Short, single-stranded RNAs known as microRNAs
(miRNAs/miRs) are found in nature. They interact with their target
messenger RNA (mRNAs) to control gene expression at the
post-transcriptional level (13),
and were first described in C. elegans by Lee et al
(14) in 1993. It has been
demonstrated that miRNAs are essential for a number of crucial
biological functions, such as cell proliferation, differentiation,
apoptosis, metabolism and immunological responses (15-19). Depending on their functions in
various types of tumors, miRNAs are categorized as either oncogenes
or tumor suppressor genes (20-22). miR-124 may function as a tumor
suppressor in a range of cancer types, according to mounting
evidence (23). In addition,
miR-124 has been linked to a number of crucial cancer-related
processes, such as proliferation, tumor spread,
epithelial-mesenchymal transition (EMT), metastasis and the
resistance of cells (24,25). For instance, breast cancer (BC)
tissues have been shown to exhibit a reduced expression of miR-124
compared with normal tissues. The proliferation and migration of
cells may be inhibited by miR-124 overexpression in MDA-MB-231 and
MCF-7 cells, and the upregulation miR-124 has been shown to be
associated with an increased overall survival (OS) of patients with
BC (26).
The present review focused on previous findings
associated with the expression patterns of miR-124 in various
cancer types, and aimed to discuss the major molecular mechanism
and potential clinical application involved in the upregulation or
downregulation of miR-124 expression, in an effort to clarify which
factors are essential to the etiology of this type of cancer. A
flowchart of the literature screening, categorization and
summarization of the literature is presented in Fig. S1.
Malignant tumors are caused by aberrant cell growth
and proliferation. Tumors are recognized by contemporary medicine
as a sort of genetic mutation (46). miRNAs have a significant influence
on the onset and progression of cancer, according to mounting data
(47). Zhang et al
(48) revealed miR-124 expression
was noticeably downregulated in primary BC tissues, and by
specifically targeting the programmed cell death protein 6 (PDCD6),
it prevented EMT and the motility of BC cells. Simultaneously,
miRNA-124 can also target EPH receptor A2 (EphA2) to inhibit cell
motility and proliferation in glioma (49). Notably, it has been found that
miR-124 is a highly conserved miRNA involved in a number of
biological tumor processes, including cellular differentiation,
invasion, apoptosis, metastasis and proliferation (50-52). The aim of the present review was
to provide a summary of the research findings on miR-124 in various
human malignancies, and to discuss its mechanisms of action and
clinical importance in the emergence and growth of tumors. The
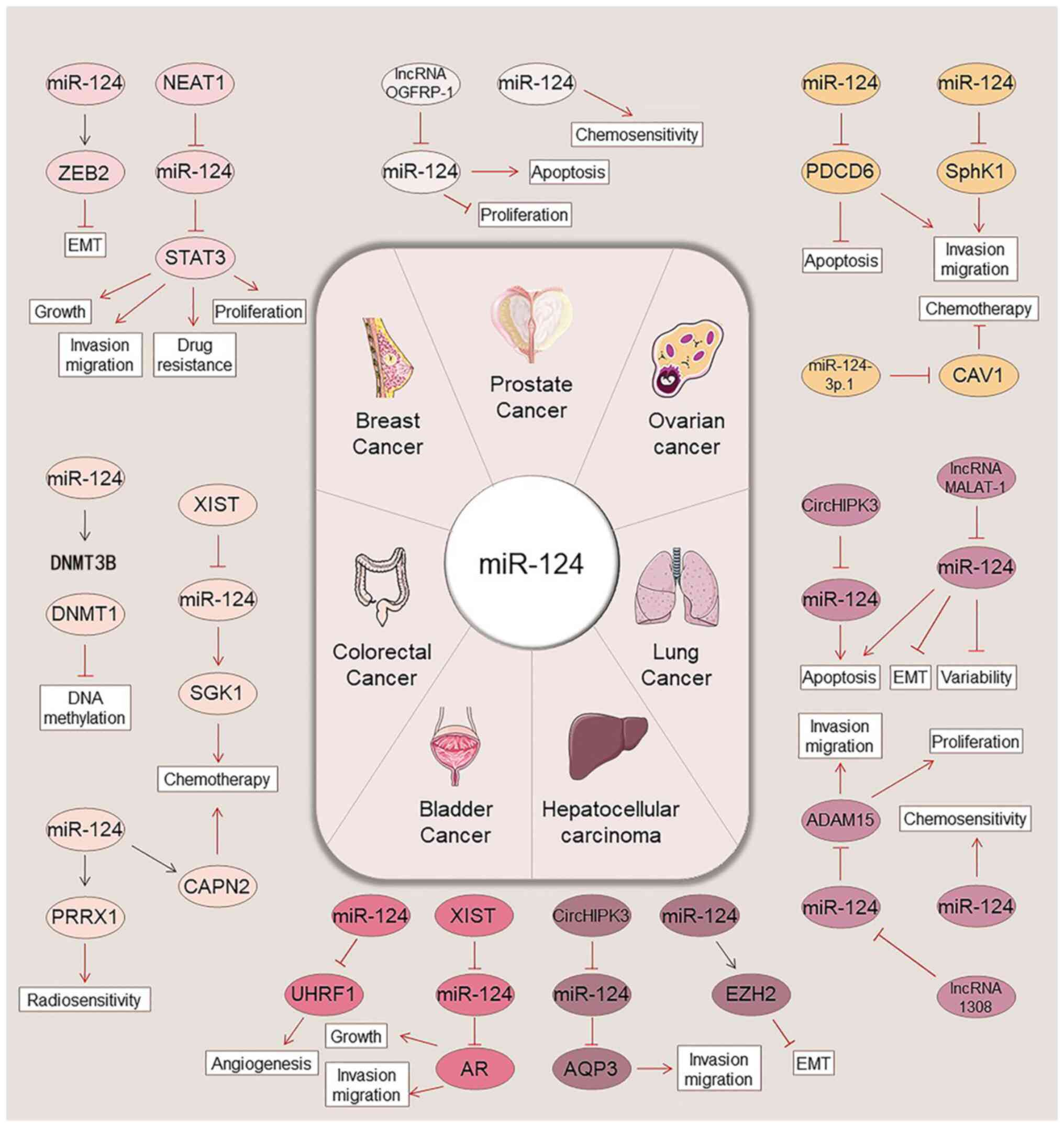
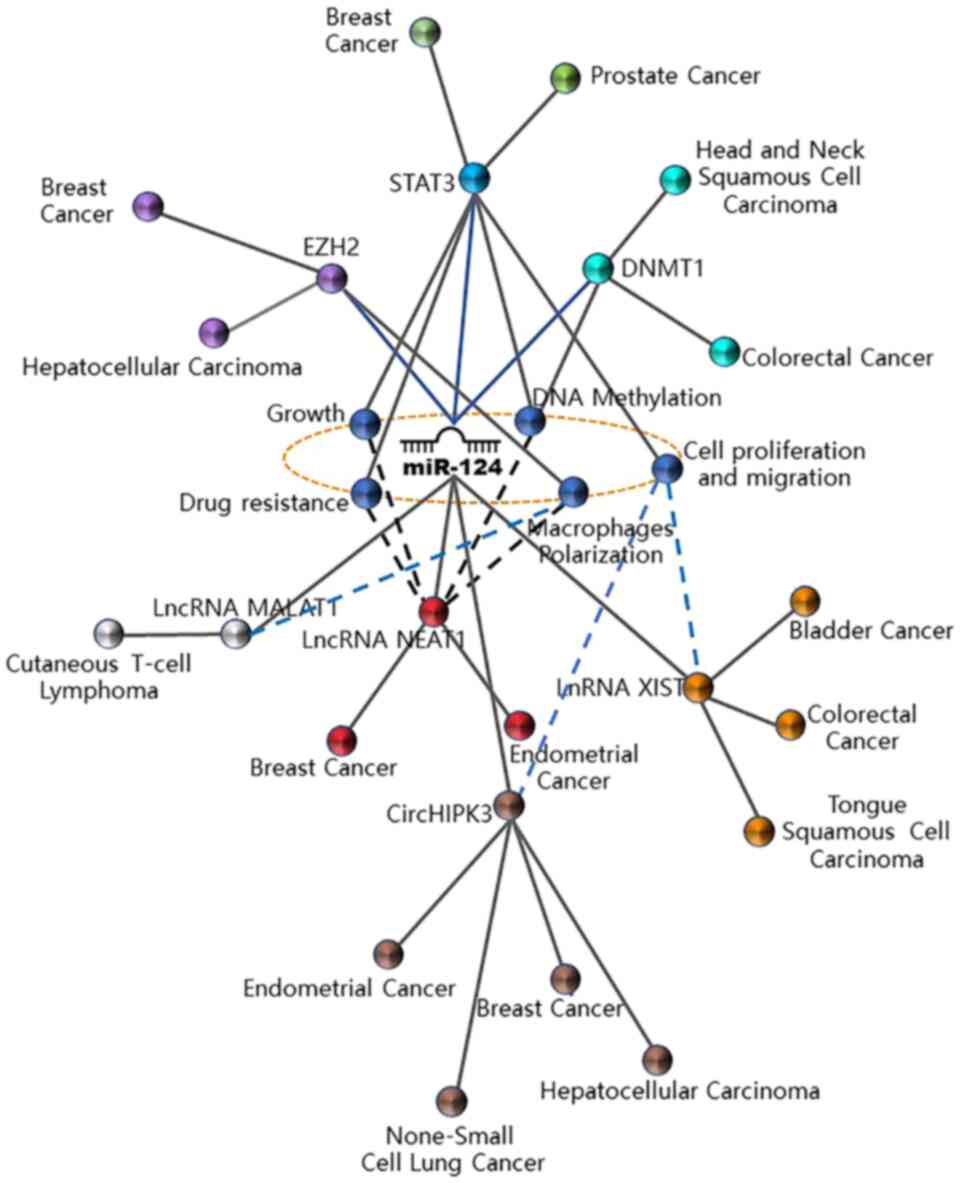
regulatory mechanisms, expression patterns and functional roles of
