Introduction
Colorectal cancer (CRC), the third most common and
second deadliest cancer worldwide with an incidence rate of 25.9
and a mortality rate 11.8/100,000 individuals in 2020 (1), is a progressive, multistep disease
that develops following phenotypical progression from adenoma to
invasive adenocarcinoma. Although environmental and genetic risk
factors have been identified (2),
the etiology of CRC is unknown. Among environmental factors,
inflammatory bowel disease is associated with higher CRC risk
(3) and higher inflammatory
activity is associated with worse prognosis (4). Of clinical relevance, higher levels
of circulating inflammatory markers C-reactive protein, IL-6), and
chitinase-3-like-1 protein have been found in patients with CRC
compared with controls and increase risk of complications after
tumor resection in older patients with CRC (5). The role of inflammation is indicated
by evidence that long-term use of aspirin or other non-steroidal
anti-inflammatory drugs decreases CRC risk (6-9).
A causal link between inflammation and cancer was
suggested in 1863 by Rudolf Virchow, who noted that cancer
originates at sites of inflammation (10). Inflammation is considered one of
the major drivers of tumor transformation and progression in CRC
(11-13). Cellular inflammation is initiated
by activation of phospholipase A2, which releases arachidonic acid
(AA) from membrane phospholipids. AA is a polyunsaturated 20-carbon
fatty acid metabolized by either the cyclooxygenase (COX) or the
lipoxygenase (LO) pathways to generate powerful inflammatory
mediators called eicosanoids, which comprise prostaglandins,
thromboxanes and leukotrienes. Eicosanoids serve as
autocrine/paracrine regulators of inflammation and modulate
cancer-associated physiopathological responses, including
suppression of immune surveillance and enhanced proliferation and
invasiveness of tumor cells (14).
A series of studies indicate that abnormal AA
metabolism is involved in cardiovascular and metabolic disease
(15) as well as in carcinogenesis
(16). Overexpression of COX-2 and
5-LO has been reported in several human malignancies, including CRC
(17). High COX-2 and 5-LO
expression is associated with more aggressive tumor phenotype and
poor survival (17,18). COX-2 can be induced by inflammatory
cytokines such as IL-1β, TNF-α and IL-6, which are abundantly
expressed in CRC (19). Expression
of 5-LO is tightly regulated by promoter methylation (20) and is induced by the demethylating
compound 5-aza-2′-deoxycytidine in cancer cell lines (21) or stimulation with
lipopolysaccharide, TGFβ and/or 1,25(OH)2D3 in monocytes
and differentiating macrophages (20,22).
Several anti-inflammatory agents have anti-cancer
properties (23). Long-term
treatment with COX-2 inhibitors significantly decreases risk of
many malignancies, including breast (24), lung (25) and prostate cancer (26,27)
and CRC (28). Similarly,
prolonged use of non-specific COX inhibitor aspirin lowers the risk
of colorectal adenoma (29) and
CRC (30-33), being especially effective in cases
with PIK3CA mutations (34) that
are found in 10-15% of all CRC cases. Inhibition of 5-LO triggers
apoptosis in breast, lung, prostate and colon cancer cell lines
(35) and combined use of COX-2
and 5-LO inhibitors have additive anti-CRC effects (36).
Emerging evidence indicates an important role of
human cytomegalovirus (HCMV) in cancers of different origins,
including CRC (37). HCMV, a
β-herpes virus with a 83% worldwide prevalence in 2019 (38), exhibits a complex genome encoding
>750 RNAs and 200 proteins, most of which promote viral
persistence and replication via dysregulation of cellular and
immunological functions (39).
Only ~50 viral proteins are considered to be essential for viral
replication (40). Studies
reported a high prevalence of HCMV antigens and/or DNA in various
types of cancer, including CRC (18,41,42),
breast (43) and prostate
(44) cancer, medulloblastoma
(45), neuroblastoma (46) and glioblastoma (47). HCMV DNA and/or protein is generally
not detectable or is expressed at low levels in adjacent
non-neoplastic tissue, which raises the question of the relevance
of this virus in tumor biology.
HCMV infection is generally asymptomatic but may
cause severe disease in immunocompromised patients. Following
primary infection, the virus establishes latency in myeloid cells,
where it can be reactivated by inflammation (48,49).
This may be autonomously promoted via virus-encoded chemokine
receptor homologue US28, an inducer of the NF-κB/COX-2/VEGF axis
(50,51). Our previous study showed that HCMV
infection induces expression of 5-LO and production of leukotriene
B4 (LTB4, 52). Our previous study reported that HCMV induces COX-2
and 5-LO expression in breast cancer cells (53) and HCMV infection is associated with
COX-2 and 5-LO expression in breast tumors and poor patient outcome
(54); there is also a correlatin
between presence of HCMV proteins and 5-LO expression in borderline
ovarian tumor (55). Furthermore,
COX-2 inhibitor celecoxib (CCX), in combination with the antiviral
drug valganciclovir (VGCV), decreases growth of HCMV-positive
medulloblastoma in a xenograft model and inhibits prostaglandin E2
(PGE2) production (45).
In 2002, Harkins et al (18) analyzed colorectal adenoma, CRC and
adjacent non-neoplastic mucosa samples from 29 patients and found
that the early and late HCMV proteins immediate early 1-72 (IE1-72)
and pp65 were present in 82 and 78% of the adenomas and 80 and 92%
of the adenocarcinomas, respectively. The presence of HCMV in
cancer cells was confirmed by detection of HCMV DNA by in
situ hybridization (ISH) and PCR. Follow up studies have
reported that HCMV proteins are frequently present in CRC and HCMV
DNA is found in tumors but rarely in non-tumorous mucosa (41,56).
Meta-analyses suggest an increased risk of CRC in patients with
HCMV (56,57). In older patients, HCMV is linked to
worse outcome, regardless of stage (58) and high levels of HCMV-specific
immunostaining in primary tumors are correlated with shorter
survival and risk of brain metastasis (54). The relationship between CRC and
inflammation is consistent with a potential effect of HCMV in
colorectal tumorigenesis. HCMV US28 induces intestinal tumors in
transgenic mice (59) and HCMV
infection induces BCL-2 and COX-2 protein expression in Caco-2
cells (18).
The present study aimed to analyze the expression of
IE and late HCMV protein pp65, together with 5-LO and COX-2 by
immunohistochemstry, in a pathologically and genetically
characterized tissue microarray (TMA) series of 146 CRC cases. It
was hypothesized that HCMV is a major driver of inflammation in CRC
and a possible target of therapy.
Materials and methods
CRC cases
The study was part of 'Epidemiology of colorectal
cancer and its risk factors in Iran', approved by the Institutional
Review Board of the Digestive Disease Research Center, Tehran
University of Medical Sciences, Tehran, Iran (approval no.
FWA00001331/DHHS-IRB00001641; March 17, 2004). The series of 146
individual, formalin-fixed, paraffin-embedded CRC cores were
derived from patients who underwent surgical resection from
February 1998 to September 2003 in two major hospitals (Atieh
Hospital and Mehr General Hospital), in Tehran, Iran (median age at
surgery 53 years; range 20-80 years). Patients included 50 (42.4%)
females and 60 (57.6%) males. The cases were previously
characterized for Duke's staging, differentiation grade
(poor/moderate/well-differentiated), microsatellite instability
(MSI)/microsatellite stable (MSS) status, tumor-associated KRAS and
TP53 gene mutations (60,61) and Ki-67 and EGFR immunostaining
(62). TMA blocks (2 mm cores
representative of morphologically relevant areas containing ≥50%
tumor cells) were obtained at the Pathology Section of the Center
for Advanced Studies and Technology, G. d'Annunzio University,
Chieti, Italy, as previously described (63) and CRC diagnosis was confirmed after
pathological review. Patients had no history of inflammatory bowel
disease, familial adenomatous polyposis or hereditary nonpolyposis
CRC and had not undergone preoperative chemotherapy or
radiotherapy. Loss of cores due to progressive exhaustion after
sectioning account for variations of the total number of cases
stained for each marker.
Immunohistochemical staining
TMA blocks were serially cut at a diameter of 5
μm and immunostained as previously reported (54,55,64).
Sections were deparaffinized in xylene (Sigma Aldrich; Merck KGaA),
rehydrated in descending ethanol gradient and washed in TBST (0.1%
Tween 20, SigmaAldrich; Merck KGaA). Antigen retrieval was
performed by heating in Citra plus pH 6.0 buffer in a pressure
cooker (Biocare Medical, LLC) at 95°C for 20 min. Endogenous
peroxidase was blocked with 3% H2O2 (Histolab
Products AB) for 15 min at room temperature in the dark. Endogenous
avidin and biotin were neutralized using Avidin-Biotin Blocking kit
(Dako; Agilent Technologies, Inc.). Slides were incubated in
Protein Block (Dako; Agilent Technologies, Inc.) for 30 min at room
temperature, then overnight at 4°C with primary antibodies against
HCMV IE (1:300; Cat. No. MAB819R, Merck KGaA), pp65 (1:100; Cat.
No. NCL-CMVpp65, Leica Biosystem), COX-2 (1:400; Cat. No. 12282,
Cell Signaling Technology, Inc.), 5-LO (1:200, Cat. No. ab169755
Abcam) diluted in antibody diluting buffer (BioGenex Laboratories).
The slides were washed in TBST, incubated with secondary anti-mouse
(1:20; Cat. No. HK325-UM, BioGenex Laboratories) or anti-rabbit
(1:20; Cat. No. HK326-UR, BioGenex Laboratories) antibody for 30
min at room temperature, then washed again in TBST and incubated
with streptavidin-biotin-peroxidase complex for up to 5 min at room
temperature (1:20; cat. No. HK320-UK,BioGenex Laboratories).
Hematoxylin (ready to use, Histolab) was used for counterstaining
for up to 10 seconds at room temperature. HCMV-infected human lung
and HCMV-negative human tonsil (positive and negative controls,
respectively). To assess cancer specificity, HCMV IE, pp65, COX-2
and 5-LO immunostaining was performed on five individual
non-tumorous colorectal mucosa samplesrun in parallel to the CRC
cases. The non-tumorous colorectal mucosa samples were previously
collected at the Department of Molecular Medicine and Surgery at
Karolinska Institutet and ethical approval was granted by Regional
Human Ethics Committee, Stockholm, Sweden (approval no2008/628-31).
Isotype controls for primary antibodies targeting HCMV-IE, HCMV
pp65, COX-2 and 5-LO were also run in all experiments. Slides
stained for Ki-67 and EGFR as previously described (62) were re-analyzed, with the newly
stained tissues. Serial sections were digitally scanned using a
Hamamatsu Nano Zoomer-XR Digital slide scanner (Hamamatsu Photonics
K.K.) and shared for analysis at CAST and Bioclinicum Karolinska
Institutet using Nano Zoomer Digital Pathology (NDP) viewer
software (Cat. No. U12388-01; Version NDP. view2 Viewing,
Hamamatsu). Digital sections were evaluated independently by a
pathologist and senior researcher to assess percentages of cells
expressing HCMV IE, pp65, COX-2, 5-LO, KI-67 and EGFR proteins. The
scoring system was as follows: Negative (0%), 1 (<25%), 2
(25-50%), 3 (51-75%) and 4 (>75%). Some tissue cores were lost
during the staining procedure.
Cell culture
Caco-2 (cat. no. HTB-37) and LS-174T (cat. no.
CL-188) cells, selected to represent a differentiation-competent
and poorly differentiated, highly invasive human CRC cell line
(65), were acquired from American
Type Culture Collection (ATCC) and cultured in RPMI-1640 (Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA) and 100 U/ml penicillin and
streptomycin at 37°C in 5% CO2/95% air. Cells were
infected at 50% confluency with HCMV strain VR1814 at multiplicity
of infection (MOI) 5 and were collected for analysis at 1, 3 and 7
days post-infection (dpi). Mock-infected cells were used as
controls.
