receptor ALK1 in non-small
cell lung cancer was reported to inhibit the growth of A549 cells
in vitro and in vivo, which was related to the
PI3K/Akt and Smad1/5 signalling pathways (38). In another study, in hereditary
haemorrhagic telangiectasia type 2, PI3K/AKT signal transduction
increased when ALK1 was absent (39). Additionally, after reducing the
binding of BMP-9 and ALK1 in osteosarcoma, the PI3K/AKT pathway was
activated (40). In the present
study, it was observed that the phosphorylation of PI3K and AKT in
the low-dose PDT group was not significantly altered. After using
the PI3K inhibitor, LY294002, the results revealed that the
expression of p-PI3K and p-AKT in the LY294002 group and PDT +
LY294002 group was significantly decreased compared with the
control group, and there was no significant difference between the
two groups; similarly, there was no significant difference in the
expression of p-PI3K and p-AKT between the PDT group and the
control group. Furthermore, the results of RT-qPCR and western blot
analysis revealed that there was no significant effect on the
PI3K/AKT pathway, indicating that PDT induced changes in ALK1 to
promote vascular proliferation, which was not through the PI3K/AKT
pathway. Notably, it was also found that AKT phosphorylation
increased after the adenovirus knockdown of ALK1, which may be due
to the blocking of BMP9 signal transduction after the ALK1
knockdown. It was also observed to regulate the location and
activity of PTEN, a lipid phosphatase upstream of PI3K-AKT, thus
activating the PI3K-AKT pathway (39). The PI3K/Akt signalling pathway
promotes tumour angiogenesis by affecting various angiogenic
factors and plays a critical role in tumour development (41). Based on the findings of the present
study, ALK1 gene therapy is not recommended in the future strategy
of tumour PDT combined with anti-ALK1, owing to its activation of
the PI3K/AKT pathway that could lead to vascular proliferation and
tumour recurrence. However, a limitation of the present study is
that no experiments were performed using inhibitors of Smad1/5, ID1
and AKT to fully validate the pathway.
In conclusion, the present study observed that
low-dose PDT enhanced the activity of tumour vascular endothelial
cells and their ability to proliferate, migrate, invade and form
tubes. However. when PDT was combined with ALK1 inhibitor or
Ad-ALK1 treatment, the aforementioned changes were reversed.
Additionally, ALK1 participates in PDT-induced tumour angiogenesis
by activating the Smad1/5-ID1 pathway, as opposed to the PI3K/AKT
pathway (illustrated in Fig. 8).
To the best of our knowledge, the present study is the first to
demonstrate that ALK1 is involved in PDT-induced tumour
angiogenesis and provides a theoretical basis for the combination
of PDT with anti-ALK1 treatment and has strong clinical
significance. However, another limitation of the present study is
the lack of in vivo experiments. The authors aim to conduct
further research on other tumour cells and also perform in
vivo studies to verify these results in the future.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, DB and KL conceptualized the study. XG, KL, WH,
YN and XH were involved in the study methodology. YZ and QC were
involved in data validation. YZ and ST were involved in the formal
analysis. QC was involved in the investigative aspects of the
study. XG was involved in data curation, and in the writing and
preparation of the original draft of the manuscript. KL was
involved in the writing, reviewing and editing of the manuscript.
DB and KL supervised the study. KL and DB were involved in funding
acquisition. XG and KL confirm the authenticity of all the raw
data. All authors have read and agreed to the published version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81871853 and 82003306), the Natural
Science Foundation of Chongqing (grant nos. cstc2019jcyj-msxmX0397
and cstc2020jcyj-msxmX0484) and the Cultivating Fund in the First
Affiliated Hospital of Chongqing Medical University (grant nos.
PYJJ2018-24 and PYJJ2019-214).
References
|
1
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L, Song D, Qi L, Jiang M, Wu Y, Gan J,
Cao K, Li Y, Bai Y and Zheng T: Photodynamic therapy induces human
esophageal carcinoma cell pyroptosis by targeting the
PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 520:143–159.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simone CB II and Cengel KA: Photodynamic
therapy for lung cancer and malignant pleural mesothelioma. Semin
Oncol. 41:820–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gamelas SR, Moura NM, Habraken Y, Piette
J, Neves M and Faustino MA: Tetracationic porphyrin derivatives
against human breast cancer. J Photochem Photobiol B.
222:1122582021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leon D, Buchegger K, Silva R, Riquelme I,
Viscarra T, Mora-Lagos B, Zanella L, Schafer F, Kurachi C, Roa JC,
et al: Epigallocatechin gallate enhances MAL-PDT cytotoxic effect
on PDT-resistant skin cancer squamous cells. Int J Mol Sci.
