It was estimated that there were >19 million new
cancer cases and 9 million cancer-related deaths in 2020 worldwide.
The top five cancer sites, based on the global cancer incidence,
are as follows: Female breast cancer (BC), lung cancer (LC),
colorectal cancer (CRC), prostate cancer (PCa) and stomach cancer,
and more than half of cancer deaths globally are attributed to
these cancer types (World Health Organization. Cancer Today. 2020;
http://gco.iarc.fr/). In China, a growing cancer
burden was unexpectedly observed, and nearly 3 million cancer
deaths and >4 million individuals were diagnosed with cancer in
2020. Accurate diagnosis and personalized cancer therapy are the
keys to improving the cancer prognosis for every patient. With the
promise of an estimated numerical prognosis for patients with
cancer, AI has been proposed as a prominent way to improve
pathological diagnostic accuracy (DA) and cancer prognostication
(CP). Over the past decades, although the exploration and
exploitation of AI in cancer pathology has evolved substantially,
their generations, interpretations and impact on patients have
remained incompletely understood by oncologists. In the present
study, the published literature on Google Scholar and PubMed were
searched using the following terms: ‘AI’ AND ‘pathology’ AND
‘cancer’, or ‘machine learning (ML)’ AND ‘pathology’ AND ‘cancer’,
or ‘deep learning’ AND ‘pathology’ AND ‘cancer’. The present review
includes an overview of the roles of AI-integrated cancer pathology
in precision medicine by summarizing the rationale for its use,
clarifying recent innovations and grouping the main applications,
as well as discussing the challenges for AI-integrated cancer
pathology-based individualized treatment.
AI is defined as the ability of a machine to
simulate, extend and expand human cognition, and has the most
features suggestive of human intelligence, to decide upon an action
to achieve the desired goal. Initially proposed in 1950 by Turing
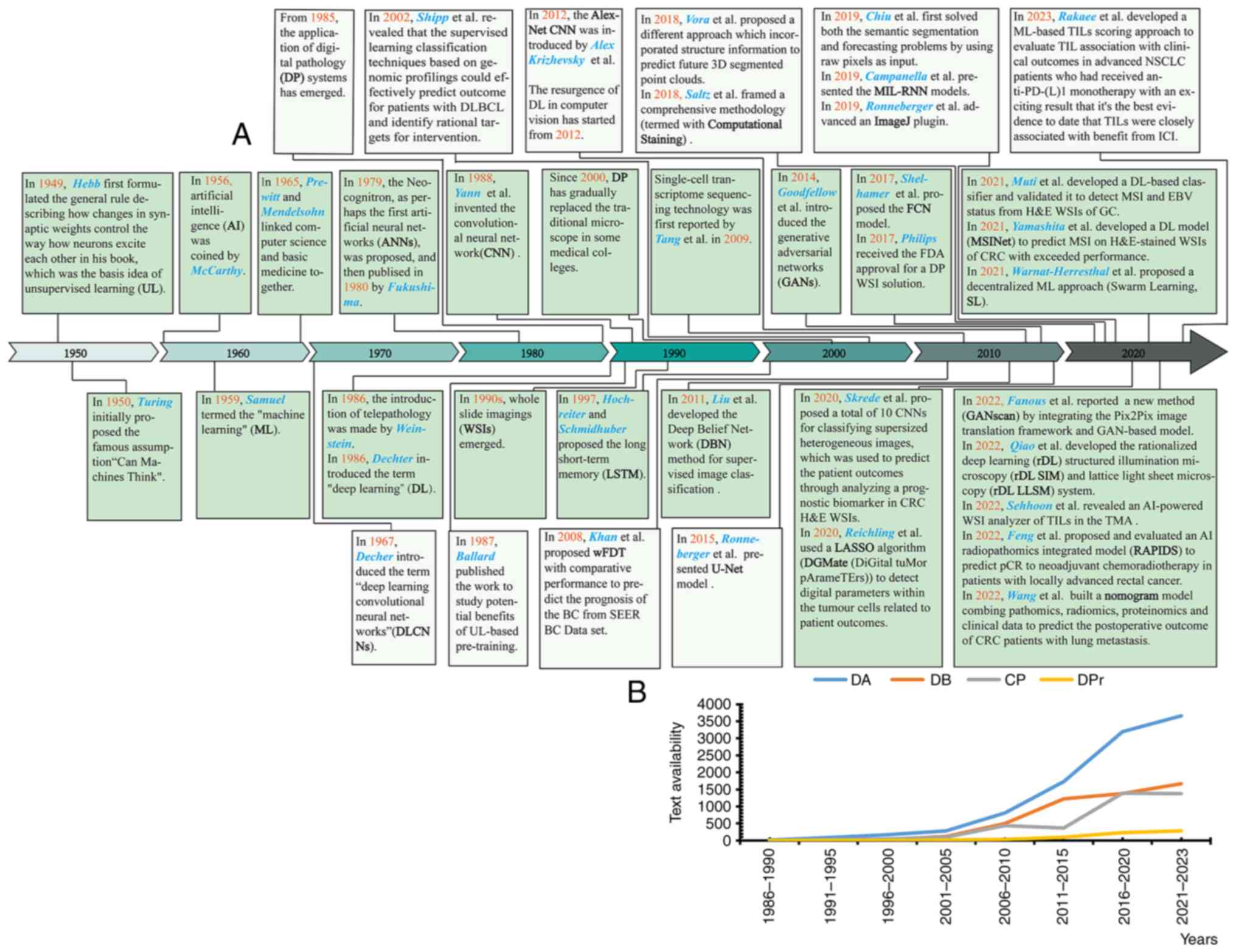
with ‘Can Machines Think’, and subsequently coined in 1956 by
McCarthy (Fig. 1), AI was
originally described as being able to ‘simulate intelligence’. With
the gradual advent of ML based on computational algorithms and the
following computational intelligence, the more power of the
decision-making pattern AI was endued with, and the more diverse,
complex or esoteric fields AI was involved in, a marked impact was
made on modern civilization, namely on Industrial Revolution 4.0
(1). Deep learning (DL) introduced
in 1986 by Dechter is by far the most common subtype of ML, and
represents computational models originating from artificial neural
networks (ANNs) to derive progressively higher-order features from
data in the form of multiple non-linear layers (Fig. 2) (1). Inspired by neurobiology, DL is
composed of units that compute a weighted sum of the multiple
inputs (referred to as the pre-activation) and transforms
the results non-linearly, which includes automatic learning and
hierarchical representation on multiple levels (2). DL has been employed in nearly all
scientific areas, especially in medical imaging, including cancer
pathology (the cornerstone of cancer medicine), which has yielded
important results that paved the way for the development of the
convolutional neural network (CNN), generative adversarial network
(GAN), auto encode and so on, to meet the demands of specific
biomedical applications for precision oncology.
Defined as an image-based approach, DP aims to
acquire, manage, interpret and distribute pathology information in
a computer-empowered manner, to extract and analyze pathological
visual data. Since the introduction of telepathology in 1986 by
Weinstein, the emergence of whole slide images (WSIs) in the 1990s,
and other digital images (Electronic microscope digital images),
has boosted the prosperity of the DP era (Fig. 1A) (3). Efforts have been devoted to improving
the scanning speed and the accuracy of images, which are the two
major issues affecting the throughout performance of WSIs. Notably,
GAN, an unsupervised technique proposed by Goodfellow et al
(4) in 2014, was composed of two
competing models: i) A generative model G capturing the data
distribution; and ii) a discriminative model D, which made
likelihood estimations on the training data rather than G. With
respect to this, Fanous and Popescu (5) reported a new method in 2022 (referred
to as GANscan) to significantly increase the scanning speed of the
whole slide scanner and to correct any defocusing by integrating
the Pix2Pix image translation framework and GAN-based model.
Notably, WSIs provide an enabling AI platform for
generating a rich variety of novel ecosystems in DP, which is known
as Pathomics. Pathomics is an AI-based multi-systems integration
methodology to understand cellular interactions and signaling by
analyzing relevant data from tissues and cells. With the emergence
of Pathomics, integration of AI into the DP workflow has achieved
some breakthroughs in cancer pathology. For example, in MICCAI 2014
(Table I), the brain tumor digital
pathology challenge, including two sub-challenges (classification
and automated segmentation), was posted. In this regard, Barker
et al (6) demonstrated a
novel method using local representative tiles, decided
diagnostically by an elastic net classifier, for automated
classification in brain cancer cases, which exhibited high accuracy
for diagnosis as well as structural stability and a robustness for
varying parameters, implying that it may be useful for automatic
differentiation of the two subtypes of brain cancer (glioblastoma
multiforme and lower grade glioma) (Table II). In another example,
multiple-instance learning (MIL), first proposed by Dietterich
et al in 1997, is a variation on supervised learning. In
2019, Campanella et al (7)
presented the MIL-RNN models in the analysis of three datasets
(including PCa, basal cell carcinoma of the skin and BC metastases
to axillary lymph nodes datasets) of 44,732 WSIs from 15,187
patients, in which MIL-based residual neural network (ResNet)34
models were used to classify tiles. Then, semantically rich
tile-level feature representations were generated, which integrated
the information across the WSIs through the RNN models and achieved
the final classification result. This system was shown to be able
to train accurate classification models at an unprecedented scale,
providing the cornerstone for the evolution of computational
decision support systems in clinical practice (Fig. 1).
