Introduction
Breast cancer is the most prevalent malignancy
affecting women worldwide, also ranking as the primary cause of
cancer-related mortality among them (1). In spite of standardized comprehensive
treatment for patients with early-stage breast cancer, ~20-30% of
patients experience fatal distant recurrence and metastasis
(2). The vast majority (~90%) of
breast cancer-related deaths are due to distant metastasis
(3). Therefore, breast cancer
metastasis is the main reason affecting the prognosis of patients
with breast cancer and the major challenge of breast cancer
treatment. In primary tumors, epithelial-to-mesenchymal transition
(EMT) plays a critical role in tumor cell invasion of the vascular
system and the induction of proteases that degrade the
extracellular matrix (4). EMT
results in changes in cell surface structure, and leads to an
increased invasive ability and a weakened adhesive ability. With
EMT, the expression of the epithelial marker, E-cadherin,
decreases, while that of the markers of interstitial cell
phenotypes, N-cadherin and Vimentin, increases (5,6).
Moreover, cells become spindle-shaped, intercellular adhesion
weakens, and the movement and mobility of the cells are enhanced.
Subsequently, the cells travel via the bloodstream to distant
organs, gradually revert back to their original shape, and
proliferate to form metastatic tumors (7).
The joining chain of multimeric IgA and IgM (JCHAIN,
also known as IGJ, as referred to herein) gene is located on 4q13.3
and the translated protein is J chain (8). IGJ protein is composed of 159 amino
acid residues with a molecular weight of ~15 kDa. Previous research
has indicated that the J chain is involved in the formation of
dimer IgA and multimer IgM, promoting their binding to secretory
components and regulating the transport process of secretory
immunoglobulins (dimer IgA and pentamer IgM) to realize exocytosis
(9). The expression of the IGJ
gene is accompanied by the differentiation and development of B-
and T-lymphocytes, particularly after B cells differentiate into
plasma cells, and the J chain is highly expressed with the
production of immunoglobulin (10). In addition, IGJ protein can also be
detected in dendritic cells, intestinal epithelial cells,
endometrial cells and mammary epithelial cells (10). Given that the IGJ gene is expressed
not only in immunoglobulin-secreting cells, but also in some
non-immunoglobulin-secreting cells, the biological function of the
J chain may extend beyond polymerized immunoglobulins (8,10).
The expression of the IGJ gene is known to vary across various
pathological conditions, with significant transcription changes in
certain infectious diseases, autoimmune diseases and hematological
tumors (11-13). In patients with acute B
lymphoblastic leukemia, a high expression of IGJ indicates a poor
disease-free survival and overall survival (OS) (13). The transcription level of IGJ in
lung squamous cell carcinoma, adenocarcinoma tissues and gastric
cancer has been found to be markedly lower than that in normal
tissues (14,15). Some breast cancer prognostic
prediction studies have found that IGJ exhibits a high accuracy in
distinguishing breast cancer tissue from normal breast tissue, and
that high levels of IGJ are associated with an improvised prognosis
of patients with breast cancer, suggesting that IGJ may be utilized
as a biomarker for breast cancer (16-19).
However, research on IGJ remains limited to a
superficial level in malignant tumors, and its potential biological
function and mechanisms underlying its involvement in malignant
tumor occurrence and development remain unclear.
The present study aimed to identify novel
therapeutic targets that can potentially inhibit breast cancer
metastasis and increase the survival rates of patients. Through the
investigation of genes that can promote or hinder tumor metastasis
in breast cancer, the present study attempted to identify IGJ as
the main target gene. It was hypothesized that IGJ may be an
independent prognostic factor in breast cancer by inhibiting tumor
metastasis and the EMT process. Verification assays were conducted
to examine this hypothesis at the cellular level and to investigate
whether the overexpression of IGJ is effective in suppressing the
proliferation, migration, invasion and EMT process of breast cancer
cell lines. Further experiments were conducted using nude mice to
verify whether IGJ can inhibit breast cancer tumor growth.
Materials and methods
The Cancer Genome Atlas (TCGA) Chip data
and Kaplan-Meier survival analysis
The TCGA high-throughput sequencing data for breast
cancer were downloaded from the UCSC database (version 2015-02-24;
available at https://genome.ucsc.edu/). To
investigate the clinical relevance of IGJ, 991 patients with breast
cancer from the TCGA database were included, whose RNA-seq data and
clinical information were complete. The information included age,
tumor size, lymph node metastasis, tumor size, tumor stage (TNM),
estrogen receptor (ER), progesterone receptor (PR), human epidermal
growth factor receptor 2 (HER2) and follow-up information.
Kaplan-Meier analysis (http://kmplot.com/analysis/) was applied to analyze
the association between IGJ expression and the prognosis of
patients with breast cancer using the dataset from the study by
Győrffy (20).
Differential gene screening
Differential gene analysis was performed for bone
and lung metastasis. Statistical analyses were performed using R
software 3.5.1 and DEseq, an R language package. An adjusted
P-value <0.05 and a fold change >2 were considered to
indicate statistically significant differences, and the difference
in gene expression was regarded as significant between the
experimental and the control groups.
Propensity score matching (PSM)
To eliminate the difference in clinical baseline
characteristics and selection bias between the metastatic group and
the non-metastatic group, PSM was conducted for eight variables:
Sex, age, tumor size, lymph node metastasis, ER, PR, HER2 and
histological type. SPSS 25.0 software (IBM Corp.) was used to
calculate individual PSM, randomly matching at a 1:1 ratio with a
caliper value set to 0.05.
Clinical tissue samples
A total of 32 pairs of breast cancer tissues and
adjacent normal tissues were collected from patients that underwent
breast cancer resection at the First Affiliated Hospital of
Chongqing Medical University (Chongqing, China) between 2014 and
2016. Moreover, 28 pairs of breast cancer tissues and normal
tissues were collected from patients with breast cancer metastasis
at follow-up. The collected tissues were used for reverse
transcription-quantitative PCR (RT-qPCR) and immunohistochemistry
(IHC), which were performed as described below. All specimens
collected were preserved in liquid nitrogen. The collection and use
of the tissues were approved by the Institutional Ethics Committee
of the First Affiliated Hospital of Chongqing Medical University
(approval no. 2017-012) and written informed consent was signed by
the patients.
Cell lines
Healthy mammary epithelial cells (MCF-10A) and human
breast cancer cells (T47D, YCC-B1, MDA-MB-231, MCF-7, SK-BR-3,
ZR-75-1 and BT-549) were used in the present study. The YCC-B1 cell
line was kindly provided as a gift from Professor Qian Tao at The
Chinese University of Hong Kong (Hong Kong, SAR, China) and
authenticated using short tandem repeat profiling. All other cell
lines were obtained from the American Type Culture Collection
(ATCC) cell bank (MCF-10A: cat. no. CRL-10317; TC7D: cat. no.
HTB-133; MDA-MB-231: cat. no. HTB-26; MFC-7: cat. no. HTB-22;
SK-BR-3: cat. no. HTB-30; ZR-75-1: cat. no. CRL-1500; BT-549: cat.
no. HTB-122). The breast cancer cell lines were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin. MCF-10A cells were cultured in
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) containing 20
ng/ml EGF (Beijing Solarbio Science & Technology Co., Ltd.),
0.5 µg/ml hydro-cortisone, 10 µg/ml insulin, and 1%
penicillin-streptomycin. All cells were in a cell incubator at 37°C
with 5% CO2.
Cell transfection
The control plasmids (vector) and IGJ overexpression
plasmids were obtained from Hanbio Biotechnology Co., Ltd.
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect the MDA-MB-231 and YCC-B1
cells. Subsequently, lipopolysaccharide (LPS; 10 µg/ml; cat.
no. L8880, Beijing Solarbio Science & Technology Co., Ltd.) was
added to the vector- and IGJ-treated cells, respectively. The cells
were cultured for 48 h following transfection and then collected
for use in subsequent experiments.
RNA isolation and RT-qPCR
As previously described (21), TRIzol® reagent (Thermo
Fisher Scientific, Inc.) we used to extract total RNA following the
manufacturer's instructions. The RT reaction was performed using
the RT reagent kit (cat. no. RR047A; Takara Bio, Inc.), and the
reaction conditions were 15 min at 37°C and 5 sec at 85°C. RT-qPCR
(cat. no. RR820A; Takara Bio, Inc.) was performed to detect IGJ
expression on 32 pairs of tissues using an ABI 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
RT-qPCR reaction conditions were set as follows: 95°C for 30 sec,
40 cycles at 95°C for 3 sec, and 60°C for 30 sec. The relative
quantification of IGJ mRNA expression was normalized to the GAPDH
expression level using the 2−ΔΔCq method (22). The primer pairs used in the present
study are listed in Table I.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene | Forward sequence
(5′ end to 3′ end) | Reverse sequence
(5′ end to 3′ end) |
|---|
| IGJ |
TCCTGGCGGTTTTTATTAAGGC |
AGTAATCCGGGCACACTTACAT |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Western blot analysis
Breast cancer cells were collected and lysed in a
pre-cooled RIPA lysis buffer (Beyotime Institute of Biotechnology)
for 20 min. The mixture was then centrifuged at high speed for 20
min at a minimal 17,383 × g, at 4°C. Following centrifugation, the
supernatant was collected and the protein concentration was
quantified using a BCA protein assay kit (Wuhan Boster Biological
Technology, Ltd.), and the protein sample was boiled and denatured
at 100°C. Electrophoresis separation was performed using 6 or 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) (Beyotime Institute of Biotechnology), and the protein
was then transferred to a polyvinylidene fluoride (PVDF) membrane
(MilliporeSigma). The membrane was blocked using 5% skim milk for 2
h at room temperature. Subsequently, diluted primary antibodies
were added for protein culture overnight at 4°C. Additionally, the
membrane was washed three times using TBST, for 3 min each time.