miR-124 in cancer are illustrated in Fig. 1, while Fig. 2 summarizes regulatory networks of
miR-124 repeatedly observed in various tumors. In addition, the
regulatory mechanisms and targets of miR-124 in various
malignancies are presented in Table
I.
Worldwide, BC is the most prevalent malignant
illness and the main cause of cancer-related mortality among women.
It affects ~12% of women worldwide, including 25% (1.7 million) of
patients newly diagnosed with cancer and is responsible for 15%
(>0.5 million) of all cancer-related deaths (53,54). Accumulating evidence has suggested
that the etiology of BC is predominantly hormonal, with an
increased lifetime exposure to endogenous and exogenous hormones
related to the development of the disease (55,56). The most severe form of BC occurs
when the tumor spreads from the breast tissue to other parts of the
body (metastasis), which markedly increases the tumor burden and
often leads to a terminal diagnosis (57,58). Recently, early diagnosis and novel
treatments for BC have achieved notable advancements, and the OS of
patients has also improved. However, tumor recurrence and
metastasis remain the main cause of mortality for a large number of
patients with BC, and represent the main obstacles for the
reduction of the mortality rates of patients with terminal BC
(59,60). In order to increase the OS and
disease-free survival (DFS) rates of patients with BC, it may be
useful to investigate the biomarkers of BC cell metastasis.
Bladder cancer is considered one of the most common
malignant urinary system cancer types. Bladder cancer is listed as
the second highest cause of cancer-related mortality among all
genitourinary tumors (74). It is
estimated that there are currently 765,950 patients in the USA with
bladder cancer (75). Urothelial
carcinomas comprise ≤90% of all bladder cancer cases, including
invasive and non-invasive bladder cancer. Approximately 15% of
urothelial bladder cancer cases may worsen to muscle-invasive
cancer (76). Surgery, often an
en bloc resection, followed by adjuvant intravenous chemotherapy is
the conventional course of treatment for non-muscle invasive
bladder cancer (77). The 5-year
survival rate for individuals with muscle-invasive cancer is 70%,
and the 10-year survival rate is significantly lower (78,79). The highly metastatic nature of
bladder cancer is the main reason for its high recurrence and
mortality rates. In order to improve the clinical treatment
efficiency and patient survival, it is essential to identify
effective therapeutic targets and to develop efficient treatment
techniques.
miR-124 has been shown to be a crucial regulator of
bladder cancer. Previous studies by Zhou et al (37) and Zo and Long (38) on miR-124 expression in bladder
cancer tissues found that its expression was significantly lower
than that in adjacent noncancerous tissues. HT-1376, T24 and 5637
bladder cancer cell lines had considerably lower levels of miR-124
than did SV-HUC-1 normal human bladder epithelial cells.