In vitro treatment
A total of ~1.5×105 Caco-2 and LS-174T
cells were plated in 6-well plates in the presence or absence of
100 μM ganciclovir (GCV; Roche Diagnostics) and/or 10
μM CCX (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C, then
infected with HCMV strain VR1814 at MOI 5 or mock infected (cell
culture medium not containing viral particles). GCV was used
instead of its pro-drug form VGCV, as VGCV needs to be hydrolyzed
into GCV to exert its antiviral activity. This is mediated by cells
in in the liver. In vivo, GCV has to be administered
intravenously/intraperitoneally while VGCV is taken perorally. GCV
is already active and the only suitable drug form for in
vitro experiments and has been used in multiple in vitro
studies (66,67). At 1, 3 and 7 dpi, the cells were
lysed and collected with TRIzol (Thermo Fisher Scientific, Inc.).
Samples were stored at -80°C for up to 1 month.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RNA of treated cells was extracted using Trizol
manufacturer's protocol (Thermo Fisher Scientific, Inc.). RNA
concentration was measured using Nanodrop (Thermo Fisher
Scientific, Inc.).
A total of ~1 μg RNA was used for cDNA
synthesis using SuperScript™ III First-Strand Synthesis System
according to manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.) and reactions were primed with random hexamers.
Expression of target genes was assessed by TaqMan PCR using primers
from Thermo Fisher Scientific, Inc. as follows: IE forward, 5′-GTG
ACC CAT GTG CTT ATG ACT CTA T-3′ and reverse, 5′-CTC AAC ATA GTC
TGC AGG AAC GT-3′, probe FAM-TTG GTC ACG GGT GTC TC), HCMV tegument
protein pp65 (on-demand, forward primer 5′-CCC AGC GTG ACG TGC ATA
A-3′ and reverse primer 5′-AGG TGT ACC TGG AGT CCT TCT G-3′, probe
FAM-CTC CGG CAA GCT CT), COX-2 (sequence not available; Cat. No.
hs00153133.m1) and 5-LO (sequence not available; Cat. No.
hs00167536.m1). PCR mixes were prepared using the Applied
Biosystems™ TaqMan™ Fast Universal PCR Master Mix (cat. No.
10702697, Applied Biosystems, Thermo Fisher Scientific, Inc.). The
7900HT Fast Real-Time PCR System (Cat. No. 4329001, Applied
Biosystems, Thermo Fisher Scientific, Inc.) was used to process the
PCR plates with the thermocyling conditions set according to the
manufacturer instruction (denaturation at 95°C for 20 seconds,
annealing 95°C for 1 second, extension 60°C for 20 seconds, 40
cycles). The results were analyzed with SDS version 2.4 software
(Cat. No. 4350490, Applied Biosystem). The 2-ΔΔCq method (68) was used to quantify the relative
expression of the targets normalized to the housekeeping gene human
β2-microglobulin (sequence not available; cat. No.
hs00187842.m1).
Immunofluorescence (IF)
HCMV-infected CRC cells LS-174T and Caco-2 were
seeded at a density of 104 cells/well and cultured
overnight on sterile eight-well chamber glass slides to allow cells
to attach, fixed with ice-cold methanol:acetone (1:1) for 20 min at
-20°C, rinsed with PBS and incubated with Dako protein Blocker
(ready to use; Agilent) and Fc receptor blocker (Innovex
Biosciences Inc.; both 30 min at room temperature). After blocking,
cells were incubated overnight at 4°C with rabbit anti-human 5-LO
(1:500; Cat. No. ab169755, Abcam), anti-COX-2 (1:500; Cat. No.
12282, Cell Signaling Technology, Inc.) and mouse anti-HCMV IE
(1:1,000; Cat. No. 11-003; Biomerieux). The primary antibodies were
detected by incubating cells with secondary antibodies (Alexa Fluor
594 donkey anti-mouse; Cat. No. R37115 and Alexa Fluor 488 donkey
anti-rabbit Cat. No. A-21206; both 1:500; both Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h and DAPI for 5
min at room temperature (Sigma-Aldrich; Merck KGaA). After mounting
slides with fluorescence mounting medium (Dako Cytomation),
confocal microscopy at 20× was performed using Zeiss LSM 700
confocal microscope. Rabbit IgG1 isotype control (1:300; Cat. No.
AB-105-C, R&D Systems, Inc.) and mouse monoclonal IgG2a (1:300,
Cat. No. ab190463 Abcam) served as negative controls.
MTT assay
A total of ~3×103 LS-174T and Caco-2
cells were seeded onto 96-well plates and assayed for proliferation
at 1, 3 and 7 dpi, either with HCMV VR1814 at MOI 5 or mock, using
MTT kit (Cat. No. 11465007001; Sigma Aldrich) according to the
manufacturer's protocol. Absorbance was read at 570 nm with a
reference wavelength of 670 nm with a Versa Max Plate reader
(Molecular Devices, LLC).
Viral infectivity assay
LS-174T, Caco-2 and MRC5 cells (ATCC; cat. no.
CCL-171) were seeded in a six-well plate at 105
cells/well and incubated at 37°C. The MRC-5 cells were used as a
positive control for the viral infectivity assay since they are
used in routine virus propagation protocols, microbiological
diagnostic, and sustain a lytic infection of HCMV in which
particles are produced (69,70).
RPMI 1640 (Thermo Fisher Scientific, Inc.) was used to culture
LS-174T, Caco-2 and EMEM (Sigma-Aldrich) supplemented with 10%
fetal bovine serum (Sigma-Aldrich; Merck KGaA) and 100 U/ml
penicillin and streptomycin was used for MRC-5 cells.
At 24 h after seeding, cells were infected either
with HCMV-VR1814 at MOI 5 for colon cancer cell lines and MOI 1 for
MRC5 or with mock controls. At 7 dpi, supernatants were harvested
from HCMV-infected and mock-infected LS-174T, Caco-2 and MRC5 cells
and underwent centrifugation at 1,200 g for 10 min at room
temperature. A total of 2 ml supernatant from one well of infected
MRC5 or colon cancer cells was used to directly infect for 6 h at
37°C in a cell culture incubator a chamber slide of uninfected MRC5
cells seeded 24 h prior at a density of 2×103
cells/well. Supernatant from infected MRC5 cells was incubated in a
humidified chamber at 37°C with 5% CO2 for 6 h. Cells
were washed three times with sterile PBS to remove the
supernatants. Fresh RPMI for LS-174T, Caco-2 and EMEM for MRC-5 was
added to each well to a total volume of 0.5 ml/well. At 7 dpi,
supernatants were washed with PBS, fixed in Paraformaldehyde (PFA)
4% in PBS for 20 min at room temperaturend then washed again in
PBS. Cell membranes were permeabilised in 0.2% Triton X-100
(Sigma-Aldrich) for 5 min. The cells were washed with PBS and
incubated with Dako protein Blocker (ready to use; Agilent) and Fc
receptor blocker (Innovex Biosciences; both 30 min at room
temperature). After blocking, cells were incubated overnight at 4°C
with mouse anti-HCMV IE (1:1,000; Cat. No. 11-003, Argene,
Biomerieux). The primary antibodies was detected by incubating
cells with secondary antibody (Alexa Fluor 594 donkey anti-mouse
Cat. No. R37115 1:500; Invitrogen, Thermo Fisher Scientific, Inc.)
at room temperature for 1 h and DAPI for 5 min at room temperature
(Sigma-Aldrich; Merck KGaA). After mounting slides with
fluorescence mounting medium (Dako Cytomation), confocal microscopy
at 20× was performed using Zeiss LSM 700 confocal microscope. Mouse
monoclonal IgG2a (1:300, Cat. No. ab190463 Abcam) served as
negative controls.
Statistical analysis
Correlation between categorical ordinal (IHC score)
and continuous variables (age) was calculated by multivariable
non-parametric Spearman analysis. Associations between binominal
values (sex, MSI, TP53 and KRAS mutation) and HCMV, 5-LO and COX-2
IHC score were calculated by Fisher's exact test. IHC scores were
grouped into 'low positivity' group (scores 0-2, <50% tumor
cells stained positive) and 'high positivity' group (scores 3 and
4; >50% tumor cell stained positive). One-way ANOVA test
followed by Tukey's post hoc test was used to analyze RT-qPCRdata.
Data are presented as the mean ± standard deviation. At least 3
indipendent experimental repeats were run. All statistical
hypotheses were two-sided. P≤0.05 was considered to indicate a
statistically significant difference. GraphPad Prism (version 9.2,
GraphPad Software, Inc.; Dotmatics) was used for statistical
analysis.
Results
Demographic, clinical and
histopathological characterization of CRC cases
TMA series of 146 Iranian CRC cases was analyzed.
Demographic and histopathological characteristics are summarized in
Table SI. The cases were
previously characterized for Duke's staging, differentiation
(poor/moderate/well-differentiated), MSI status and KRAS and TP53
mutation (60,61). A total of two tumors (0.2%) were
rated as Duke's stage A, 65 (55.1%) as stage B, 43 (36.4%) as stage
C and 8 (6.8%) as stage D. Most tumors showed moderate
differentiation (84, 71.2%) while 17 were well differentiated
(14.4%) and 17 were poorly differentiated (14.4%). Mutations in
KRAS and TP53 were detected in 41/113 (36.3%) and 52/117 (44.4%)
cases, respectively; 28 cases were MSI+ (23.7%) and 90
cases were MSS (76.3%).
Immunohistochemical staining for nuclear Ki-67 and
cell membrane EGFR were performed previously (62); IHC data are summarized in Table I. Canonic nuclear Ki-67 was
expressed in 127 of 140 (90.7%) cases, of which 76 (54.3%) had
>50% Ki-67-positive cancer cells (IHC score 3 and 4). EGFR
exhibited canonical membranous localization in 65/140 (46.4%) of
cases. Representative images for EGFR and Ki-67 immunostaining are
shown in Fig. S1.
 | Table IImmunohistochemical staining score in
colorectal cancer cases. |
Table I
Immunohistochemical staining score in
colorectal cancer cases.