Subsequently, a diluted secondary antibody was added and the
membrane was cultured at room temperature for 40 min, and the
membrane was then rinsed with TBST three times, for 3 min each
time. Protein bands were developed using an enhanced
chemiluminescence (ECL) kit (Beyotime Institute of Biotechnology).
GAPDH was used as an endogenous control. The primary antibodies
used were the following: IGJ (1:1,000; cat. no. ab269855), β-actin
(1:1,000; cat. no. ab8226) (both from Abcam), E-cadherin (1:1,000;
cat. no. 14472), N-cadherin (1:1,000; cat. no. 13116), Vimentin
(1:1,000; cat. no. 5741), Claudin-1 (1:1,000; cat. no. 13255),
matrix metalloproteinase (MMP)9 (1:1,000; cat. no. 13667s), MMP7
(1:1,000; cat. no. 3801), p65 (1:1,000; cat. no. 8242),
phosphorylated (p-)-p65 (Ser536; 1:1,000; cat. no. 3033), IκBα
(1:1,000; cat. no. 4818), p-IκBα (Ser32; 1:1,000; cat. no. 5209),
proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no. 13110),
and GAPDH (1:1,000; cat. no. 51332) (all from Cell Signaling
Technology, Inc.). The secondary antibodies used were the
following: Rabbit secondary antibody (1:4,000; cat. no. 7074) and
mouse secondary antibody (1:4,000; cat. no. 7076) (both from Cell
Signaling Technology, Inc.).
IHC
The procedures to conduct IHC followed the
description of previous studies (21,23).
The samples underwent formalin fixation and paraffin embedding
before being sliced into 4-µm-thick sections and mounted
onto glass slides. IHC was performed on the slides following
deparaffinization and rehydration with xylene and a graded ethanol
series for 0.5 h. Antigen retrieval was carried out by microwaving
the samples in a sodium citrate-hydrochloric acid buffer solution
at 95°C for 20 min. To block endogenous horseradish peroxidase
activity, the sections were then treated with 3% hydrogen peroxide
and subsequently washed with phosphate-buffered saline (PBS) three
times. Normal goat serum was applied to the sections as a blocking
agent at 25°C for 30 min. The anti-IGJ rabbit polyclonal antibody
(1:100; cat. no. ab105229, Abcam) or Ki-67 mouse monoclonal
antibody (1:1,000, cat. no. 9449, Cell Signaling Technology, Inc.)
was added to the sections, followed by overnight incubation at 4°C.
Another round of PBS washing preceded incubation with goat
anti-rabbit HRP secondary antibody (1:100; cat. no. SPN9001,
OriGene Technologies, Inc.). After washing, the sections were
treated with streptavidin-biotin-conjugated horseradish peroxidase
(HRP), which was followed by visualization of signals using
diaminobenzidine. Hematoxylin (cat. no. G1004, Wuhan Servicebio
Technology Co., Ltd.) was applied to counterstain the sections at
25°C for 1 min. A total of 28 pairs of tissues were subjected to
IHC. IHC staining scores were used to indicate the intensity of
staining: 0 represents no staining, 1 represents weak staining, 2
represents moderate staining, and 3 represents strong staining. The
scores for determining the proportion of positive tumor cells were
0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; and 4, >75%. The
total score was calculated by multiplying the intensity scores and
the percentage scores.
Immunofluorescence (IF) staining
The tumor tissue sections were boiled in citrate
antigen retrieval solution (P0081, Beyotime Institute of
Biotechnology) for 30 min. Subsequently, 0.5% Triton X-100 (cat.
no. T8200, Beijing Solarbio Science & Technology Co., Ltd.) was
added to the sections, followed by a 30-min incubation at room
temperature. The sections were blocked with goat serum (cat. no.
16210064, Thermo Fisher Scientific, Inc.) at 25°C for 30 min. The
sections were then incubated overnight at 4°C with E-cadherin
(1:200; cat. no. 14472) or Vimentin (1:100; cat. no. 5741)
antibodies (both from Cell Signaling Technology, Inc.). After
washing with PBS, the sections were incubated at 25°C in the dark
for 1.5 h with Cy3 goat anti-rabbit IgG (1:50; cat. no. AS007) and
FITC goat anti-rabbit IgG (1:50; cat. no. AS011) antibodies (both
from ABclonal Technology Co., Ltd.). DAPI (cat. no. C1005, Beyotime
Institute of Biotechnology) was then added to the sections and
incubated in the dark for 5 min. The excess DAPI was washed away
with PBS, and the sections were mounted using an anti-fade mounting
medium (cat. no. P0126, Beyotime Institute of Biotechnology) to
prevent fluorescence quenching. Finally, the sections were observed
and photographed under an inverted fluorescent microscope [ICX41,
Sunny Optical Technology (Group) Co Ltd.].
Cell Counting Kit-8 (CCK-8) assay
The cells in each group were collected in the
logarithmic phase to prepare a single-cell suspension and cell
density was adjusted after cell count calculation. The cells were
then inoculated into 96-well plates (1×103 cells/well)
and 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was dripped into each well on days 1, 2 and 3,
respectively. At 1 h following incubation at 37°C, the absorbance
of each well was measured at 450 nm wavelength using a xMark™
microplate spectrometer (Bench markPlus™ system, Bio-Rad
Laboratories, Inc.). After 3 days, the cell proliferation curve was
plotted based on the absorbance value.
Transwell assay
A Transwell chamber (MilliporeSigma) was used for
the Transwell assays. The chamber for detecting cell invasion was
covered with a layer of Matrigel (MilliporeSigma) and air-dried
overnight. After the cells were resuspended in a serum-free medium
at 1×105 cells/ml, 200 µl of the breast cancer
cell suspension were added to the upper chamber, and 600 µl
medium containing 10% FBS was added to the lower chamber. Following
24 h of culture, the upper chamber was removed, and the cells were
fixed with 4% paraformaldehyde at 25°C for 20 min and stained with
a 0.1% crystal violet solution (cat. no. DZ0055, Leagene
Biotechnology) at 25°C for 20 min. Additionally, after being washed
with PBS three times, the cells remaining on the surface of the
membrane were wiped off using a wet swab. The cells were
subsequently counted under an inverted fluorescent microscope
[ICX41, Sunny Optical Technology (Group) Co Ltd.].
Scratch test
The cells were seeded into six-well plates
(5×106/well). When the plate was fully grown with cells,
a straight line was marked using the tip of a sterile 200-µl
pipette to create a direct scratch between the fused monolayer
cells. The cells were then gently rinsed with a serum-free medium
and scratched three times, and the scratches were observed and
photographed under an inverted fluorescent microscope [0 h; ICX41,
Sunny Optical Technology (Group) Co Ltd.]. Subsequently, the cells
were cultured in a serum-free medium for 24 h, observed the
scratches again, and photographed under an inverted fluorescent
microscope [ICX41, Sunny Optical Technology (Group) Co Ltd.]. The
healing of the scratches in each group indicated the migratory
ability of the cells in each group.
Animal experiments
Healthy female BALB/c nude mice (4 weeks old, n=20)
were purchased from Chongqing Ensiweier Biological Co., Ltd. The
mice were raised in a pathogen-free environment with a temperature
of 25°C and a humidity range of 50 to 60%, and had free access to
food and water. The nude mice were randomly divided into a control
group and an IGJ overexpression group (n=10 per group). The
MDA-MB-231 cells were subcutaneously injected into the right
inguinal region or caudal vein as the control group. The tumor
volume of the right inguinal region was measured, and the mouse
body weights were assessed every 3 days for 5 consecutive weeks.
After 30 days, 10 nude mice that had developed tumors in the right
inguinal region were administered anesthesia by the intraperitoneal
injection of 100 mg/kg ketamine and 5 mg/kg valium. Once it was
confirmed that the anesthesia had taken effect, the mice were
euthanized by an intraperitoneal injection of an overdose of
pentobarbital sodium (200 mg/kg). Tumor tissues were then collected
for weight and volume measurements. On day 33, the remaining 10
nude mice with tumors in the caudal vein were anesthetized by gas
inhalation (2% isoflurane at 0.4 l/min gas flow) and photographed
under a real-time imager (IVIS Lumina III, PerkinElmer, Inc.). Some
tumor tissue samples were fixed in 4% paraformaldehyde at 25°C for
24 h, and then stained with hematoxylin and eosin (H&E) at 25°C
for 5 min, while the remaining tissues were stored at -80°C for
further use. All experimental procedures were approved by the
Animal Ethics Committee of Chongqing Medical University (approval
no. 2022-K121).
Gene Set Enrichment Analysis (GSEA)
GSEA (http://software.broadinstitute.org/gsea) was applied
to analyze the association between IGJ expression and biological
processes/Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways
according to the instructions of users. The microarray data set was
GSE1456 and the gene set (c2 all.v6.0 symbols.gmt) for analyses was
obtained from the Molecular Signatures Database (http://software.broadinstitute.org/gsea/msigdb/index.jsp).
A false discovery rate (FDR) <0.25 and a P-value <0.05 were
considered to indicate statistically significant differences.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism (version 8.0; Dotmatics) or SPSS software (Version
23.0; IBM Corp.). Cox regression analysis was used to estimate the
association between IGJ expression and the prognosis [OS and
distant metastasis-free survival (DMFS)] of patients with breast
cancer. An unpaired Student's t-test was used to evaluate the
difference between the two groups. One-way ANOVA with post hoc
analysis using Tukey's test was used for comparisons between
multiple groups. The Chi-squared test or Fisher's test was used to
evaluate the significance between categorical data, and the
non-parametric Mann-Whitney U test (Wilcoxon rank-sum test) was
used to analyze the continuous data. A P-value <0.05 was
considered significant to indicate a statistically significant
difference.
