Introduction
Bladder cancer is one of the most common cancers in
the world population and the most common urological malignancy
(1). Its pathogenesis is complex
with the risk varying by sex, intrinsic genetic factors and age. In
addition, it is heavily influenced by the degree of exposure to a
number of carcinogens due to different life circumstances, such as
smoking (2). Treatment of early
bladder cancer is primarily by surgical resection, but surgical
intervention is general ineffective for patients with advanced
disease or developed metastases (3). Further understanding of bladder
cancer through molecular biology and genetics studies has led to
the development of diagnostic and therapeutic modalities for
localized and advanced bladder cancer. Notable breakthroughs
include intravesical infusion of bacilli Calmette-Guerin vaccine,
immunotherapy with checkpoint inhibition, targeted therapy and
antibody-drug adjuvants (4).
However, bladder cancer is a molecularly heterogeneous disease,
where genomic expression analysis has shown that different
expression profiles are associated with different profiles of
cancer progression and metastasis (5). Based on the different gene
expression profiles, selection of the optimal therapeutic approach
has become a key factor for increasing the efficacy of bladder
cancer treatment.
In a variety of solid tumors, such as breast
(6), lung (7), colon (8), ovarian, bladder, prostate, head and
neck tumors (9,10). EGFR has been reported to serve an
important role in cell origin, apoptosis, tumor vascularization and
metastasis (11,12). In particular, receptor
tyrosine-protein kinase erbB HER-1 and HER-2, being similar in
structure and function, serves key roles in tumorigenesis and share
homologous sequences with expressed oncogenes and/or viral
oncogenes (13).
CUDC-101, a multipathway inhibitor, has been
reported to inhibit both histone deacetylases (HDAC) and receptor
kinases (14). Additionally,
CUDC-101 combined with HDAC inhibition was found to block the key
regulator of the EGFR/HER2 signaling pathway synergistically and
attenuated multiple surrogate pathways. This enhanced the potential
for treating heterogeneous and resistant tumors that cannot be
controlled by single-target drugs (15,16). It also showed a favorable
preclinical safety profile in a Phase I clinical trial and had no
effect on the growth of normal urinary tract epithelial cells
(17) and CUDC-101 was developed
as a novel anti-cancer drug (18).
At present, targeted therapies for bladder cancer in
the presence of EGFR are poorly studied. In order to explore
targeted agents for the treatment of bladder cancer, the present
study chose this multipathway inhibitor to test its effect on the
growth of EGFR overexpressing bladder cancer cells.
Materials and methods
Drugs and reagents
Human bladder cancer T24 cell was purchased from
Procell Life Science & Technology Co., Ltd. Human embryonic
kidney cell 293T was purchased from Gaining Biological. CUDC-101
was purchased from Selleck Chemicals. All molecular cloning
reagents were purchased from New England Biolabs, Inc. MTT,
protease inhibitor cocktail, Halt phosphatase inhibitor cocktail,
BCA protein quantification kit were purchased from Beijing Solarbio
Science & Technology Co., Ltd. BeyoClick EdU cell proliferation
kit with Alexa Fluor 555 (cat. no. C0075S), mitochondrial membrane
potential and apoptosis detection kit with Mito-Tracker Red CMXRos
(cat. no. C1071M), Annexin V-FITC, FITC annexin V solution and PI
(cat. no. ST512) were purchased from Beyotime Institute of
Biotechnology. All antibodies were purchased from Proteintech
Group, Inc. Blasticidin (BSD) was purchased from Thermo Fisher
Scientific, Inc. RPMI1640 complete medium, DMEM, FBS, penicillin
and streptomycin were purchased from Gibco (Thermo Fisher
Scientific, Inc.). The fluorescent microscope used was Eclipse Ti
(Nikon Corporation). The flow cytometer used was BD FACSCanto (BD
Biosciences).
Cell culture
The medium used for 293T cells was DMEM containing
10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The
medium used for the human bladder cancer cell lines T24 (bladder
cell lines) was RPMI1640 complete medium containing 10% FBS, 100
U/ml penicillin and 100 μg/ml streptomycin. The medium used
for T24-EGFR-everexpressing (EGFR-OE) cells was RPMI1640 complete
medium containing 10% FBS, 100 U/ml penicillin, 100 μg/ml
streptomycin and 10 μg/ml BSD-HCl. All cells were cultured
in a cell culture incubator at 37°C, and 5% CO2 with
saturated humidity. Cells were passaged at logarithmic growth
phases and frozen for storage according to the experimental
requirement.
Establishment of the T24-EGFR-OE cell
line
The 293T cells were cultured in six-well plates and
co-transfected with the pLenti-EF1α plasmid containing the
wild-type EGFR gene (deposited in the Laboratory of the
Translational Center of Huaihe Hospital, Henan University) and two
packaging plasmids psPAX2 and pMD2.G (deposited in the Laboratory
of the Translational Center of Huaihe Hospital, Henan University)
at a 1:1:1 molar ratio using the cationic polymer transfection
reagent (EZ Trans cell transfection reagent; Life-iLab) when cell
confluency reached 80%. Cells were grown for 16 h at 37°C in a
humidified atmosphere with 5% CO2 before being replaced
with fresh medium. After a further 48 h, the cell supernatant
containing the viral particles were collected and filtered through
a 0.45-μM filter before being added to T24 cells when they
reached 80% confluency. At 24 h later, the culture medium of T24
cells was replaced with a fresh medium. After another 48 h, a
medium containing 10 μg/ml BSD was added for 14 days for
screening.
Cell survival and viability assays
MTT assay was used for cell survival and viability
assays. T24 and T24-EGFR-OE cells in the logarithmic growth phase
were seeded into 96-well plates at a density of 5,000 cells per
well. After 18 h, the medium from each well was removed, before 100
μl of medium containing different concentrations of CUDC-101
was added and incubated for 48 h. Subsequently, 20 μl of MTT
was added, before incubation continued for a further 4 h in an
incubator at 37°C. The supernatant was then discarded at the end of
the culture and 100 μl DMSO solution was added into each
well. The plates were placed in a shaker at 37°C under light-proof
conditions for 15 min, before the optical density (OD) was measured
using a microplate reader.
Transcriptome sequencing
Cells in the logarithmic growth phase were seeded
into 10-cm dishes at a density of ~5 million cells per well, where
they were left to stably attached to the bottom of the dish. The
medium was then aspirated before 1 μM of the CUDC-101 was
added. The reaction was terminated after 48 h of drug treatment,
before total cellular RNA was extracted by TRIzol®
lysis, chloroform separation, and RNA precipitation with isoamyl
alcohol and anhydrous ethanol. In total, three independent
replicates were made for each sample and the extracted RNA was
stored on dry ice.
Integrity of the test sample was detected by nucleic
acid gel electrophoresis using a NanoDrop 2000 (Thermo Fisher
Scientific, Inc.). The concentrations of each sample in the present
study was >50 ng/μl and library concentration was ~7 pM
with a volume of 30 μl for sequencing. The length bit was
150 bp and the sequencing direction was 'paired end'. The
transcriptome data was performed using the Illumina sequencing
platform [VAHTS Universal V8 RNA-seq Library Prep Kit for Illumina
(Vazyme Biotech Co., Ltd.)] and the raw data were optimized to
obtain high-quality clean reads. Differential gene analysis among
the samples was performed using DESeq2 (all software provided by
GoldViz Biotechnology Co., Ltd. https://www.genewiz.com.cn/). Fold Change (FC) was
used to represents the ratio of expression between two sample
groups, where the screening criteria were Log2FC >2
and P≤0.05. Multiple testing for statistically significant
differences were corrected according to the false discovery rate.
The differentially expressed genes were enriched in Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases
(all software provided by GoldViz Biotechnology Co., Ltd.,
https://www.genewiz.com.cn/) to analyze
the biochemical metabolic pathways and signal transduction pathways
with which they are associated.
Cell proliferation assay
EdU was used for cell proliferation assay. Cells in
the logarithmic growth phase were seed into 96-well plates at a
density of 5,000 cells per well, before the medium in the wells was
changed to CUDC-101-containing medium after 18 h before further
culturing for 48 h at 37°C. A total of 100 μl of EdU was
added into each well, then cultured at 37°C for 1 h. Afterwards,
the medium was removed and the cells were fixed with 4%
paraformaldehyde at 37°C for 15 min, blocked with 3% bovine serum
albumin in PBS at room temperature for 5 min and permeabilized in
0.3% Triton X-100 in PBS at room temperature for 5 min. The cells
were then washed sequentially and 50 μl prepared click
reaction solution was added to label proliferating cells in the
dark at room temperature for 30 min. The medium was then removed
and the cells were washed again, before 50 μl Hoechst was
added to label all nuclei and incubated in the dark at room
temperature for 10 min. The cells were then immediately observed by
fluorescence microscopy (CX31; Olympus Corporation).