| Target protein | Negative (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | Total (%) |
|---|
| HCMV IE | 7 (4.9) | 12 (8.3) | 25 (17.4) | 51 (35.4) | 49 (34.0) | 144 (100) |
| HCMV pp65 | 20 (14.1) | 40 (28.2) | 24 (16.9) | 38 (26.8) | 20 (14.1) | 142 (100) |
| 5-LO | 2 (1.4) | 5 (3.5) | 15 (10.3) | 33 (22.8) | 90 (62.1) | 145 (100) |
| COX-2 | 7 (3.5) | 32 (22.1) | 29 (20.0) | 39 (26.9) | 37 (25.5) | 145 (100) |
|
Ki-67a | 13 (9.3) | 13 (9.3) | 38 (27.1) | 42 (30.0) | 34 (24.3) | 140 (100) |
|
EGFRa | 80 (55.2) | 45 (31.0) | 16 (11.0) | 4 (2.8) | 0 (0.0) | 145 (100) |
High expression of HCMV and inflamatory
proteins is found in CRC
A total of 146 individual TMA cores were available
for HCMV IE, HCMV pp65, COX-2 and 5-LO immunostaining. Some cores
were lost during staining. As sumarized in Table I, HCMV IE expression was detected
in 137 of 144 (95.1%) individual cores, of which 51 (38.3%) scored
3 and 49 (30.8%) 4. HCMV pp65 was detected in 122 of 142 (85.9%)
biopsies, approximately half of which scored 1 or 2 (n=40 and n=24,
respectively) and the rest had score of 3 (n=38) or 4 (n=20). IE
and pp65 proteins were predominantly found in the cytoplasm of
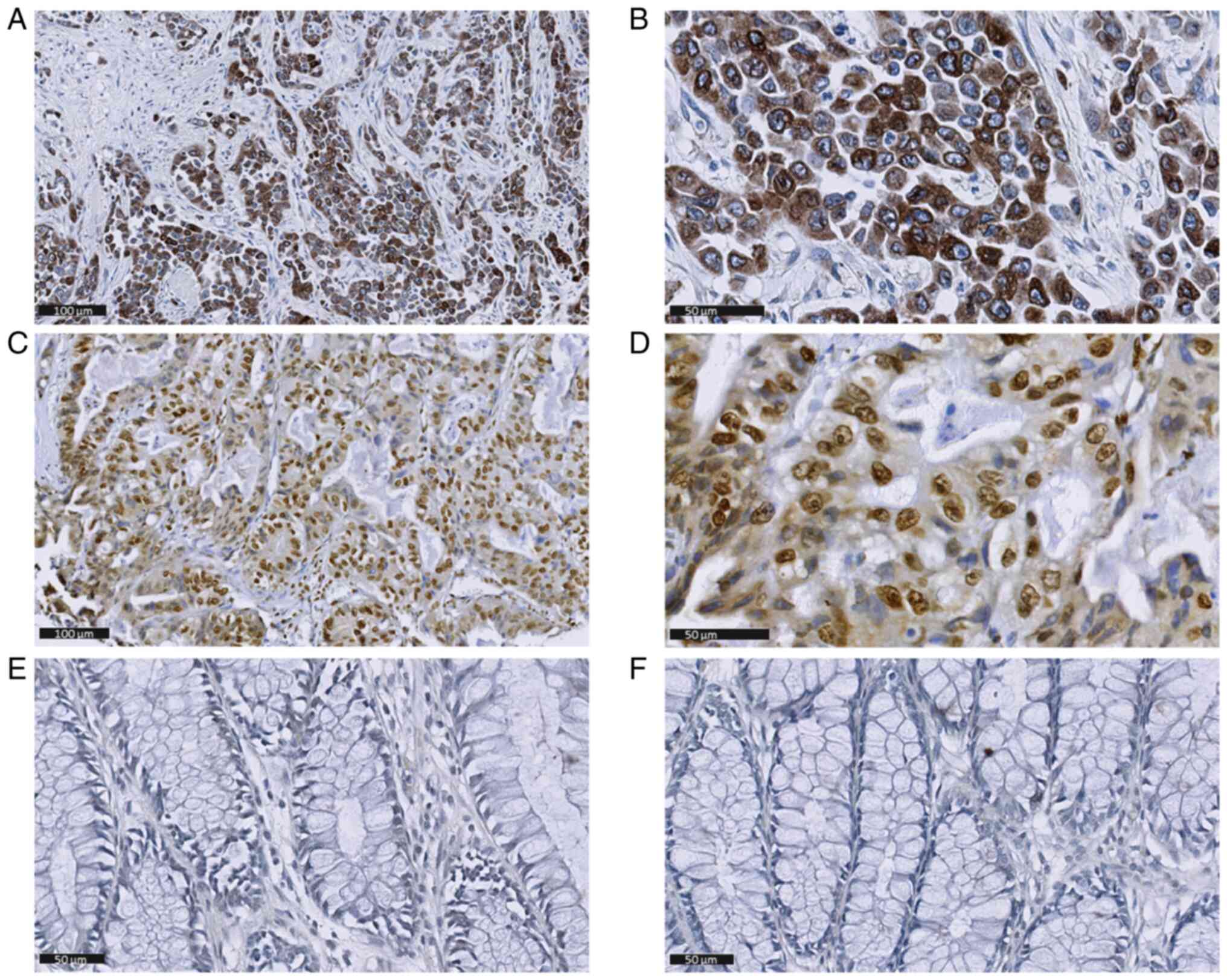
tumor cells (Fig. 1A, B, D and E)
and were not detectable in non-tumorous mucosa (Fig. 1C and F respectively). In certain
cases, IE was also found in endothelial cells (Fig. 1A) and pp65 in tumor-associated
interstitial cells (Fig. 1D).
COX-2 was localized in the cytoplasm and expressed in 137 of 144
(95%) individual cores: 32 (22.2%) had score 1, 29 (20.1%) score 2,
39 (27%) score 3 and 37 (25.7%) score 4 (Fig. 2A, B). No COX-2 immunostaining was
found in non-tumorous mucosa (Fig.
2C). Canonical nuclear 5-LO staining was observed in 143 of 145
(96.6%) individual cores (Fig. 2D and
E), most of which exhibited a high grade (90, 62.9% scored 4),
while no immunostaining was apparent in non-tumorous mucosa
(Fig. 2F).
Expression of HCMV proteins is correlated
with inflammatory and proliferation markers
Spearman correlation tests were performed for
immunohistopathological and clinical variables (Table II). The expression of IE and pp65
was correlated positively in the tumor tissue specimens. The
inflammatory marker COX-2 was positively correlated with both IE
and pp65. Positive correlations were also observed between 5-LO and
IE and and pp65. Additionally, expression of viral proteins IE and
pp65 was correlated with the proliferation marker Ki-67, suggesting
that increased cellular proliferation might be linked to higher
viral load. No correlation was found between IE or pp65 and Duke's
stage, histological grade, EGFR expression and age. 5-LO and COX-2
staining was correlated positively (P<0.0001). COX-2 staining
was positively correlated with Ki-67 labeling; 5-LO was not
significantly positively correlated with Ki-67. A higher
differentiation grade was correlated with older age.
 | Table IISpearman correlation analysis of
viral and inflammatory proteins and immunohistopathological and
clinical variables. |
Table II
Spearman correlation analysis of
viral and inflammatory proteins and immunohistopathological and
clinical variables.
A, IE
|
|---|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|---|
| n | 144 | 142 | 143 | 144 | 140 | 1 43 | 116 | 116 | 116 |
| ρ | 1 | 0.3487 | 0.4347 | 0.5415 | 0.2958 | -0.0672 | 0.0334 | 0.0489 | 0.0656 |
| P | NA | <0.0001 | <0.0001 | <0.0001 | 0.0004 | 0.4249 | 0.7219 | 0.6022 | 0.4839 |
|
| B, pp65 |
|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|
| n | 142 | 142 | 141 | 142 | 138 | 141 | 114 | 114 | 114 |
| ρ | 0.3487 | 1 | 0.1706 | 0.3200 | 0.2913 | 0.0571 | 0.0530 | -0.1238 | 0.0444 |
| P | <0.0001 | NA | 0.0432 | 0.0001 | 0.0005 | 0.5013 | 0.5751 | 0.1893 | 0.6391 |
|
| C, 5-LO |
|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|
| n | 143 | 141 | 145 | 143 | 140 | 144 | 118 | 118 | 118 |
| ρ | 0.4347 | 0.1706 | 1 | 0.3573 | 0.1553 | 0.0378 | -0.1273 | -0.1070 | -0.0055 |
| P | <0.0001 | 0.0432 | NA | <0.0001 | 0.0669 | 0.6529 | 0.1695 | 0.2487 | 0.9529 |
|
| D, COX-2 |
|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|
| n | 144 | 1 42 | 1 43 | 144 | 1 40 | 1 43 | 116 | 116 | 116 |
| ρ | 0.5415 | 0.3200 | 0.3573 | 1 | 0.2311 | -0.0784 | -0.0164 | -0.0298 | 0.1486 |
| P | <0.0001 | 0.0001 | <0.0001 | NA | 0.0060 | 0.3519 | 0.8610 | 0.7504 | 0.1114 |
|
| E, Ki-67 |
|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|
| n | 140 | 138 | 140 | 140 | 140 | 140 | 114 | 114 | 114 |
| ρ | 0.2958 | 0.2913 | 0.1553 | 0.2311 | 1 | 0.1284 | -0.0732 | -0.0899 | -0.0362 |
| P | 0.0004 | 0.0005 | 0.0669 | 0.0060 | NA | 0.1307 | 0.4387 | 0.3417 | 0.7019 |
|
| F, EGFR |
|
| Value | IE | pp65 | 5-LO | COX-2 | Ki-67 | EGFR | Differentiation
grade | Duke's stage | Age |
|
| n | 143 | 141 | 144 | 143 | 140 | 145 | 118 | 118 | 118 |
| ρ | -0.0672 | 0.0571 | 0.0378 | -0.0784 | 0.1284 | 1 | -0.0383 | -0.0402 | 0.0465 |
| P | 0.4249 | 0.5013 | 0.6529 | 0.3519 | 0.1307 | NA | 0.6804 | 0.6659 | 0.6170 |
|
| G, Differentiation
grade |
|
| n | 116 | 114 | 118 | 116 | 114 | 118 | 118 | 118 | 118 |
| ρ | 0.0334 | 0.0530 | -0.1273 | -0.0164 | -0.0732 | -0.0383 | 1 | -0.0267 | -0.1837 |
| P | 0.7219 | 0.5751 | 0.1695 | 0.8610 | 0.4387 | 0.6804 | NA | 0.7742 | 0.0465 |
|
| H, Duke's
stage |
|
| n | 116 | 114 | 118 | 116 | 114 | 118 | 118 | 118 | 118 |
| ρ | 0.0489 | -0.1238 | -0.1070 | -0.0298 | -0.0899 | -0.0402 | -0.0267 | 1 | -0.0027 |
| P | 0.6022 | 0.1893 | 0.2487 | 0.7504 | 0.3417 | 0.6659 | 0.7742 | NA | 0.9771 |
|
| I, Age |
|
| n | 118 | 114 | 118 | 116 | 114 | 118 | 118 | 118 | 118 |
| ρ | -0.005 | 0.0444 | -0.0055 | 0.1486 | -0.0362 | 0.0465 | -0.1837 | -0.0027 | 1 |
| P | 0.9529 | 0.6391 | 0.9529 | 0.1114 | 0.7019 | 0.6170 | 0.0465 | 0.9771 | NA |
To investigate whether HCMV and inflammatory protein
expression was correlated with sex, MSI status and mutations in
TP53 or KRAS, CRC samples stained for HCMV IE, HCMV pp65, 5-LO and
COX-2 by IHC were grouped according to the number of positive tumor
cells (low, score 0-2, <50% tumor cells positive and high, score
3 and 4, >50% tumor cell positive). Fisher's exact test was used
to compare low and high positive cases according to sex (male vs.
female), MSI status (MSS vs. MSI+) and TP53 and KRAS
(wild-type vs. mutated). No statistical significance was found
between groups (data not shown).
Antiviral GCV and/or anti-COX-2 CCX
therapy decreases viral transcript expression in HCMV-infected CRC
cells
Caco-2 and LS-174T cells were infected in
vitro with the HCMV strain VR1814, harvested at 1, 3, 7 dpi and
subjected to RT-qPCR for HCMV IE and pp65 transcript analysis.
Mock-infected cells did not express any viral transcripts, while
HCMV IE transcripts were detected at 3 and 7 dpi in infected Caco-2
cells (Fig. 3B and C) and 1-7 dpi
in infected LS174T cells (Fig.
3E-G). The late transcript pp65 was detected in both cell lines
but only at 7 dpi (Fig. 3D and H),
indicating slow or defective HCMV replicaton cycle in colon cancer
cells. To assess whether anti-viral or COX-2 inhibitory drugs
affected HCMV replication, infected cells were treated with GCV at
100 μM and/or CCX at 10 μM. GCV and/or CCX
significantly decreased IE and pp65 transcript levels in both
infected cell lines at all time points except CCX in LS-174T at 1
and 3 dpi and GCV in LS-174T at 3 dpi. The combination of drugs was
more effective in decreasing HCMV IE transcripts in LS-174T cells
at 1 dpi compared with GCV or CCX alone.