Results
Screening of genes closely related to
metastasis in patients with breast cancer
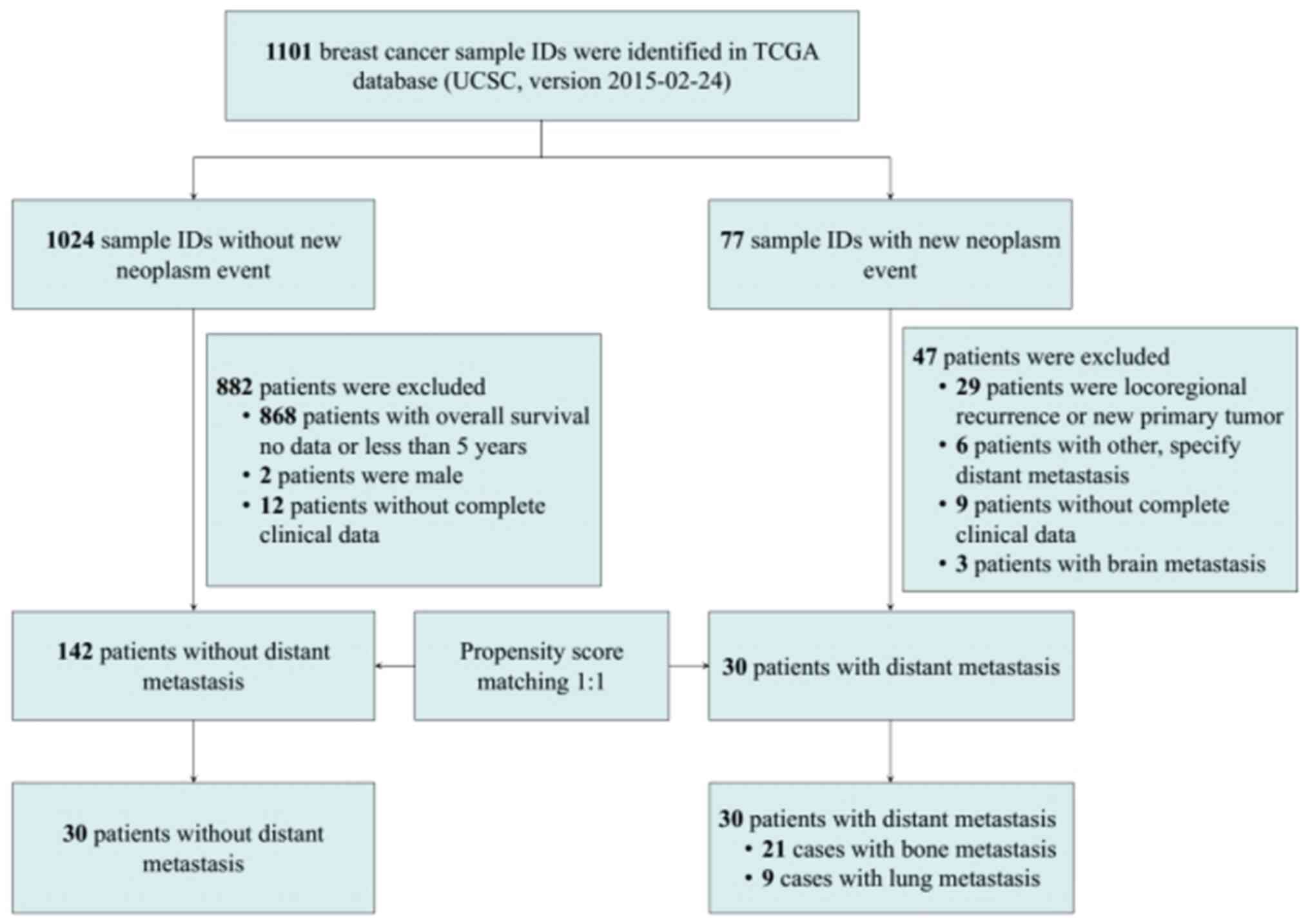
Through TCGA analysis, the high-throughput RNA
sequencing data were obtained and 30 patients with non-metastatic
breast cancer at diagnosis who had developed confirmed bone and
lung metastasis during follow-up (21 with bone metastasis and 9
with lung metastasis) were screened. Subsequently, PSM was
performed on these patients with breast cancer and those with
breast cancer without metastasis at diagnosis and who developed no
distant metastasis during the follow-up period (>5 years)
according to clinical characteristics, such as age and tumor size
(Fig. 1). No significant
difference in was observed in the clinical characteristics between
the two groups after matching (Table
II). Subsequently, the differential genes between the
metastatic and non-metastatic patients were analyzed. Through
differential gene analysis, there were 587 differential genes in
the bone metastasis group and 439 differential genes in the lung
metastasis group. When analyzing the differential genes between the
metastatic and non-metastatic groups, the bone metastasis group and
the lung metastasis group simultaneously included 23 downregulated
genes and one upregulated gene (Fig.
2A-C). The Kaplan-Meier plotter database was employed to verify
the association between the expression of these 24 genes and the
prognosis of patients with breast cancer. Among the downregulated
genes in the metastasis group, 13 genes (IGJ, ADAM6, POU2AF1, CD38,
LAX1, SLAMF7, TNFRSF17, ZNF238, GBP4, CCR2, STAP1, MEI1 and IFNG)
were associated with DMFS. Furthermore, the high expression of such
genes indicated an improved OS (Table III). The high expression of IGJ
was closely associated with an improved DMFS [hazard ratio (HR),
0.69; 95% confidence interval (CI), 0.59-0.81; P<0.001] and OS
(HR, 0.55; 95% CI, 0.46-0.67, P<0.001) (Fig. 2D and E), and IGJ was determined as
the target gene in the present study. The clinical association
between IGJ and breast cancer in 991 patients with breast cancer
was further analyzed in TCGA database; this revealed that IGJ
expression was associated with age (P<0.001), lymph node
metastasis (P<0.001) and ER status (P=0.027) (Table IV). Multivariate Cox regression
analyses revealed that IGJ was an independent prognostic factor for
OS (HR, 0.59; 95% CI, 0.421-0.866, P=0.007) and relapse-free
survival (HR, 0.651; 95% CI, 0.461-0.837, P<0.001) in breast
cancer (Table V).
 | Table IIBaseline characteristics of
metastatic and non-metastatic patients following propensity score
matching. |
Table II
Baseline characteristics of
metastatic and non-metastatic patients following propensity score
matching.
|
Characteristics | Cohort 1
| Cohort 2
|
|---|
| Non-metastasis | Bone
metastasis | P-value | Non-metastasis | Lung
metastasis | P-value |
|---|
| Sex/female, n | 21 | 21 | 0.999 | 9 | 9 | 0.999 |
| Age, median
(range), years | 54 (29-85) | 56 (39-85) | NA | 54 (42-62) | 52 (49-69) | NA |
| Pathologic tumor
size | | | | | | |
| T1 | 6 | 4 | 0.80 | 0 | 2 | NA |
| T2 | 7 | 10 | | 8 | 6 | |
| T3 | 7 | 6 | | 1 | 1 | |
| T4 | 1 | 1 | | 0 | 0 | |
| Pathologic lymph
node status | | | | | | |
| Negative | 4 | 6 | 0.72 | 5 | 3 | 0.64 |
| Positive | 17 | 15 | | 4 | 6 | |
| ER status | | | | | | |
| Negative | 16 | 18 | 0.70 | 5 | 6 | 0.999 |
| Positive | 5 | 3 | | 4 | 3 | |
| PR status | | | | | | |
| Negative | 13 | 15 | 0.74 | 5 | 6 | 0.999 |
| Positive | 8 | 6 | | 4 | 3 | |
| HER2 | | | | | | |
| Negative | 4 | 2 | 0.66 | 8 | 8 | 0.999 |
| Positive | 17 | 19 | | 1 | 1 | |
| Histological
type | | | | | | |
| IDC | 15 | 12 | 0.61 | 6 | 6 | 0.55 |
| ILD | 3 | 4 | | 0 | 1 | |
| Other | 3 | 5 | | 3 | 2 | |
 | Table IIIThe association between the 24
differentially expressed genes and breast cancer prognosis.\ |
Table III
The association between the 24
differentially expressed genes and breast cancer prognosis.\
| Gene symbol | Probe ID | DMFS
| OS
|
|---|
| HR | 95% CI | Log-rank
P-value | HR | 95% CI | Log-rank
P-value |
|---|
| Downregulated | | | | | | | |
| IGJ | 212592_at | 0.66 | 0.54-0.81 | <0.001 | 0.56 | 0.45-0.69 | <0.001 |
| LOC96610 | NA | | | | | | |
| ADAM6 | 237909_at | 0.58 | 0.40-0.83 | 0.003 | 0.72 | 0.52-1.00 | 0.048 |
| FCRL5 | 224406_s_at | 0.71 | 0.49-1.03 | 0.072 | 0.62 | 0.44-0.87 | 0.006 |
| IDO1 | 210029_at | 1.29 | 1.02-1.63 | 0.036 | 0.81 | 0.65-1.00 | 0.053 |
| POU2AF1 | 205267_at | 0.75 | 0.61-0.92 | 0.005 | 0.72 | 0.58-0.90 | 0.004 |
| CD38 | 205692_s_at | 0.75 | 0.60-0.95 | 0.015 | 0.73 | 0.58-0.92 | 0.007 |
| LAX1 | 207734_at | 0.74 | 0.60-0.90 | 0.003 | 0.69 | 0.54-0.87 | 0.001 |
| C8orf80 | NA | | | | | | |
| SLAMF7 | 222838_at | 0.64 | 0.44-0.95 | 0.025 | 0.53 | 0.37-0.75 | <0.001 |
| CXCL10 | 204533_at | 1.34 | 1.09-1.65 | 0.005 | 0.76 | 0.60-0.98 | 0.030 |
| TNFRSF17 | 206641_at | 0.60 | 0.47-0.77 | <0.001 | 0.67 | 0.54-0.83 | <0.001 |
| ZNF238 | 212774_at | 0.66 | 0.54-0.80 | <0.001 | 0.60 | 0.48-0.74 | <0.001 |
| ELOVL7 | 227180_at | 1.25 | 0.89-1.77 | 0.200 | 0.82 | 0.60-1.12 | 0.220 |
| GBP4 | 235175_at | 0.68 | 0.48-0.97 | 0.031 | 0.52 | 0.38-0.71 | <0.001 |
| ERAP2 | 227462_at | 0.74 | 0.53-1.03 | 0.075 | 0.67 | 0.48-0.93 | 0.017 |
| CCR2 | 207794_at | 0.78 | 0.62-0.97 | 0.028 | 0.64 | 0.52-0.79 | <0.001 |
| KCNA3 | 207237_at | 0.8 | 0.63-1.01 | 0.064 | 0.83 | 0.64-1.07 | 0.142 |
| AMPD1 | 206121_at | 0.82 | 0.65-1.04 | 0.106 | 0.80 | 0.64-0.99 | 0.040 |
| LOC400759 | NA | | | | | | |
| STAP1 | 1554343_a_at | 0.67 | 0.46-0.97 | 0.032 | 0.63 | 0.45-0.87 | 0.005 |
| MEI1 | 230011_at | 0.66 | 0.43-0.99 | 0.043 | 0.58 | 0.43-0.80 | <0.001 |
| IFNG | 210354_at | 0.76 | 0.63-0.93 | 0.006 | 0.66 | 0.53-0.82 | <0.001 |
| Upregulated | | | | | | | |
| DSCAML1 | 234908_s_at | 1.36 | 0.97-1.90 | 0.072 | 1.73 | 1.23-2.43 | 0.002 |
 | Table IVTCGA clinical correlation analysis of
patients with breast cancer. |
Table IV
TCGA clinical correlation analysis of
patients with breast cancer.