Furthermore, miR-124 overexpression could effectively suppress
bladder cancer cell proliferation, while miR-124 downregulation
markedly improved the proliferative ability of bladder cancer
cells. Compared with the control cells, silencing of miR-124
promoted the invasive and migratory abilities of bladder cancer
cells, while these migratory and invasive capacities were impeded
by the high expression of miR-124, suggesting that bladder cancer
growth and invasion may be inhibited by miR-124. In addition,
miR-124 was confirmed to be associated with angiogenesis (80). Vasculogenic mimicry (VM), MMP-2,
VEGF and MMP-9 are valid indications of angiogenesis that have been
described in previous research (81,82). Wang et al (83) observed that miR-124 overexpression
notably inhibited the tubular channels formed, and considerably
decreased VEGF, MMP-9 and MMP-2 expression levels, thus
highlighting the suppressive effects of miR-124 overexpression on
bladder cancer angiogenesis. Moreover, the knockdown of miR-124
exerted opposite effects on VM, as well as on the protein level of
VEGF, MMP-2 and MMP-9, thus indicating that the ectopic expression
if miR-124 can inhibit the angiogenesis of bladder cancer cells
(83). Mechanistic analyses
demonstrated that miR-124 could interact with ubiquitin-like with
PHD and RING finger domain 1 (UHRF1), acting as an important
modulator of epigenetic modifications, and significantly decreasing
UHRF1 expression in bladder cancer cells. In contrast to UHRF1
overexpression, the aforementioned results demonstrated that
miR-124 reduces bladder cancer development, migration, invasion and
angiogenesis (83). In several
cancer types, lncRNA X-inactive specific transcript (XIST) is
associated with cancer progression (84). By controlling the expression of
AR, Xiong et al (85)
demonstrated that the bladder cancer cell proliferation and
migration may be affected by XIST; AR is a ligand-dependent
transcription factor that regulates biological functions (86). To inhibit the formation of
miR-124, XIST can function as a ceRNA. miR-124 may also bind to the
protein's 3'-UTR in order to regulate the production of AR
(85). miR-124 suppression cab
partially override the impact of XIST downregulation on the
expression level of AR, c-Myc, p27, MMP-13 and MMP-9 (85), indicating that the newly
identified XIST/miR-124/AR axis may regulate bladder cancer cell
proliferation, invasion, and migration and may act as a marker and
target for bladder cancer patients. The pattern of miR-124
expression in bladder cancer remains unclear, and further studies
are required to determine the predictive value of miR-124 and the
association between its expression and clinical indicators.
CRC, which affects >1.2 million individuals per
year and is the third highest cause of cancer-related mortality
globally, has a 5-year survival rate of 63.5% (87). In 2020, the number of confirmed
cases of CRC reached 147,950, and there were 53,200 related deaths.
Among patients <50 years of age, 17,930 patients were diagnosed
with CRC, and 3,640 patients succumbed to the disease (88). Despite the existence of screening
and prevention strategies, due to the frequent occurrence of CRC,
it has long been a severe global wellness issue. Despite recent
advancements in surgical techniques and comprehensive therapy, CRC
is still associated with a poor prognosis, particularly the low
long-term survival rate of patients with advanced-stage CRC
(89,90). Thus, precise prognosis estimation
is essential for physicians, in order to improve and individualize
therapeutic strategies. Any novel prognostic signal needs to be
verified due to the poor prognosis of patients with CRC.
Liver cancer is the sixth most prevalent solid
cancer worldwide, particularly in China. There are ~841,000 new
cases and 782,000 deaths associated with this disease worldwide,
rendering it a serious health concern (4). HCC is the most common type of liver
cancer and comprises >90% of all instances of primary liver
cancer. Patients typically succumb to intrahepatic or extrahepatic
metastasis in the absence of suitable therapy. Metastasis is often
coupled with the development of new tumors (101,102). Consequently, further research on
the metastatic mechanisms of HCC may help to elucidate HCC
metastasis factors and to identify novel therapeutic targets.
A family of transmembrane channels termed aquaporins
(AQPs) transport solutes, such as glycerol and water (110,111). Previous studies have reported a
critical role for AQPs in tumorigenesis and cancer progression
(112,113). AQP3 has been shown to be
overexpressed in HCC, and patients with elevated levels of AQP3
have a negative prognosis (114). Moreover, Chen et al
(109) confirmed that AQP3 was a
target of miR-124 and inhibited the expression of AQP3. The
knockdown of circHIPK3 reduced the proliferation and migration
abilities of HCC cells, which could be rescued by a miR-124
inhibitor. Furthermore, the circHIPK3-induced suppression of AQP3
expression may be reversed by miR-124 inhibition. These results
demonstrated that circHIPK3 controlled the migration and
proliferation of HCC cells by sponging miR-124, which in turn
controlled the expression of AQP3 (109). Further research on the
circHIPK3-miR-124-AQP3 axis may provide new perspectives for the
future therapy of HCC. Yu et al (115) suggested that circHIPK3
accelerated cell proliferation and invasion by sponging miR-124.
MALAT1, a lncRNA linked with lung adenocarcinoma, may promote the
growth of HBx-induced malignancy (116). The 3'-UTR of miR-124 in cancer
stem cells (CSCs) can specifically bind caveolin-1 (CAV1), which
indicates that miR-124 can be overexpressed by targeting CAV1.
Therefore, the high expression of CAV1 can inhibit the self-renewal
and tumorigenesis of liver CSCs. When miR-124 is absent, this
effect disappears. Other lncRNAs, such as DS cell adhesion
molecule-antisense RNA 1 and miR-124 have also been found to
interact in HCC (117). Taken
together, these findings may provide new perspectives on the
pathogenesis of HCC and may provide underlying strategies for
miRNA-directed therapy. However, future studies are required to
determine and elucidate the in vivo implications and other
potential mechanisms of miR-124.
Lung cancer is the leading cause of
cancer-associated mortality worldwide, accounting for ~1.4 million
deaths each year (118). The two
main types of lung cancer are small cell lung cancer (SCLC) and
non-small cell lung cancer (NSCLC) (119). NSCLC is diagnosed in ~85% of
patients, while the remaining ~15% of patients are diagnosed with
SCLC (120). Despite the
therapeutic progress that has recently been made, the 5-year OS
rates of patients with NSCLC remain low (121). Increasing evidence has indicated
that cancer metastasis or recurrence is frequent in NSCLC therapy,
and is mainly responsible for the low 5-year survival rate
(122,123). Thus, understanding the
mechanisms of NSCLC progression is critical.
With a high mortality rate observed over the past
few decades, ovarian cancer continues to be one of the major causes
of mortality among women worldwide (134-136). The primary factor behind this
high mortality rate is the fact that >70% of patients are
diagnosed at an advanced stage of the disease and have already
developed distant tumors (137,138). According to previous studies,
complex changes in the genome, particularly in the expression and
function of several miRNAs, are linked to ovarian cancer (137,139). The exact mechanisms through
which miRNAs are associated with the invasion and migration of
ovarian cancer cells remain unclear, despite the fact that multiple
studies have suggested the potential utility of miRNAs in ovarian
cancer diagnosis, prognosis and therapy (140,141). Therefore, it would be crucial
for clinical practice to identify effective biomarkers for ovarian
cancer diagnosis and therapy.