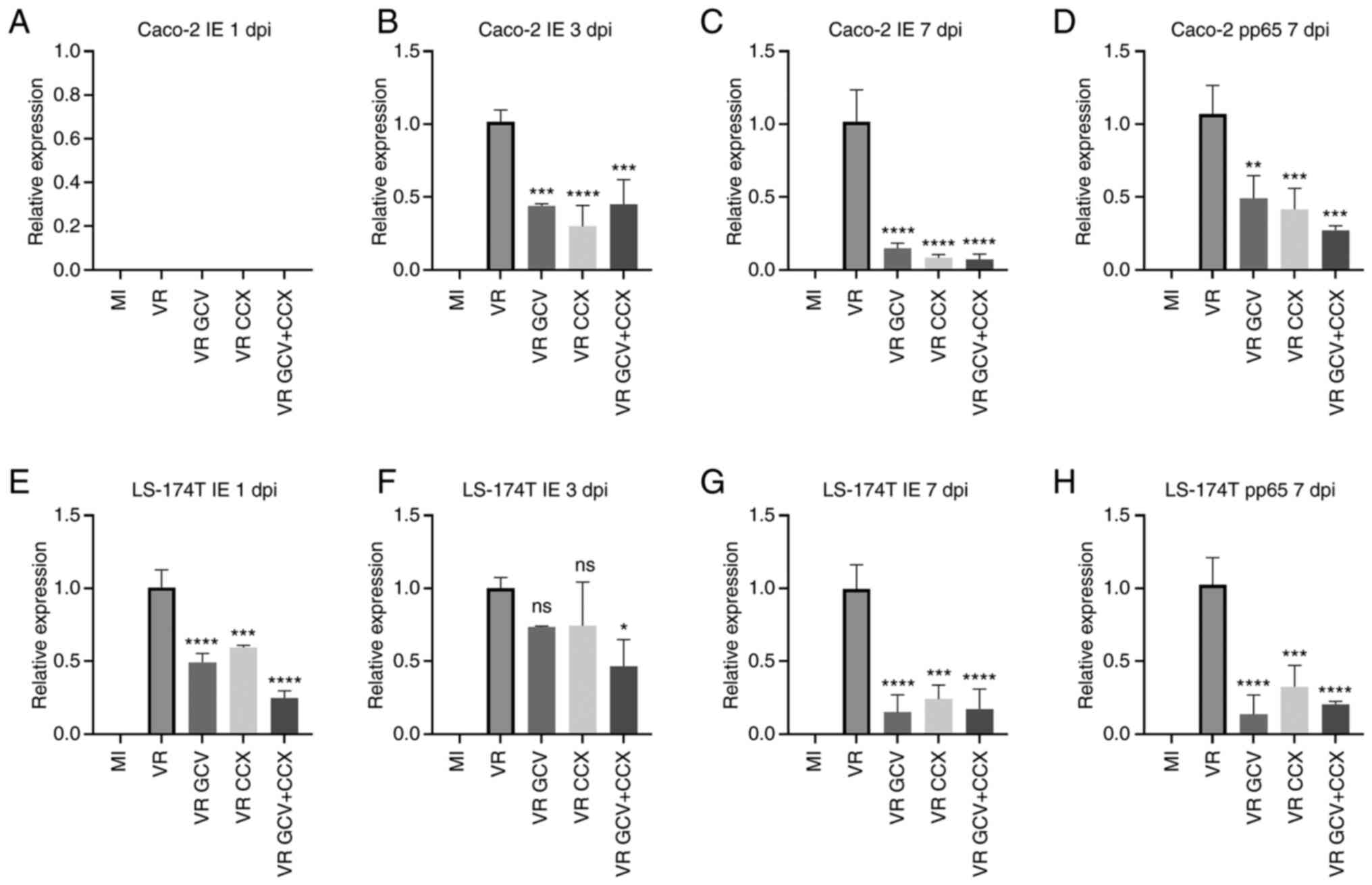 | Figure 3HCMV IE and pp65 transcript
expression in HCMV-infected Caco-2 and LS-174T cells is reduced by
GCV and CCX. Relative IE and pp65 expression levels were determined
by reverse transcription-quantitative PCR. The bars represent COX-2
relative expression to the housekeeping gene. (A) No IE transcripts
were detected at 1 dpi in HCM-infected or mock-infected Caco-2
cells; (B) IE transcripts were detected at 3 dpi in HCMV infected
Caco-2 cells (VR) and they were reduced by treamtents with GCV and
CCX. (C) IE transcripts were detected at 7 dpi in VR) and they were
reduced by treamtent with GCV and CCX. (D) Pp65 transcripts were
detected at 7 pi in HCMV-infected Caco-2 cells and reduced by GCV
and CCX treatments. Only the 7 dpi timepoint is shown for pp65
since no transcripts were detected at earlier timepoints (E) No IE
transcripts were detected at 1 dpi in HCM-infected or mock-infected
LS-174T cells; (F) IE transcripts were detected at 3 dpi in HCMV
infected LS-174T cells (VR) and they were reduced by treamtents
with GCV and CCX; (G) IE transcripts were detected at 7 dpi in HCMV
infected LS-174T cells (VR) and they were reduced by treamtents
with GCV and CCX; (H) Pp65 transcripts were detected at 7 pi in
HCMV-infected LS-174T cells and reduced by GCV and CCX treatments.
Only the 7 dpi timepoint is shown for pp65 since no transcripts
were detected at earlier timepoints. Data are presented as the mean
± SD. Statistical significance was determined by ANOVA test.
*P≤0.05, **P≤0.01, ***P≤0.001,
****P≤0.0001 vs. VR. HCMV, human cytomegalovirus; IE,
immediate early; pp65, posphoprotein 65; GCV, ganciclovir; CCX,
celecoxib; MI, mock-infected; VR, virus-infected; dpi, days post
infection; ns, not significant. |
HCMV induces COX-2 and 5-LO expression in
CRC cells
To assess whether HCMV could activate
pro-inflammatory pathways, COX-2 and 5-LO transcripts were analyzed
by RT-qPCR at 1, 3 and 7 dpi in VR1814-infected Caco-2 and LS-174T
cells (Fig. 4). In Caco-2 cells,
HCMV significantly induced COX-2 expression at 1, 3 and 7 dpi
compared with mock-infected cells. VR1814-infected Caco-2 cells
treated with GCV and/or CCX had lower COX-2 transcript levels
compared with untreated infected cells (Fig. 4A-C). Likewise, in LS-174T cells,
HCMV VR1814 infection significantly induced COX-2 expression at 1,
3 and 7 dpi compared with mock-infected cells (Fig. 4D-F). LS-174T cells treated with GCV
and/or CCX showed a significant decrease in COX-2 transcript levels
compared with untreated infected cells at all time point.
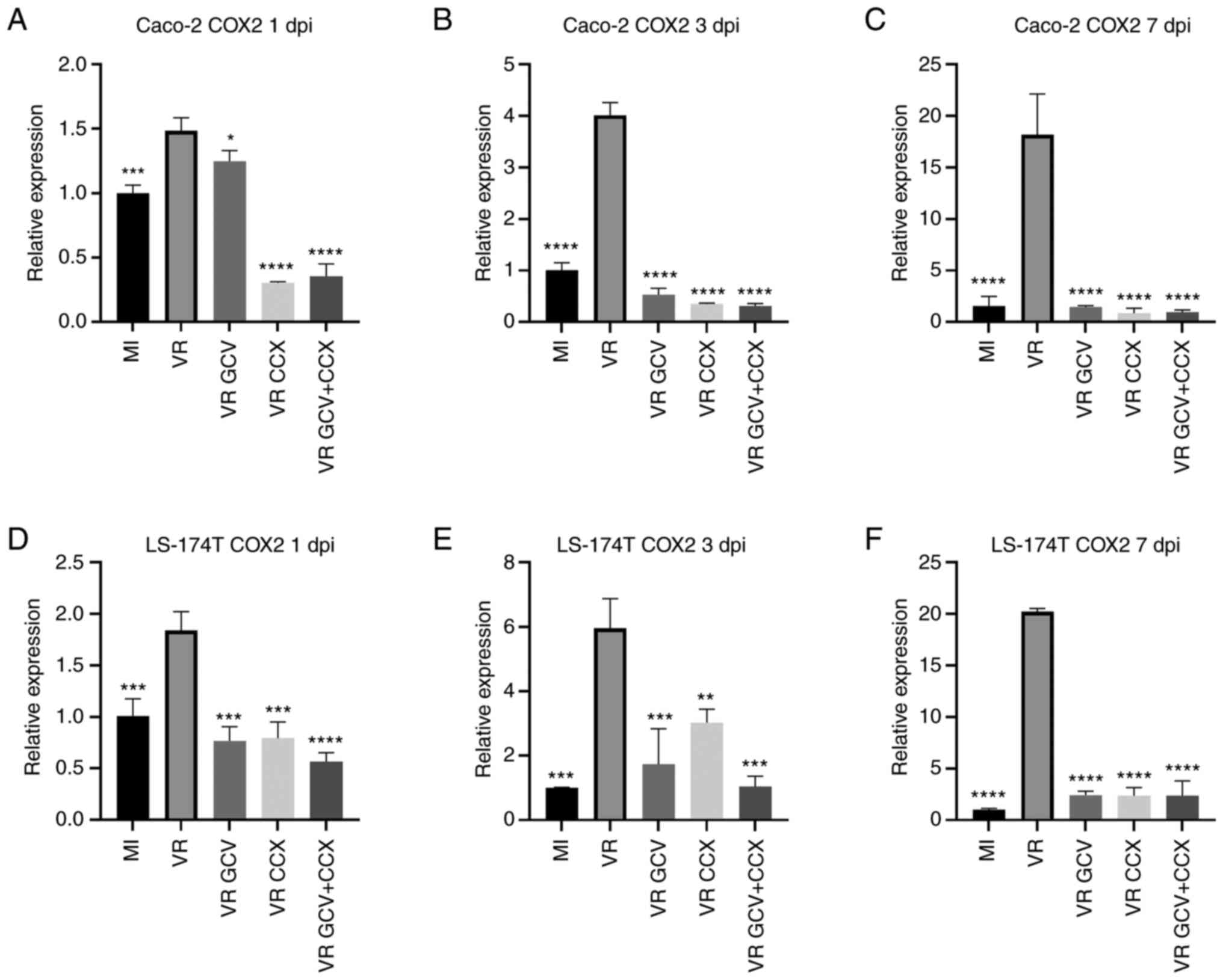 | Figure 4HCMV-infected Caco-2 and LS-174T
cells exhibit increased COX-2 expression. Relative COX-2 expression
was determined by reverse transcription-quantitative PCR at 1, 3
and 7 dpi. Data are presented as the mean ± SD. Caco-2 infected
with HCMV (VR) shows higher COX-2 transcripts than mock-infected
cells (MI) at 1 dpi (A), 3 dpi (B) and 7 dpi (C). Treatment with
GCV, CCX or a combination of the two drugs significantly reduced
COX-2 transcripts at all time points in Caco-2 HCMV-infected cells.
LS-174 cells infected with HCMV (VR) shows higher COX-2 transcripts
than mock-infected cells (MI) at 1 dpi (D), 3 dpi (E) and 7 dpi
(F). Treatment with GCV, CCX or a combination of the two drugs
significantly reduced COX-2 transcripts at all time points in
LS-174 HCMV-infected cells. Statistical significance was determined
by ANOVA test. Statistical significance was determined by
ANOVAtest. *P≤0.05, **P≤0.01,
***P≤0.001, ****P≤0.0001 vs. VR. HCMV, human
cytomegalovirus; COX-2, cyclooxygenase-2; GCV, ganciclovir; CCX,
celecoxib; MI, mock-infected; VR, virus-infected; dpi, days post
infection. |
5-LO transcripts were analyzed by RT-qPCR at 1, 3
and 7 dpi in mock- and HCMV-infected Caco-2 and LS-174T cells
(Fig. 5). In Caco-2 cells, HCMV
infection significantly upregulated 5-LO expression at 3 and 7 dpi
compared with mock-infected cells but not at 1 dpi. Infected Caco-2
cells treated with GCV showed decreased 5-LO transcript levels at 3
and 7 but not at 1 dpi This was coherent with the fact that the
HCMV-infected cells did not reveal increased 5-LO transcript levels
at 1 dpi compared with mock-infected cells and confirms specificity
of GCV antiviral action. CCX and CCX + GCV downregulated 5-LO
levels at all time points in infected Caco-2 cells. CCX was more
effective than GCV in reducing 5-LO transcripts at 7 dpi.