| Characteristic | No. of cases | High (n) | Low (n) | P-value |
|---|
| Age, years | | | | |
| <50 | 280 | 179 | 101 | <0.001 |
| ≥50 | 711 | 368 | 343 | |
| Tumor size | | | | |
| T1 | 266 | 159 | 107 | 0.087 |
| T2 | 577 | 309 | 268 | |
| T3 | 119 | 68 | 51 | |
| T4 | 29 | 11 | 18 | |
| Lymph node
metastasis | | | | |
| Negative | 474 | 226 | 248 | <0.001 |
| Positive | 517 | 321 | 196 | |
| TNM stage | | | | |
| I | 179 | 109 | 70 | 0.12 |
| II | 575 | 306 | 269 | |
| III | 225 | 128 | 97 | |
| IV | 12 | 4 | 8 | |
| ER | | | | |
| Positive | 769 | 410 | 359 | 0.027 |
| Negative | 222 | 137 | 85 | |
| PR | | | | |
| Positive | 672 | 367 | 305 | 0.592 |
| Negative | 319 | 180 | 139 | |
| HER2 | | | | |
| Positive | 202 | 107 | 95 | 0.476 |
| Negative | 789 | 440 | 349 | |
| Triple-negative
breast cancer | | | | |
| Yes | 166 | 100 | 66 | 0.152 |
| No | 825 | 447 | 378 | |
 | Table VUnivariate and multivariate COX
regression analysis of IGJ in the TCGA cohort. |
Table V
Univariate and multivariate COX
regression analysis of IGJ in the TCGA cohort.
| Variant | OS
| RFS
|
|---|
Univariate analysis
| Multivariate
analysis
| Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (<50
vs. ≥50) | 0.675 | 0.463-0.924 | 0.031 | 0.554 | 0.269-0.904 | 0.027 | 0.783 | 0.442-0.913 | 0.044 | 0.758 | 0.422-1.511 | 0.21 |
| Tumor size (T1/T2
vs. T3/T4) | 0.914 | 0.593-1.380 | 0.464 | | | | 0.864 | 0.322-1.348 | 0.232 | | | |
| Lymph node (N0 vs.
N1/N2/N3) | 0.476 | 0.345-0.863 | 0.001 | 0.673 | 0.414-1.031 | 0.873 | 0.698 | 0.343-1.321 | 0.317 | | | |
| TNM stage (I/II vs.
III/IV) | 0.682 | 0.311-0.1.132 | 0.065 | | | | 0.548 | 0.332-0.804 | 0.001 | 0.5497 | 0.347-0.843 | 0.011 |
| ER (negative vs.
positive) | 1.274 | 0.883-1.614 | 0.174 | | | | 1.126 | 0.532-1.895 | 0.743 | | | |
| PR (negative vs.
positive) | 1.163 | 0.715-1.331 | 0.763 | | | | 0.994 | 0.416-1.769 | 0.897 | | | |
| HER2 (negative vs.
positive) | 1.48 | 0.945-2.317 | 0.086 | | | | 1.203 | 0.678-2.875 | 0.512 | | | |
| IGJ (high vs.
low) | 0.62 | 0.423-0.906 | 0.014 | 0.59 | 0.421-0.866 | 0.007 | 0.634 | 0.412-0.858 | 0.021 | 0.651 | 0.461-0.837 | 0.001 |
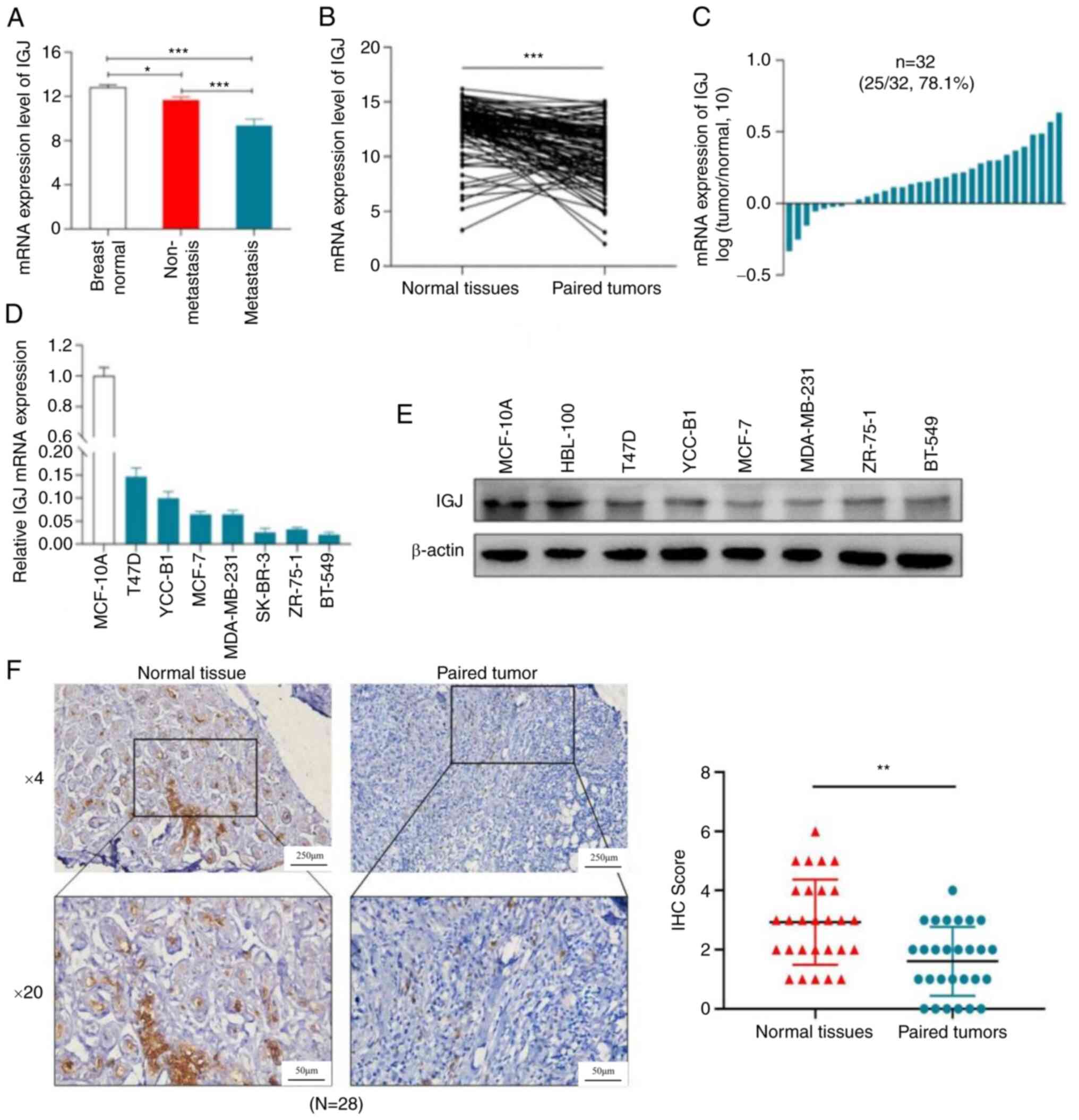
Expression of IGJ in breast cancer
The present study first analyzed the expression of
IGJ in breast cancer patients in TCGA database, which indicated
that IGJ had the highest expression in adjacent tissues, but the
lowest in metastatic breast cancer (Fig. 3A). In 114 cases of breast cancer in
TCGA, the expression of IGJ in normal tissues was markedly higher
than that in cancer tissues (Fig.