Notably, one of the biggest challenges in
neuroanatomy [the automatic segmentation of neuronal structures
presented in stacks of electron microscopy (EM) images] at ISBI
2012 was won by Ciresan et al (8) by employing a special type of deep ANN
as a pixel classifier (Table I).
However, there were two key disadvantages to the strategy raised by
Ciresan et al, which were that it was slow and required a
trade-off between localization accuracy and the use of context.
Fortunately, a noteworthy finding was reported by Shelhamer et
al (9) in 2017, where the
fully convolutional networks (FCN) model could convert
semantic-level images into pixel-level images using a convolution
layer, instead of the full connection layer of the original
segmentation network, which promoted the rapid development of image
segmentation. For instance, Signaevsky et al (10) revealed that the FCN implemented in
PyTorch was efficient and well suited for the practical application
of WSIs derived from 22 autopsy brains from patients with
tauopathies, which produced high precision and recall in naïve WSI
semantic segmentation. Yi et al (11) also demonstrated that a finely-tuned
FCN could be applied to analyze the microvessels of H&E stained
histology images from patients with lung adenocarcinoma (LUAD) for
prognostication. As a result of this FCN development, the U-Net
model was presented in 2015 by Ronneberger et al (12) who, through modifying and extending
the FCN, which supported automatic recognition and precise
segmentation of characteristic lesions in EM stacks, beat the
network presented by Ciresan et al (8) at ISBI 2015 (Table I). Furthermore, building on past
efforts, Ronneberger et al developed an ImageJ plugin that
enabled non-ML practitioners to solve problems with U-Net on either
a local computer or a remote server/cloud service, which provided a
generic DL-based software package for 2D and 3D cell detection and
segmentation (12,13). A case in point, Lee et al
(14) in 2020 used the modified
U-Net to perform spatial analysis for the tumor microenvironment
(TME) in WSIs from breast invasive carcinoma cases to replenish
cancer classification and prediction, which demonstrated the
effectiveness of the U-Net approach and the quantitative estimates
derived from the spatial analysis. In addition, U-Net was proposed
to aid the epidermis segmentation task on WSIs from malignant
melanoma by Oskal et al (15), in which a superior performance of
U-Net compared with existing techniques was observed.
In short, AI has been involved in the growth of DP,
which has indicated that AI may bring a future of intelligent
diagnostic robots.
In addition to the use of conventional pathology as
the basis for DP, AI has also demonstrated unparalleled advantages
and potential in MP, which is a sub-microscopic discipline of
pathology. MP predicts risk, facilitates diagnosis and improves
prognostication based on a complete understanding of the biological
impact of specific molecular variants, mutations and dysregulations
in diseases. MP techniques, which were rooted in fundamental
molecular biology discoveries of the 1940s to 1980s (for example,
PCR developed by Mullis in the 1980s), have moved from genomics to
proteomics [such as next-generation sequencing, single cell RNA
sequencing, fluorescence in situ hybridization, NMR spectroscopy
and iTRAQ-MALDI-MS/MS], which has been driven by an increasing
number of druggable targets and predictive biomarkers. Undeniably,
AI fostered the development of MP, which was mainly encompassed by
molecular profiles for risk prediction and molecular diagnostics,
especially genomic diagnostics (16). For example, Woerl et al
(17) formulated a novel
ResNet-based approach (termed mibCNN) to predict the molecular
subtype of muscle-invasive bladder cancer (MIBC) by analyzing two
datasets of WSIs from H&E staining alone. The results
demonstrated that DL could be trained to predict the significant
molecular characteristics of MIBC from digital images derived from
H&E slides only, potentially resulting in an improvement in
managing this disease. Similarly, Schrammen et al (18) described a comparative performance
with a state-of-the-art method (referred to as the slide-level
assessment model), which simultaneously detected tumors and
predicted the genetic alterations (including microsatellite
instability/mismatch repair deficiency status and BRAF mutational
status) of CRC from H&E-stained histopathological WSIs.
Numerous studies have focused on the risk
stratification for cancer, especially for the globally leading
cause of cancer deaths, LC. For instance, Choi and Na (19) proposed a novel DL-based risk
stratification model to predict prognosis for patients with LUAD
based on a gene co-expression network, which exhibited a
significant association with patient prognosis independent of other
clinicopathological features. Moreover, Choi et al (20) designed a robust Genomic Sequencing
Classifier (GSC; a second-generation risk stratification algorithm)
based on whole transcriptome RNA sequencing to validate the gene
expression values predicting clinicopathological features of
several LC cohorts. Compared with the Bronchial Genomic Classifier
(the first generation), the GSC presented an optimized risk
stratification across several independent LC cohorts, which aided
physicians in capturing the optimal actionable information for the
precision medicine of patients.
Growing evidence has shown that the TME, which is a
complex physical and biochemical system comprising tumor cells,
tumor stromal cells, vascular cells, immune cells and cytokines,
plays an important role in tumor initiation, progression,
metastasis and drug resistance. One of the most notable advances
facilitated by AI has been in the understanding of the
tumor-infiltrating lymphocytes (TILs) of the TME. Based on
information regarding the TME from multiple studies in which immune
content response data from The Cancer Genome Atlas (TCGA) were
comprehensively analyzed, Saltz et al (21) framed a comprehensive methodology
(termed Computational Staining) based on CNNs to map TILs and
analyze the molecular and clinical correlation of TILs from
H&E-stained WSIs containing 13 TCGA cancer types (Table II). In the present study, TIL map
structural patterns with competitive performance were generated and
it was revealed that the TIL patterns were associated with tumor
and immune molecular features, cancer type and patient survival,
which laid a new milestone for TME research (Fig. 3). Rakaee et al (22) described an attempt to associate an
ML-based assessment of TILs from standard histological images with
the outcomes of anti-PD-L1 monotherapy in patients with advanced
non-small cell LC (NSCLC), which provided evidence that AI-based
patient TIL assessment may enhance precision therapy.
As another key component of the TME,
tumor-associated macrophages (TAMs) were studied by Bao et
al (23) in triple-negative BC
through an integrated analysis of single-cell and bulk RNA-seq. A
DL approach based on the neural network, PyTorch, for the
TAM-related gene signature was observed to display high accuracy in
predicting the immunotherapy response (Fig. 3). Moreover, Cancian et al
(24) demonstrated that CNN-based
DeepLab-v3 could accurately recognize TAMs from the background, and
separate different TAMs in marked contrast to the other two
CNN-based models, which may satisfy the requirements of clinical
practice for the characterization of TAM-related metrics in human
colorectal liver metastases. In addition, Bian et al
(25) designed a DL-based
computational framework (termed ImmunoAIzer) to analyze the spatial
distribution of immune cells and cancer cells in the TME and to
detect gene mutations in colon cancer, which provided comprehensive
information of cell distribution and tumor gene mutation status
more efficiently and less expensively.
Moreover, tumor heterogeneity (including the
contribution of the TME), an unneglectable factor affecting tumor
evolution and therapy resistance, has also rapidly become a hot
field of tumor phenotype research facilitated by AI. Undoubtedly,
AI has made it easier to capture information from heterogeneous
datasets to achieve accurate predictions and recognition patterns
(26). For instance, since
single-cell transcriptome sequencing technology was first reported
by Tang et al (27) in
2009, and it has been widely applied in understanding molecular
mechanisms and cellular properties of numerous biological
processes, especially the tumor heterogeneity in various tumor
types, such as CRC (28), ovarian
cancer (29,30), head and neck cancer (31) and hepatocellular carcinoma (HCC)
(32). In the light of this, Del
Giudice et al (26)
attempted to comprehensively review AI-based single-cell RNA-seq
experiments in cancer from six network databases including the
Genomic Data Commons Data Portal, ENCODE, Gene Expression Omnibus,
Sequence Read Archive, Human Cell Atlas and Single Cell Portal, and
sorted these experiments according to the tasks of AI models. These
experiments, as well as the latest data, were categorized as
defining cancer subtypes and cell clones (33–36),
parsing tumor immune microenvironment (37–39),
identifying new drug biomarkers (40), assessing and predicting disease
recurrence and patient survival (41–45),
detecting new putative actionable vulnerabilities (44,46)
and predicting tumor immunoprofiling (47) (Table
II) (26). More notably, the
contributions of AI to the needs of cancer genomics for improving
patient management were highlighted (26).
With the promise of continuing advances in the
autonomous systems, explainable AI (XAI; the emerging generation of
AI), which modified DL techniques to learn explainable features,
has come to the fore in precision medicine, guaranteeing that
predictions were more comprehensible and robust both in DP and MP.
For example, Altini et al (48) tested the performance of NDG class
activation mapping (NDG-CAM), a gradient-weighted CAM (one of the
XAI applications) based method for nuclei detection in
histopathology WSIs. NDG-CAM was observed to outperform in five
datasets compared with other state-of-the-art methods. Moreover,
Gimeno et al (49)
developed a novel XAI method (multi-dimensional module
optimization; MOM), which was applied to an acute myeloid leukemia
(AML) cohort of 319 ex vivo tumor samples with 122 screened drugs
and whole-exome sequencing. The predictive performance of MOM in
AML was successfully validated in three different large-scale
screening experiments with a therapeutic strategy based on the
FLT3, CBFβ-MYH11 and NRAS status, which predicted
patient response to quizartinib, trametinib, selumetinib and
crizotinib (49). In addition,
Meena and Hasija (50) described
the outperformance of XGBoost ML models in identifying 23
significant genes of squamous cell cancer (SCC) of the skin, which
may be the diagnostic and prognostic biomarkers of patients with
SCC.