Cell cycle assay
Cells in the logarithmic growth phase were seeded
into six-well plates at a density of 6×105 cells per
well. After the cells completely adhered to the bottom of the dish,
a medium containing different concentrations of CUDC-101 was added
before culturing continued for 48 h at 37°C. The medium was then
removed and the cells were washed with PBS, before they were
digested with 0.25% trypsin. These digested cells were collected
and centrifuged at 600 × g for 5 min to remove the supernatant,
before the tubes were transferred onto ice and the cells were
slowly resuspended with pre-chilled 70% ethanol and left at -20°C
for 24 h. Cells were removed the next day and centrifuged at 1,000
× g for 5 min at 4°C. The ethanol was removed and cells were
resuspended in 1 ml ice-cold PBS, before they cells were
centrifuged at 500 × g for 10 min at 4°C and the PBS was removed.
Cells were then resuspended in 0.5 ml of staining solution and
incubated at 37°C for 30 min in the dark. The cells were washed
twice with PBS and resuspend with pre-chilled PBS. Cells were
analyzed within 24 h by flow cytometry [CytoFLEX S; Bellman
Coulter, Inc.; FlowJo v10.6.2 (FlowJo LLC) was used to analyze the
data].
Colony formation assay
Cells in the logarithmic growth phase were seeded
into six-well plates at a density of 500 cells per well and left to
fully attach to the bottom. A medium containing different
concentrations of CUDC-101 was added. The cells were incubated at
37°C for 2 weeks, during which the medium was changed once every 3
days. When the colonies became visible in the dishes to with the
naked eye, the culture was terminated and the medium was removed.
The cells were fixed with 4% paraformaldehyde for 10 min and washed
twice with PBS, before 1X Giemsa stain was added for 10 min at room
temperature. Finally, PBS was rinsed and dried for imaging using a
gel imager (ChemiDoc; Bio-Rad Laboratories, Inc.).
Cell morphology observation
Cells in the logarithmic growth phase were seeded
into 12-well plates with cell crawling sheets at a density of
5×104 per well. After the cells attached to the bottom,
different concentrations of CUDC-101 were added to continue the
culturing for 48 h. Subsequently, the cell supernatant was removed,
the cells were washed with PBS for 5 min and fixed with
paraformaldehyde for 10 min, before being treated with
TRITC-phalloidin solution and DAPI solution for 10 min at room
temperature. The cells were placed on cover slides in the bottom of
the petri dish, before being imaged and observed by fluorescence
microscopy after the adsorption was stabilized.
Mitochondrial membrane potential and
apoptosis assay
Cells were seed into 96-well plates at a density of
5,000 per well and incubated at 37°C for 18 h. The supernatant was
then removed, before culture medium containing different
concentrations of CUDC-101 was added and incubation continued for
48 h. The 96-well plates were removed and the cells were
centrifuged at 1,000 × g for 5 min at room temperature, before the
culture medium was removed. In total, 100 μl PBS was added
to each well for washing, followed by centrifugation at 1,000 × g
for 5 min at room temperature before removing the PBS. After
removing PBS, 32 μl Annexin V-FITC conjugate was added into
each well, before add 0.84 μl Annexin V-FITC staining
solution, 0.34 μl Mito-Tracker Red CMXRos staining solution
and 0.84 μl Hoechst 33342 staining solution were mixed
gently and added into each well for incubation at room temperature
in the dark for 20 min. Images were captured under a fluorescent
microscope.
Flow cytometry for apoptosis
Cells in the logarithmic growth phase were seeded
into six-well plates at a density of 6×105 cells per
well, before they were allowed adhered to the bottom completely. A
medium containing different concentrations of CUDC-101 was then
added culture at 37°C for 48 h. Subsequently, the supernatant was
aspirated into Eppendorf tubes, before the cells were washed with
PBS and digested with 0.25% trypsin. The digested cells were then
collected, mixed with supernatant and centrifuged in 1,000 × g for
5 min at room temperature, washed and resuspended again with PBS,
stained with Annexin V-FITC and PI at room temperature for 15 min
to label the apoptotic cells. Finally, the percentage of apoptosis
was detected. Calculation of apoptosis rate was by the percentage
of early + late apoptotic cells. Flow cytometer CytoFLEX S was used
for the detection (Bellman Coulter, Inc.) and FlowJo v10.6.2
(FlowJo LLC) was used to analyze the data.
Western blot analysis
Cells in the logarithmic growth phase were seeded
into six-well plates at a density of 6×105 cells per
well and were allowed to adhere to the bottom completely. Media
containing different CUDC-101 concentrations was added for
incubation for 48 h and the control group was only treated with
1640 medium. The cells were then lysed with RIPA lysis buffer
(Shanghai Biyuntian Biotechnology Research Institute) to extract
total cellular proteins. Protein determination was by BCA from
Beijing Kangwei Century Co., Ltd. A total of 50 μg of
protein was loaded per lane and separated on 10% gel before
transfer to NC membrane. Blocking was by 5X loading buffer from
Shanghai Biyuntian Biotechnology Co., Ltd at 100°C for 5 min The
following antibodies were used: Anti-Bcl-XL (1:2,000), anti-Bad
(1:2,000), anti-heme oxygenase-1 (HO-1; 1:2,000), anti-ERK
(1:2,000), anti-phosphorylated (p-) ERK (1:2,000), anti-AKT
(1:1,000), anti-p-AKT (1:2,000), anti-EGFR (1:1,000), anti-p-EGFR
(Cell Signaling Technology, 1:1,000, USA), anti-GAPDH (1:50,000),
Goat Anti-Rabbit IgG (1:5,000), Goat Anti-Mouse IgG (1:5,000). All
antibodies were purchased from Wuhan Sanying Biotechnology Co.,
Ltd. Primary antibody incubation was at 4°C for 12 h and secondary
antibody incubation was at room temperature for 1 h. ECL
chemiluminescence kit purchased from Shanghai Biyuntian
Biotechnology Co., Ltd. and ImageJ (version 1.53f51; National
Institutes of Health) was used for densitometry.
Statistical analysis
All data are presented as the mean ± SEM. One-way
ANOVA (with Bonferroni for post hoc test) was used for statistical
comparisons between the groups. Tests were performed out using SPSS
21.0 software (IBM Corp.) and drawn using GraphPad Prism 8.0
software (GraphPad Software, Inc.; Dotmatics). Cutadapt (V1.9.1),
Hisat2 (v2.0.1), HTSeq (v0.6.1), were used for data processing and
gene analysis. GOSeq (v1.34.1) was used for GO and KEGG analysis;
all software provided by GoldViz Biotechnology Co., Ltd.
(https://www.genewiz.com.cn/). Pearson
correlation analysis was used to analyze RNA sample correlation on
transcriptome sequencing. Raw data generated by Illumina
transcriptome sequencing has been submitted to the NCBI public
database (BioProject ID: PRJNA1010990).