3B). Moreover, through the expression analyses of 32 pairs of
normal breast tissues and paired cancer tissues collected from
patients for the present study, the expression of IGJ in normal
tissues was markedly higher than that in cancer tissues (Fig. 3C). IGJ mRNA and protein expression
in the normal breast epithelial cell line, MCF-10A, was markedly
higher than that in breast cancer cells (Fig. 3D and E). Furthermore, the
pathological section analysis of 28 pairs of breast cancer using
IHC revealed that IGJ had the highest expression in normal tissues,
whereas it had the lowest expression in breast cancer (Fig. 3F), suggesting that IGJ functions as
a tumor suppressor gene in breast cancer.
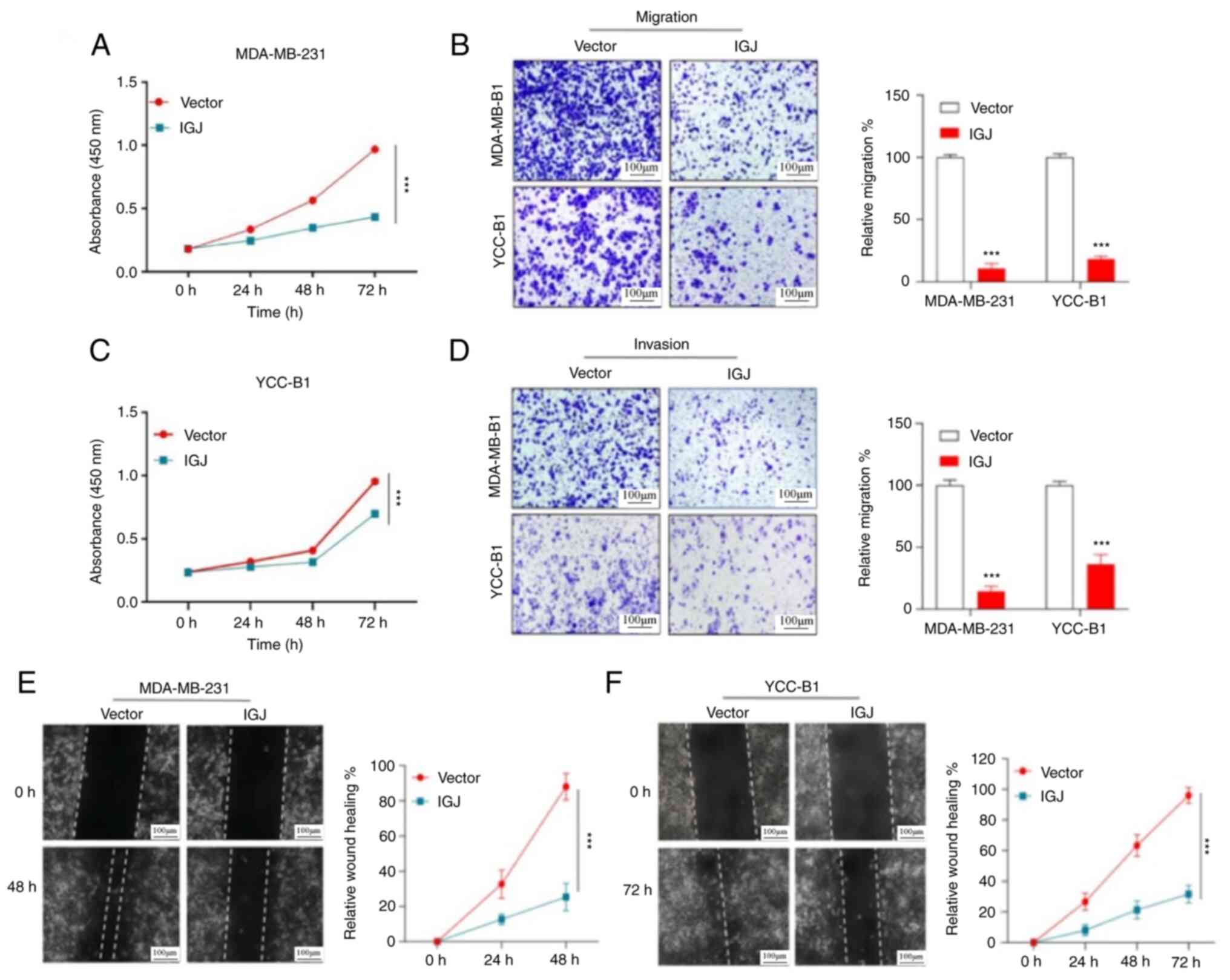
Overexpression of IGJ inhibits the
invasion and metastasis of breast cancer in vitro and in vivo
To explore the biological function of IGJ in breast
cancer cells, the MDA-MB-231 and YCC-B1 cells were transfected with
synthesized IGJ lentivirus or empty lentivirus vector and
verification was conducted (Fig.
S1). CCK-8 cell proliferation assay revealed that IGJ
overexpression markedly inhibited the proliferation of breast
cancer cells (Fig. 4A and C). In
addition, Transwell assays revealed that the number of invasive or
migratory breast cancer cells following the overexpression of IGJ
was markedly reduced compared with the control group (Fig. 4B and D). The wound scratch tests
also demonstrated that IGJ evidently inhibited the migration of
breast cancer cells (Fig. 4E and
F). Through subcutaneous tumorigenesis experiments in nude
mice, it was found that compared with the vector group, the tumor
size of the stable overexpression IGJ group was markedly smaller
(Fig. 5A-D). The IHC detection of
the expression of Ki-67 was conducted in the two groups with tumors
(Fig. 5E). The in vivo
imaging results revealed that the lung metastatic nodules of the
nude mice in the IGJ overexpression group were markedly lower in
number than those in the control group (Fig. 5F-H). These results indicated that
IGJ inhibited the proliferation, invasion and metastasis of breast
cancer cells in vivo.
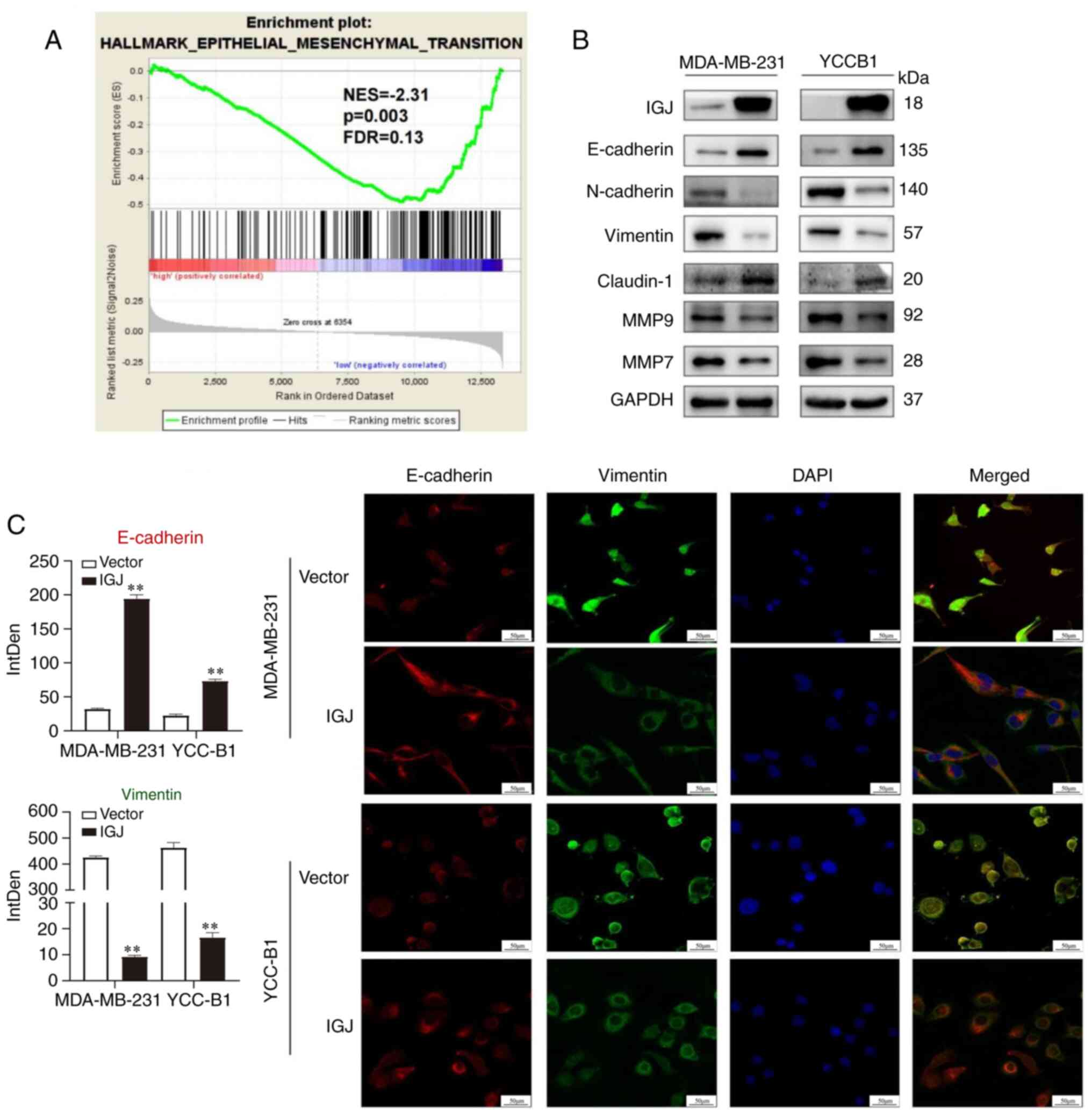
IGJ inhibits EMT in breast cancer
Following GSEA analysis, it was found that the
expression of IGJ in breast cancer was negatively associated with
the occurrence of EMT (normalized enrichment score, -2.31; Fig. 6A). Since GSEA analysis revealed
that IGJ may be associated with EMT, the expression levels of the
EMT markers, E-cadherin, N-cadherin, Clautin-1 and vimentin, were
detected using western blot analysis. The results obtained
confirmed that the overexpression of IGJ increased the expression
of E-cadherin and claudin-1, while it decreased that of vimentin,
N-cadherin, MMP9 and MMP7, thereby reversing EMT in breast cancer
cells (Fig. 6B). Furthermore,
additional evidence supporting the inhibitory effects of IGJ on EMT
was provided by demonstrating that the overexpression of IGJ
reversed EMT in breast cancer cells using IF staining (Fig. 6C).