Taken together, AI has empowered MP by challenging
itself, which mitigated various broken promises of precision
medicine to improve the poor prognosis of different cancer
types.
To date, integration of AI and the cancer pathology
workflow has achieved some breakthroughs for precision medicine,
these applications were summarized and classified into four groups
according to their principal tasks in precision medicine: DA, CP,
drug benefit (DB) and disease prevention (DPr) (Figs. 1B and 2). A review of the literature from Google
scholar and PubMed was conducted using the following terms: ‘AI’
AND ‘pathology’ AND ‘cancer’ AND ‘diagnosis’, or ‘AI’ AND
‘pathology’ AND ‘cancer’ AND ‘therapy’, or ‘AI’ AND ‘pathology’ AND
‘cancer’ AND ‘prognosis’. or ‘AI’ AND ‘pathology’ AND ‘cancer’ AND
‘prevention’. In total, ~20,000 available texts (Fig. 1B) were retrieved (most published
studies had an impact factor of ≥5) and these principal
applications of AI-integrated cancer pathology were listed
chronologically for the first time (to the best of our knowledge)
in Table II.
While reducing the workload of oncologists, the vast
majority of these studies were concerned with integration of AI
methods into cancer pathology to improve DA, including
identification, classification, detection and discrimination of
cancer and other malignancies. This means that integration of AI
and cancer pathology has been used primarily as an aid to cancer
diagnosis, and a great deal of effort has been devoted to address
the bottlenecks in improving DA, such as low speed and efficiency
of scanning slides, image distortion, spatial distribution
complexities, limited recognition or auto-recognition ability and
inefficient analytical capabilities, as well as the shortfalls for
process integration (i.e., tradeoffs between performance and new
features), etc. (Table II). Thus,
these reasonable efforts have served to enhance the roles of cancer
pathology in precision medicine, as far as possible, but they are
not sufficient to keep pace with the ever-growing needs of
personalized treatment.
Although the body of literature in the field of
integration of AI into cancer pathology for CP is relatively small,
the prediction of cancer prognosis has gained more attention, which
is mainly attributed to the growth of MP. It is well-known that a
cancer prognosis typically involves multiple physicians from
different specialties using different subsets of biomarkers and
multiple clinical factors, including the age and general health of
the patient, the location, type, grade and size of the cancer, as
well as the tumor-node-metastasis staging of the tumor. There are
three predictive foci concerned with cancer prognostication,
including the prediction of cancer susceptibility (i.e., risk
assessment), the prediction of cancer recurrence and the prediction
of cancer survivability. Differentiating these cancer predictive
foci from biomarkers and factors may pose a problem. In this
regard, as a consequence of the outperformance of AI integrated
into MP driven by the rapid development of cancer biomarkers, which
cover a broad range of biochemical entities, such as nucleic acids,
proteins, sugars, small metabolites and cytogenetic and cytokinetic
parameters, as well as entire tumor cells found in bodily fluids,
improving predictions of prognosis via these biomarkers were
observed in 10 types of TCGA cancer (41), soft tissue sarcomas (44), BC (46), cervical cancer (CC) (61), bladder cancer (73), glioma (82,127), AML (129) and chronic myelogenous leukemia
(130) (Table II). Therefore, AI has helped
clinicians to accurately stratify risk in patients via the
assessment of various cancer biomarkers.
Similarly, encouraged by the emergence of cancer
biomarkers and corresponding technologies, there has been a gradual
increase in the studies related to the integrations of AI and
cancer pathology in predicting drug benefits by evaluating novel
cancer biomarkers, including TIGIT (38), activation of BH3-only proteins
(39), amino acid synthesis and
interconversion (39), tumor human
leukocyte antigen peptide (47),
CTLA4 (66), oncotype DX
and other gene expression (69),
21 genes (73), dysplasia
(129), TOP1, PDIA4 and
OGN (141) (Table II). Accordingly, these studies
have provided compelling evidence for the feasibility of developing
targeted therapies against these molecules for patients to obtain
optimized medical treatment.
Regardless of the number of papers focusing on DPr
in terms of integration of AI and cancer pathology is the least
among the four main tasks, the influence of these studies on cancer
precision medicine remains vast. As a part of precision medicine,
cancer prevention, which is generalized as being ‘founded on
describing the burden of cancer, identifying the causes, and
evaluating and implementing preventive interventions’, means there
is ‘a significant amount of work to be conducted’. Cancer
prevention has achieved some success in reducing the global cancer
incidence. The most convincing evidence is that tobacco control
efforts and smoking cessation efforts as well as additional
efforts, such as computed tomography for LC screening, since the
1960′s will continue to reduce global LC incidence (150). Due to the advances in
understanding cancer biology, precision prevention approaches have
been made feasible in various types of cancer with the assistance
of AI, such as in CRC (80,128),
BC (91), CC (96,107), liver cancer (128), LUAD (128) and renal cell cancer (131) (Table II). It is hypothesized that these
attempts will reduce global cancer burden in the future.
The integration of AI into these practices has
allowed technologies to handle large amounts of data related to
cancer pathology, which has not only alleviated the workload
pressure of oncologists but has also improved the accuracy of
diagnosis and prediction of cancer prognosis and drug benefits, as
well as promoted DPr for precision medicine.
At present, cancer remains a major public health
problem worldwide, and cancer death was adversely affected by the
coronavirus 2019 (COVID-19) global pandemic. Thus, there is a
pressing need to develop novel and effective strategies for cancer
precision medicine. Although growing evidence indicates that a
combination of cancer pathology and AI can bring exciting changes
to cancer precision medicine, a large number of technical, ethical
and legal challenges still need to be appropriately addressed.
Firstly, there are four main limitations in terms of
technology level in the development of AI in cancer pathology,
including: The quality and standardization of pathological images
and WSIs (especially the images from 2D to 3D, if possible, or even
4D), the optimization of data, the interpretability of data and the
verification of algorithms. Therefore, more efforts than could
possibly be reviewed in the present review have been devoted to
address these problems. For example, Zheng et al (151) created a Graph-Transformer fusing
a graph-based representation of a WSI, and developed a graph-based
digital pathology visual transducer to predict disease grade, which
outperformed compared with current state-of-the-art methods for WSI
classification. It is hypothesized that these behaviors could
benefit cancer pathology ultimately with faster and more accurate
diagnoses, as well as faster screening. Moreover, Qiao et al
(152) described the rationalized
DL-structured illumination microscopy and lattice light sheet
microscopy system to build a minimally invasive 2D/3D live cell
imaging system for following intracellular dynamics and trajectory,
which provided the possibility that a novel system may be created
in the future by extending the aforementioned capabilities to
monitor the therapeutic effect of the treatments to tumor cells
in vivo safely. In addition, owing to the fact that the
effectiveness of these systems was limited by the inability of the
machine to explain its thoughts and actions to human users, XAI, a
suite of ML techniques, was created to promote safety and clarity
by showing how decisions are made in AI models, especially in
critical tasks, such as drug screening with genetic events
(49,50) and drug-drug interaction predictions
(153).
Nevertheless, cancer is a highly complex disease
involving a cascade of microscopic and macroscopic changes with
mechanisms and interactions that are not yet fully understood. With
the continuous discovery of novel cancer biomarkers and the
innovation of technologies, cancer biomarkers that provide insights
into the state and course of disease in the form of quantitative or
qualitative measurements have made the fusion of multiple medical
perspectives to guide patient management feasible, which AI has
promoted in multi-modal patterns. Since Chaudhary et al
(83) first employed a DL-based
model to identify multi-omics features to predict HCC survival
risk, AI-guided research linked to multi-omics has emerged. For
instance, Feng et al (135) proposed and evaluated an AI
radiopath-omics integrated model (termed RAPIDS) to predict
pathological complete response (pCR) to neoadjuvant
chemoradiotherapy in patients with locally advanced rectal cancer
via pretreatment MRI and H&E stained WSIs. The results
suggested that RAPIDS was able to predict pCR to neoadjuvant
chemoradiotherapy, based on pretreatment radiopath-omics images,
with high accuracy and robustness, which demonstrated a successful
fusion of imagen-omics, path-omics and clinical data to predict
patient prognosis (Fig. 4).
Furthermore, Wang et al (137) built a nomogram model combining
path-omics, radio-omics, protein-omics and clinical data to predict
the postoperative outcome of patients with CRC and lung metastasis,
which demonstrated an outperformance in risk prediction. Moreover,
Vanguri et al (144)
recently developed an ML-based multimodal model to predict
immunotherapy response to PD-L1 blockade by capturing the
information through a combination of imagen-omics, path-omics,
genomics and clinical data from patients with advanced NSCLC. The
model provided a quantitative rationale for using AI-guided
multimodal integration to improve prediction of the drug benefits
and outcomes in patients with NSCLC. Hence, synergies of cancer
pathology with other medical tools could provide more information
to the clinic to make an accurate and rapid decision in
personalized treatments for patients. However, the well-known
failed implementation of AI in oncology (the IBM Watson program at
MD Anderson) is a reminder that multiple deployment challenges must
be overcome prior to the integration of a pathology AI system into
a clinical work environment (154,155). It is hypothesized that exploring
the potential advantages of multimodal integration of path-omics,
genomics, imagen-omics, protein-omics, epigenomics and other omics,
as well as clinical data to make appropriate management decisions
and improve patient outcomes may be the most challenging issue of
cancer precision medicine in the future.