Similarly, in LS-174T cells, HCMV infection significantly induced
5-LO transcript expression at 1, 3 and 7 dpi compared with
mock-infected cells. Compared with untreated infected cells,
infected LS-174T cells treated with GCV, CCX or GCV + CCX had
significantly lower levels of 5-LO transcripts at each time point.
GCV + CCX was more effective in reducing 5-LO transcript expression
than GCV or CCX alone at 1 and 3 dpi. At 7 dpi, CCX alone or in
combination with GCV was more effective than GCV alone.
 | Figure 5HCMV increases 5-LO expression in
Caco-2 and LS-174T cells. Relative 5-LO expression was determined
by qPCR at 1, 3 and 7 dpi. The bars represent 5-LO relative
expression to the housekeeping gene. Data are presented as the mean
± SD. (A) At 1 dpi Caco-2 infected with HCMV (VR) do not show
higher 5-LO transcripts than MI); GCV treatment on infected cells
did not decrease 5-LO transcripts at 1 dpi, on the contrary CCX and
a combination of GCV and CCX reduce 5-LO transcripts in
HCMV-infected cells. At 3 dpi (B) and 7 dpi (C) Caco-2 infected
with HCMV (VR) shows higher 5-LO transcripts than mock-infected
cells (MI). Treatment with GCV, CCX or a combination of the two
drugs significantly reduced 5-LO transcripts at 3 dpi (B) and 7 dpi
(C) in Caco-2 HCMV-infected cells. LS-174 cells infected with HCMV
(VR) shows higher 5-LO transcripts than mock-infected cells (MI) at
1 dpi (D), 3 dpi (E) and 7 dpi (F). Treatment with GCV, CCX or a
combination of the two drugs significantly reduced 5-LO transcripts
at all time points in LS-174 HCMV-infected cells. Statistical
significance was determined by ANOVA test. ***P≤0.001,
****P≤0.0001 vs. VR. HCMV, human cytomegalovirus; 5-LO,
5-lipoxygenase; GCV, ganciclovir; CCX, celecoxib; MI,
mock-infected; VR, virus-infected; dpi, days post infection; ns,
not significant. |
HCMV induces expression of the
inflammatory proteins 5-LO and COX-2 in CRC cells
Caco-2 and LS-174T cells were plated onto chamber
slides and infected with HCMV strain VR1814 at MOI 5 or
mock-infected. At 7 dpi the cells were fixed and stained for IE,
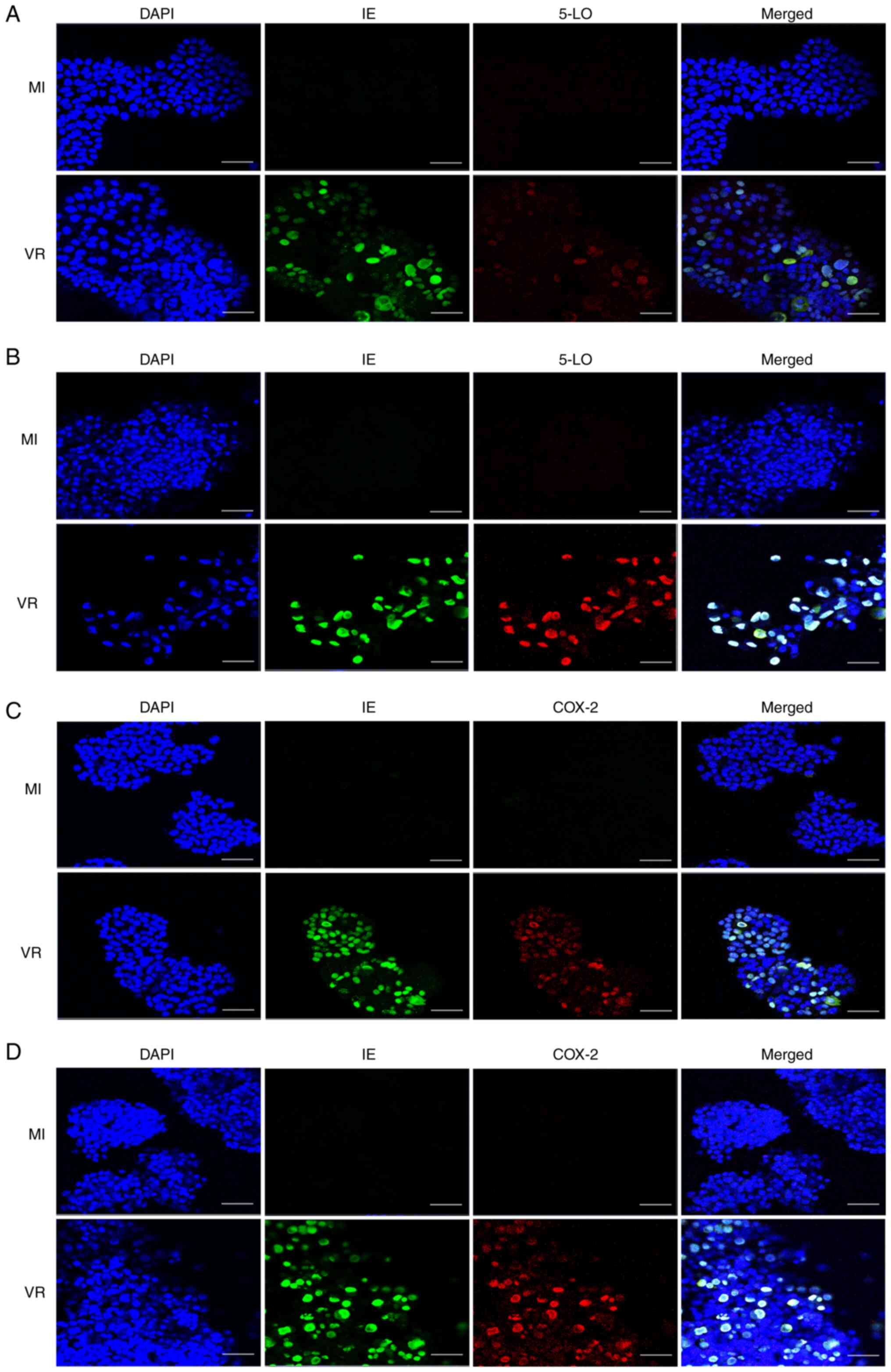
COX-2 and 5-LO and subsequently analyzed by IF. Infected cells
exhibited higher expression of both COX-2 and 5-LO compared with
mock-infected cells (Fig. 6).
HCMV induces proliferation of CRC
cells
To assess whether HCMV infection altered the
proliferation rate of colon cancer cells, MTT assay was performed
to assess proliferation in mock- and HCMV-infected CRC cell lines.
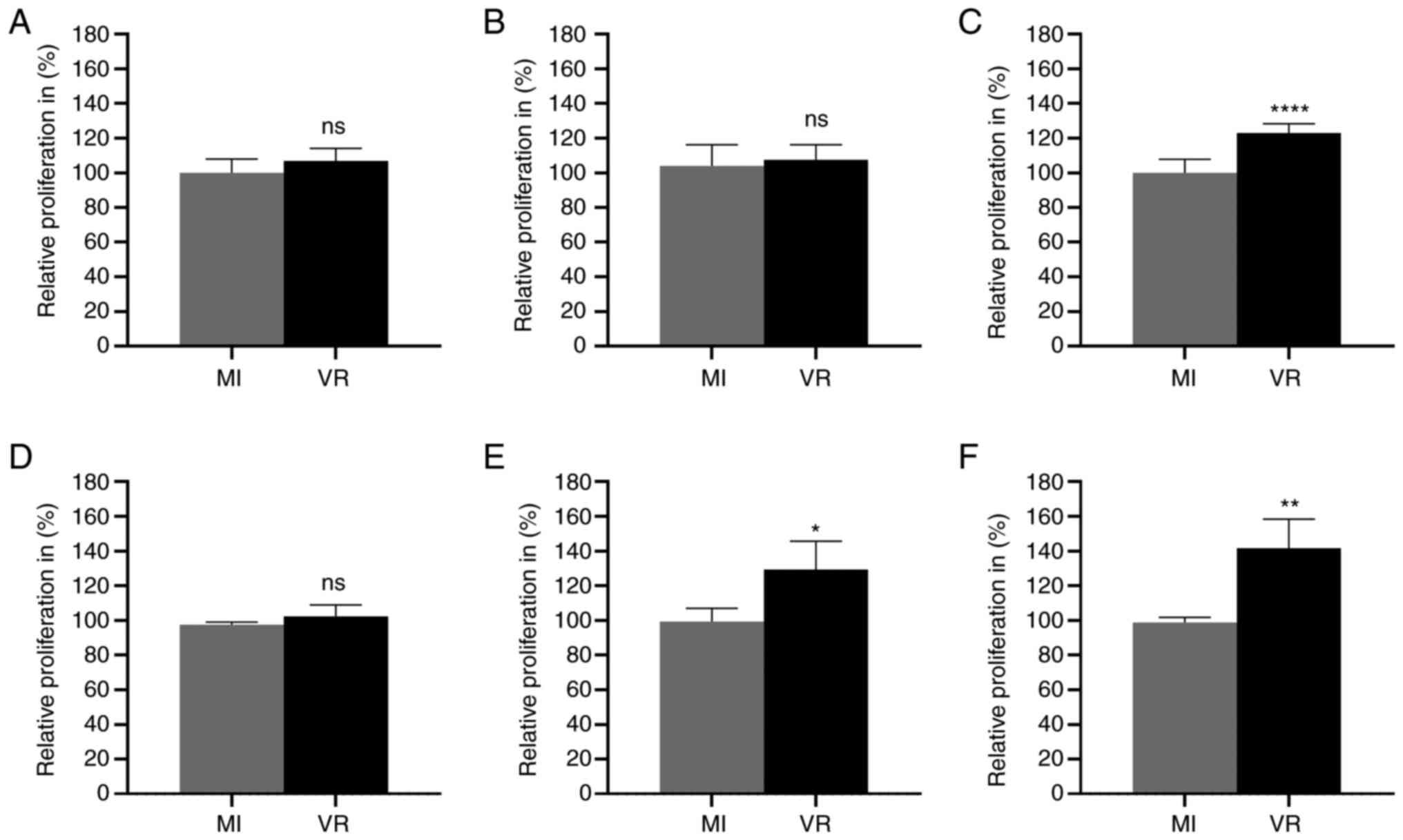
HCMV-infected CRC cells had a higher proliferation than
mock-infected cells at late time points (Fig. 7). HCMV-infected Caco-2 cells showed
higher proliferation capacity at 7 dpi (Fig. 7C). HCMV-infected LS-174T cells
showed higher proliferative capacity compared with mock-infected
cells at 3 and 7 dpi (Fig. 7E and
F). No significant difference in proliferation activity was
observed at 1 or 3 dpi in Caco-2 cells (Fig. 7A and B) or at 1 dpi in LS-174T
(Fig. 7D).
HCMV-infected CRC cells do not produce
infective viral particles
The present study tested the effect of antiviral
therapy with GCV and COX-2 inhibitor CCX on production of
infectious virus particles in Caco-2 and LS-174T cells. No
IE-positive staining was observed by IF in MRC5 cells incubated
with supernatant from infected Caco-2 and LS-174T cells (data not
shown), thus no evidence of virions produced in these colorectal
cancer cells was found at 7 dpi.
Discussion
The presence of HCMV in cancer has been extensively
studied (37,71) but there is no consensus regarding
the clinical relevance of these observations. Recently,
Peredo-Harvey et al (72)
published a systematic review on diagnostic methods for detection
of HCMV in glioblastoma and concluded that the discordant data
reflect unresolved technical issues as optimized techniques to
detect HCMV nucleic acids and proteins in tumors have not been
developed (72-74). The high expression of HCMV in
tumors may serve a key role in tumorigenesis and tumor progression
as this virus can cause all ten hallmarks of cancer (75,76),
promote tumor cell proliferation and serve as a target for
therapy.
Taher et al (54) reported that HCMV DNA, transcripts
and protein are present not only in primary CRC but also in brain
metastases of patients with CRC and that high grade of HCMV
infection in primary tumors is associated with shorter time to
development of brain metastases and shorter overall survival time.