IGJ regulates the NF-κB signaling
pathway
IGJ was verified to be poorly expressed in breast
cancer tissues and cells, and it inhibited the growth, invasion and
metastasis of breast cancer, as well as the occurrence of EMT in
breast cancer. These results suggested that IGJ functions as a
tumor suppressor gene in breast cancer; however, the underlying
mechanisms remained unclear. Therefore, the mechanisms of IGJ in
regulating breast cancer metastasis were further explored. To
identify the possible IGJ-related pathways, KEGG analysis was
conducted, indicating that the NF-κB signaling pathway may be one
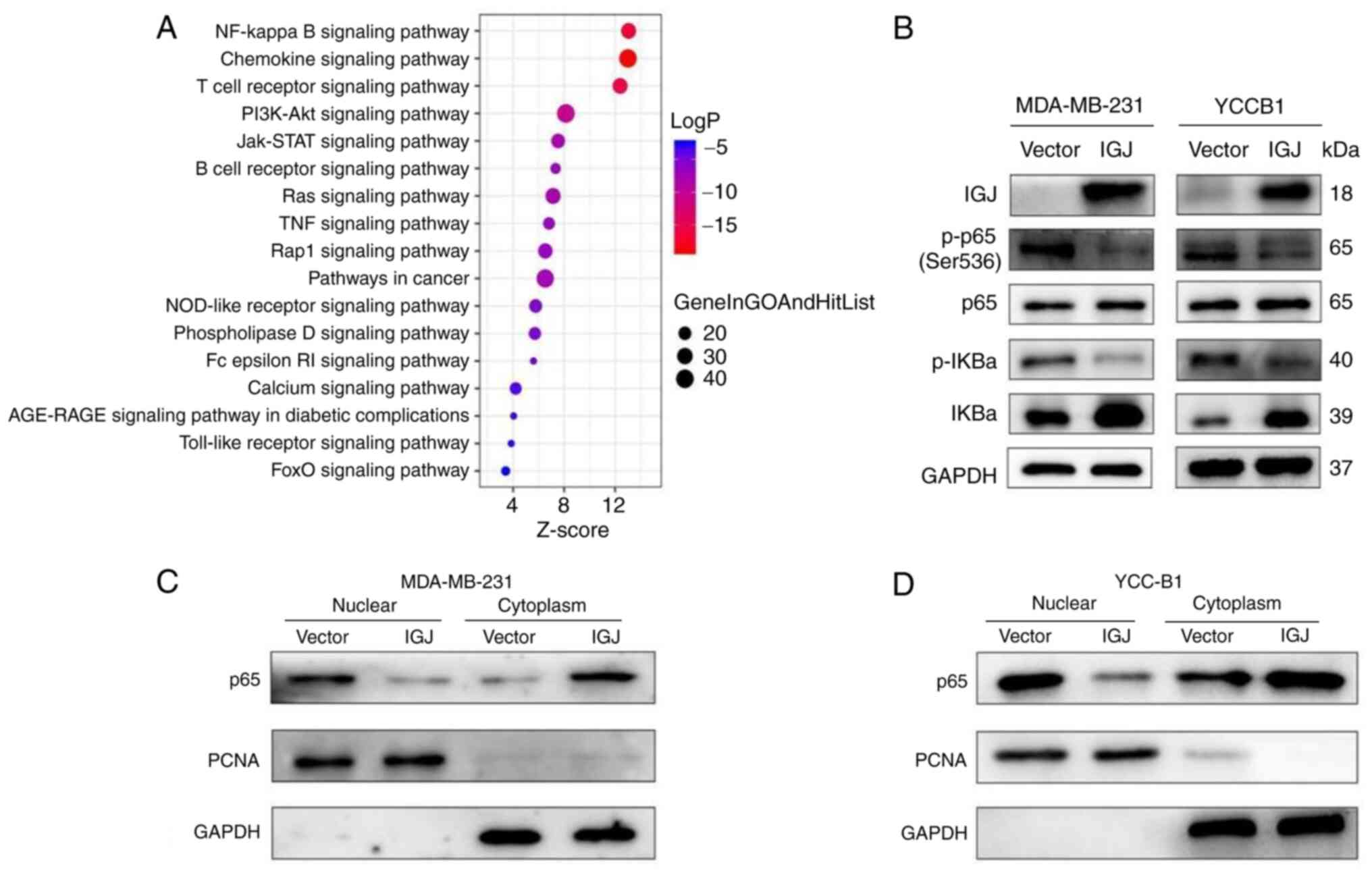
of the major enrichment pathways associated with IGJ (Fig. 7A). To determine the role of IGJ in
the NF-κB pathway, the expression of the NF-κB pathway-related
proteins, p65 and p-p65, were detected using western blot analysis.
The overexpression of IGJ decreased the expression of p-p65 and
p-IκBα, whereas it increased the expression of IκBα. However, the
protein level of p65 was not markedly altered (Fig. 7B). To further reveal the mechanisms
underlying the effects of IGJ on the NF-κB signaling pathway,
nuclear and cytoplasmic proteins were extracted from
IGJ-overexpressing and control cells to detect the expression of
p65 using western blot analysis. The results indicated that the
overexpression of IGJ markedly inhibited the nuclear accumulation
of p65 in the MDA-MB-231 and YCC-B1 cells (Fig. 7C and D). Taken together, these
findings indicate that IGJ inhibits the NF-κB signaling pathway by
preventing the translocation of p65 into the nucleus.
IGJ inhibits the invasion and metastasis
of breast cancer through the NF-κB signaling pathway
The present study demonstrated that IGJ inhibited
the proliferation, invasion and metastasis of breast cancer cells,
and IGJ was also found to inhibit the activation of the NF-κB
signaling pathway. Since the NF-κB signaling pathway has been
reported to be essential for the occurrence and development of
tumors, it was hypothesized that IGJ inhibited the proliferation,
invasion and metastasis of breast cancer cells by mediating the
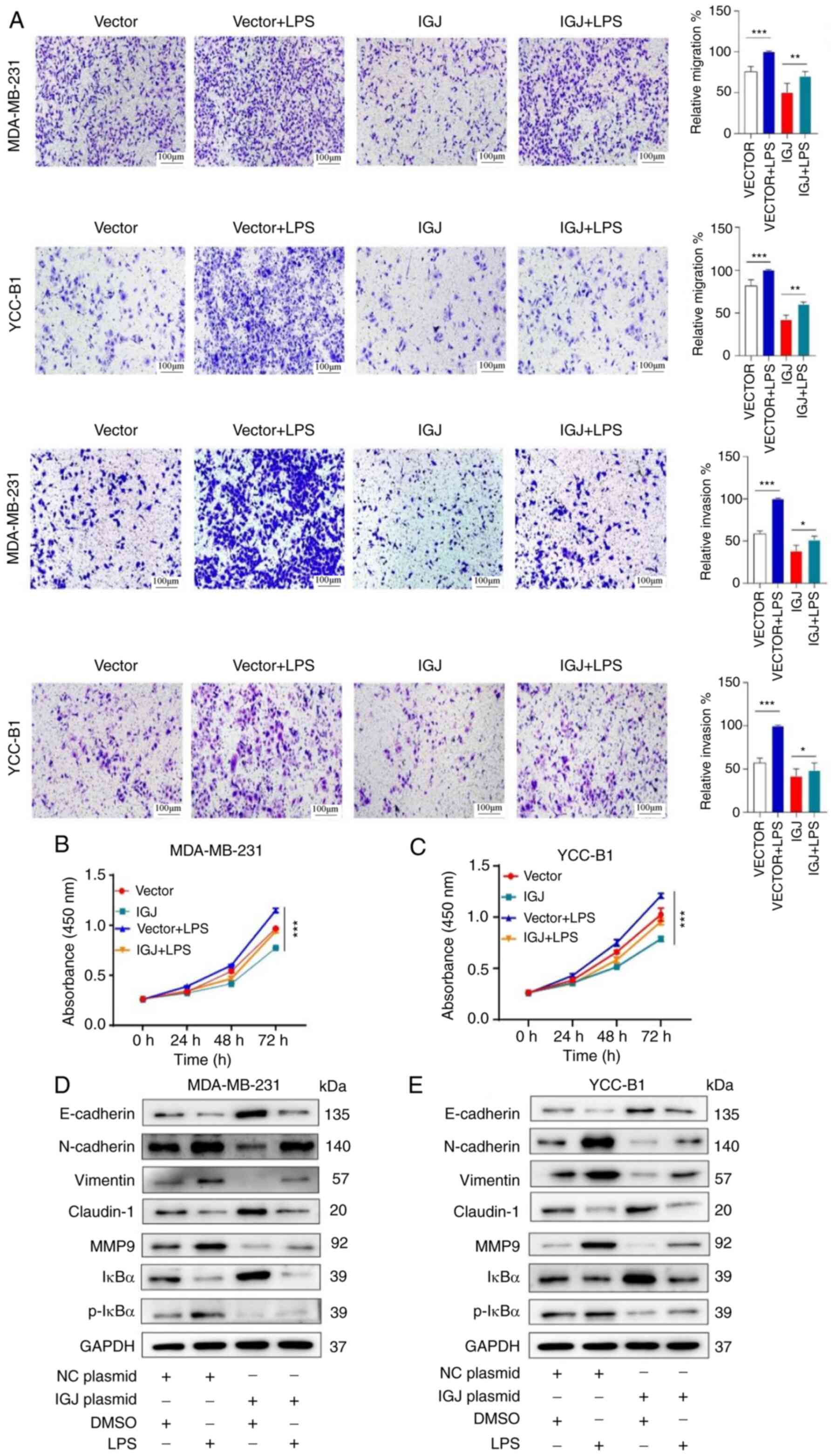
NF-κB pathway. Therefore, the NF-κB pathway agonist, LPS, we
applied to conduct rescue experiments. It was found that the
overexpression of IGJ markedly inhibited breast cancer cell
proliferation following exposure to LPS; however, without LPS, cell
proliferation was more markedly inhibited by IGJ overexpression;
thus, LPS reduced the suppressive ability of IGJ on breast cancer
(Fig. 8B and C). Similarly,
Transwell assays revealed that LPS also reduced the ability of IGJ
overexpression to inhibit the invasion and metastasis of breast
cancer cells (Fig. 8A). These
results suggested that IGJ played a role in breast cancer by
regulating the NF-κB signaling pathway. Subsequently, the
expression of various markers of EMT and protein molecules of the
NF-κB signaling pathway was detected (Fig. 8D and E). These results indicated
that the activation of the NF-κB signaling pathway was closely
related to IGJ gene expression.