Notably, recent attempts to verify the capability of
ChatGPT in effecting precision medicine have been reported since
ChatGPT was released at the end of 2022, as it was reasonable to
assume that using larger language models to enhance relationships
through better communication would have a beneficial impact on
patients (156,157). Sinha et al (158) assessed the capability of ChatGPT
in solving higher-order reasoning regarding pathology and found
that ChatGPT may offer meaningful responses but also current
limitations.
Secondly, the vulnerability of ethics in the
development of AI in cancer pathology cannot be ignored. With the
introduction of WSI scanners, slides (as high-resolution digital
images) can be captured, stored and transmitted electronically, and
oncologists can annotate and label images as part of their reports.
When the images and reports are stored in the cloud, the process
allows third parties to share the information and associated data
for research or other purposes. Moreover, oncologists have an
obligation to keep identifiable patient information confidential
(except with expressed consent), however, we consider that more
could be done to ensure patient privacy and data security in this
process. For instance, Chauhan and Gullapalli (159) posed three key foundational
principles of ethical AI in the context of cancer pathology:
Transparency, accountability and governance, to guide future
practice. This viewpoint is appreciated, and it deserves
popularizing in the clinic.
Thirdly, it has been recognized that the adoption of
a new technology cannot be accomplished without some changes to the
human and social aspects of the organization. The lack of global
standardization has prevented the reusing of AI-integrated cancer
pathology data to meet the broad range of patient safety and
quality reporting requirements, which has been attributed to the
following potential factors: i) The standardization for storage and
retrieval of medical images; ii) the high standards and regulations
that surround the cancer pathology laboratory; iii) the adoption of
a global diagnostic system (i.e., as with the World Health
Organisation); iv) the normalization of validation systems; v) the
establishment of corresponding legal liabilities related to AI; and
vi) the oncologist, etc. To facilitate the integration of any
medical data from any data owner worldwide without violating
privacy laws, Warnat-Herresthal et al (160) proposed a decentralized ML
approach termed Swarm Learning (SL), which united edge computing,
blockchain-based peer-to-peer networking and coordination, while
maintaining confidentiality without the need for a central
coordinator, thereby going beyond federated learning. They chose
four cases of heterogeneous diseases (COVID-19, tuberculosis,
leukemia and lung pathologies) with >16,400 blood transcriptomes
derived from 127 clinical studies with non-uniform distributions of
cases and controls and substantial study biases, as well as
>95,000 chest X-ray images to validate the feasibility of using
SL to develop disease classifiers using distributed data. The
results demonstrated that SL classifiers outperform those developed
at individual sites, which allows for further study in more cancer
types (160). It is hoped that in
addition to establishing new rules and laws to provide a
comprehensive guarantee for the development of AI in oncology
pathology, more options could be explored to integrate the medical
data for precision medicine to the extent allowed by laws (Fig. 4).
In conclusion, the 21st century has witnessed
tremendous breakthroughs in AI and cancer pathology, and most of
these creations and innovations have improved and advanced
understanding of these systems. As such, it is hoped to improve
understanding of the concepts related to these systems and to help
others realize the goals of precision medicine.
Not applicable.
The present study was partly supported by 2017 Elite Talents
Training Plan of the Third Affiliated Hospital of Guangzhou Medical
University and 2023 Plan on enhancing scientific research in
GMU.
Not applicable.
JP designed the review, wrote and revised the
manuscript with input from BL, JF, QZ, QJ. BL, JF, QZ, ND, QJ and
JP performed and analyzed the literature search. JF and BL made the
figures. QZ, BL and JP completed the major part of tables. QJ
participated in revising the review critically for the important
intellectual content. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
LeCun Y, Bengio Y and Hinton G: Deep
learning. Nature. 521:436–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kriegeskorte N and Golan T: Neural network
models and deep learning. Curr Biol. 29:R231–R236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barisoni L, Lafata KJ, Hewitt SM,
Madabhushi A and Balis UGJ: Digital pathology and computational
image analysis in nephropathology. Nat Rev Nephrol. 16:669–685.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodfellow IJ, Pouget-Abadie J, Mirza M,
Xu B, Warde-Farley D, Ozair S, Courville A and Bengio Y: Generative
Adversarial Networks. 10–Jun;2014.doi:
10.48550/arXiv.1406.2661.
|
|
5
|
Fanous MJ and Popescu G: GANscan:
Continuous scanning microscopy using deep learning deblurring.
Light Sci Appl. 11:2652022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barker J, Hoogi A, Depeursinge A and Rubin
DL: Automated classification of brain tumor type in whole-slide
digital pathology images using local representative tiles. Med
Image Anal. 30:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campanella G, Hanna MG, Geneslaw L,
Miraflor A, Werneck Krauss Silva V, Busam KJ, Brogi E, Reuter VE,
Klimstra DS and Fuchs TJ: Clinical-grade computational pathology
using weakly supervised deep learning on whole slide images. Nat
Med. 25:1301–1309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ciresan D, Giusti A, Gambardella L and
Schmidhuber J: Deep neural net-works segment neuronal membranes in
electron microscopy images. NIPS. 2852–2860. 2012.
|
|
9
|
Shelhamer E, Long J and Darrell T: Fully
Convolutional networks for semantic segmentation. IEEE Trans
Pattern Anal Mach Intell. 39:640–651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Signaevsky M, Prastawa M, Farrell K,
Tabish N, Baldwin E, Han N, Iida MA, Koll J, Bryce C, Purohit D, et
al: Artificial intelligence in neuropathology: Deep learning-based
assessment of tauopathy. Lab Invest. 99:1019–1029. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi F, Yang L, Wang S, Guo L, Huang C, Xie
Y and Xiao G: Microvessel prediction in H&E Stained Pathology
Images using fully convolutional neural networks. BMC
Bioinformatics. 19:642018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronneberger O, Fischer P and Brox T:
U-net: Convolutional networks for biomedical image segmentation.
Medical Image Computing and Computer Assisted Intervention
(MICCAI). Springer, LNCS; pp. 9351pp. 234–241. 2015
|
|
13
|
Falk T, Mai D, Bensch R, Çiçek Ö,
Abdulkadir A, Marrakchi Y, Böhm A, Deubner J, Jäckel Z, Seiwald K,
et al: U-Net: Deep learning for cell counting, detection, and
morphometry. Nat Methods. 16:67–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S, Zhao Y, Masoud M and Belkasim S:
Quantitative spatial analysis on whole slide images using U-net.
Computational Biol Bioinform. 8:90–96. 2020. View Article : Google Scholar
|
|
15
|
Oskal KRJ, Risdal M, Janssen EAM,
Undersrud ES and Gulsrud TO: A U-net based approach to epidermal
tissue segmentation in whole slide histopathological images. SN
Applied Sciences. 1:6722019. View Article : Google Scholar
|
|
16
|
Echle A, Rindtorff NT, Brinker TJ, Luedde
T, Pearson AT and Kather JN: Deep learning in cancer pathology: A
new generation of clinical biomarkers. Br J Cancer. 124:686–696.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woerl AC, Eckstein M, Geiger J, Wagner DC,
Daher T, Stenzel P, Fernandez A, Hartmann A, Wand M, Roth W, et al:
Deep learning predicts molecular subtype of muscle-invasive bladder
cancer from conventional Histopathological Slides. Eur Urol.
78:256–264. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schrammen PL, Ghaffari Laleh N, Echle A,
Truhn D, Schulz V, Brinker TJ, Brenner H, Chang-Claude J, Alwers E,
Brobeil A, et al: Weakly supervised annotation-free cancer
detection and prediction of genotype in routine histopathology. J
Pathol. 256:50–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi H and Na KJ: A Risk stratification
model for lung cancer based on gene coexpression network and deep
learning. Biomed Res Int. 16:29142802018.PubMed/NCBI
|
|
20
|
Choi Y, Qu J, Wu S, Hao Y, Zhang J, Ning
J, Yang X, Lofaro L, Pankratz DG, Babiarz J, et al: Improving lung
cancer risk stratification leveraging whole transcriptome RNA
sequencing and machine learning across multiple cohorts. BMC Med
Genomics. 13:1512020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saltz J, Gupta R, Hou L, Kurc T, Singh P,
Nguyen V, Samaras D, Shroyer KR, Zhao T, Batiste R, et al: Spatial
organization and molecular correlation of tumor-infiltrating
lymphocytes using deep learning on pathology images. Cell Rep.
23:181–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rakaee M, Adib E, Ricciuti B, Sholl LM,
Shi W, Alessi JV, Cortellini A, Fulgenzi CAM, Viola P, Pinato DJ,
et al: Association of machine learning-based assessment of
tumor-infiltrating lymphocytes on standard histologic images with
outcomes of immunotherapy in patients with NSCLC. JAMA Oncol.