HCMV protein expression is confined to tumor cells and metastases
and is not present in healthy surrounding tissue. Harkins et
al (18) reported that HCMV
IE72 and COX-2 are co-expressed in CRC and HCMV infection in
vitro induces COX-2 expression in Caco-2 cells. HCMV DNA and
proteins are rarely found in non-tumorous tissue surrounding
primary CRC or metastases, which suggests a tumor-specific
connection and hence potential role of this virus in CRC (18). The present study examined 146
Iranian CRC cases, of which 144 were analyzed by IHC for IE and
pp65, key HCMV proteins representative of IE and late phase of
viral replication. Both viral proteins were diffusely expressed in
most of cases, consistent with previous studies (18,56).
The association between levels of immunostaining for HCMV proteins,
immunohistochemical expression of COX-2, 5-LO, Ki-67 and EGFR, TP53
and KRAS mutation and MSI status was assessed. HCMV proteins were
similarly expressed in tumors regardless of Duke's grades, TP53
and/or KRAS mutation, MSI status and EGFR membrane expression. By
contrast, HCMV IE and pp65 expression levels were positively
correlated with 5-LO, COX-2 and Ki-67, suggesting that higher HCMV
load was associated with more aggressive pro-inflammatory tumor
phenotype, regardless of tumor genetic and molecular
background.
Inflammation is a hallmark of cancer and is
considered to be essential for carcinogenesis. HCMV promotes
inflammation (50,77-78);
inflammation is linked to HCMV reactivation and replication
(79-82). The HCMV major immediate early
promoter that controls expression of IE proteins is activated by
inflammatory cytokines such as TNF-α and IL-6, which induce
reactivation of latent HCMV in myeloid lineage cells (79,83).
HCMV also induces expression of 5-LO and COX-2 and the production
of PGE2 and LTB4, which are potent inflammatory mediators that
attract inflammatory cells to sites of infection (51,77,78,84);
this promotes inflammation that can drive HCMV replication. The
viral chemokine receptor homologue US28 induces STAT3 expression
and IL-6 production and IL-1β is produced via activation of absent
in melanoma 2 inflammasomes (85).
HCMV infection induces production of pro-inflammatory cytokines,
such as IL-1, IL-2, IL-6, IL-8, IL-17, TNF-α granulocyte
colony-stimulating factor (CSF), granulocyte-macrophage CSF and
chemokines such as monocyte chemoattractant protein, macrophage
inflammatory protein-1 and IFN-inducing protein 10 (86).
In the present study, both 5-LO and COX-2 were found
to be expressed in HCMV-infected CRC specimens and were associated
with higher Ki-67 index. HCMV induces cell proliferation and
increase tumor aggressiveness (87), which may reflect its strong
connection with inflammatory signaling. HCMV-encoded tumorigenic
chemokine receptor homologue US28 (88,89)
directly induces COX-2 expression (50). Transgenic mice expressing US28 in
intestinal epithelial cells develop intestinal adenomas and
adenocarcinoma by 40 weeks of age, a process that is significantly
enhanced by a combined treatment with azoxymethane and dextran
sodium sulfate, which promote inflammation (59).
Our previous study reported that HCMV contributes to
inflammation in breast cancer and medulloblastoma, potentially by
driving COX-2 and/or 5-LO expression (45,53).
HCMV DNA and proteins co-localize with inflammatory mediator IL-6
(90) and HCMV-infected vascular
smooth muscle cells express high levels of 5-LO protein in tissue
samples from patients with inflammatory bowel disease (77), which suggests a broader role of
HCMV in the intestine. The present study demonstrated that HCMV
infection induced COX-2 and 5-LO expression and proliferation in
Caco-2 cells, which maintains enterocytic differentiation
potential, and LS-174, an aggressive, poorly differentiated cell
line (65). Caco-2 cells have
similar behavior to normal differentiated villus cells and are
well-characterized in the Wnt pathway and its role in confluency
and cancer (91,92). The fact that HCMV induces
inflammation and proliferation in both Caco-2 and LS-174 cells
strengthen the potential role of HCMV as inflammation occurs in the
normal tissues and is critical in the process of CRC development
(11-13). Here, anti-viral therapy with GCV
effectively reduced virus-induced COX-2 and 5-LO transcript
expression in both Caco-2 and LS-174T cells. Similarly, COX-2
inhibitor CCX decreased expression of COX-2 and 5-LO as well as
HCMV IE and pp65 transcripts, suggesting a mutual loop. VGCV, a
prodrug of GCV, significantly decreases growth of HCMV-positive
medulloblastoma xenografts in mice and combination with CCX results
in a significant additive effect, decreasing tumor growth by 72%
(45). HCMV benefits from a
pro-inflammatory environment and anti-inflammatory drugs
effectively decrease replication in infected cells (51,93,94)
and tumor cell proliferation
Viral infectivity assay showed that no infectious
virions were produced in HCMV infected Caco-2 and LS-174 cells,
consistent with previous literature reporting that HCMV replicates
poorly or not at all in cancer cells (95). This may be dependent on the virus
inability to arrest cells in G1/G2 phase, which is important for to
initiate DNA replication. There was no evidence of virions produced
in supernatant of these colon cancer cells at 7 dpi, indicating
that the effects of HCMV on colon cancer cells may be associated
with virus ability to express proteins and thereby affect cell
function (95).
HCMV is not able to fully replicate in cancer cells
may be one of the driving forces of oncomodulation as, for example,
HCMV infection fuels genomic instability in non-permissive cells
such as cancer cells (95).
Future studies should investigate how HCMV controls
the cell cycle in normal and cancer cells, how COX-2 and 5-LO
expression is regulated in different cancer cells and the
mechanisms that HCMV uses to induce their expression in cancer
cells.
Taken together with literature (18,41,56),
the present results confirmed that HCMV was present in CRC,
regardless of stage and molecular/genetic background, with higher
viral activity associated with an aggressive pro-inflammatory
phenotype, and suggested that anti-viral drugs, alone or in
combination with anti-inflammatory treatment, could be effective in
patients with CRC. Here, HCMV infection resulted in an upregulation
of COX-2 and 5-LO in CRC cells. These inflammatory mediators
promote tumor aggressiveness and hence therapies that interfere
with their release or effects on neighboring tumor cells may affect
tumor growth. Aspirin reduces risk of colon cancer and metastastic
disease (6). Aspirin, initially
given to decrease platelet formation and risk of reinfarction in
patients with myocardial infarction, significantly decreases cancer
risk (96). Other anti-platelet
therapy that interferes with production and release of AA may exert
negative downstream effects on cancer cells (97,98).
Examples of these include the P2Y12 inhibitor tricagrelor that
inhibits tumor-platelet interaction and metastasis formation
(99), as well as clopidogrel
(100) and flavonoids with
anti-COX and anti-cancer effects (101,102).
Limitations of the present study include the lack
of clinical data such as therapy, recurrence and survival.
Moreover, the present in vitro findings need to be validated
in animal models before possible investigation of the potential
therapeutic effect of antiviral drugs, alone or in combination with
anti-inflammatory agents in patients with CRC. Further studies need
to assess the impact of anti-inflammatory and anti-viral drugs in
preventing malignant transformation, tumor proliferation and
metastasis formation.
The efficacy of antiviral therapy against HCMV has
been already addressed in patients with newly diagnosed, recurrent
and secondary glioblastoma, where VGCV given as add on to standard
therapy shows good tolerability and significantly improved survival
(103,104,105). Dendritic cell vaccination with
HCMV pp65 mRNA also improves survival rates among patients with
glioblastoma (106,107). Both strategies are currently
being evaluated in randomized phase II trials (trial nos.
NCT04116411 and NCT00639639), which could provide proof of concept
for the use of HCMV-targeted therapies in patients with
glioblastoma and other HCMV-positive tumors, such as CRC. This may
provide potential novel therapeutic options for patients with
HCMV-positive cancer, including combined therapy with drugs
targeting both the virus and COX-2/5-LO pathways.
In conclusion, the present study detected high
levels of HCMV IE, pp65, COX-2 and 5-LO in most CRC samples but not
in non-malignant mucosa. There was a significant correlation
between higher expression of HCMV proteins (IE and pp65) and higher
COX-2 and 5-LO protein levels. Ki-67 index was correlated with
higher load of viral proteins, suggesting that HCMV infection was
associated with more aggressive, proliferative tumor phenotype. The
in vitro results demonstrated that HCMV induced expression
of COX-2 and 5-LO in CRC cell lines that represent different tumor
phenotypes. Antiviral therapy with GCV effectively reduced
transcript levels of HCMV genes encoding IE and pp65 and the
cellular genes encoding COX-2 and 5-LO. The anti-inflammatory drug
CCX decreased transcript levels of 5-LO and COX-2 as well as HCMV
IE and pp65 viral transcripts. A combination of GCV and CCX was
more effective than monotherapy. These results provide a basis for
mechanistic studies in vitro and preclinical in vivo
validation. Combined anti-HCMV and anti-inflammatory agents may
serve as a novel therapeutic options for patients with CRC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MRP, AR, RMC, CSN and CT conceived the study. MRP,
CSN and RMC wrote and edited the manuscript. MRP, NMA, RL, MM, SV,
CT and SDF performed experiments. RMC, RL, AR, and MRP analyzed the
data. FV, FB and MM edited the manuscript and colleted the data.
RMC, AR and CSN supervised the study. MRP and NMA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki for human medical research and approved by
the ethics committee of the Digestive Disease Research Center of
Tehran University of Medical Sciences (approval no. #91033718830).
The requirement for patient consent was waived as samples were
anonymized and had been stored for ~20 years.
Ethics approval for the non-tumorous colorectal
mucosa samples, previously collected at the Department of Molecular
Medicine and Surgery at Karolinska Institutet, was obtained from
Regional Human Ethics Committee, Stockholm, Sweden
(No2008/628-31).
Patient consent for publication
Not applicable.
Competing interests
CSN holds a patent on diagnostics and treatment of
a CMV variant strain found in cancer (patent no. US9701943B2). The
other authors declare that they have no competing interests.
Abbreviations:
|
HCMV
|
human cytomegalovirus
|
|
CRC
|
colorectal cancer
|
|
5-LO
|
5-lipoxygenase
|
|
COX-2
|
cyclooxygenase-2
|
|
GCV
|
ganciclovir
|
|
CCX
|
celecoxib
|
|
MSS
|
microsatellite stability
|
|
MSI
|
microsatellite instability
|
|
dpi
|
days post-infection
|
|
IE
|
immediate early
|
Acknowledgments
Not applicable.
Funding
The present study was supported by Swedish Medical Research
Council (grant no. 2019-01736), The Cure Cancer Foundation and
Italian Association for Cancer Research (grant no. 24501).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeon J, Du M, Schoen RE, Hoffmeister M,
Newcomb PA, Berndt SI, Caan B, Campbell PT, Chan AT, Chang-Claude
J, et al: Determining risk of colorectal cancer and starting age of
screening based on lifestyle, environmental, and genetic factors.
Gastroenterology. 154:2152–2164.e2119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulaylat MN and Dayton MT: Ulcerative
colitis and cancer. J Surg Oncol. 101:706–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jensen AB, Larsen M, Gislum M, Skriver MV,
Jepsen P, Nørgaard B and Sørensen HT: Survival after colorectal
cancer in patients with ulcerative colitis: A nationwide
population-based Danish study. Am J Gastroenterol. 101:1283–1287.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dolin TG, Christensen IJ, Johansen AZ,
Nielsen HJ, Jakobsen HL, Klein MF, Lund CM, Bojesen SE, Nielsen DL,
Jensen BV and Johansen JS: Pre- and perioperative inflammatory
biomarkers in older patients resected for localized colorectal
cancer: Associations with complications and prognosis. Cancers
(Basel). 14:1612022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giovannucci E: The prevention of
colorectal cancer by aspirin use. Biomed Pharmacother. 53:303–308.