Results
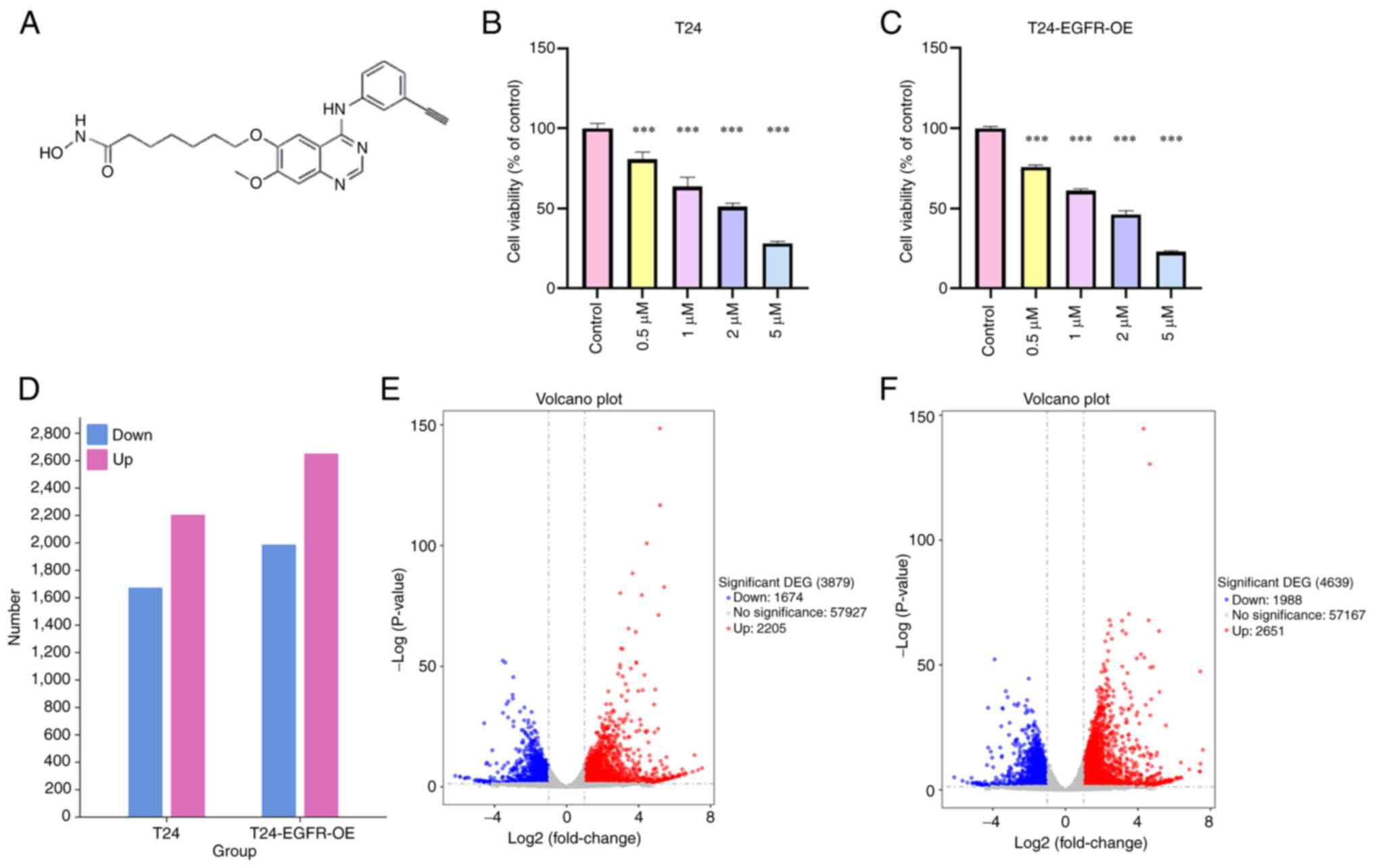
CUDC-101 decreases T24 cell
viability
To investigate the effect of CUDC-101 on cell
viability, T24 and T24-EGFR-OE cells were treated with different
concentrations of CUDC-101 before being assayed using MTT method
after 48 h. The results are shown in Fig. 1B and C. The cell viability of both
cell types were decreased by CUDC-101 treatment in a dose-dependent
manner, with the difference being statistically significant
compared with that in the control group.
CUDC-101 can regulate gene expression in
T24 cells
RNA was extracted from the drug-treated cells,
before transcriptome sequencing of the cells was completed using
the Illumina sequencing platform (Figs. 1 and S1; Tables SI-IV), RNA was extracted from
the drug-treated cells, before transcriptome sequencing of the
cells was completed using the Illumina sequencing platform
(Figs. 1 and S1; Tables SI-IV). After the drug treatment
of T24 cells, the expression of 2,205 genes were found to be
upregulated, including cytochrome P450 family 1 subfamily A member
1 (CYP1A1), transmembrane protein 59-like (TMEM59L) and
TGFβ-induced (TGFBI). By contrast, the expression of 1,674 genes
were found to be decreased, including erythroferrone (ERFE), UNC-5
netrin receptor B (UNC5B) and microRNA-503 host gene (MIR503HG). In
T24-EGFR-OE cells, the expression of 2,651 genes [including
retbindin (RTBDN), CYP1A1, TMEM59L and TGFBI] were found to be
upregulated, whereas 1,988 genes [such as pappalysin 2, IL-1
receptor-like 1, ERFE, spectrin repeat containing nuclear envelope
family member 3 (SYNE3) and thrombomodulin (THBD)] were found to be
downregulated. As shown in Tables
SI-IV, a number of genes with similar functions or with close
associations with each other had their expression levels altered
before and after drug administration in both groups of cells. All
differentially expressed genes were then analyzed by GO and KEGG
enrichment, where the top 30 most significantly enriched GO terms
were selected (Figs. S2 and S3).
In addition, the most significantly enriched pathway entries are
shown in Figs. S4 and S5. The
concentration and quality of the RNA samples are shown in Tables SV and SVI. The Pearson's
correlation coefficients of the RNA samples are shown in Fig. S6.
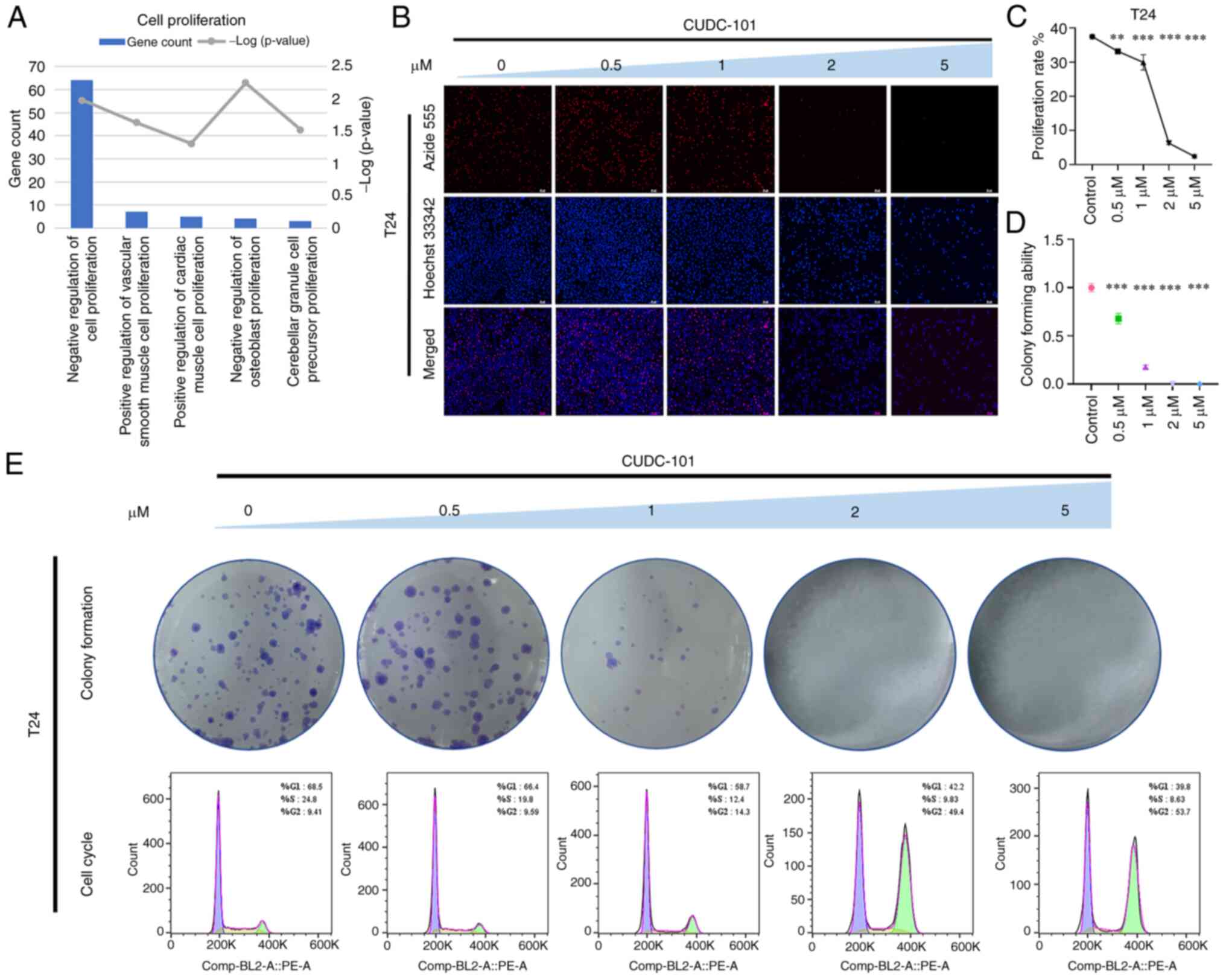
CUDC-101 decreased the proliferative
capacity of T24-EGFR-OE cells
GO enrichment analysis of genes that were
significantly differentially expressed in T24-EGFR-OE cells
following CUDC-101 treatment revealed that the effects mediated by
CUDC-101 was significantly associated with cell proliferation
biological processes (Fig. 2A).