9:51–60. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao X, Shi R, Zhao T, Wang Y, Anastasov N,
Rosemann M and Fang W: Integrated analysis of single-cell RNA-seq
and bulk RNA-seq unravels tumour heterogeneity plus M2-like
tumour-associated macrophage infiltration and aggressiveness in
TNBC. Cancer Immunol Immunother. 70:189–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cancian P, Cortese N, Donadon M, Di Maio
M, Soldani C, Marchesi F, Savevski V, Santambrogio MD, Cerina L,
Laino ME, et al: Development of a deep-learning pipeline to
recognize and characterize macrophages in colo-rectal liver
metastasis. Cancers (Basel). 13:33132021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bian C, Wang Y, Lu Z, An Y, Wang H, Kong
L, Du Y and Tian J: ImmunoAIzer: A deep learning-based
computational framework to characterize cell distribution and gene
mutation in tumor microenvironment. Cancers (Basel). 13:16592021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Del Giudice M, Peirone S, Perrone S,
Priante F, Varese F, Tirtei E, Fagioli F and Cereda M: Artificial
intelligence in bulk and single-cell RNA-sequencing data to foster
precision oncology. Int J Mol Sci. 22:45632021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang F, Barbacioru C, Wang Y, Nordman E,
Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, et al: mRNA-Seq
whole-transcriptome analysis of a single cell. Nat Methods.
6:377–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D,
Cheng Y, Huang S, Liu Y, Jiang S, et al: Spatiotemporal immune
landscape of colorectal cancer liver metastasis at single-cell
level. Cancer Discov. 12:134–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geistlinger L, Oh S, Ramos M, Schiffer L,
LaRue RS, Henzler CM, Munro SA, Daughters C, Nelson AC, Winterhoff
BJ, et al: Multiomic analysis of subtype evolution and
heterogeneity in high-grade serous ovarian carcinoma. Cancer Res.
80:4335–4345. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao Q, Li J, Zhang Q, Xu F, Xie B, Lu H,
Wu X and Zhou X: Single-cell transcriptomes reveal heterogeneity of
high-grade serous ovarian carcinoma. Clin Transl Med. 11:e5002021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puram SV, Tirosh I, Parikh AS, Patel AP,
Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et
al: Single-Cell transcriptomic analysis of primary and metastatic
tumor ecosystems in head and neck cancer. Cell. 171:1611–1624.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao
R, Modak M, Carotta S, Haslinger C, Kind D, et al: Landscape and
dynamics of single immune cells in hepatocellular carcinoma. Cell.
179:829–845.e20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cascianelli S, Molineris I, Isella C,
Masseroli M and Medico E: Machine learning for RNA sequencing-based
intrinsic subtyping of breast cancer. Sci Rep. 10:140712020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Z, Wang Z, Yu X and Zhang Z:
RNA-Seq-Based breast cancer subtypes classification using machine
learning approaches. Comput Intell Neurosci. 2020:47379692020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valle F, Osella M and Caselle M: A Topic
modeling analysis of TCGA breast and lung cancer transcriptomic
data. Cancers (Basel). 12:37992020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao F, Wang W, Tan M, Zhu L, Zhang Y,
Fessler E, Vermeulen L and Wang X: DeepCC: A novel deep
learning-based framework for cancer molecular subtype
classification. Oncogenesis. 8:442019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen YP, Yin JH, Li WF, Li HJ, Chen DP,
Zhang CJ, Lv JW, Wang YQ, Li XM, Li JY, et al: Single-cell
transcriptomics reveals regulators underlying immune cell diversity
and immune subtypes associated with prognosis in nasopharyngeal
carcinoma. Cell Res. 30:1024–1042. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Y, Yang D, Yang Q, Lv X, Huang W,
Zhou Z, Wang Y, Zhang Z, Yuan T, Ding X, et al: Single-cell RNA
landscape of intratumoral heterogeneity and immunosuppressive
microenvironment in advanced osteosarcoma. Nat Commun. 11:63222020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorin M, Rezanejad M, Karimi E, Fiset B,
Desharnais L, Perus LJM, Milette S, Yu MW, Maritan SM, Doré S, et
al: Single-cell spatial landscapes of the lung tumour immune
microenvironment. Nature. 614:548–554. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kong J, Lee H, Kim D, Han SK, Ha D, Shin K
and Kim S: Network-based machine learning in colorectal and bladder
organoid models predicts anti-cancer drug efficacy in patients. Nat
Commun. 11:54852020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ching T, Zhu X and Garmire LX: Cox-nnet:
An artificial neural network method for prognosis prediction of
high-throughput omics data. PLoS Comput Biol. 14:e10060762018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Katzman JL, Shaham U, Cloninger A, Bates
J, Jiang T and Kluger Y: DeepSurv: Personalized treatment
recommender system using a Cox proportional hazards deep neural
network. BMC Med Res Methodol. 18:242018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Z, Johnson TS, Han Z, Helm B, Cao S,
Zhang C, Salama P, Rizkalla M, Yu CY, Cheng J, et al: Deep
learning-based cancer survival prognosis from RNA-seq data:
Approaches and evaluations. BMC Med Genomics. 13:412020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
van IJzendoorn DGP, Szuhai K, Briaire-de
Bruijn IH, Kostine M, Kuijjer ML and Bovée JVMG: Machine learning
analysis of gene expression data reveals novel diagnostic and
prognostic biomarkers and identifies therapeutic targets for soft
tissue sarcomas. PLoS Comput Biol. 15:e10068262019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haider S, Yao CQ, Sabine VS, Grzadkowski
M, Stimper V, Starmans MHW, Wang J, Nguyen F, Moon NC, Lin X, et
al: Pathway-based subnetworks enable cross-disease biomarker
discovery. Nat Commun. 9:47462018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tabl AA, Alkhateeb A, ElMaraghy W, Rueda L
and Ngom A: A machine learning approach for identifying gene
biomarkers guiding the treatment of breast cancer. Front Genet.
10:2562018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bulik-Sullivan B, Busby J, Palmer CD,
Davis MJ, Murphy T, Clark A, Busby M, Duke F, Yang A, Young L, et
al: Deep learning using tumor HLA peptide mass spectrometry
datasets improves neoantigen identification. Nat Biotechnol.
37:55–63. 2019. View Article : Google Scholar
|
|
48
|
Altini N, Brunetti A, Puro E, Taccogna MG,
Saponaro C, Zito FA, De Summa S and Bevilacqua V: NDG-CAM: Nuclei
detection in histopathology images with semantic segmentation
networks and Grad-CAM. Bioengineering (Basel). 9:4752022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gimeno M, San José-Enériz E, Villar S,
Agirre X, Prosper F, Rubio A and Carazo F: Explainable artificial
intelligence for precision medicine in acute myeloid leukemia.
Front Immunol. 13:9773582022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meena J and Hasija Y: Application of
explainable artificial intelligence in the identification of
Squamous Cell Carcinoma biomarkers. Comput Biol Med.
146:1055052022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paul A and Prasad Mukherjee D: Mitosis
detection for invasive breast cancer grading in histopathological
images. IEEE Transactions on Image Processing. 24:4041–4054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Veta M, van Diest PJ, Willems SM, Wang H,
Madabhushi A, Cruz-Roa A, Gonzalez F, Larsen AB, Vestergaard JS,
Dahl AB, et al: Assessment of algorithms for mitosis detection in
breast cancer histopathology images. Med Image Anal. 20:237–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ehteshami Bejnordi B, Veta M, Johannes van
Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak
JAWM; the CAMELYON16 Consortium, ; Hermsen M, Manson QF, et al:
Diagnostic assessment of deep learning algorithms for detection of
lymph node metastases in women with breast cancer. JAMA.
318:2199–2210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang CW, Lee YC, Calista E, Zhou F, Zhu H,
Suzuki R, Komura D, Ishikawa S and Cheng SP: A benchmark for
comparing precision medicine methods in thyroid cancer diagnosis
using tissue microarrays. Bioinformatics. 34:1767–1773. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shipp MA, Ross KN, Tamayo P, Weng AP,
Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, et
al: Diffuse large B-cell lymphoma outcome prediction by
gene-expression profiling and supervised machine learning. Nat Med.
8:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khan U, Shin H, Choi JP and Kim M:
wFDT-Weighted Fuzzy Decision Trees for prognosis of breast cancer
survivability. AusDM ‘08: Proceedings of the 7th Australasian Data
Mining Conference. Vol 87:pp141–152. 2008.
|
|
57
|
Fatakdawala H, Xu J, Basavanhally A,
Bhanot G, Ganesan S, Feldman M, Tomaszewski JE and Madabhushi A:
Expectation-maximization-driven geodesic active contour with
overlap resolution (EMaGACOR): application to lymphocyte
segmentation on breast cancer histopathology. IEEE Trans Biomed
Eng. 57:1676–1689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sertel O, Lozanski G, Shana'ah A and
Gurcan MN: Computer-aided detection of centroblasts for follicular
lymphoma grading using adaptive likelihood-based cell segmentation.
IEEE Trans Biomed Eng. 57:2613–2616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dundar MM, Badve S, Bilgin G, Raykar V,
Jain R, Sertel O and Gurcan MN: Computerized classification of
intraductal breast lesions using histopathological images. IEEE
Trans Biomed Eng. 58:1977–1984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tuominen VJ, Tolonen TT and Isola J:
ImmunoMembrane: A publicly available web application for digital
image analysis of HER2 immunohistochemistry. Histopathology.
60:758–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gertych A, Joseph AO, Walts AE and Bose S:
Automated detection of dual p16/Ki67 nuclear immunoreactivity in
liquid-based Pap tests for improved cervical cancer risk
stratification. Ann Biomed Eng. 40:1192–1204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Doyle S, Feldman MD, Shih N, Tomaszewski J
and Madabhushi A: Cascaded discrimination of normal abnormal, and
confounder classes in histopathology: Gleason grading of prostate
cancer. BMC Bioinformatics. 13:2822012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang H, Cruz-Roa A, Basavanhally A,
Gilmore H, Shih N, Feldman M, Tomaszewski J, Gonzalez F and
Madabhushi A: Mitosis detection in breast cancer pathology images
by combining handcrafted and convolutional neural network features.