21:33272020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broekgaarden M, Weijer R, van Gulik TM,
Hamblin MR and Heger M: Tumor cell survival pathways activated by
photodynamic therapy: A molecular basis for pharmacological
inhibition strategies. Cancer Metastasis Rev. 34:643–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan Y, Fu LH, Li C, Lin J and Huang P:
Conquering the hypoxia limitation for photodynamic therapy. Adv
Mater. 33:e21039782021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yue D, Cai X, Fan M, Zhu J, Tian J, Wu L,
Jiang Q and Gu Z: An alternating irradiation strategy-driven
combination therapy of PDT and RNAi for highly efficient inhibition
of tumor growth and metastasis. Adv Healthc Mater. 10:e20018502021.
View Article : Google Scholar
|
|
9
|
De Sanctis F, Ugel S, Facciponte J and
Facciabene A: The dark side of tumor-associated endothelial cells.
Semin Immunol. 35:35–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrario A, von Tiehl KF, Rucker N,
Schwarz MA, Gill PS and Gomer CJ: Antiangiogenic treatment enhances
photodynamic therapy responsiveness in a mouse mammary carcinoma.
Cancer Res. 60:4066–4069. 2000.PubMed/NCBI
|
|
11
|
Peng CL, Lin HC, Chiang WL, Shih YH,
Chiang PF, Luo TY, Cheng CC and Shieh MJ: Anti-angiogenic treatment
(Bevacizumab) improves the responsiveness of photodynamic therapy
in colorectal cancer. Photodiagnosis Photodyn Ther. 23:111–118.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olivo M, Bhuvaneswari R, Lucky SS,
Dendukuri N and Soo-Ping Thong P: Targeted therapy of cancer using
photodynamic therapy in combination with multi-faceted anti-tumor
modalities. Pharmaceuticals (Basel). 3:1507–1529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizu K, Asai T and Oku N:
Antineovascular therapy, a novel antiangiogenic approach. Expert
Opin Ther Targets. 9:63–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss A, den Bergh Hv, Griffioen AW and
Nowak-Sliwinska P: Angiogenesis inhibition for the improvement of
photodynamic therapy: The revival of a promising idea. Biochim
Biophys Acta. 1826:53–70. 2012.PubMed/NCBI
|
|
15
|
Hu-Lowe DD, Chen E, Zhang L, Watson KD,
Mancuso P, Lappin P, Wickman G, Chen JH, Wang J, Jiang X, et al:
Targeting activin receptor-like kinase 1 inhibits angiogenesis and
tumorigenesis through a mechanism of action complementary to
anti-VEGF therapies. Cancer Res. 71:1362–1373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Vinuesa AG, Bocci M, Pietras K and Ten
Dijke P: Targeting tumour vasculature by inhibiting activin
receptor-like kinase (ALK)1 function. Biochem Soc Trans.
44:1142–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roman BL and Hinck AP: ALK1 signaling in
development and disease: New paradigms. Cell Mol Life Sci.
74:4539–4560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhatt RS and Atkins MB: Molecular
pathways: Can activin-like kinase pathway inhibition enhance the
limited efficacy of VEGF inhibitors? Clin Cancer Res. 20:2838–2845.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cunha SI, Pardali E, Thorikay M, Anderberg
C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P and
Pietras K: Genetic and pharmacological targeting of activin
receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp
Med. 207:85–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hawinkels LJ, de Vinuesa AG, Paauwe M,
Kruithof-de Julio M, Wiercinska E, Pardali E, Mezzanotte L,
Keereweer S, Braumuller TM, Heijkants RC, et al: Activin
receptor-like kinase 1 ligand trap reduces microvascular density
and improves chemotherapy efficiency to various solid tumors. Clin
Cancer Res. 22:96–106. 2016. View Article : Google Scholar
|
|
21
|
Cunha SI, Bocci M, Lövrot J, Eleftheriou
N, Roswall P, Cordero E, Lindström L, Bartoschek M, Haller BK,
Pearsall RS, et al: Endothelial ALK1 is a therapeutic target to
block metastatic dissemination of breast cancer. Cancer Res.
75:2445–2456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goff LW, Cohen RB, Berlin JD, de Braud FG,
Lyshchik A, Noberasco C, Bertolini F, Carpentieri M, Stampino CG,
Abbattista A, et al: A Phase I study of the Anti-Activin
Receptor-Like Kinase 1 (ALK-1) monoclonal antibody PF-03446962 in
patients with advanced solid tumors. Clin Cancer Res. 22:2146–2154.