As shown in Fig. 2B, the overall
proportion of surviving cells after drug treatment was decreased
compared with that in the control group, where number of
proliferative cells 1 h after treatment was also decreased compared
with that in the control group. Furthermore, the total number of
cells and the number of proliferative cells decreased in magnitude
as the drug concentration increased. The number of proliferating
cells compared with the overall number of cells was used as the
metric to calculate the proliferation rate of cells (Fig. 2C). The proliferation rate of the
cells in the drug-treated groups was found to be lower compared
with that in the control group, in a concentration-dependent
manner.
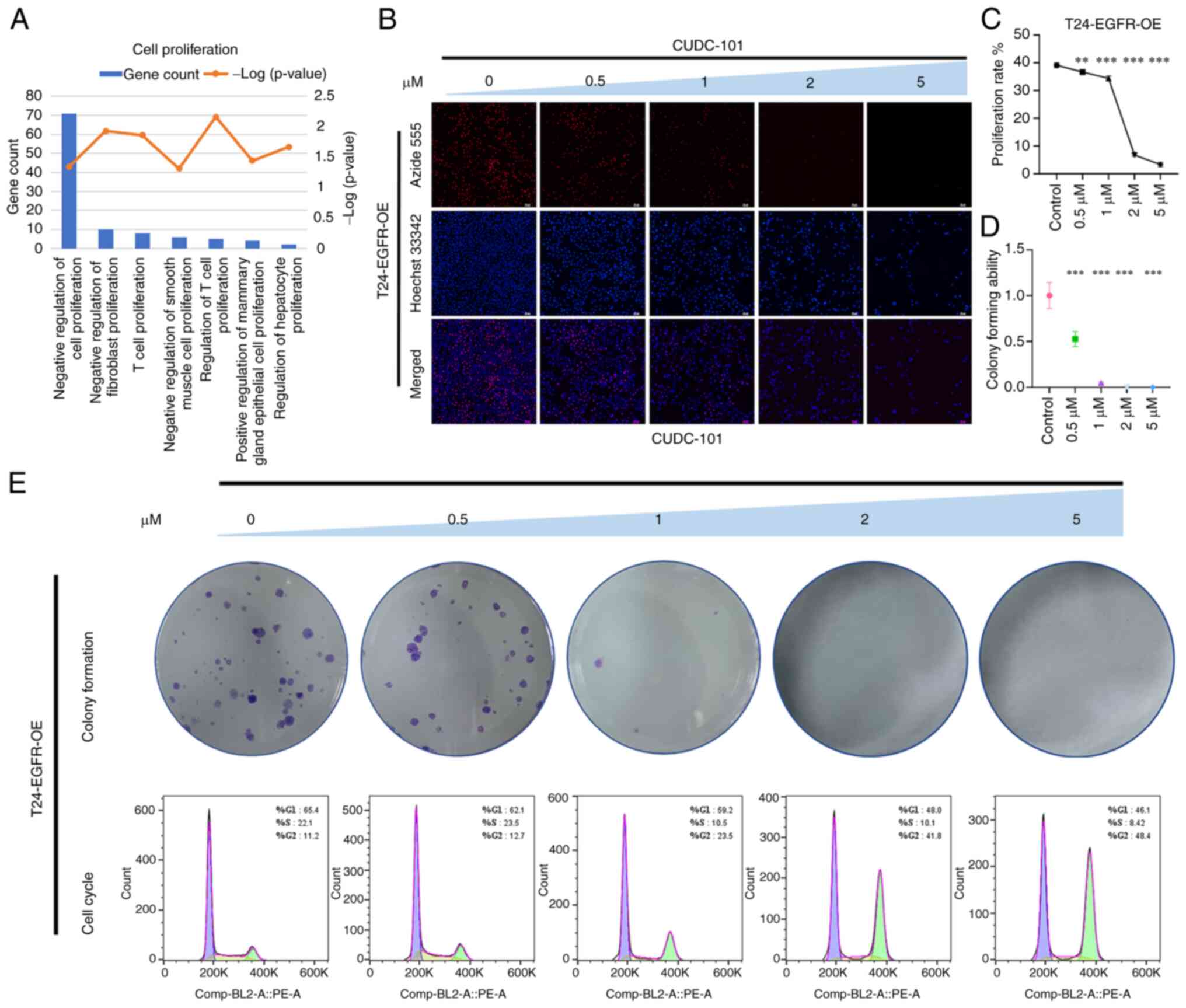 | Figure 2Effect of CUDC-101 on the
proliferative capacity of T24-EGFR-OE cells. (A) The results of
Gene Ontology enrichment analysis of the differentially expressed
genes after CUDC-101 treatment on T24-EGFR-OE cells. (B) The
results of EdU staining, Azide 555 was used to label the
proliferative cells and Hoechst 33342 was used to label all nuclei.
Magnification, ×100; Scale bar, 50 μm. (C) Effects of
CUDC-101 on the growth rates of T24-EGFR-OE cells at 0, 0.5, 1, 2
and 5 μM. The proliferation rate was obtained from the
results of EdU experiment, where the concentrations were presented
from high to low. Data are presented as mean ± SEM from three
independent experiments. (D and E) The colony formation assay.
Comparing the CUDC-101 groups with the control group, the
percentage of the clone number in each group was calculated by
normalizing to the control group, with the latter identified as
'1'. Data are presented as the mean ± SEM from three independent
experiments. **P<0.01 and ***P<0.001
vs. control. In the lower part of Fig. 2E are the cell cycle results. OE,
overexpression. |
As shown in Fig.
2E, the number of colonies in the experimental group was found
to be lower compared with that in the control group after treatment
with CUDC-101, also in a concentration-dependent manner. The number
of colonies in the control group was set to 1, before the
clonogenic capacity of cells in the drug-treated groups was
obtained by dividing the number of colonies drugs-treated groups by
that in the control group. As the concentration increased, the
clonogenic capacity of the cells correspondingly decreased, with
the difference between the CUDC-101-treated groups and the control
group being significant (Fig.
2D). In particular, the 2 μM CUDC-101 group did not show
any colony formation. The cell cycle assay results are shown in
Fig. 2E. CUDC-101 treatment was
found to decrease the number of cells in G1- and
S-phases whilst inducing G2/M-phase cell aggregation in
a dose-dependent manner. This suggested that CUDC-101 may have
caused cell cycle arrest, which may be one of the causes of the
inhibited cell proliferation.
CUDC-101 reduces the proliferative
capacity of T24 cells
In T24 cells, the effects mediated by CUDC-101 was
found to be significantly associated with cell proliferation or
growth biological processes (Fig.
3A). The effect of CUDC-101 on the proliferation of T24 cells
was also examined. As shown in Fig.
3B and C, the number of proliferative cells and the
proliferation rate of cells showed a concentration-dependent
decrease with increasing drug concentration. As shown in Fig. 3E, cell cycle was similarly blocked
at the G2/M phase, which may have caused the inhibition
of cell proliferation. In addition, the number of T24 cell colonies
was also found to be decreased compared with that in the control
group after treatment with CUDC-101, which increased in magnitude
decreased as the concentration of CUDC-101 increased (Fig. 3E). The colony-forming ability of
the cells in the treated group was significantly lower compared
with that in the control group (Fig.
3D). At 2 μM, the T24 cells were not able to form any
colonies.