J Med Imaging (Bellingham). 1:0340032014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lewis JS Jr, Ali S, Luo J, Thorstad WL and
Madabhushi A: A quantitative histomorphometric classifier (QuHbIC)
identifies aggressive versus indolent p16-positive oropharyngeal
squamous cell carcinoma. Am J Surg Pathol. 38:128–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sirinukunwattana K, Raza SEA, Tsang YW,
Snead DR, Cree IA and Rajpoot NM: A Spatially Constrained Deep
Learning Framework for Detection of Epithelial Tumor Nuclei in
Cancer Histology Images. Conference: 1st International Workshop on
Patch-based Techniques in Medical Imaging, Held in Conjunction with
MICCAI. 2015.doi: 10.1007/978-3-319-28194-0_19.
|
|
66
|
Yuan Y: Modelling the spatial
heterogeneity and molecular correlates of lymphocytic infiltration
in triple-negative breast cancer. J R Soc Interface.
12:201411532015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xie Y, Kong X, Xing F, Liu F, Su H and
Yang L: Deep Voting: A robust approach toward nucleus localization
in microscopy images. Med Image Comput Comput Assist Interv.
9351:374–382. 2015.PubMed/NCBI
|
|
68
|
Sirinukunwattana K, Ahmed Raza SE, Yee-Wah
Tsang, Snead DR, Cree IA and Rajpoot NM: Locality sensitive deep
learning for detection and classification of nuclei in routine
colon cancer histology images. IEEE Trans Med Imaging.
35:1196–1206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Romo-Bucheli D, Janowczyk A, Gilmore H,
Romero E and Madabhushi A: Automated tubule nuclei quantification
and correlation with oncotype DX risk categories in ER+ breast
cancer whole slide images. Sci Rep. 6:327062016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xu J, Xiang L, Liu Q, Gilmore H, Wu J,
Tang J and Madabhushi A: Stacked Sparse Autoencoder (SSAE) for
nuclei detection on breast cancer histopathology images. IEEE Trans
Med Imaging. 35:119–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Turkki R, Linder N, Kovanen PE, Pellinen T
and Lundin J: Antibody-supervised deep learning for quantification
of tumor-infiltrating immune cells in hematoxylin and eosin stained
breast cancer samples. J Pathol Inform. 7:382016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ali HR, Dariush A, Provenzano E, Bardwell
H, Abraham JE, Iddawela M, Vallier AL, Hiller L, Dunn JA, Bowden
SJ, et al: Computational pathology of pre-treatment biopsies
identifies lymphocyte density as a predictor of response to
neoadjuvant chemotherapy in breast cancer. Breast Cancer Res.
18:212016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bartsch G Jr, Mitra AP, Mitra SA, Almal
AA, Steven KE, Skinner DG, Fry DW, Lenehan PF, Worzel WP and Cote
RJ: Use of artificial intelligence and machine learning algorithms
with gene expression profiling to predict recurrent nonmuscle
invasive urothelial carcinoma of the bladder. J Urol. 195:493–498.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yu KH, Zhang C, Berry GJ, Altman RB, Ré C,
Rubin DL and Snyder M: Predicting non-small cell lung cancer
prognosis by fully automated microscopic pathology image features.
Nat Commun. 7:124742016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ali HR, Dariush A, Thomas J, Provenzano E,
Dunn J, Hiller L, Vallier AL, Abraham J, Piper T, Bartlett JMS, et
al: Lymphocyte density determined by computational pathology
validated as a predictor of response to neoadjuvant chemotherapy in
breast cancer: Secondary analysis of the ARTemis trial. Ann Oncol.
28:1832–1835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lu C, Lewis JS Jr, Dupont WD, Plummer WD
Jr, Janowczyk A and Madabhushi A: An oral cavity squamous cell
carcinoma quantitative histomorphometric-based image classifier of
nuclear morphology can risk stratify patients for disease-specific
survival. Mod Pathol. 30:1655–1665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gecer B, Aksoy S, Mercan E, Shapiro LG,
Weaver DL and Elmore JG: Detection and classification of cancer in
whole slide breast histopathology images using deep convolutional
networks. Pattern Recognit. 84:345–356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yoshida H, Shimazu T, Kiyuna T, Marugame
A, Yamashita Y, Cosatto E, Taniguchi H, Sekine S and Ochiai A:
Automated histological classification of whole-slide images of
gastric biopsy specimens. Gastric Cancer. 21:249–257. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bo L, Huang M, Zhang J, Li Y and Li R:
Gastric Pathology Image Recognition Based on Deep Residual
Networks. 2018 IEEE 42nd Annual Computer Software and Applications
Conference (COMPSAC). 408–412. 2018.
|
|
80
|
Ichimasa K, Kudo SE, Mori Y, Misawa M,
Matsudaira S, Kouyama Y, Baba T, Hidaka E, Wakamura K, Hayashi T,
et al: Artificial intelligence may help in predicting the need for
additional surgery after endoscopic resection of T1 colorectal
cancer. Endoscopy. 50:230–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mezheyeuski A, Bergsland CH, Backman M,
Djureinovic D, Sjöblom T, Bruun J and Micke P: Multispectral
imaging for quantitative and compartment-specific immune
infiltrates reveals distinct immune profiles that classify lung
cancer patients. J Pathol. 244:421–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mobadersany P, Yousefi S, Amgad M, Gutman
DA, Barnholtz-Sloan JS, Velázquez Vega JE, Brat DJ and Cooper LAD:
Predicting cancer outcomes from histology and genomics using
convolutional networks. Proc Natl Acad Sci USA. 115:E2970–E2979.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chaudhary K, Poirion OB, Lu L and Garmire
LX: Deep learning-based multi-omics integration robustly predicts
survival in liver cancer. Clin Cancer Res. 24:1248–1259. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Günakan E, Atan S, Haberal AN, Küçükyıldız
İA, Gökçe E and Ayhan A: A novel prediction method for lymph node
involvement in endometrial cancer: Machine learning. Int J Gynecol
Cancer. 29:320–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Turkki R, Byckhov D, Lundin M, Isola J,
Nordling S, Kovanen PE, Verrill C, von Smitten K, Joensuu H, Lundin
J, et al: Breast cancer outcome prediction with tumour tissue
images and machine learning. Breast Cancer Res Treat. 177:41–52.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fang M, Zhang W, Dong D, Zhou J and Tian
J: Predicting histopathological findings of gastric cancer via deep
generalized multi-instance learning. Proceedings of the SPIE. Vol
10949:id. 109491Q. pp62019.
|
|
87
|
Mori H and Miwa H: A histopathologic
feature of the behavior of gastric signet-ring cell carcinoma; an
image analysis study with deep learning. Pathol Int. 69:437–439.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Leon F, Gelvez M, Jaimes Z, Gelvez T and
Arguello H: Supervised Classification of Histopathological Images
Using Convolutional Neuronal Networks for Gastric Cancer Detection.
STSIVA; 2019, View Article : Google Scholar
|
|
89
|
Wang S, Wang T, Yang L, Yang DM, Fujimoto
J, Yi F, Luo X, Yang Y, Yao B, Lin S, et al: ConvPath: A software
tool for lung adenocarcinoma digital pathological image analysis
aided by a convolutional neural network. EBioMedicine. 50:103–110.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Aprupe L, Litjens G, Brinker TJ, van der
Laak J and Grabe N: Robust and accurate quantification of
biomarkers of immune cells in lung cancer micro-environment using
deep convolutional neural networks. PeerJ. 7:e63352019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dihge L, Vallon-Christersson J, Hegardt C,
Saal LH, Häkkinen J, Larsson C, Ehinger A, Loman N, Malmberg M,
Bendahl PO, et al: Prediction of lymph node metastasis in breast
cancer by gene expression and clinicopathological models:
Development and validation within a population-based cohort. Clin
Cancer Res. 25:6368–6381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Nir G, Davood K, Goldenberg SL, Fazli L,
Skinnider BF, Tavassoli P, Turbin D, Villamil CF, Wang G..Thompson
DJS, et al: Comparison of artificial intelligence techniques to
evaluate performance of a classifier for automatic grading of
prostate cancer from digitized histopathologic images. JAMA Netw
Open. 2:e1904422019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Courtiol P, Maussion C, Moarii M, Pronier
E, Pilcer S, Sefta M, Manceron P, Toldo S, Zaslavskiy M, Le Stang
N, et al: Deep learning-based classification of mesothelioma
improves prediction of patient outcome. Nat Med. 25:1519–1525.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Achi HE, Belousova T, Chen L, Wahed A,
Wang I, Hu Z, Kanaan Z, Rios A and Nguyen AND: Automated diagnosis
of lymphoma with digital pathology images using deep learning. Ann
Clin Lab Sci. 49:153–160. 2019.PubMed/NCBI
|
|
95
|
Rodner E, Bocklitz T, von Eggeling F,
Ernst G, Chernavskaia O, Popp J, Denzler J and Guntinas-Lichius O:
Fully convolutional networks in multimodal nonlinear microscopy
images for automated detection of head and neck carcinoma: Pilot
study. Head Neck. 41:116–121. 2019.PubMed/NCBI
|
|
96
|
Hu L, Bell D, Antani S, Xue Z, Yu K,
Horning MP, Gachuhi N, Wilson B, Jaiswal MS, Befano B, et al: An
observational study of deep learning and automated evaluation of
cervical images for cancer screening. J Natl Cancer Inst.