1999. View Article : Google Scholar
|
|
7
|
Watson AJ: Chemopreventive effects of
NSAIDs against colorectal cancer: Regulation of apoptosis and
mitosis by COX-1 and COX-2. Histol Histopathol. 13:591–597.
1998.PubMed/NCBI
|
|
8
|
Bus PJ, Verspaget HW, Lamers CB and
Griffioen G: Chemoprevention of colorectal cancer by non-steroidal
anti-inflammatory drugs. Scand J Gastroenterol Suppl. 2000:101–104.
2000.
|
|
9
|
Andersen V, Halekoh U, Tjønneland A, Vogel
U and Kopp TI: Intake of red and processed meat, use of non-steroid
anti-inflammatory drugs, genetic variants and risk of colorectal
cancer: A prospective study of the danish 'Diet, Cancer and Health'
cohort. Int J Mol Sci. 20:11212019. View Article : Google Scholar
|
|
10
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmitt M and Greten FR: The inflammatory
pathogenesis of colorectal cancer. Nat Rev Immunol. 21:653–667.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lichtenstern CR, Ngu RK, Shalapour S and
Karin M: Immunotherapy, inflammation and colorectal cancer. Cells.
9:6182020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Percario R, Panaccio P, di Mola FF,
Grottola T and Di Sebastiano P: The complex network between
inflammation and colorectal cancer: A systematic review of the
literature. Cancers (Basel). 13:62372021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khanapure SP, Garvey DS, Janero DR and
Letts LG: Eicosanoids in inflammation: Biosynthesis, pharmacology,
and therapeutic frontiers. Curr Top Med Chem. 7:311–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sonnweber T, Pizzini A, Nairz M, Weiss G
and Tancevski I: Arachidonic acid metabolites in cardiovascular and
metabolic diseases. Int J Mol Sci. 19:32852018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Sood S, Yang CS, Li N and Sun Z:
Five-lipoxygenase pathway of arachidonic acid metabolism in
carcino-genesis and cancer chemoprevention. Curr Cancer Drug
Targets. 6:613–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang W, Sanidad KZ, Shih PA, Zhao
X and Zhang G: Eicosanoid signaling in carcinogenesis of colorectal
cancer. Cancer Metastasis Rev. 37:257–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harkins L, Volk AL, Samanta M, Mikolaenko
I, Britt WJ, Bland KI and Cobbs CS: Specific localisation of human
cytomegalovirus nucleic acids and proteins in human colorectal
cancer. Lancet. 360:1557–1563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kany S, Vollrath JT and Relja B: Cytokines
in inflammatory disease. Int J Mol Sci. 20:60082019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radmark O and Samuelsson B:
5-Lipoxygenase: Mechanisms of regulation. J Lipid Res. 50(Suppl):
S40–S45. 2009. View Article : Google Scholar :
|
|
21
|
Uhl J, Klan N, Rose M, Entian KD, Werz O
and Steinhilber D: The 5-lipoxygenase promoter is regulated by DNA
methylation. J Biol Chem. 277:4374–4379. 2002. View Article : Google Scholar
|
|
22
|
Lee SJ, Seo KW and Kim CD: LPS increases
5-LO expression on monocytes via an activation of Akt-Sp1/NF-kappaB
pathways. Korean J Physiol Pharmacol. 19:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zappavigna S, Cossu AM, Grimaldi A,
Bocchetti M, Ferraro GA, Nicoletti GF, Filosa R and Caraglia M:
Anti-inflammatory drugs as anticancer agents. Int J Mol Sci.
21:26052020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris RE, Beebe-Donk J and Alshafie GA:
Reduction in the risk of human breast cancer by selective
cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 6:272006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris RE, Beebe-Donk J and Alshafie GA:
Reduced risk of human lung cancer by selective cyclooxygenase 2
(Cox-2) blockade: Results of a case control study. Int J Biol Sci.
3:328–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobs EJ, Rodriguez C, Mondul AM, Connell
CJ, Henley SJ, Calle EE and Thun MJ: A large cohort study of
aspirin and other nonsteroidal anti-inflammatory drugs and prostate
cancer incidence. J Natl Cancer Inst. 97:975–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel MI, Subbaramaiah K, Du B, Chang M,
Yang P, Newman RA, Cordon-Cardo C, Thaler HT and Dannenberg AJ:
Celecoxib inhibits prostate cancer growth: Evidence of a
cyclooxygenase-2-independent mechanism. Clin Cancer Res.
11:1999–2007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maniewska J and Jeżewska D: Non-steroidal
anti-inflammatory drugs in colorectal cancer chemoprevention.
Cancers (Basel). 13:5942021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cole BF, Logan RF, Halabi S, Benamouzig R,
Sandler RS, Grainge MJ, Chaussade S and Baron JA: Aspirin for the
chemoprevention of colorectal adenomas: Meta-analysis of the
randomized trials. J Natl Cancer Inst. 101:256–266. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cook NR, Lee IM, Zhang SM, Moorthy MV and
Buring JE: Alternate-day, low-dose aspirin and cancer risk:
Long-term observational follow-up of a randomized trial. Ann Intern
Med. 159:77–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: Analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar
|
|
32
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo CG, Ma W, Drew DA, Cao Y, Nguyen LH,
Joshi AD, Ng K, Ogino S, Meyerhardt JA, Song M, et al: Aspirin use
and risk of colorectal cancer among older adults. JAMA Oncol.
7:428–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coyle C, Cafferty FH and Langley RE:
Aspirin and colorectal cancer prevention and treatment: Is it for
everyone? Curr Colorectal Cancer Rep. 12:27–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Avis I, Hong SH, Martinez A, Moody T, Choi
YH, Trepel J, Das R, Jett M and Mulshine JL: Five-lipoxygenase
inhibitors can mediate apoptosis in human breast cancer cell lines
through complex eicosanoid interactions. FASEB J. 15:2007–2009.
2001. View Article : Google Scholar
|
|
36
|
Rao CV, Janakiram NB and Mohammed A:
Lipoxygenase and cyclooxygenase pathways and colorectal cancer
prevention. Curr Colorectal Cancer Rep. 8:316–324. 2012. View Article : Google Scholar
|
|
37
|
Nauclér CS, Geisler J and Vetvik K: The
emerging role of human cytomegalovirus infection in human
carcinogenesis: A review of current evidence and potential
therapeutic implications. Oncotarget. 10:4333–4347. 2019.
View Article : Google Scholar :
|
|
38
|
Zuhair M, Smit GSA, Wallis G, Jabbar F,
Smith C, Devleesschauwer B and Griffiths P: Estimation of the
worldwide seroprevalence of cytomegalovirus: A systematic review
and meta-analysis. Rev Med Virol. 29:e20342019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stern-Ginossar N, Weisburd B, Michalski A,
Le VT, Hein MY, Huang SX, Ma M, Shen B, Qian SB, Hengel H, et al:
Decoding human cytomegalovirus. Science. 338:1088–1093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murphy E, Rigoutsos I, Shibuya T and Shenk
TE: Reevaluation of human cytomegalovirus coding potential. Proc
Natl Acad Sci USA. 100:13585–13590. 2003. View Article : Google Scholar
|
|
41
|
Chen HP, Jiang JK, Chen CY, Chou TY, Chen
YC, Chang YT, Lin SF, Chan CH, Yang CY, Lin CH, et al: Human
cytomegalovirus preferentially infects the neoplastic epithelium of
colorectal cancer: A quantitative and histological analysis. J Clin
Virol. 54:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang ES and Roche JK: Cytomegalovirus
D.N.A. and adenocarcinoma of the colon: Evidence for latent viral
infection. Lancet. 1:957–960. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harkins LE, Matlaf LA, Soroceanu L, Klemm
K, Britt WJ, Wang W, Bland KI and Cobbs CS: Detection of human
cytomegalovirus in normal and neoplastic breast epithelium.
Herpesviridae. 1:82010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samanta M, Harkins L, Klemm K, Britt WJ
and Cobbs CS: High prevalence of human cytomegalovirus in prostatic
intraepithelial neoplasia and prostatic carcinoma. J Urol.
170:998–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baryawno N, Rahbar A, Wolmer-Solberg N,
Taher C, Odeberg J, Darabi A, Khan Z, Sveinbjörnsson B, FuskevÅg
OM, Segerström L, et al: Detection of human cytomegalovirus in
medulloblastomas reveals a potential therapeutic target. J Clin
Invest. 121:4043–4055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wolmer-Solberg N, Baryawno N, Rahbar A,
Fuchs D, Odeberg J, Taher C, Wilhelmi V, Milosevic J, Mohammad AA,
Martinsson T, et al: Frequent detection of human cytomegalovirus in
neuroblastoma: A novel therapeutic target? Int J Cancer.
133:2351–2361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cobbs CS, Harkins L, Samanta M, Gillespie
GY, Bharara S, King PH, Nabors LB, Cobbs CG and Britt WJ: Human
cytomegalovirus infection and expression in human malignant glioma.
Cancer Res. 62:3347–3350. 2002.PubMed/NCBI
|
|
48
|
Forte E, Zhang Z, Thorp EB and Hummel M:
Cytomegalovirus latency and reactivation: An intricate interplay
with the host immune response. Front Cell Infect Microbiol.
10:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Diggins NL, Skalsky RL and Hancock MH:
Regulation of latency and reactivation by human cytomegalovirus
miRNAs. Pathogens. 10:2002021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maussang D, Langemeijer E, Fitzsimons CP,
Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A,
Michel D, Tensen CP, et al: The human cytomegalovirus-encoded
chemokine receptor US28 promotes angiogenesis and tumor formation
via cyclooxygenase-2. Cancer Res. 69:2861–2869. 2009. View Article : Google Scholar
|
|
51
|
Zhu H, Cong JP, Yu D, Bresnahan WA and
Shenk TE: Inhibition of cyclooxygenase 2 blocks human
cytomegalovirus replication. Proc Natl Acad Sci USA. 99:3932–3937.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Benard M, Straat K, Omarsdottir S,
Leghmari K, Bertrand J, Davrinche C, Duga-Neulat I,
Söderberg-Nauclér C, Rahbar A and Casper C: Human cytomegalovirus
infection induces leukotriene B4 and 5-lipoxygenase expression in
human placentae and umbilical vein endothelial cells. Placenta.
35:345–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Costa H, Touma J, Davoudi B, Benard M,
Sauer T, Geisler J, Vetvik K, Rahbar A and Söderberg-Naucler C:
Human cytomegalovirus infection is correlated with enhanced
cyclooxygenase-2 and 5-lipoxygenase protein expression in breast
cancer. J Cancer Res Clin Oncol. 145:2083–2095. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Taher C, Frisk G, Fuentes S, Religa P,
Costa H, Assinger A, Vetvik KK, Bukholm IR, Yaiw KC, Smedby KE, et
al: High prevalence of human cytomegalovirus in brain metastases of
patients with primary breast and colorectal cancers. Transl Oncol.
7:732–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rahbar A, Pantalone MR, Religa P, Rådestad
AF and Söderberg-Naucler C: Evidence of human cytomegalovirus
infection and expression of 5-lipoxygenase in borderline ovarian
tumors. J Med Virol. 93:4023–4027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bai B, Wang X, Chen E and Zhu H: Human
cytomegalovirus infection and colorectal cancer risk: A
meta-analysis. Oncotarget. 7:76735–76742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lv YL, Han FF, An ZL, Jia Y, Xuan LL, Gong
LL, Zhang W, Ren LL, Yang S, Liu H and Liu LH: Cytomegalovirus
infection is a risk factor in gastrointestinal cancer: A
cross-sectional and meta-analysis study. Intervirology. 63:10–16.