Discussion
Tumor metastasis is a complex, multi-step process
involving several genes and biomolecules (24). At present, the understanding of the
mechanisms underlying breast cancer metastasis is incomplete. A
deeper insight into this process is crucial for improving the
treatment efficacy in and prognosis of patients with clinical
breast cancer. Current strategies for the treatment of breast
cancer involve gene-specific, tissue-specific and genome-wide
approaches to identify specific genes associated with specific
breast cancer types, which can be applied to optimize the treatment
of tumors for specific patients (25).
Given that the bone and lungs are the most common
metastatic sites for breast cancer, the present study first
collected data of breast cancer patients from TCGA database who
initially reported no metastasis at diagnosis, but experienced it
during follow-up. Moreover, non-metastatic breast cancer patients
were matched with a series of clinical features similar to this
group of patients. Through differential gene screening, a total of
24 genes with notable changes in expression were identified and IGJ
was finally determined as the target gene of the present study via
survival analysis. Further analysis revealed that IGJ was poorly
expressed in breast cancer tissues and cells. The expression of IGJ
was linked to age, lymph node metastasis and ER status.
Multivariate COX regression analysis revealed that IGJ functioned
as an independent prognostic factor for patients with breast
cancer.
The present study also focused on the exploration of
the biological role of IGJ in breast cancer. IGJ was revealed to
inhibit the proliferation, invasion and metastasis of breast cancer
in vitro and in vivo. Furthermore, a negative
association was found between IGJ and EMT. A key change in
promoting the migration and invasion of breast cancer cells has
been recognized as the epithelial-EMT process (24,26).
Over the past decade, multiple studies have reported the role of
EMT in the invasion and metastasis of breast cancer (27-29).
Epithelial cells are characterized by a complete intercellular
interaction through adhesion molecules within tight junctions,
adhesion junctions, desmosomes and gap junctions (30,31).
However, due to various extracellular and tissue-specific
EMT-induced signal stimuli, epithelial cells can upregulate
EMT-induced transcription factors to coordinate all morphological,
cellular and molecular changes during EMT, thereby promoting tumor
metastasis (32). The tumor
microenvironment plays a crucial role in determining the phenotype
of epithelial cancer cells through a series of heterotypic cell
signaling molecules. The Wnt, TGF-β and Notch signaling pathways
have been identified as important components of the process of EMT
(33). As research on the role of
EMT in tumors continues to progress, its occurrence process and
mechanisms have been gradually elucidated. In addition to the
previously described classical pathways, certain growth factors,
including epidermal growth factor, insulin growth factor,
hepatocyte growth factor, fibroblast growth factor and
platelet-derived growth factor have also been found to trigger the
EMT program (33-35). Moreover, it has been indicated that
hypoxia-inducible factor 1α, inflammatory signals (NF-κB) and
cytokines (IL-1β), and TNFα also induce the occurrence of EMT
(36). Such pathways and cytokines
act together in the occurrence of EMT in tumors. The occurrence and
development of EMT in cancer cells may be regulated by numerous
factors. Breast cancer cells may respond differently to signals
from different pathways that cause EMT depending on their
phenotypic status (35). Studies
have indicated that triple-negative breast cancer cells can respond
quickly to signals that induce EMT, particularly those transmitted
by TGF-β (37,38). However, epithelial cancer cells of
luminal breast cancer do not respond to the same EMT-induced signal
and maintain the same state. The successful induction of EMT
requires appropriate signaling and responsive target cells
(39). Conversely, this suggests
that the response of tumor cells to EMT-induced signals is
applicable for the prediction of the future biological behavior of
tumor cells at early stages of carcinogenesis (39,40).
MMP is a type of protease, which has a number of biological
functions in the development and progression of cancer (41). MMP9, also known as gelatinase B,
plays a crucial role in extracellular matrix remodeling and protein
cleavage, involving tumor invasion, metastasis, and the regulation
of the tumor microenvironment (42). MMP9 is capable of breaking down
collagen, and promoting migration, invasion and metastasis in
basement membrane degradation (43). MMP7 is a secreted zinc and calcium
dependent endopeptidase that is one of the most important
downstream target genes of the Wnt/β-catenin signaling
transduction. Its expression is associated with the poor prognosis
of patients with breast cancer (43,44).
The present study discovered that IGJ was closely
related to the NF-κB pathway and the expression of proteins p65 and
p-p65 related to the NF-κB pathway was determined using western
blot analysis. The results revealed that the overexpression of IGJ
decreased the expression of p-p65 and p-IκBα, whereas it increased
the expression of IκBα. The overexpression of IGJ markedly
inhibited the nuclear accumulation of p65 in the MDA-MB-231 and
YCC-B1 cells. NF-κB is one of the most complex transcription
factors, consisting of five subunits (p50, p52, RelA, RelB and
c-Rel), NF-κB inhibitor family (IκBs) and upstream activated kinase
complex (IKKα, IKKβ and IKKγ)/NEMO (45,46).
Normally, NF-κB forms heterodimers or homodimers isolated by IκB in
the cytoplasm during the quiescent phase. Upon stimulation, IκB is
phosphorylated by upstream kinases and is degraded through the
ubiquitination-proteasome pathway, thereby releasing the active
NF-κB dimer to regulate gene transcription inside the nucleus
(47,48). Among several key factors in the
progression of EMT, the NF-κB signaling pathway is a crucial factor
in mediating the inflammatory process, and manipulating the
occurrence and development of breast cancer (33). In this process, NF-κB may induce
transcription factors SNAIL, TWIST and ZEB2 to enhance the
occurrence of EMT and tumor metastasis (48-50).
Several factors such as TGF-β1, ROS and TNF-α and hypoxia can also
induce EMT in vitro and in vivo. EMT plays a role in
AKT/GSK or NF-κB-mediated Snail expression, and promotes invasion
and migration in various types of cancer, including breast, kidney
and colon cancer (51,52). The loss of E-cadherin, a known
adhesive cell surface protein expressed in epithelial cells, is the
major feature of EMT (53). The
key transcription factors, Snail and Slug, downregulate E-cadherin
expression by binding to the E-box in the E-cadherin promoter,
leading to the upregulation of MMP9 expression and the promotion of
cell invasion (54).
Taken together, the present study demonstrated that
IGJ suppressed the invasion and metastasis of breast cancer by
inhibiting the occurrence of EMT and the NF-κB signaling pathway.
These findings may provide novel biomarkers and therapeutic targets
for the treatment of metastatic breast cancer.
While the present study provides valuable insight
into breast cancer metastasis and the tole of IGJ, it is important
to acknowledge certain limitations. Firstly, the present study was
primarily based on bioinformatics analyses and in vitro
experiments, which may not fully reflect the complex biological
processes that occur in the human body. To confirm the therapeutic
potential of IGJ as a target for breast cancer treatment, further
in vivo experiments and clinical trials are warranted.
Secondly, the sample size of the study was relatively small, and
the tissue samples were all collected from a single center. The
replication of these findings in larger and more diverse cohorts is
crucial to ensure the generalizability of the results and their
applicability to a broader population.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW and SL conceptualized the study. MW, YW, XL and
MD carried out the formal analysis and investigations. MW wrote the
original draft of the manuscript. MW, YW, and SL wrote, reviewed
and edited the manuscript. MW, YW and SL confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The collection and use of the tissues in the present
study were approved by the Institutional Ethics Committee of the
First Affiliated Hospital of Chongqing Medical University and
written informed consent was signed by the patients (approval no.