The most frequent disease among males and the
subsequent largest reason for cancer-related mortality is prostate
cancer, with >29,000 males succumbing to prostate cancer in the
USA in 2020 (145). Despite the
fact that prostate cancer may be treated with cutting-edge
techniques, such as androgen deprivation therapy and docetaxel
chemotherapy (146,147), the survival rate of patients
with prostate cancer remains low, and the 5-year survival rate is
only 29% (74,148). In addition, 30% of patients
relapse after receiving first treatment (149). Thus, identifying novel
diagnostic biomarkers and therapeutic targets for enhancing the
survival rate of patients with prostate cancer is of utmost
importance.
With ~600,000 new cases and a mortality rate of
223,000-300,000 fatalities per year, head and neck cancer is the
sixth most common type of cancer worldwide (153,154). Squamous cell carcinomas, which
develop in the squamous epithelial cells of the nasal cavity,
paranasal sinuses, oral cavity, oropharynx, larynx and hypopharynx,
account for the majority of instances of head and neck cancer
(153). Currently, alcohol
misuse, long-term cigarette use and human papillomavirus infection
are the primary risk factors for HNSCC and are connected to age,
sex and ethnicity (155). The
relative frequency of these risk factors causes a variance in the
distribution of HNSCC internationally. The most hazardous
characteristic of HNSCC is that cancer is commonly identified at
late stages (T3 or T4), when the survival rate is decreased to 20%,
resulting in high mortality and morbidity rates (156,157). By contrast, the 5-year survival
rate of patients is 80% if HCSCC is identified at an early stage
(T1 or T2) (158,159). Therefore, including biomarkers
unique to various ethnic groups holds promise for screening, early
identification and tracking therapy response in HNSCC, rendering it
an indispensable aspect of modern clinical practice.
It is widely acknowledged that improving the
patient survival rate requires an enhanced of understanding the
related mechanisms and effective biomarkers for determining tumor
occurrence and metastasis. Furthermore, the early detection of
these aggressive cancer types during the course of the illness and
the development of effective therapeutics are expected to decrease
cancer mortality rates. Previous studies have demonstrated that
multiple signal transduction pathways are associated with cancer
occurrence and development (162-164). The aim of the present review was
to summarize the associations between several regulatory systems
that are involved in the initiation and development of tumors.
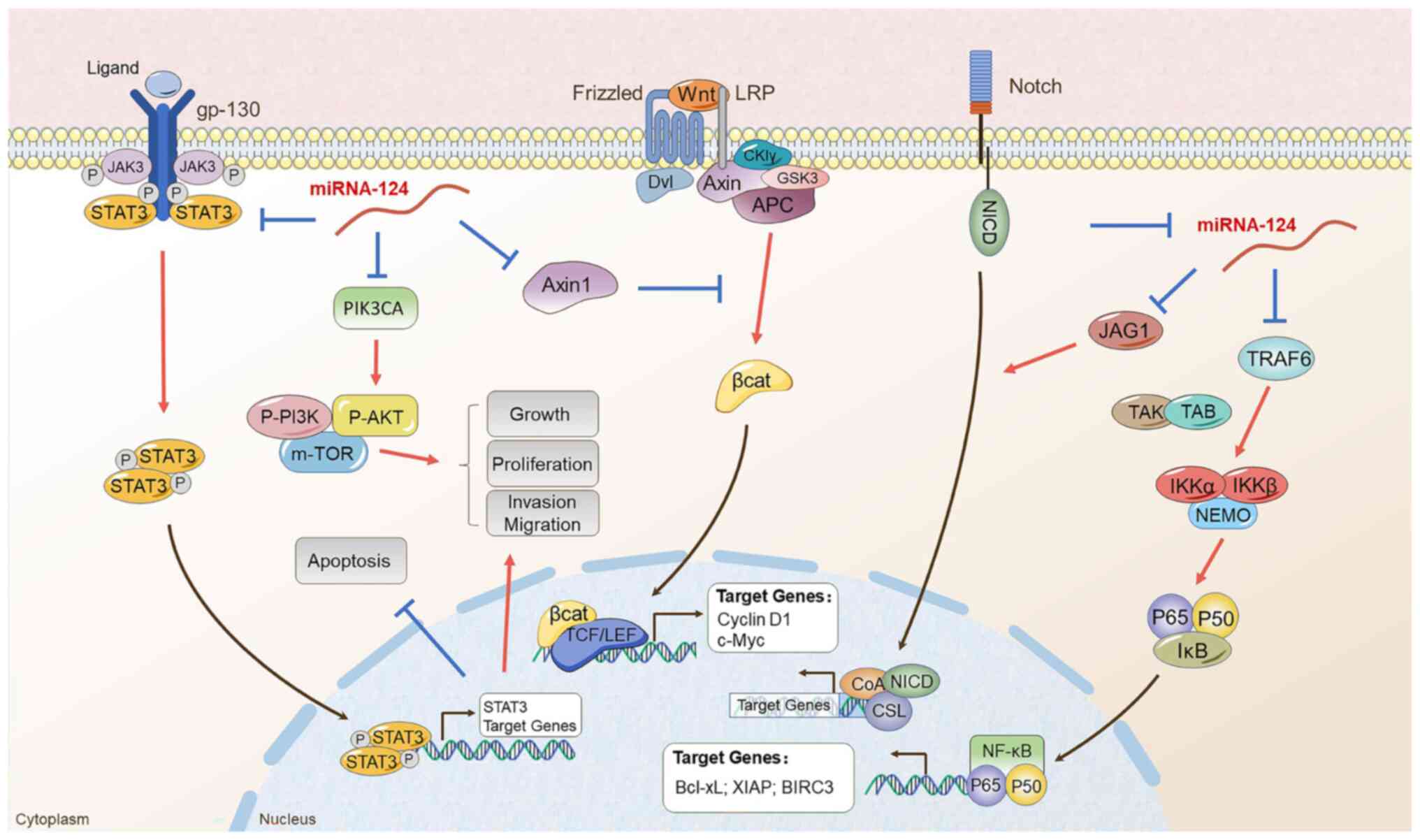
Firstly, it was reported that miR-124 can directly influence the
progression of various cancer types via several signaling pathways
(Fig. 3). Adult tissue
homeostasis and embryonic development have both been demonstrated
to be dependent on Wnt/β-catenin signaling, which is often
dysregulated in a number of malignancies (165). Classical Wnt ligands initiate
Wnt/β-catenin signaling, which causes the protein complex comprised
of APC, Axin and GSK3 to degrade, inhibiting β-catenin (166). Stable β-catenin moves into the
nucleus to begin the process of the transcription of genes
downstream, tightly regulating cell division and the development of
tumors. It has been proposed that aberrant PI3K/Akt signaling
exists in human cancer (167,168). Epidemiological and experimental
research has established abnormal PI3K/Akt signaling pathway
activation as a critical stage in the development and maintenance
of human cancer. Previous research has demonstrated that PI3K/Akt
triggers a signaling cascade that modulates tumor cell
proliferation, invasion, metastasis and survival, in addition to
affecting patient prognosis (169,170). PIK3CA is an oncogene part of the
PI3K signaling pathway, and it is connected to cell proliferation
and carcinogenesis in multiple tumor types (171,172). The NF-κB signaling pathway is
regarded as a vital factor in numerous steps of carcinogenesis and
progression (173). The critical
function of the NF-B signaling system in mediating angiogenic
neovascularization, EMT, and cancer cell 'stemness', as well as the
mechanisms through which it contributes to chemoresistance,
radioresistance and endocrine resistance, have been emphasized in
previous research, which are associated with invasive phenotypes
that cause early relapse, late disease stages and a poor OS
(174,175). The JAK/STAT signaling system is
a widely expressed intracellular signal transduction route that is
involved in a variety of significant biological processes,
including the control of immune response, cell division, apoptosis
and differentiation (176).