2020. View Article : Google Scholar
|
|
58
|
Chen HP, Jiang JK, Lai PY, Chen CY, Chou
TY, Chen YC, Chan CH, Lin SF, Yang CY, Chen CY, et al: Tumoral
presence of human cytomegalovirus is associated with shorter
disease-free survival in elderly patients with colorectal cancer
and higher levels of intratumoral interleukin-17. Clin Microbiol
Infect. 20:664–671. 2014. View Article : Google Scholar
|
|
59
|
Bongers G, Maussang D, Muniz LR, Noriega
VM, Fraile-Ramos A, Barker N, Marchesi F, Thirunarayanan N, Vischer
HF, Qin L, et al: The cytomegalovirus-encoded chemokine receptor
US28 promotes intestinal neoplasia in transgenic mice. J Clin
Invest. 120:3969–3978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mahdavinia M, Bishehsari F, Verginelli F,
Cumashi A, Lattanzio R, Sotoudeh M, Ansari R, Semeraro D, Hormazdi
M, Fakheri H, et al: P53 mutations in colorectal cancer from
northern Iran: Relationships with site of tumor origin,
microsatellite instability and K-ras mutations. J Cell Physiol.
216:543–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bishehsari F, Mahdavinia M, Malekzadeh R,
Verginelli F, Catalano T, Sotoudeh M, Bazan V, Agnese V, Esposito
DL, De Lellis L, et al: Patterns of K-ras mutation in colorectal
carcinomas from Iran and Italy (a Gruppo Oncologico dell'Italia
Meridionale study): Influence of microsatellite instability status
and country of origin. Ann Oncol. 17(Suppl 7): vii91–vii96. 2006.
View Article : Google Scholar
|
|
62
|
Esposito DL, Aru F, Lattanzio R, Morgano
A, Abbondanza M, Malekzadeh R, Bishehsari F, Valanzano R, Russo A,
Piantelli M, et al: The insulin receptor substrate 1 (IRS1) in
intestinal epithelial differentiation and in colorectal cancer.
PLoS One. 7:e361902012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lattanzio R, Marchisio M, La Sorda R,
Tinari N, Falasca M, Alberti S, Miscia S, Ercolani C, Di Benedetto
A, Perracchio L, et al: Overexpression of activated phospholipase
Cγ1 is a risk factor for distant metastases in T1-T2, N0 breast
cancer patients undergoing adjuvant chemotherapy. Int J Cancer.
132:1022–1031. 2013. View Article : Google Scholar
|
|
64
|
Rahbar A, Orrego A, Peredo I, Dzabic M,
Wolmer-Solberg N, Strååt K, Stragliotto G and Söderberg-Nauclér C:
Human cytomegalovirus infection levels in glioblastoma multiforme
are of prognostic value for survival. J Clin Virol. 57:36–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bu XD, Li N, Tian XQ and Huang PL: Caco-2
and LS174T cell lines provide different models for studying mucin
expression in colon cancer. Tissue Cell. 43:201–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Al-Badr AA and Ajarim TDS: Ganciclovir.
Profiles Drug Subst Excip Relat Methodol. 43:1–208. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cai H, Kapoor A, He R, Venkatadri R,
Forman M, Posner GH and Arav-Boger R: In vitro combination of
anti-cytomegalovirus compounds acting through different targets:
Role of the slope parameter and insights into mechanisms of action.
Antimicrob Agents Chemother. 58:986–994. 2014. View Article : Google Scholar :
|
|
68
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
69
|
Sinzger C, Digel M and Jahn G:
Cytomegalovirus cell tropism. Curr Top Mirobiol Immunol. 325:63–83.
2008.
|
|
70
|
Fields BN, Knipe DM and Howley PM: Fields
virology. Wolters Kluwer Health/Lippincott Williams & Wilkins;
Philadelphia: 2007
|
|
71
|
Herbein G: Tumors and cytomegalovirus: An
intimate interplay. Viruses. 14:8122022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Peredo-Harvey I, Rahbar A and
Söderberg-Nauclér C: Presence of the human cytomegalovirus in
glioblastomas-a systematic review. Cancers (Basel). 13:50512021.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cobbs CS, Matlaf L and Harkins LE: Methods
for the detection of cytomegalovirus in glioblastoma cells and
tissues. Methods Mol Biol. 1119:165–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dziurzynski K, Chang SM, Heimberger AB,
Kalejta RF, Dallas SR, Smit M, Soroceanu L and Cobbs CS; HCMV and
Gliomas Symposium: Consensus on the role of human cytomegalovirus
in glioblastoma. Neuro Oncol. 14:246–255. 2012. View Article : Google Scholar :
|
|
75
|
Herbein G: The human cytomegalovirus, from
oncomodulation to oncogenesis. Viruses. 10:4082018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cobbs C: Cytomegalovirus is a
tumor-associated virus: Armed and dangerous. Curr Opin Virol.
39:49–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qiu H, Straat K, Rahbar A, Wan M,
Soderberg-Naucler C and Haeggstrom JZ: Human CMV infection induces
5-lipoxygenase expression and leukotriene B4 production in vascular
smooth muscle cells. J Exp Med. 205:19–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hooks JJ, Chin MS, Srinivasan K, Momma Y,
Hooper LC, Nagineni CN, Chan CC and Detrick B: Human
cytomegalovirus induced cyclooxygenase-2 in human retinal pigment
epithelial cells augments viral replication through a prostaglandin
pathway. Microbes Infect. 8:2236–2244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Söderberg-Nauclér C, Fish KN and Nelson
JA: Reactivation of latent human cytomegalovirus by allogeneic
stimulation of blood cells from healthy donors. Cell. 91:119–126.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Reeves M and Sinclair J: Aspects of human
cytomegalovirus latency and reactivation. Curr Top Microbiol
Immunol. 325:297–313. 2008.PubMed/NCBI
|
|
81
|
Söderberg-Nauclér C, Fish KN and Nelson
JA: Interferon-gamma and tumor necrosis factor-alpha specifically
induce formation of cytomegalovirus-permissive monocyte-derived
macrophages that are refractory to the antiviral activity of these
cytokines. J Clin Invest. 100:3154–3163. 1997. View Article : Google Scholar
|
|
82
|
Reeves MB and Compton T: Inhibition of
inflammatory interleukin-6 activity via extracellular
signal-regulated kinase-mitogen- activated protein kinase signaling
antagonizes human cytomegalovirus reactivation from dendritic
cells. J Virol. 85:12750–12758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Griffiths P, Baraniak I and Reeves M: The
pathogenesis of human cytomegalovirus. J Pathol. 235:288–297. 2015.
View Article : Google Scholar
|
|
84
|
Yi HA, Kim MS, Jang SY, Lee YM, Ahn JH and
Lee CH: Cellular signals involved in cyclooxygenase-2 expression
induced by human cytomegalovirus. Virus Res. 146:89–96. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang Y, Ma D, Huang H, Lu Y, Liao Y, Liu
L, Liu X and Fang F: Interaction between HCMV pUL83 and human AIM2
disrupts the activation of the AIM2 inflammasome. Virol J.
14:342017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Söderberg-Nauclér C: Does cytomegalovirus
play a causative role in the development of various inflammatory
diseases and cancer? J Intern Med. 259:219–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Teo WH, Chen HP, Huang JC and Chan YJ:
Human cytomegalovirus infection enhances cell proliferation,
migration and upregulation of EMT markers in colorectal
cancer-derived stem cell-like cells. Int J Oncol. 51:1415–1426.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Maussang D, Verzijl D, van Walsum M, Leurs
R, Holl J, Pleskoff O, Michel D, van Dongen GA and Smit MJ: Human
cytomegalovirus-encoded chemokine receptor US28 promotes
tumorigenesis. Proc Natl Acad Sci USA. 103:13068–13073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Heukers R, Fan TS, de Wit RH, van Senten
JR, De Groof TWM, Bebelman MP, Lagerweij T, Vieira J, de Munnik SM,
Smits-de Vries L, et al: The constitutive activity of the virally
encoded chemokine receptor US28 accelerates glioblastoma growth.
Oncogene. 37:4110–4121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rahbar A, Boström L, Lagerstedt U,
Magnusson I, Söderberg-Naucler C and Sundqvist VA: Evidence of
active cytomegalovirus infection and increased production of IL-6
in tissue specimens obtained from patients with inflammatory bowel
diseases. Inflam Bowel Dis. 9:154–161. 2003. View Article : Google Scholar
|
|
91
|
Sääf AM, Halbleib JM, Chen X, Yuen ST,
Leung SY, Nelson WJ and Brown PO: Parallels between global
transcriptional programs of polarizing Caco-2 intestinal epithelial
cells in vitro and gene expression programs in normal colon and
colon cancer. Mol Biol Cell. 18:4245–4260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Creff J, Malaquin L and Besson A: In vitro
models of intestinal epithelium: Toward bioengineered systems. J
Tissue Eng. 12:20417314209852022021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Schröer J and Shenk T: Inhibition of
cyclooxygenase activity blocks cell-to-cell spread of human
cytomegalovirus. Proc Natl Acad Sci USA. 105:19468–19473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Speir E, Yu ZX, Ferrans VJ, Huang ES and
Epstein SE: Aspirin attenuates cytomegalovirus infectivity and gene
expression mediated by cyclooxygenase-2 in coronary artery smooth
muscle cells. Circ Res. 83:210–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Merchut-Maya JM, Bartek J Jr, Bartkova J,
Galanos P, Pantalone MR, Lee M, Cui HL, Shilling PJ, Brøchner CB,
Broholm H, et al: Human cytomegalovirus hijacks host stress
response fueling replication stress and genome instability. Cell
Death Differ. 29:1639–1653. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guirguis-Blake JM, Evans CV, Perdue LA,
Bean SI and Senger CA: Aspirin use to prevent cardiovascular
disease and colorectal cancer: Updated evidence report and
systematic review for the US preventive services task force. JAMA.
327:1585–1597. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rovati G, Contursi A, Bruno A, Tacconelli
S, Ballerini P and Patrignani P: Antiplatelet agents affecting GPCR
signaling implicated in tumor metastasis. Cells. 11:7252022.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wojtukiewicz MZ, Hempel D, Sierko E,
Tucker SC and Honn KV: Antiplatelet agents for cancer treatment: A
real perspective or just an echo from the past? Cancer Metastasis
Rev. 36:305–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gareau AJ, Brien C, Gebremeskel S, Liwski
RS, Johnston B and Bezuhly M: Ticagrelor inhibits platelet-tumor
cell interactions and metastasis in human and murine breast cancer.
Clin Exp Metastasis. 35:25–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hobson AR, Qureshi Z, Banks P and Curzen
NP: Effects of clopidogrel on 'aspirin specific' pathways of
platelet inhibition. Platelets. 20:386–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Landolfi R, Mower RL and Steiner M:
Modification of platelet function and arachidonic acid metabolism
by bioflavonoids. Structure-activity relations. Biochem Pharmacol.
33:1525–1530. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ribeiro D, Freitas M, Tome SM, Silva AM,
Laufer S, Lima JL and Fernandes E: Flavonoids inhibit COX-1 and
COX-2 enzymes and cytokine/chemokine production in human whole
blood. Inflammation. 38:858–870. 2015. View Article : Google Scholar
|
|
103
|
Stragliotto G, Pantalone MR, Rahbar A,
Bartek J and Söderberg-Naucler C: Valganciclovir as add-on to
standard therapy in glioblastoma patients. Clin Cancer Res.
26:4031–4039. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Stragliotto G, Pantalone MR, Rahbar A and
Söderberg-Nauclér C: Valganciclovir as add-on to standard therapy
in secondary glioblastoma. Microorganisms. 8:14712020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pantalone MR, Rahbar A, Söderberg-Naucler
C and Stragliotto G: Valganciclovir as Add-on to second-line
therapy in patients with recurrent glioblastoma. Cancers (Basel).
14:19582022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Batich KA, Reap EA, Archer GE,
Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon
JE II, Healy P, et al: Long-term survival in glioblastoma with
cytomegalovirus pp65-targeted vaccination. Clin Cancer Res.
23:1898–1909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Batich KA, Mitchell DA, Healy P, Herndon
JE II and Sampson JH: Once, twice, three times a finding:
Reproducibility of dendritic cell vaccine trials targeting
cytomegalovirus in glioblastoma. Clin Cancer Res. 26:5297–5303.
2020. View Article : Google Scholar : PubMed/NCBI
|