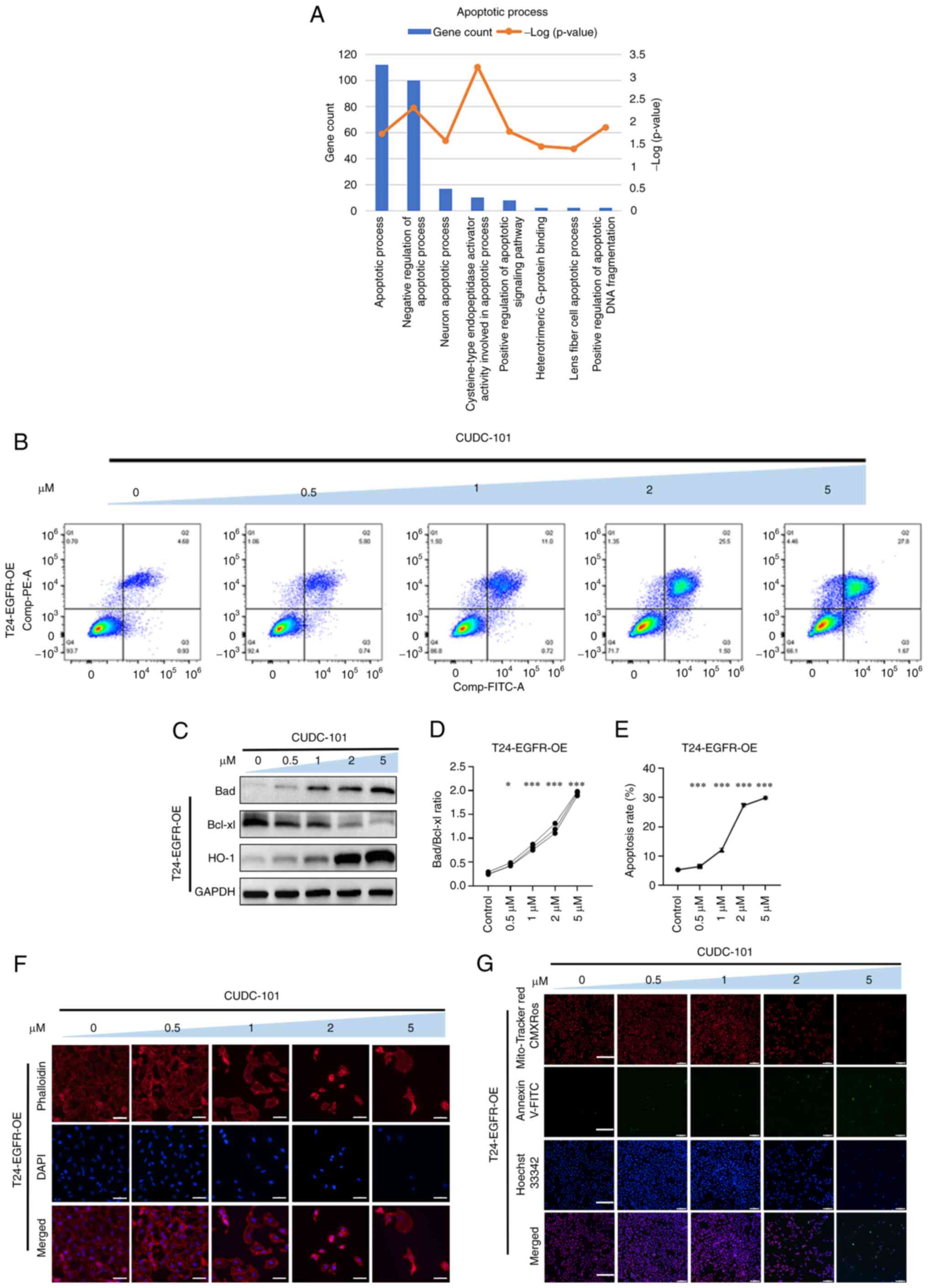
CUDC-101 promotes the apoptosis of
T24-EGFR-OE cells
GO enrichment analysis of genes that were
significantly differentially expressed in T24-EGFR-OE cells
following CUDC-101 treatment showed that the effects mediated by
CUDC-101 were significantly associated with apoptosis or death
biological processes (Fig.
4A).
As shown in Fig.
4F, the cells in the control group exhibited normal cell shape.
Furthermore, their microfilaments appear normal and evenly
distributed with intact nuclei, where some cells also showed a
number of microfilament tentacles. Following CUDC-101 treatment,
the cell numbers decreased, with cells becoming aggregated and
adherent. A number of cells also became irregular morphologically.
In addition, various cells ruptured with their microfilaments
either maldistributed or wrinkled, with disrupted nuclei. These
observations were found to be increase as dose of CUDC-101
increased. These results suggested that CUDC-101 can reduce the
number of T24-EGFR-OE cells, alter the cell morphology and
redistribute the cytoskeletal microfilament structure in the cells,
in addition to inducing nucleus fragmentation or even cell
death.
As shown in Fig.
4G, the overall number of cells was decreased after drug
treatment compared with that the control group. Specifically, the
degree of red fluorescence was decreased, suggesting that the
mitochondrial membrane potential decreased after drug
administration. Furthermore, the number of cells with green
fluorescence was increased, suggesting that apoptosis was increased
and that CUDC-101 exerted pro-apoptotic effects on T24-EGFR-OE
cells. CUDC-101 also appeared to have reduced the mitochondrial
membrane potential of T24 cells.
Apoptosis was next detected by Annexin V-FITC/PI
fluorescent double-staining, and results showed that the percentage
of apoptotic cells increased after drug treatment compared with the
control group. This increase in apoptosis was also found to be
dependent on the drug concentration (Fig. 4B and E).
As shown in Fig.
4C, the expression of the anti-apoptotic protein Bcl-xl
decreased whereas that of Bad increased after drug treatment. The
ratio of Bad/Bcl-xl was then obtained by calculation, where the
difference was observed to be statistically significant between the
drug-treated groups and the control group, suggesting that the
apoptosis ratio was increased as the CUDC-101 concentration
increased (Fig. 4D). The
expression of the oxidative stress protein HO-1 was also increased
in a concentration-dependent manner (Fig. 4C). These results suggested that
CUDC-101 treatment promoted apoptosis in a concentration-dependent
manner in T24-EGFR-OE cells.
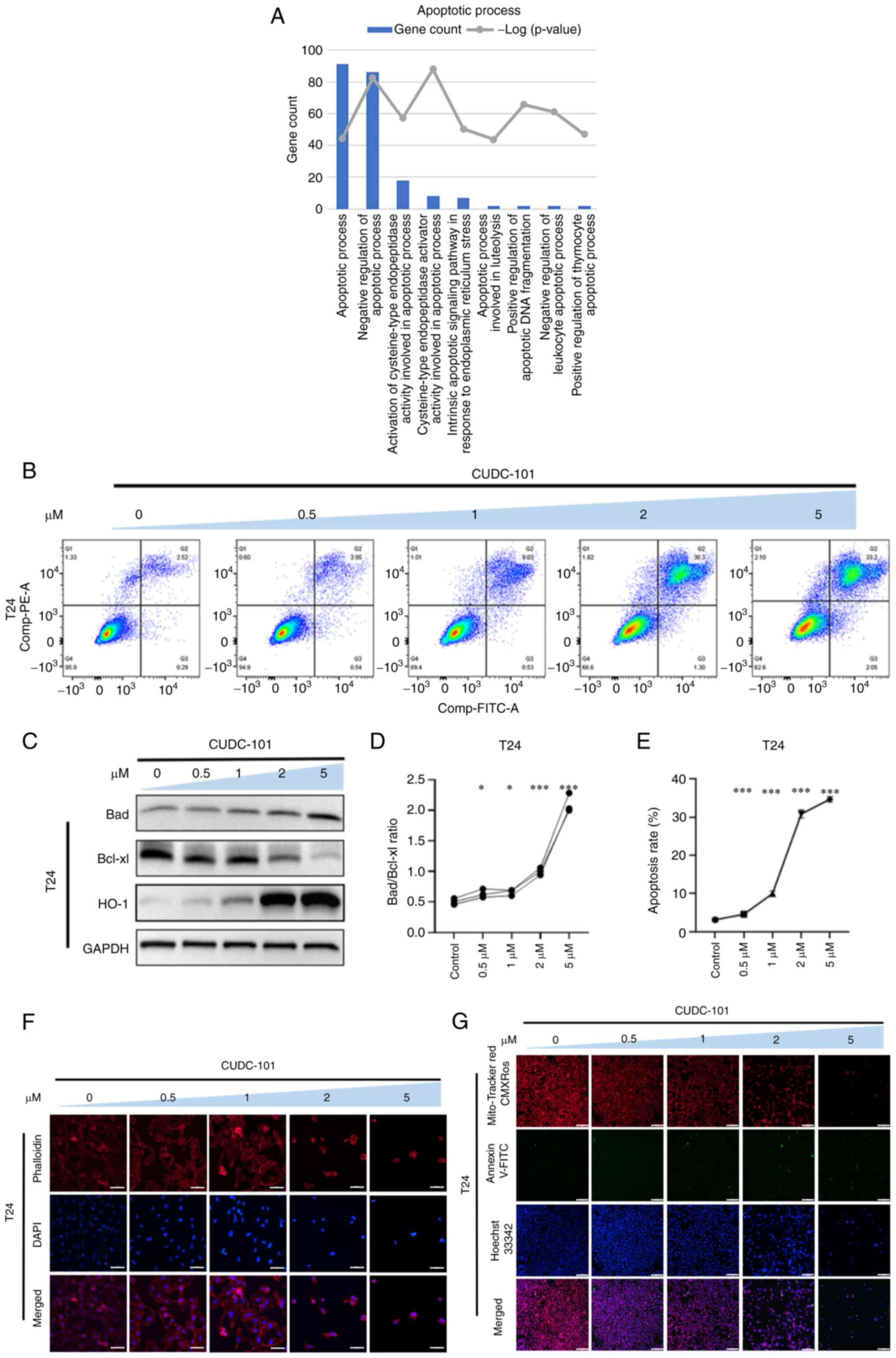
CUDC-101 promotes the apoptosis of T24
cells
In T24 cells, the effects of CUDC-101 were
significantly associated with apoptosis or death biological
processes (Fig. 5A). As shown in
Fig. 5F, T24 cells treated with
CUDC-101 also changed in a similar manner to T24-EGFR-OE cells, in
that a decreases in cell viability, cell aggregation and adhesion,
some cells became exhibiting irregular morphology, cell rupture,
poor microfilament distribution, wrinkled peripheral microfilaments
and disrupted nuclei were all observed following CUDC-101
treatment. In addition, as the concentration of CUDC-101 increased,
the aforementioned observations correspondingly worsened.