2016. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Alzeibak R, Mishchenko TA, Shilyagina NY,
Balalaeva IV, Vedunova MV and Krysko DV: Targeting immunogenic
cancer cell death by photodynamic therapy: Past, present and
future. J Immunother Cancer. 9:e0019262021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weijer R, Clavier S, Zaal EA, Pijls MM,
van Kooten RT, Vermaas K, Leen R, Jongejan A, Moerland PD, van
Kampen AH, et al: Multi-OMIC profiling of survival and metabolic
signaling networks in cells subjected to photodynamic therapy. Cell
Mol Life Sci. 74:1133–1151. 2017. View Article : Google Scholar :
|
|
26
|
Dougherty TJ, Gomer CJ, Henderson BW, Jori
G, Kessel D, Korbelik M, Moan J and Peng Q: Photodynamic therapy. J
Natl Cancer Inst. 90:889–905. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blazquez-Castro A, Carrasco E, Calvo MI,
Jaén P, Stockert JC, Juarranz A, Sánz-Rodríguez F and Espada J:
Protoporphyrin IX-dependent photodynamic production of endogenous
ROS stimulates cell proliferation. Eur J Cell Biol. 91:216–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Espada J, Galaz S, Sanz-Rodríguez F,
Blázquez-Castro A, Stockert JC, Bagazgoitia L, Jaén P, González S,
Cano A and Juarranz A: Oncogenic H-Ras and PI3K signaling can
inhibit E-cadherin-dependent apoptosis and promote cell survival
after photodynamic therapy in mouse keratinocytes. J Cell Physiol.
219:84–93. 2009. View Article : Google Scholar
|
|
29
|
Udartseva OO, Zhidkova OV, Ezdakova MI,
Ogneva IV, Andreeva ER, Buravkova LB and Gollnick SO: Low-dose
photodynamic therapy promotes angiogenic potential and increases
immunogenicity of human mesenchymal stromal cells. J Photochem
Photobiol B. 199:1115962019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Jiang F, Zhang ZG, Kalkanis SN,
Hong X, deCarvalho AC, Chen J, Yang H, Robin AM and Chopp M:
Low-dose photodynamic therapy increases endothelial cell
proliferation and VEGF expression in nude mice brain. Lasers Med
Sci. 20:74–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muñoz-Félix JM, González-Núñez M and
López-Novoa JM: ALK1-Smad1/5 signaling pathway in fibrosis
development: Friend or foe? Cytokine Growth Factor Rev. 24:523–537.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson DW, Berg JN, Baldwin MA, Gallione
CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA,
Diamond A, et al: Mutations in the activin receptor-like kinase 1
gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet.
13:189–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urness LD, Sorensen LK and Li DY:
Arteriovenous malformations in mice lacking activin receptor-like
kinase-1. Nat Genet. 26:328–331. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scharpfenecker M, van Dinther M, Liu Z,
van Bezooijen RL, Zhao Q, Pukac L, Löwik CW and ten Dijke P: BMP-9
signals via ALK1 and inhibits bFGF-induced endothelial cell
proliferation and VEGF-stimulated angiogenesis. J Cell Sci.
120:964–972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu Y, Wang H, Dai H, Zhu Q, Cui CP, Sun X,
Li Y, Deng Z, Zhou X, Ge Y, et al: OTULIN allies with LUBAC to
govern angiogenesis by editing ALK1 linear polyubiquitin. Mol Cell.
81:3187–3204.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Somekawa S, Imagawa K, Hayashi H, Sakabe
M, Ioka T, Sato GE, Inada K, Iwamoto T, Mori T, Uemura S, et al:
Tmem100, an ALK1 receptor signaling-dependent gene essential for
arterial endothelium differentiation and vascular morphogenesis.
Proc Natl Acad Sci USA. 109:12064–12069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Salmon RM, Jiang H and Morrell NW:
Regulation of the ALK1 ligands, BMP9 and BMP10. Biochem Soc Trans.
44:1135–1141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou X, Peng Y, Liu J, Zhong Q, Yu Z and
Zhang L: Bone morphogenetic protein-9 promotes the proliferation of
non-small cell lung cancer cells by activating PI3K/Akt and Smad1/5
pathways. RSC Adv. 10:7214–7220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ola R, Dubrac A, Han J, Zhang F, Fang JS,
Larrivée B, Lee M, Urarte AA, Kraehling JR, Genet G, et al: PI3
kinase inhibition improves vascular malformations in mouse models
of hereditary haemorrhagic telangiectasia. Nat Commun. 7:136502016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H, Pan R, Li H, Zhang W, Ren C, Lu Q,
Chen H, Zhang X and Nie Y: CHRDL2 promotes osteosarcoma cell
proliferation and metastasis through the BMP-9/PI3K/AKT pathway.
Cell Biol Int. 45:623–632. 2021. View Article : Google Scholar :
|
|
41
|
Gong T, Cui L, Wang H, Wang H and Han N:
Knockdown of KLF5 suppresses hypoxia-induced resistance to
cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis
through inactivation of the PI3K/Akt/mTOR pathway. J Transl Med.
16:1642018. View Article : Google Scholar
|