Since the overactivation of the JAK/STAT signaling system is
intimately related to the development, progression, invasion and
metastasis of several tumors, it is acknowledged as a novel
therapeutic target for a range of cancer types (177-179). The Notch signaling system is
essential for cell survival, differentiation, death and
proliferation, particularly in cancer cells. Notch may become
activated by connecting with the cell-bound ligands. JAG1, JAG2,
DLL-1, DLL-3 and DLL-4 are known as five Notch ligands exist in
mammals (180). In the usual
Notch signaling pathway, a Notch ligand expressed on the cell
surface interacts with a Notch receptor expressed on the surface of
a neighboring cell to activate Notch signaling (181). Many recent studies have shown
(as discussed below) that miRNA-124 can play an inhibitory role in
the development of various tumors through the action of the
aforementioned signaling pathways
It was discovered that the Wnt/β-catenin signaling
pathway controls the proliferation, invasion and metastasis of CRC
cells (182,183). miR-124 regulates Wnt4, a
component of the Wnt/β-catenin signaling system, decreasing CRC
cell proliferation in vitro and inhibiting tumor development
in vivo (182). A recent
study indicated that miR-124 suppresses CRC proliferation,
migration and invasion by downregulating the DNMT3B and DNMT1 level
(184). The inhibition of DNMT1
and DNMT3b activity has been demonstrated to regulate the
expression of genes involved in the Wnt/β-catenin pathway (185). Thus, miR-124 may be involved in
the Wnt/β-catenin pathway by regulating DNMT3B and DNMT1, leading
to the inhibition of CRC development.
It is widely recognized that the Axin family, which
includes Axin1 and Axin2, regulates the amount of Wnt/β-catenin to
negatively modify the Wnt/β-catenin signaling pathway, and is
crucial for the development and pathophysiology of a number of
human illnesses, including cancer (165,186,187). The study by Yang et al
(62) revealed that miR-124-3p.1
targeted the 3′-UTR of Axin1 mRNA in BC cells. miR-124-3p.1
overexpression promoted cell proliferation, which was suppressed by
the overexpression of Axin1 in BC cells. Therefore, utilizing
miR-124-3p.1 mimics to target Wnt/β-catenin signaling pathway
genes, including cyclin D1 and c-Myc prevented Axin1 from being
overexpressed (62). They also
found that miR-124-3p.1 mimic therapy mimicked the effects of Axin1
knockdown by upregulating β-catenin and Bcl-2, while downregulating
β-catenin phosphorylation and the levels of the apoptosis-related
protein, Bax. Taken together, these findings demonstrated that
miR-124 regulated growth of BC by regulating Wnt/β-catenin
signaling (62).
The early diagnosis of cancer is a difficult task,
and thus the loss of the optimal opportunity for curative surgery
leads to low survival rates (88). Specific tumor biomarkers that
reflect the molecular differences related to cancer, which has a
great utility for early detection, are essential, and may be
valuable for selecting the most effective treatment options and
obtaining vital therapeutic time for patients with cancer.
Prognostic indicators may be useful in clinical practice for
predicting the clinical prognosis of patients with cancer who have
not yet received therapy. Previous studies have shown that the
expression profiles of miRNAs can predict the development of cancer
or the differentiation of cancer subtypes. However, due to the
uncertainty of the molecular functions of miRNAs, it is difficult
to precisely predict the development of cancer. Generally, ideal
and convenient biomarkers should have some typical and crucial
qualities. Previous studies have revealed that miR-124 is strongly
associated with a variety of biological functions in cancer cells,
and may also function as a possible diagnostic biomarker and
therapeutic target in human cancer in the future (46,200,201).
Patients with bladder cancer are divided into two
distinct categories: One with high levels of expression and one
with low levels of expression based on the average level of miR-124
expression. It has been shown that bladder cancers with lymph node
metastasis (LNM) and advanced-stage cancers clinically have a lower
expression of miR-124 (202). In
addition, patients with bladder cancer with a low expression of
miR-124 exhibit a shorter OS time than those with miR-124
overexpression (37). As shown by
Xie et al (99), the OS
has been shown to be worse in patients with a low miR-124
expression than in those with a high miR-124 expression.
Previous studies have shown that miR-124 expression
is significantly downregulated in CRC compared to normal mucosa and
that the downregulated expression of miR-124 is significantly
associated with a poorer prognosis (98). Chen et al (96) suggested that miR-124 may be a
valuable marker for CRC prognosis. According to Gao et al
(203), the pri-miR-124 rs531564
polymorphism was significantly linked to a lower incidence of CRC.
They demonstrated a strong correlation between differentiation
status and LNM in patients with CRC and the pri-miR-124 rs531564
polymorphism.
Thus, finding ideal cancer biomarkers is crucial to
improving the early diagnosis rate. The aforementioned findings
indicate that miR-124 may be used as a potential marker for the
diagnosis of multiple cancer types. However, the precise molecular
mechanisms by which miR-124 functions in several types of cancer
remain unclear. Therefore, the function of miR-124 in cancer needs
to be further investigated and confirmed, particularly in clinical
applications.