As shown in Fig.
5G, the total number of T24 cells was decreased and the number
of apoptotic cells showing green fluorescence increased, whereas
the mitochondrial membrane potential decreased, following drug
administration, compared with those in the control group. As the
concentration of CUDC-101 increased, the ratio of Bad/Bcl-xl also
increased significantly compared with that in the control group.
Expression of the oxidative stress protein HO-1 was also increased
by CUDC-101 in a concentration-dependent manner (Fig. 5C). As shown in Fig. 5B, the number of Annexin
V-FITC-positive cells was increased, suggesting that CUDC-101 can
mediate similar pro-apoptotic effects on T24 cells.
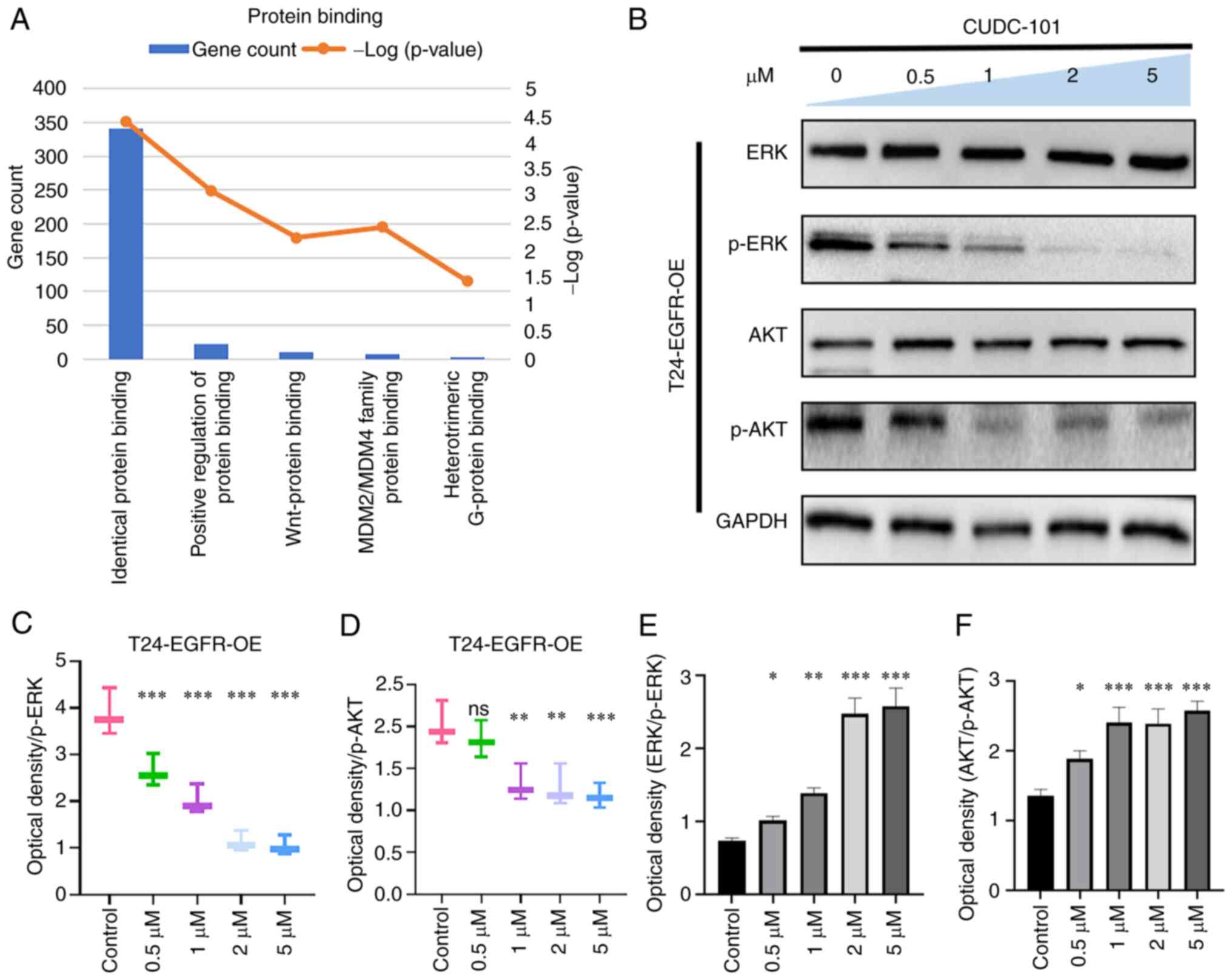
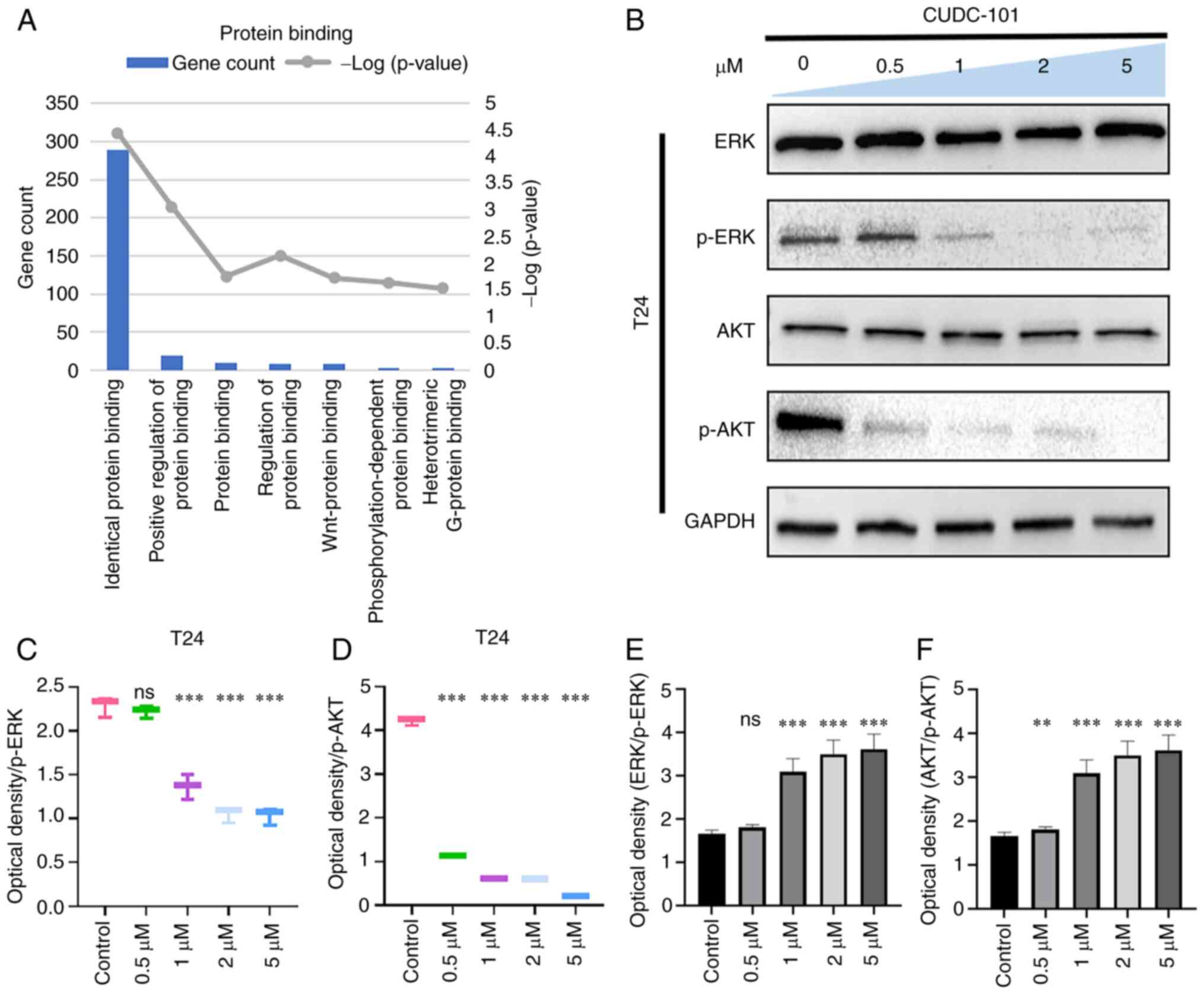
Effects of CUDC-101 on AKT and ERK
phosphorylation
As shown in Fig.