2017-012) and was bound by the Declaration of Helsinki. All
experimental procedures were approved by the Animal Ethics
Committee of Chongqing Medical University (approval no.
2022-K121).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to express their sincere
gratitude to Professor Qian Tao at The Chinese University of Hong
Kong (Hong Kong, SAR, China) for generously providing the YCC-B1
cell line used in the present study.
Funding
No funding was received.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie J, Ying YY, Xu B, Li Y, Zhang X and Li
C: Metastasis pattern and prognosis of male breast cancer patients
in US: A population-based study from SEER database. Ther Adv Med
Oncol. 11:17588359198890032019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim MY: Breast cancer metastasis. Adv Exp
Med Biol. 1187:183–204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das V, Bhattacharya S, Chikkaputtaiah C,
Hazra S and Pal M: The basics of epithelial-mesenchymal transition
(EMT): A study from a structure, dynamics, and functional
perspective. J Cell Physiol. 234:14535–14555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johansen FE, Braathen R and Brandtzaeg P:
Role of J chain in secretory immunoglobulin formation. Scand J
Immunol. 52:240–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johansen FE, Braathen R and Brandtzaeg P:
The J chain is essential for polymeric Ig receptor-mediated
epithelial transport of IgA. J Immunol. 167:5185–5192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bertrand FE III, Billips LG, Gartland GL,
Kubagawa H and Schroeder HW Jr: The J chain gene is transcribed
during B and T lymphopoiesis in humans. J Immunol. 156:4240–4244.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bjercke S and Brandtzaeg P: Glandular
distribution of immunoglobulins, J chain, secretory component, and
HLA-DR in the human endometrium throughout the menstrual cycle. Hum
Reprod. 8:1420–1425. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gui S, O'Neill WQ, Teknos TN and Pan Q:
Plasma cell marker, immunoglobulin J polypeptide, predicts early
disease-specific mortality in HPV+ HNSCC. J Immunother Cancer.
9:e0012592021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cruz-Rodriguez N, Combita AL, Enciso LJ,
Quijano SM, Pinzon PL, Lozano OC, Castillo JS, Li L, Bareño J,
Cardozo C, et al: High expression of ID family and IGJ genes
signature as predictor of low induction treatment response and
worst survival in adult Hispanic patients with B-acute
lymphoblastic leukemia. J Exp Clin Cancer Res. 35:642016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slizhikova DK, Zinov'eva MV, Kuz'min DV,
Snezhkov EB, Shakhparonov MI, Dmitriev RI, Antipova NV, Zavalova LL
and Sverdlov ED: Decrease in expression of human J-chain in lung
squamous cell cancer and adenocarcinoma. Mol Biol (Mosk).
41:659–665. 2007.In Russian. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang B, Li S, Jiang Z and Shao P: Gastric
cancer associated genes identified by an integrative analysis of
gene expression data. Biomed Res Int. 2017:72590972017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larsson C, Ehinger A, Winslow S,
Leandersson K, Klintman M, Dahl L, Vallon-Christersson J, Häkkinen
J, Hegardt C, Manjer J, et al: Prognostic implications of the
expression levels of different immunoglobulin heavy chain-encoding
RNAs in early breast cancer. NPJ Breast Cancer. 6:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong Z, Jiang W, Zhang J, Li Z and Fan F:
Identification and validation of a novel 16-gene prognostic
signature for patients with breast cancer. Sci Rep. 12:123492022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Junjun S, Yangyanqiu W, Jing Z, Jie P,
Jian C, Yuefen P and Shuwen H: Prognostic model based on six PD-1
expression and immune infiltration-associated genes predicts
survival in breast cancer. Breast Cancer. 29:666–676. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Zhu M, Guo F, Song Y, Fan X and
Qin G: Identification of tumor microenvironment-related prognostic
biomarkers in luminal breast cancer. Front Genet. 11:5558652020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Győrffy B: Survival analysis across the
entire transcriptome identifies biomarkers with the highest
prognostic power in breast cancer. Comput Struct Biotechnol J.
19:4101–4109. 2021. View Article : Google Scholar
|
|
21
|
Wang M, Dai M, Wu YS, Yi Z, Li Y and Ren
G: Immunoglobulin superfamily member 10 is a novel prognostic
biomarker for breast cancer. PeerJ. 8:e101282020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Li Y, Huang J, Zeng B, Yang D, Sun J, Yin
X, Lu M, Qiu Z, Peng W, Xiang T, et al: PSMD2 regulates breast
cancer cell proliferation and cell cycle progression by modulating
p21 and p27 proteasomal degradation. Cancer Lett. 430:109–122.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kozłowski J, Kozłowska A and Kocki J:
Breast cancer metastasis-insight into selected molecular mechanisms
of the phenomenon. Postepy Hig Med Dosw (Online). 69:447–451. 2015.
View Article : Google Scholar
|
|
25
|
Stark AM, Tongers K, Maass N, Mehdorn HM
and Held-Feindt J: Reduced metastasis-suppressor gene
mRNA-expression in breast cancer brain metastases. J Cancer Res
Clin Oncol. 131:191–198. 2005. View Article : Google Scholar
|
|
26
|
Chakrabarti R, Hwang J, Blanco MA, Wei Y,
Lukačišin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T, et al:
Elf5 inhibits the epithelial-mesenchymal transition in mammary
gland development and breast cancer metastasis by transcriptionally
repressing Snail2. Nat Cell Biol. 14:1212–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li CJ, Chu PY, Yiang GT and Wu MY: The
molecular mechanism of epithelial-mesenchymal transition for breast
carcinogenesis. Biomolecules. 9:4762019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park M, Kim D, Ko S, Kim A, Mo K and Yoon
H: Breast cancer metastasis: Mechanisms and therapeutic
implications. Int J Mol Sci. 23:68062022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karamanou K, Franchi M, Vynios D and
Brézillon S: Epithelial-to-mesenchymal transition and invadopodia
markers in breast cancer: Lumican a key regulator. Semin Cancer
Biol. 62:125–133. 2020. View Article : Google Scholar
|
|
31
|
Wang H, Guo S, Kim SJ, Shao F, Ho JWK,
Wong KU, Miao Z, Hao D, Zhao M, Xu J, et al: Cisplatin prevents
breast cancer metastasis through blocking early EMT and retards
cancer growth together with paclitaxel. Theranostics. 11:2442–2459.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lüönd F, Sugiyama N, Bill R, Bornes L,
Hager C, Tang F, Santacroce N, Beisel C, Ivanek R, Bürglin T, et
al: Distinct contributions of partial and full EMT to breast cancer
malignancy. Dev Cell. 56:3203–3221.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaffer CL, Juan BP, Lim E and Weinberg
RA: EMT, cell plasticity and metastasis. Cancer Metastasis Rev.
35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ho GY, Kyran EL, Bedo J, Wakefield MJ,
Ennis DP, Mirza HB, Vandenberg CJ, Lieschke E, Farrell A, Hadla A,
et al: Epithelial-to-mesenchymal transition supports ovarian
carcinosarcoma tumorigenesis and confers sensitivity to
microtubule-targeting with eribulin. Cancer Res. 83:4457–4473.
2022. View Article : Google Scholar
|
|
36
|
Hasmim M, Xiao M, Van Moer K, Kumar A,
Oniga A, Mittelbronn M, Duhem C, Chammout A, Berchem G, Thiery JP,
et al: SNAI1-dependent upregulation of CD73 increases extracellular
adenosine release to mediate immune suppression in TNBC. Front
Immunol. 13:9828212022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:172016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chaffer CL, Marjanovic ND, Lee T, Bell G,
Kleer CG, Reinhardt F, D'Alessio AC, Young RA and Weinberg RA:
Poised chromatin at the ZEB1 promoter enables breast cancer cell
plasticity and enhances tumorigenicity. Cell. 154:61–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashemi M, Moosavi MS, Abed HM, Dehghani
M, Aalipour M, Ali Heydari E, Behroozaghdam M, Entezari M,
Salimimoghadam S, Gunduz ES, et al: Long non-coding RNA (lncRNA)
H19 in human cancer: From proliferation and metastasis to therapy.
Pharmacol Res. 184:1064182022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
42
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DS and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31(sup1):
S177–S183. 2016. View Article : Google Scholar
|
|
45
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Muaddi H, Majumder M, Peidis P, Papadakis
AI, Holcik M, Scheuner D, Kaufman RJ, Hatzoglou M and Koromilas AE:
Phosphorylation of eIF2α at serine 51 is an important determinant
of cell survival and adaptation to glucose deficiency. Mol Biol
Cell. 21:3220–3231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pal S, Bhattacharjee A, Ali A, Mandal NC,
Mandal SC and Pal M: Chronic inflammation and cancer: potential
chemoprevention through nuclear factor kappa B and p53 mutual
antagonism. J Inflamm (Lond). 11:232014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsubaki M, Komai M, Fujimoto SI, Itoh T,
Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, et
al: Activation of NF-κB by the RANKL/RANK system up-regulates snail
and twist expressions and induces epithelial-to-mesenchymal
transition in mammary tumor cell lines. J Exp Clin Cancer Res.
32:622013. View Article : Google Scholar
|
|
50
|
Katoh M and Katoh M: Integrative genomic
analyses of ZEB2: Transcriptional regulation of ZEB2 based on
SMADs, ETS1, HIF1alpha, POU/OCT, and NF-kappaB. Int J Oncol.
34:1737–1742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated
stabilization of snail in colorectal cancer. PLoS One.
8:e566642013. View Article : Google Scholar
|
|
54
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|