Since miRNAs can inhibit tumor development through
a variety of mechanisms, the notion of treating tumors through
miRNAs was born (164,208-212). In addition to its role in
diagnosis and prognosis as a potential marker for cancer, miR-124
can make tumors more sensitive to treatment through different
targets, thereby improving patient survival. For example, miR-124
can reverse radioresistance in esophageal cancer radiotherapy by
targeting CDK4 to increase cancer cell sensitivity to radiation,
and also plays a role in immunotherapy (213,214). It has been demonstrated that
miR-124 not only reverses the resistance of BC stem cells to DOX by
targeting STAT3 to control the hypoxia-inducible factor-1 signaling
pathway (67), but also increases
the sensitivity of BC cells to adriamycin by reducing DNA
strand-break repair (215). A
previous study inferred that miR-124 inhibited the expression of
EphA2, which was beneficial for attenuating K-RAS mutation-mediated
resistance to erlotinib in pancreatic cancer (216). In retinoblastoma, the
miR-124/c-Myc axis exerts an oncogenic effect on disease
progression, suggesting that miR-124 may be a promising therapeutic
target (217). A previous study
demonstrated that bio-carrier neural stem cell-derived exosomes
(NSC-EXOs) loaded with miR-124-3p suppressed glioma growth via the
EXO-miR-124-3p/FLOT2/AKT1 pathway, thus providing a possible
measure for glioma treatment (218). miR-124 should be explored for
the reversal of drug resistance in tumor treatments in the
future.
The development of several innovative therapeutic
strategies may result from a better knowledge of miR-124's function
in the treatment of cancer. Hence, further studies on miRNA are
required. According to previous research, in BC, bladder cancer,
HCC, CRC, and lung, ovarian and prostate cancer cell lines, miR-124
expression is downregulated. In addition, miR-124 can prevent
certain cancer cell types from migrating and proliferating.
Furthermore, an abnormal miR-124 expression in cancer has been
reported to be significantly associated with diagnosis, prognosis,
tumorigenesis and metastasis via signaling pathways. Various
studies have confirmed that miR-124 may be a potential target for
cancer diagnosis and may function as a novel cancer therapeutic
target or biomarker. Nevertheless, the clinical usefulness of
miR-124 remains unknown, and the understanding of its biological
functions is only partial. Consequently, further research on miRNAs
is required (Fig. 4).
Extracellular vesicles (EVs) refers to a group of
organelles with lipid bilayer membranes that are released from
cells into the environment (219). It has been reported that a
number of cargos are transferred across cells via EVs (191,193). More importantly, the cells of
origin can be affected by the cargo of EVs. Previous studies have
suggested that, compared with parental cells, exosomes carry
different types of RNA (220-222). Unprotected ncRNAs in the blood
are known to be susceptible to degradation by blood RNases.
Nevertheless, EVs prevent the degradation of ncRNAs, and therefore
maintain their activity and integrity in the circulation. miRNAs
are considered to be gene expression regulators, since they can
bind to mRNAs directly and subsequently suppress the translation or
degradation of target mRNAs, thus inhibiting the progression and
development of tumors (223,224). It has been suggested that
EV-miRNAs establish a bridge between the tumor microenvironment and
cancer cells, meaning that EV-miRNAs play vital roles in both the
tumor microenvironment and cancer cells (225). miRNAs are carefully packaged in
EVs and sent to nearby or distant recipient cells in order to
modify their gene expression. For example, in various solid cancer
types, the let-7 miRNA family is secreted via EVs and is
downregulated (226), which also
functioned as targeting oncogenes, including high mobility group A2
and RAS (227), and tumor
suppressor genes. Metastatic GC cells can reduce their
intracellular antitumor ability to maintain their invasive and
tumorigenic behavior, which is achieved by EVs secreting the tumor
suppressor let-7 miRNA into the extracellular space (226). Kanlikilicer et al
(228) demonstrated that ovarian
cancer cells released the EV-miRNA miR-6126, which enhanced their
capacity to metastasize by directly targeting integrin-1, a crucial
regulator of cancer cell metastasis. It has also been shown that
EVs produced by CRC promote immune escape via the upregulation of
PD-L1 in TAMs, and by inducing macrophage M2 polarization via
miR-21-5p and miR-200a, suggesting that EVs and miRNAs may serve as
novel targets for CRC immunotherapy (229). In addition, in prostate cancer,
miRNAs have been shown to inhibit the production of EVs by
targeting genes associated with EV secretion, thereby mediating
tumor suppression, which has led to new hypotheses for prostate
cancer treatment (230).
Overall, these findings demonstrated that EV-miRNAs can serve as
miRNAs with either oncogenic or tumor suppressor roles, and that
understanding these mechanisms may help to develop new systems that
regulate the sorting of EV-miRNAs to reduce their effects on the
development of cancer. It may be helpful to identify and alter the
composition of cancer EVs to develop novel diagnostic, preventative
and therapeutic strategies with perhaps less intrusive techniques.
As such, miR-124 appears to pay a vital role in cancer diagnosis,
prevention and treatment, and its expression level can be
upregulated by the administration of exosomal miR-124.
As a versatile editing tool, clustered regularly
interspaced short palindromic repeats (CRISPR)-Cas9-mediated genome
editing technology has attracted extensive attention worldwide
(231-233). Single guide RNA (sgRNA) and DNA
endonuclease Cas9 form the CRISPR-Cas9 system, and Cas9 and sgRNA
conduct site-specific cleavage of double stranded DNA by guiding
Cas9 to a specific DNA sequence (234). It has been demonstrated that
CRISPR-Cas9 is promising for cancer treatment (235). For instance,
CRISPR-Cas9-mediated CD133 deletion can prevent colon cancer
invasion by lowering EMT as a possible CSC marker (236). Notably, Zhou et al
(237) used the CRISPR/Cas9
method to successfully knockdown miR-3188 in an HCC cell line. As
it efficiently inhibited the proliferation, invasion and migration
of nude mice cells, as well as the development of xenograft tumors,
it was observed that the CRISPR/Cas9 system played a key role in
the editing and control of miRNA-related genomes. It has been shown
that miR-124 downregulation inhibits tumor invasion and migration
(238), suggesting that, in the
future, CRISPR-Cas9 may be used to downregulate miR-124 and thus
control tumor development. Moreover, due to its precision,
effectiveness, simplicity and adaptability, CRISPR/Cas9, as an
effective genomic engineering tool, has played a vital role in
treating various diseases (239). For example, Huo et al
(240) disrupted the precursor
miRNA sequence using CRISPR/Cas9, and ovarian cancer cell invasion,
migration and proliferation were decreased once miR-21 expression
was suppressed. The efficacy of cancer therapy is increased by the
use of CRISPR/Cas9 gene editing technologies. To better elucidate
the function of miR-124 and related therapeutic interventions, the
authors aim to use CRISPR technology for miR-124 editing in future
studies, with the aim of reducing the delivery failure rate of the
CRISPR/Cas system in cancer cells through the combination of
components.