111:923–932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tian R, Cui Z, He D, Tian X, Gao Q, Ma X,
Yang JR, Wu J, Das BC, Severinov K, et al: Risk stratification of
cervical lesions using capture sequencing and machine learning
method based on HPV and human integrated genomic profiles.
Carcinogenesis. 40:1220–1228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Newman AM, Steen CB, Liu CL, Gentles AJ,
Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA,
Steiner D, et al: Determining cell type abundance and expression
from bulk tissues with digital cytometry. Nat Biotechnol.
37:773–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Saillard C, Schmauch B, Laifa O, Moarii M,
Toldo S, Zaslavskiy M, Pronier E, Laurent A, Amaddeo G, Regnault H,
et al: Predicting survival after hepatocellular carcinoma resection
using deep learning on histological slides. Hepatology.
72:2000–2013. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Dietz C, Rueden CT, Helfrich S, Dobson
ETA, Horn M, Eglinger J, Evans EL III, McLean DT, Novitskaya T,
Ricke WA, et al: Integration of the ImageJ Ecosystem in the KNIME
Analytics Platform. Front Comput Sci. 2:82020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bulten W, Pinckaers H, van Boven H, Vink
R, de Bel T, van Ginneken B, van der Laak J, Hulsbergen-van de Kaa
C and Litjens G: Automated deep-learning system for Gleason grading
of prostate cancer using biopsies: A diagnostic study. Lancet
Oncol. 21:233–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Han W, Johnson C, Gaed M, Gómez JA, Moussa
M, Chin JL, Pautler S, Bauman GS and Ward AD: Histologic tissue
components provide major cues for machine learning-based prostate
cancer detection and grading on prostatectomy specimens. Sci Rep.
10:99112020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jaber MI, Song B, Taylor C, Vaske CJ, Benz
SC, Rabizadeh S, Soon-Shiong P and Szeto CW: A deep learning
image-based intrinsic molecular subtype classifier of breast tumors
reveals tumor heterogeneity that may affect survival. Breast Cancer
Res. 22:122020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Valieris R, Amaro L, Osório CABT, Bueno
AP, Rosales Mitrowsky RA, Carraro DM, Nunes DN, Dias-Neto E and
Silva ITD: Deep learning predicts underlying features on pathology
images with therapeutic relevance for breast and gastric cancer.
Cancers (Basel). 12:36872020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Skrede OJ, De Raedt S, Kleppe A, Hveem TS,
Liestøl K, Maddison J, Askautrud HA, Pradhan M, Nesheim JA,
Albregtsen F, et al: Deep learning for prediction of colorectal
cancer outcome: A discovery and validation study. Lancet.
395:350–360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Reichling C, Taieb J, Derangere V,
Klopfenstein Q, Le Malicot K, Gornet JM, Becheur H, Fein F,
Cojocarasu O, Kaminsky MC, et al: Artificial intelligence-guided
tissue analysis combined with immune infiltrate assessment predicts
stage III colon cancer outcomes in PETACC08 study. Gut. 69:681–690.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bao H, Sun X, Zhang Y, Pang B, Li H, Zhou
L, Wu F, Cao D, Wang J, Turic B, et al: The artificial
intelligence-assisted cytology diagnostic system in large-scale
cervical cancer screening: A population-based cohort study of 0.7
million women. Cancer Med. 9:6896–6906. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xu-Monette ZY, Zhang H, Zhu F, Tzankov A,
Bhagat G, Visco C, Dybkaer K, Chiu A, Tam W, Zu Y, et al: A refined
cell-of-origin classifier with targeted NGS and artificial
intelligence shows robust predictive value in DLBCL. Blood Adv.
4:3391–3404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Foersch S, Eckstein M, Wagner DC, Gach F,
Woerl AC, Geiger J, Glasner C, Schelbert S, Schulz S, Porubsky S,
et al: Deep learning for diagnosis and survival prediction in soft
tissue sarcoma. Ann Oncol. 32:1178–1187. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lagree A, Shiner A, Alera MA, Fleshner L,
Law E, Law B, Lu FI, Dodington D, Gandhi S, Slodkowska EA, et al:
Assessment of digital pathology imaging biomarkers associated with
breast cancer histologic grade. Curr Oncol. 28:4298–4316. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bychkov D, Linder N, Tiulpin A, Kücükel H,
Lundin M, Nordling S, Sihto H, Isola J, Lehtimäki T,
Kellokumpu-Lehtinen PL, et al: Deep learning identifies
morphological features in breast cancer predictive of cancer ERBB2
status and trastuzumab treatment efficacy. Sci Rep. 11:40372021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li F, Yang Y, Wei Y, He P, Chen J, Zheng Z
and Bu H: Deep learning-based predictive biomarker of pathological
complete response to neoadjuvant chemotherapy from histological
images in breast cancer. J Transl Med. 19:3482021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chakraborty D, Ivan C, Amero P, Khan M,
Rodriguez-Aguayo C, Başağaoğlu H and Lopez-Berestein G: Explainable
artificial intelligence reveals novel insight into tumor
microenvironment conditions linked with better prognosis in
patients with breast cancer. Cancers (Basel). 13:34502021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fitzgerald J, Higgins D, Mazo Vargas C,
Watson W, Mooney C, Rahman A, Aspell N, Connolly A, Aura Gonzalez C
and Gallagher W: Future of biomarker evaluation in the realm of
artificial intelligence algorithms: Application in improved
therapeutic stratification of patients with breast and prostate
cancer. J Clin Pathol. 74:429–434. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ayyad SM, Shehata M, Shalaby A, Abou
El-Ghar M, Ghazal M, El-Melegy M, Abdel-Hamid NB, Labib LM, Ali HA
and El-Baz A: Role of AI: histopathological images in detecting
prostate cancer: A survey. Sensors (Basel). 21:25862021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Haggenmüller S, Maron RC, Hekler A, Utikal
JS, Barata C, Barnhill RL, Beltraminelli H, Berking C,
Betz-Stablein B, Blum A, et al: Skin cancer classification via
convolutional neural networks: Systematic review of studies
involving human experts. Eur J Cancer. 156:202–216. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kiehl L, Kuntz S, Höhn J, Jutzi T,
Krieghoff-Henning E, Kather JN, Holland-Letz T, Kopp-Schneider A,
Chang-Claude J, Brobeil A, et al: Deep learning can predict lymph
node status directly from histology in colorectal cancer. Eur J
Cancer. 157:464–473. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yamashita R, Long J, Longacre T, Peng L,
Berry G, Martin B, Higgins J, Rubin DL and Shen J: Deep learning
model for the prediction of microsatellite instability in
colorectal cancer: A diagnostic study. Lancet Oncol. 22:132–141.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Krause J, Grabsch HI, Kloor M, Jendrusch
M, Echle A, Buelow RD, Boor P, Luedde T, Brinker TJ, Trautwein C,
et al: Deep learning detects genetic alterations in cancer
histology generated by adversarial networks. J Pathol. 254:70–79.
2021.PubMed/NCBI
|
|
120
|
Saito A, Toyoda H, Kobayashi M, Koiwa Y,
Fujii H, Fujita K, Maeda A, Kaneoka Y, Hazama S, Nagano H, et al:
Prediction of early recurrence of hepatocellular carcinoma after
resection using digital pathology images assessed by machine
learning. Mod Pathol. 34:417–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yamashita R, Long J, Saleem A, Rubin DL
and Shen J: Deep learning predicts postsurgical recurrence of
hepatocellular carcinoma from digital histopathologic images. Sci
Rep. 11:20472021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yang H, Chen L, Cheng Z, Yang M, Wang J,
Lin C, Wang Y, Huang L, Chen Y, Peng S, et al: Deep learning-based
six-type classifier for lung cancer and mimics from
histopathological whole slide images: A retrospective study. BMC
Med. 19:802021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen CL, Chen CC, Yu WH, Chen SH, Chang
YC, Hsu TI, Hsiao M, Yeh CY and Chen CY: An annotation-free
whole-slide training approach to pathological classification of
lung cancer types using deep learning. Nat Commun. 12:11932021.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Park J, Jang BG, Kim YW, Park H, Kim BH,
Kim MJ, Ko H, Gwak JM, Lee EJ, Chung YR, et al: A prospective
validation and observer performance study of a deep learning
algorithm for pathologic diagnosis of gastric tumors in endoscopic
biopsies. Clin Cancer Res. 27:719–728. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang X, Chen Y, Gao Y, Zhang H, Guan Z,
Dong Z, Zheng Y, Jiang J, Yang H, Wang L, et al: Predicting gastric
cancer outcome from resected lymph node histopathology images using
deep learning. Nat Commun. 12:16372021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Muti HS, Heij LR, Keller G, Kohlruss M,
Langer R, Dislich B, Cheong JH, Kim YW, Kim H, Kook MC, et al:
Development and validation of deep learning classifiers to detect
Epstein-Barr virus and microsatellite instability status in gastric
cancer: A retrospective multicentre cohort study. Lancet Digit
Health. 3:e654–e664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yan J, Zhao Y, Chen Y, Wang W, Duan W,
Wang L, Zhang S, Ding T, Liu L, Sun Q, et al: Deep learning
features from diffusion tensor imaging improve glioma
stratification and identify risk groups with distinct molecular
pathway activities. EBioMedicine. 72:1035832021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bao H, Wang Z, Ma X, Guo W, Zhang X, Tang
W, Chen X, Wang X, Chen Y, Mo S, et al: Letter to the Editor: An
ultra-sensitive assay using cell-free DNA fragmentomics for
multi-cancer early detection. Mol Cancer. 21:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Duchmann M, Wagner-Ballon O, Boyer T,
Cheok M, Fournier E, Guerin E, Fenwarth L, Badaoui B, Freynet N,
Benayoun E, et al: Machine learning identifies the independent role
of dysplasia in the prediction of response to chemotherapy in AML.