S7, compared with those in T24 cells, the expression of EGFR
and phosphorylation of EGFR were increased in the
EGFR-overexpressing T24 cell line, both groups were cultured with
1640 medium and no special treatment, suggesting that the
T24-EGFR-OE cell line was successfully constructed. As shown in
Figs. 6 and 7, the expression of ERK and AKT did not
change in T24 or T24-EGFR-OE cells, whilst the expression of
phosphorylation of ERK and AKT decreased in response to CUDC-101
treatment in a concentration-dependent manner. This suggested that
CUDC-101 can inhibit cell proliferation by promoting apoptosis
through a number of signaling pathways.
Discussion
Bladder cancer is the most common malignancy in the
genitourinary system, with high rates of morbidity and mortality
(19). The majority of cases have
poor prognosis with complex etiology (20). Although advanced diagnostic
techniques and surgical treatment have greatly improved the
prognosis of bladder cancer (21,22), the treatment strategy for bladder
cancers with certain genetic and expression mutation profiles
remain less clear. Therefore, precise molecular targeting is not
possible on certain bladder cancer cases where surgical treatment
is also not possible (23).
In a previous study, bladder cancer with EGFR
overexpression was found to be susceptible to targeted TKI
afatinib, which can affect the proliferation and apoptosis of
bladder cancer cells (24).
However, the role of other targeted drugs on bladder cancer remain
to be elucidated. Therefore, the present study explored a novel
molecularly targeted therapeutic agent to enrich the number
molecular treatment options for bladder cancer.
In the present study, the bladder cancer cell lines
T24 was chosen as the model; this contain mutations in the RAS
gene, the most common mutation among bladder cancer mutation types
(25) and there is a large
variation in positive EGFR expression in bladder cancer (26), which in T24 cells is shown in
Fig. S7. A wild-type EGFR gene
fragment was introduced into this cell line using the viral
packaging method described previously (27) to create a bladder cancer cell
model with EGFR overexpression for simulating patients with this
type of bladder cancer. Subsequently, CUDC-101, a multi-targeted
inhibitor of HDAC, EGFR and HER2 (28), was applied. CUDC-101 has been
previously shown to inhibit cell proliferation in head and neck
cancer (29), thyroid cancer
(30) and pancreatic cancer
(31), in addition to serving a
role in reversing drug resistance and preventing cancer cell
migration (32).
In the present study, MTT assay was used to detect
the effects of CUDC-101 on cell survival and viability. It was
found that CUDC-101 could inhibit cell proliferation in a dose- and
time-dependent manner. EdU and colony assays were used to detect
the effects of CUDC-101 on cell proliferation and it was found that
it could inhibit cell proliferation in both T24 and T24-EGFR-OE
cells in a dose-dependent manner. Flow cytometry was then used to
detect the extent of apoptosis, which found that the degree of
apoptosis was increased by CUDC-101 in a dose-dependent manner.
These results suggested that CUDC-101 reduced cell viability and
vigor while promoting apoptosis.
The present study additionally detected changes in
cell morphology and internal structure. The cytoskeleton is the
main mechanical structure within cells, which is formed by a
complex network of dynamic biopolymers, such as microtubules, actin
and intermediate filaments (33).
In response to stimulation, cytoskeletal reorganization,
microfilament bundle breakage and shrinkage will occur, ultimately
leading to apoptotic cell death (34,35). The present study found that the
cell membrane was uneven, where the nuclei appeared crinkled and
fragmented following CUDC-101 treatment. In addition, the actin
microfilament structure changed following drug treatment,
suggesting that CUDC-101 could alter the distribution of
cytoskeletal structures in both the T24 and T24-EGFR-OE cell lines,
in turn promoting apoptosis (36). A normal transmembrane
mitochondrial potential is necessary for maintaining mitochondrial
function (37). Significant
decreases in the mitochondrial transmembrane potential will
irreversibly initiate the apoptotic process (38). In the present study, CUDC-101 was
found to promote apoptosis in both cell lines. Flow cytometry
results confirmed that CUDC-101 was able to significantly promote
apoptosis in T24 and T24-EGFR-OE cells in a dose-dependent manner.
These results suggested that CUDC-101 acted on bladder cancer cells
by decreasing cell survival and viability, altering cytoskeletal
structure, decreasing mitochondrial membrane potential, and
ultimately promoting apoptosis.
Performed signaling pathways modulate the
equilibrium between cell viability and apoptosis. In
CUDC-101-treated bladder cancer cells, the effect of CUDC-101 on
apoptosis via AKT and ERK signaling pathways was evaluated. The
EGFR signaling pathway induces the mobilization of a number of
pathways downstream, including PI3K, AKT, MAPK/ERK, protein kinase
C (PKC), and JAK/STAT pathways (39). The results of the present study
also suggested that in CUDC-101-treated bladder cancer cells,
CUDC-101 could inhibit differentiation and enhanced apoptotic cell
death through the PI3K/AKT and ERK cascade pathways. The PI3K/Akt
and ERK signaling pathways are closely associated with tumor cell
survival, invasion and metastasis (40) and PI3K/AKT inhibitors have been
explored as therapeutic agents for tumor treatment (41). The results of the present study
showed effects on the inactivation of PI3K/AKT and ERK pathways,
thus demonstrating the potential ability of CUDC-101 to be used as
a PI3K/AKT inhibitor for the treatment of bladder cancer.
However, the mechanisms of the CUDC-101-mediated
pathway remain to be elucidated. In the present study,
RNA-sequencing was used for further exploration and supplementary
material provided the expression profiles of differentiated
expressed genes in CUDC-101-treated cells. Transcriptome sequencing
showed similar changes on the expression profiles in T24 and
T24-EGFR-OE, suggesting that the regulation genes by CUDC-101 could
be responsible for affecting cell growth, inhibiting cell
proliferation. The next step in our research program is to refine
the validation of the effects of CUDC-101 on the expression of
these genes and data using a variety of cell lines, and to perform
animal studies to eliminate the current limitations of this study
due to the lack of in vivo experiments.
In conclusion, results from the present study
suggest that CUDC-101 can reduce cell viability, inhibit cell
proliferation, alter cell morphology and promote cell apoptosis on
both T24 cells and T24 EGFR-overexpressed cells. These findings
support a novel strategy for using CUDC-101 as a targeted inhibitor
for EGFR-overexpressing bladder cancer clinical treatment.
Supplementary Data
Availability of data and materials
The SRA records will be accessible with the
following link after the indicated release date: https://www.ncbi.nlm.nih.gov/sra/PRJNA1010990.
Accession to cite for these SRA data: PRJNA1010990. Temporary
Submission ID: SUB13808738.
Authors' contributions
LA, XL and YW conceived and designed the study. ZW,
LL and CC performed the experiments. XW, QL, RW, GZ and GW analyzed
the data. LA and XL wrote the manuscript. ZW, LL and CC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Completing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Teaching reform research
and practice project of Henan University (grant no. 146) and the
Research Project of Institutes of Traditional Chinese Medicine of
Henan University (grant no. 2021YJYJZ06).