The present review had certain strengths and
limitations. The present review described current research on
miR-R124 in various tumors. By summarizing these studies, the
targets of action of miR-124 that are currently being studied were
discussed. In addition to this, the current regulatory mechanisms
of miR-124 in tumors were elaborated in-depth, and found the five
most studied pathways (as described above) were summarized.
Finally, by summarizing the current and future clinical
applications of miR-124, it can be concluded that miR-124 is
expected to be a potential marker and target for adjuvant therapy
in cancer. However, due to the limited information available from
miR-124 studies in tumors, many of which are still in the
preliminary stage, further studies are required in the future.
miRNAs may have an impact on the etiology of
malignancies. miRNAs inhibit the expression of tumor suppressors
and oncogenes (241). miR-124 is
abnormally expressed in various malignant tumors, including HCC,
BC, NSCLC, CRC and bladder, ovarian and prostate cancer. The
miR-124 expression level was confirmed to be similar in various
cancer types. Furthermore, previous research (as discussed above)
has revealed that miR-124 is linked to OS, LNM, DFS, clinical stage
and distant metastasis, and can function as a tumor suppressor. The
growth, migration, spread and death of the aforementioned cancer
types may be controlled by miR-124. The characterization of
specific alterations of miR-124 expression in cancer has potential
for identifying biomarkers for cancer detection in the early
stages, and for therapeutic intervention during cancer treatment.
Previous studies have shown that miR-124 can control molecular
pathways, such as the JAK/STAT signaling pathway, which is
associated with tumor growth and development.
In conclusion, the present review addresses the
mechanisms through which miR-124 controls cancer cell migration,
proliferation, invasion and death by inhibiting the expression of
oncogenic mRNAs, as well as the importance of miR-124 and its
target genes in diverse malignancies. However, further
investigations are required to elucidate the molecular mechanisms
of miR-124 in additional human malignancies.
Not applicable.
YL, YY and XW drafted the manuscript. BL, SY, YZ
and MF revised the manuscript. ZF, CS, YH and BC reviewed and
modified the manuscript. QZ put forward constructive opinions on
the topic selection of the article. All authors have read and
approved the final version. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have competing
interests.
Not applicable.
The present study was supported by Scientific Research Projects
at the Affiliated Bozhou Hospital of Anhui Medical University
(By202111).
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.
|
|
2
|
Zaimy MA, Saffarzadeh N, Mohammadi A,
Pourghadamyari H, Izadi P, Sarli A, Moghaddam LK, Paschepari SR,
Azizi H, Torkamandi S and Tavakkoly-Bazzaz J: New methods in the
diagnosis of cancer and gene therapy of cancer based on
nanoparticles. Cancer Gene Ther. 24:233–243. 2017.
|
|
3
|
Vineis P and Wild CP: Global cancer
patterns: Causes and prevention. Lancet. 383:549–557. 2014.
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.
|
|
5
|
Basuroy R, Bouvier C, Ramage JK, Sissons
M, Kent A and Srirajaskanthan R: Presenting Symptoms and Delay in
Diagnosis of Gastrointestinal and Pancreatic Neuroendocrine
Tumours. Neuroendocrinology. 107:42–49. 2018.
|
|
6
|
Koo MM, Hamilton W, Walter FM, Rubin GP
and Lyratzopoulos G: Symptom signatures and diagnostic timeliness
in cancer patients: A review of current evidence. Neoplasia.
20:165–174. 2018.
|
|
7
|
Smith JS, Lindsay L, Hoots B, Keys J,
Franceschi S, Winer R and Clifford GM: Human papillomavirus type
distribution in invasive cervical cancer and high-grade cervical
lesions: A meta-analysis update. Int J Cancer. 121:621–632.
2007.
|
|
8
|
Yan L, Xu F and Dai CL: Relationship
between epithelial-to-mesenchymal transition and the inflammatory
microenvironment of hepatocellular carcinoma. J Exp Clin Cancer
Res. 37:2032018.
|
|
9
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016.
|
|
10
|
Roy PS and Saikia BJ: Cancer and cure: A
critical analysis. Indian J Cancer. 53:441–442. 2016.
|
|
11
|
Wang JJ, Lei KF and Han F: Tumor
microenvironment: Recent advances in various cancer treatments. Eur
Rev Med Pharmacol Sci. 22:3855–3864. 2018.
|
|
12
|
Okamoto A, Watanabe T, Kamata K, Minaga K
and Kudo M: Recent updates on the relationship between cancer and
autoimmune pancreatitis. Intern Med. 58:1533–1539. 2019.
|
|
13
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355
|
|
14
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
15
|
Asli NS, Pitulescu ME and Kessel M:
MicroRNAs in organogenesis and disease. Curr Mol Med. 8:698–710.
2008.
|
|
16
|
Bueno MJ, Perez de Castro I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008.
|
|
17
|
Lu LF and Liston A: MicroRNA in the immune
system, microRNA as an immune system. Immunology. 127:291–298.
2009.
|
|
18
|
Maatouk D and Harfe B: MicroRNAs in
development. ScientificWorldJournal. 6:1828–1840. 2006.
|
|
19
|
Wang Y and Lee CG: MicroRNA and
cancer-focus on apoptosis. J Cell Mol Med. 13:12–23. 2009.
|
|
20
|
Wu SG, Huang YJ, Bao B, Wu LM, Dong J, Liu
XH, Li ZH, Wang XY, Wang L, Chen BJ and Chen W: miR-508-5p acts as
an anti-oncogene by targeting MESDC1 in hepatocellular carcinoma.
Neoplasma. 64:40–47. 2017.
|
|
21
|
Gao W, Li W, Xiao T, Liu XS and Kaelin WG
Jr: Inactivation of the PBRM1 tumor suppressor gene amplifies the
HIF-response in VHL-/-clear cell renal carcinoma. Proc Natl Acad
Sci USA. 114:1027–1032. 2017.
|
|
22
|
Xiao X, Tang C, Xiao S, Fu C and Yu P:
Enhancement of proliferation and invasion by MicroRNA-590-5p via
targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res.
20:537–544. 2013.
|
|
23
|
Wan HY, Li QQ, Zhang Y, Tian W, Li YN, Liu
M, Li X and Tang H: MiR-124 represses vasculogenic mimicry and cell
motility by targeting amotL1 in cervical cancer cells. Cancer Lett.