Leukemia. 36:656–663. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yen R, Grasedieck S, Wu A, Lin H, Su J,
Rothe K, Nakamoto H, Forrest DL, Eaves CJ and Jiang X:
Identification of key microRNAs as predictive biomarkers of
Nilotinib response in chronic myeloid leukemia: A sub-analysis of
the ENESTxtnd clinical trial. Leukemia. 36:2443–2452. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Laukhtina E, Schuettfort VM, D'Andrea D,
Pradere B, Quhal F, Mori K, Sari Motlagh R, Mostafaei H, Katayama
S, Grossmann NC, et al: Selection and evaluation of preoperative
systemic inflammatory response biomarkers model prior to
cytoreductive nephrectomy using a machine-learning approach. World
J Urol. 40:747–754. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Cheng N, Ren Y, Zhou J, Zhang Y, Wang D,
Zhang X, Chen B, Liu F, Lv J, Cao Q, et al: Deep Learning-based
classification of hepatocellular nodular lesions on whole-slide
histopathologic images. Gastroenterology. 162:1948–1961. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kleppe A, Skrede OJ, De Raedt S, Hveem TS,
Askautrud HA, Jacobsen JE, Church DN, Nesbakken A, Shepherd NA,
Novelli M, et al: A clinical decision support system optimising
adjuvant chemotherapy for colorectal cancers by integrating deep
learning and pathological staging markers: A development and
validation study. Lancet Oncol. 23:1221–1232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Byeon SJ, Park J, Cho YA and Cho BJ:
Automated histological classification for digital pathology images
of colonoscopy specimen via deep learning. Sci Rep. 12:128042022.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Feng L, Liu Z, Li C, Li Z, Lou X, Shao L,
Wang Y, Huang Y, Chen H, Pang X, et al: Development and validation
of a radiopathomics model to predict pathological complete response
to neoadjuvant chemoradiotherapy in locally advanced rectal cancer:
A multicentre observational study. Lancet Digit Health. 4:e8–e17.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ding K, Zhou M, Wang H, Zhang S and
Metaxas DN: Spatially aware graph neural networks and cross-level
molecular profile prediction in colon cancer histopathology: A
retrospective multi-cohort study. Lancet Digit Health. 4:e787–e795.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang R, Dai W, Gong J, Huang M, Hu T, Li
H, Lin K, Tan C, Hu H, Tong T, et al: Development of a novel
combined nomogram model integrating deep learning-pathomics,
radiomics and immunoscore to predict postoperative outcome of
colorectal cancer lung metastasis patients. J Hematol Oncol.
15:112022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sundar R, Barr Kumarakulasinghe N, Huak
Chan Y, Yoshida K, Yoshikawa T, Miyagi Y, Rino Y, Masuda M, Guan J,
Sakamoto J, et al: Machine-learning model derived gene signature
predictive of paclitaxel survival benefit in gastric cancer:
Results from the randomised phase III SAMIT trial. Gut. 71:676–685.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Pfob A, Sidey-Gibbons C, Rauch G, Thomas
B, Schaefgen B, Kuemmel S, Reimer T, Hahn M, Thill M, Blohmer JU,
et al: Intelligent Vacuum-assisted biopsy to identify breast cancer
patients with pathologic complete response (ypT0 and ypN0) after
neoadjuvant systemic treatment for omission of breast and axillary
surgery. J Clin Oncol. 40:1903–1915. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Prat A, Guarneri V, Pascual T,
Brasó-Maristany F, Sanfeliu E, Paré L, Schettini F, Martínez D,
Jares P, Griguolo G, et al: Development and validation of the new
HER2DX assay for predicting pathological response and survival
outcome in early-stage HER2-positive breast cancer. EBioMedicine.
75:1038012022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Farinella F, Merone M, Bacco L, Capirchio
A, Ciccozzi M and Caligiore D: Machine Learning analysis of
high-grade serous ovarian cancer proteomic dataset reveals novel
candidate biomarkers. Sci Rep. 12:30412022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Park S, Ock CY, Kim H, Pereira S, Park S,
Ma M, Choi S, Kim S, Shin S, Aum BJ, et al: Artificial
Intelligence-powered spatial analysis of tumor-infiltrating
lymphocytes as complementary biomarker for immune checkpoint
inhibition in Non-Small-Cell lung cancer. J Clin Oncol.
40:1916–1928. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Cheng G, Zhang F, Xing Y, Hu X, Zhang H,
Chen S, Li M, Peng C, Ding G, Zhang D, et al: Artificial
intelligence-assisted score analysis for predicting the expression
of the immunotherapy biomarker PD-L1 in lung cancer. Front Immunol.
13:8931982022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Vanguri RS, Luo J, Aukerman AT, Egger JV,
Fong CJ, Horvat N, Pagano A, Araujo-Filho JAB, Geneslaw L, Rizvi H,
et al: Multimodal integration of radiology, pathology and genomics
for prediction of response to PD-(L)1 blockade in patients with
non-small cell lung cancer. Nat Cancer. 3:1151–1164. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu Y, Jia Y, Hou C, Li N, Zhang N, Yan X,
Yang L, Guo Y, Chen H, Li J, et al: Pathological prognosis
classification of patients with neuroblastoma using computational
pathology analysis. Comput Biol Med. 149:1059802022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Foersch S, Glasner C, Woerl AC, Eckstein
M, Wagner DC, Schulz S, Kellers F, Fernandez A, Tserea K, Kloth M,
et al: Multistain deep learning for prediction of prognosis and
therapy response in colorectal cancer. Nat Med. 29:430–439. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Liu XP, Jin X, Seyed Ahmadian S, Yang X,
Tian SF, Cai YX, Chawla K, Snijders AM, Xia Y, van Diest PJ, et al:
Clinical significance and molecular annotation of cellular
morphometric subtypes in lower-grade gliomas discovered by machine
learning. Neuro Oncol. 25:68–81. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Cao R, Nelson SD, Davis S, Liang Y, Luo Y,
Zhang Y, Crawford B and Wang LV: Label-free intraoperative
histology of bone tissue via deep-learning-assisted ultraviolet
photoacoustic microscopy. Nat Biomed Eng. 7:124–134. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wu S, Hong G, Xu A, Zeng H, Chen X, Wang
Y, Luo Y, Wu P, Liu C, Jiang N, et al: Artificial
intelligence-based model for lymph node metastases detection on
whole slide images in bladder cancer: A retrospective, multicentre,
diagnostic study. Lancet Oncol. 24:360–370. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Barnett R: Lung cancer. Lancet.
390:9282017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zheng Y, Gindra RH, Green EJ, Burks EJ,
Betke M, Beane JE and Kolachalama VB: A Graph-transformer for whole
slide image classification. IEEE Trans Med Imaging. 41:3003–3015.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Qiao C, Li D, Liu Y, Zhang S, Liu K, Liu
C, Guo Y, Jiang T, Fang C, Li N, et al: Rationalized deep learning
super-resolution microscopy for sustained live imaging of rapid
subcellular processes. Nat Biotechnol. 41:367–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Vo TH, Nguyen NTK, Kha QH and Le NQK: On
the road to explainable AI in drug-drug interactions prediction: A
systematic review. Comput Struct Biotechnol J. 20:2112–2123. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cheng JY, Abel JT, Balis UGJ, McClintock
DS and Pantanowitz L: Challenges in the development, deployment,
and regulation of artificial intelligence in anatomic pathology. Am
J Pathol. 191:1684–1692. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Schmidt C: M. D. Anderson Breaks with IBM
Watson, Raising Questions about artificial intelligence in
oncology. J Natl Cancer Inst. 1092017.doi: 10.1093/jnci/djx113.
|
|
156
|
Lecler A, Duron L and Soyer P:
Revolutionizing radiology with GPT-based models: Current
applications, future possibilities and limitations of ChatGPT.
Diagn Interv Imaging. 104:269–274. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Will ChatGPT transform healthcare? Nat
Med. 29:505–506. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Sinha RK, Deb Roy A, Kumar N and Mondal H:
Applicability of ChatGPT in assisting to solve higher order
problems in pathology. Cureus. 15:e352372023.PubMed/NCBI
|
|
159
|
Chauhan C and Gullapalli RR: Ethics of AI
in pathology: Current paradigms and emerging issues. Am J Pathol.
91:1673–1683. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Warnat-Herresthal S, Schultze H, Shastry
KL, Manamohan S, Mukherjee S, Garg V, Sarveswara R, Händler K,
Pickkers P, Aziz NA, et al: Swarm Learning for decentralized and
confidential clinical machine learning. Nature. 594:265–270. 2021.
View Article : Google Scholar : PubMed/NCBI
|