References
|
1
|
Dobruch J and Oszczudlowski M: Bladder
cancer: Current challenges and future directions. Medicina
(Kaunas). 57:7492021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim IH and Lee HJ: Perioperative systemic
treatment for muscle-invasive bladder cancer: Current evidence and
future perspectives. Int J Mol Sci. 22:72012021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo CC and Czerniak B: Bladder cancer in
the genomic era. Arch Pathol Lab Med. 143:695–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Leary C, Gasper H, Sahin KB, Tang M,
Kulasinghe A, Adams MN, Richard DJ and O'Byrne KJ: Epidermal growth
factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC).
Pharmaceuticals (Basel). 13:2732020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaiswal BS, Kljavin NM, Stawiski EW, Chan
E, Parikh C, Durinck S, Chaudhuri S, Pujara K, Guillory J, Edgar
KA, et al: Oncogenic ERBB3 mutations in human cancers. Cancer Cell.
23:603–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar
|
|
10
|
Arteaga CL and Engelman JA: ERBB
receptors: From oncogene discovery to basic science to
mechanism-based cancer therapeutics. Cancer Cell. 25:282–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabbah DA, Hajjo R and Sweidan K: Review
on epidermal growth factor receptor (EGFR) structure, signaling
pathways, interactions, and recent updates of EGFR inhibitors. Curr
Top Med Chem. 20:815–834. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar :
|
|
13
|
Wang Z: ErbB receptors and cancer. Methods
Mol Biol. 1652:3–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai CJ, Bao R, Tao X, Wang J, Atoyan R, Qu
H, Wang DG, Yin L, Samson M, Forrester J, et al: CUDC-101, a
multitargeted inhibitor of histone deacetylase, epidermal growth
factor receptor, and human epidermal growth factor receptor 2,
exerts potent anticancer activity. Cancer Res. 70:3647–3656. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Pursell NW, Samson ME, Atoyan R,
Ma AW, Selmi A, Xu W, Cai X, Voi M, Savagner P and Lai CJ:
Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and
HER2 inhibitor, in treating drug resistance and preventing cancer
cell migration and invasion. Mol Cancer Ther. 12:925–936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu T, LoRusso PM, Papadopoulos KP,
Patnaik A, Beeram M, Smith LS, Rasco DW, Mays TA, Chambers G, Ma A,
et al: Phase I first-in-human study of CUDC-101, a multitargeted
inhibitor of HDACs, EGFR, and HER2 in patients with advanced solid
tumors. Clin Cancer Res. 20:5032–5040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai X, Zhai HX, Wang J, Forrester J, Qu H,
Yin L, Lai CJ, Bao R and Qian C: Discovery of
7-[4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy]-N-hydroxyheptanamide
(CUDc-101) as a potent multi-acting HDAC, EGFR, and HER2 inhibitor
for the treatment of cancer. J Med Chem. 53:2000–2009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galloway TJ, Wirth LJ, Colevas AD, Gilbert
J, Bauman JE, Saba NF, Raben D, Mehra R, Ma AW, Atoyan R, et al: A
Phase I study of CUDC-101, a multitarget inhibitor of HDACs, EGFR,
and HER2, in combination with chemoradiation in patients with head
and neck squamous cell carcinoma. Clin Cancer Res. 21:1566–1573.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez Rodriguez RH, Buisan Rueda O and
Ibarz L: Bladder cancer: Present and future. Med Clin (Barc).
149:449–455. 2017.In English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dobruch J, Daneshmand S, Fisch M, Lotan Y,
Noon AP, Resnick MJ, Shariat SF, Zlotta AR and Boorjian SA: Gender
and bladder cancer: A collaborative review of etiology, biology,
and outcomes. Eur Urol. 69:300–310. 2016. View Article : Google Scholar
|
|
21
|
Pham A and Ballas LK: Trimodality therapy
for bladder cancer: Modern management and future directions. Curr
Opin Urol. 29:210–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmadi H, Duddalwar V and Daneshmand S:
Diagnosis and staging of bladder cancer. Hematol Oncol Clin North
Am. 35:531–541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seidl C: Targets for therapy of bladder
cancer. Semin Nucl Med. 50:162–170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Y, Zhang X, Qi F, Chen M, Li Y, Liu
L, He W, Li Z and Zu X: Afatinib inhibits proliferation and
invasion and promotes apoptosis of the T24 bladder cancer cell
line. Exp Ther Med. 9:1851–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Przybojewska B, Jagiello A and Jalmuzna P:
H-RAS, K-RAS, and N-RAS gene activation in human bladder cancers.
Cancer Genet Cytogenet. 121:73–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denny WA: The 4-anilinoquinazoline class
of inhibitors of the erbB family of receptor tyrosine kinases.
Farmaco. 56:51–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An L, Wang Y, Wu G, Wang Z, Shi Z, Liu C,
Wang C, Yi M, Niu C, Duan S, et al: Defining the sensitivity
landscape of EGFR variants to tyrosine kinase inhibitors. Transl
Res. 255:14–25. 2023. View Article : Google Scholar
|
|
28
|
Sun H, Mediwala SN, Szafran AT, Mancini MA
and Marcelli M: CUDC-101, a novel inhibitor of full-length androgen
receptor (flAR) and androgen receptor variant 7 (AR-V7) activity:
Mechanism of action and in vivo efficacy. Horm Cancer. 7:196–210.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira-Silva RJ, Carolina de Carvalho A,
de Souza Viana L, Carvalho AL and Reis RM: Anti-EGFR therapy:
Strategies in head and neck squamous cell carcinoma. Recent Pat
Anticancer Drug Discov. 11:170–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Zhang Y, Mehta A, Boufraqech M,
Davis S, Wang J, Tian Z, Yu Z, Boxer MB, Kiefer JA, et al: Dual
inhibition of HDAC and EGFR signaling with CUDC-101 induces potent
suppression of tumor growth and metastasis in anaplastic thyroid
cancer. Oncotarget. 6:9073–9085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moertl S, Payer S, Kell R, Winkler K,
Anastasov N and Atkinson MJ: Comparison of radiosensitization by
HDAC inhibitors CUDC-101 and SAHA in pancreatic cancer cells. Int J
Mol Sci. 20:35292019. View Article : Google Scholar
|
|
32
|
Bass AKA, El-Zoghbi MS, Nageeb EM, Mohamed
MFA, Badr M and Abuo-Rahma GEA: Comprehensive review for anticancer
hybridized multitargeting HDAC inhibitors. Eur J Med Chem.
209:1129042021. View Article : Google Scholar
|
|
33
|
Pegoraro AF, Janmey P and Weitz DA:
Mechanical properties of the cytoskeleton and cells. Cold Spring
Harb Perspect Biol. 9:a0220382017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bottone MG, Santin G, Aredia F, Bernocchi
G, Pellicciari C and Scovassi AI: Morphological features of
organelles during apoptosis: An overview. Cells. 2:294–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu C, Wang Z, Liu Q, Wu G, Chu C, Li L,
An L and Duan S: Sensitivity analysis of EGFR L861Q mutation to six
tyrosine kinase inhibitors. Clin Transl Oncol. 24:1975–1985. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Annesley SJ and Fisher PR: Mitochondria in
health and disease. Cells. 8:6802019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakamuru S, Attene-Ramos MS and Xia M:
Mitochondrial membrane potential assay. Methods Mol Biol.
1473:17–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hussain S: Measurement of
nanoparticle-induced mitochondrial membrane potential alterations.
Methods Mol Biol. 1894:123–131. 2019. View Article : Google Scholar
|
|
39
|
Chong CR and Jänne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yue X, Li M, Chen D, Xu Z and Sun S:
UNBS5162 induces growth inhibition and apoptosis via inhibiting
PI3K/AKT/mTOR pathway in triple negative breast cancer MDA-MB-231
cells. Exp Ther Med. 16:3921–3928. 2018.PubMed/NCBI
|
|
41
|
Yang Q, Modi P, Newcomb T, Quéva C and
Gandhi V: Idelalisib: First-in-class PI3K delta inhibitor for the
treatment of chronic lymphocytic leukemia, small lymphocytic
leukemia, and follicular lymphoma. Clin Cancer Res. 21:1537–1542.
2015. View Article : Google Scholar : PubMed/NCBI
|