355:148–158. 2014.
|
|
24
|
Peng XH, Huang HR, Lu J, Liu X, Zhao FP,
Zhang B, Lin SX, Wang L, Chen HH, Xu X, et al: MiR-124 suppresses
tumor growth and metastasis by targeting Foxq1 in nasopharyngeal
carcinoma. Mol Cancer. 13:1862014.
|
|
25
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013.
|
|
26
|
Yan G, Li Y, Zhan L, Sun S, Yuan J, Wang
T, Yin Y, Dai Z, Zhu Y, Jiang Z, et al: Decreased miR-124-3p
promoted breast cancer proliferation and metastasis by targeting
MGAT5. Am J Cancer Res. 9:585–596. 2019.
|
|
27
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739.
2002.
|
|
28
|
Clark AM, Goldstein LD, Tevlin M, Tavare
S, Shaham S and Miska EA: The microRNA miR-124 controls gene
expression in the sensory nervous system of Caenorhabditis elegans.
Nucleic Acids Res. 38:3780–3793. 2010.
|
|
29
|
Zhou Q, Long L, Shi G, Zhang J, Wu T and
Zhou B: Research of the methylation status of miR-124a gene
promoter among rheumatoid arthritis patients. Clin Dev Immunol.
2013:5242042013.
|
|
30
|
Ben Gacem R, Ben Abdelkrim O, Ziadi S, Ben
Dhiab M and Trimeche M: Methylation of miR-124a-1, miR-124a-2, and
miR-124a-3 genes correlates with aggressive and advanced breast
cancer disease. Tumour Biol. 35:4047–4056. 2014.
|
|
31
|
Ando T, Yoshida T, Enomoto S, Asada K,
Tatematsu M, Ichinose M, Sugiyama T and Ushijima T: DNA methylation
of microRNA genes in gastric mucosae of gastric cancer patients:
Its possible involvement in the formation of epigenetic field
defect. Int J Cancer. 124:2367–2374. 2009.
|
|
32
|
Gao C, Shen J, Meng ZX and He XF:
Sevoflurane Inhibits Glioma cells proliferation and metastasis
through miRNA-124-3p/ROCK1 axis. Pathol Oncol Res. 26:947–954.
2020.
|
|
33
|
Zhang TH, Liang LZ, Liu XL, Wu JN, Su K,
Chen JY, Zheng QY, Huang HZ and Liao GQ: [Retracted] Long
non-coding RNA MALAT1 interacts with miR-124 and modulates tongue
cancer growth by targeting JAG1. Oncol Rep. 40:31122018.
|
|
34
|
Wang D, Zhang H, Li M, Frid MG, Flockton
AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, et
al: MicroRNA-124 controls the proliferative, migratory, and
inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res.
114:67–78. 2014.
|
|
35
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010.
|
|
36
|
Cao J, Qiu J, Wang X, Lu Z, Wang D, Feng
H, Li X, Liu Q, Pan H, Han X, et al: Identification of microRNA-124
in regulation of Hepatocellular carcinoma through BIRC3 and the
NF-κB pathway. J Cancer. 9:3006–3015. 2018.
|
|
37
|
Zhou W, He L, Dai Y, Zhang Y, Wang J and
Liu B: MicroRNA-124 inhibits cell proliferation, invasion and
migration by targeting CAV1 in bladder cancer. Exp Ther Med.
16:2811–2820. 2018.
|
|
38
|
Zo RB and Long Z: MiR-124-3p suppresses
bladder cancer by targeting DNA methyltransferase 3B. J Cell
Physiol. 234:464–474. 2018.
|
|
39
|
Zhou L, Xu Z, Ren X, Chen K and Xin S:
MicroRNA-124 (MiR-124) inhibits cell proliferation, metastasis and
invasion in colorectal cancer by downregulating Rho-associated
protein kinase 1(ROCK1). Cell Physiol Biochem. 38:1785–1795.
2016.
|
|
40
|
Taniguchi K, Sugito N, Kumazaki M,
Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama
K and Akao Y: MicroRNA-124 inhibits cancer cell growth through
PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett.
363:17–27. 2015.
|
|
41
|
Yuan L, Li S, Zhou Q, Wang D, Zou D, Shu J
and Huang Y: MiR-124 inhibits invasion and induces apoptosis of
ovarian cancer cells by targeting programmed cell death 6. Oncol
Lett. 14:7311–7317. 2017.
|
|
42
|
Shi XB, Xue L, Ma AH, Tepper CG,
Gandour-Edwards R, Kung HJ and deVere White RW: Tumor suppressive
miR-124 targets androgen receptor and inhibits proliferation of
prostate cancer cells. Oncogene. 32:4130–4138. 2013.
|
|
43
|
Gan H, Liu H, Zhang H, Li Y, Xu X, Xu X
and Xu J: SHh-Gli1 signaling pathway promotes cell survival by
mediating baculoviral IAP repeat-containing 3 (BIRC3) gene in
pancreatic cancer cells. Tumour Biol. 37:9943–9950. 2016.
|
|
44
|
Smolewski P and Robak T: Inhibitors of
apoptosis proteins (IAPs) as potential molecular targets for
therapy of hematological malignancies. Curr Mol Med. 11:633–649.
2011.
|
|
45
|
Frazzi R: BIRC3 and BIRC5: Multi-faceted
inhibitors in cancer. Cell Biosci. 11:82021.
|
|
46
|
Jia X, Wang X, Guo X, Ji J, Lou G, Zhao J,
Zhou W, Guo M, Zhang M, Li C, et al: MicroRNA-124: An emerging
therapeutic target in cancer. Cancer Med. 8:5638–5650. 2019.
|
|
47
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
|
|
48
|
Zhang L, Chen X, Liu B and Han J:
MicroRNA-124-3p directly targets PDCD6 to inhibit metastasis in
breast cancer. Oncol Lett. 15:984–990. 2018.
|
|
49
|
Wu Q, Xu L, Wang C, Fan W, Yan H and Li Q:
MicroRNA-124-3p represses cell growth and cell motility by
targeting EphA2 in glioma. Biochem Biophys Res Commun.
503:2436–2442. 2018.
|
|
50
|
Yu B, Jiang K and Zhang J: MicroRNA-124
suppresses growth and aggressiveness of osteosarcoma and inhibits
TGF-beta-mediated AKT/GSK-3beta/SNAIL-1 signaling. Mol Med Rep.
17:6736–6744. 2018.
|
|
51
|
Moghadasi M, Alivand M, Fardi M, Moghadam
KS and Solali S: Emerging molecular functions of microRNA-124:
Cancer pathology and therapeutic implications. Pathol Res Pract.
216:1528272020.
|
|
52
|
Wang P, Zhang LD, Sun MC, Gu WD and Geng
HZ: Over-expression of mir-124 inhibits MMP-9 expression and
decreases invasion of renal cell carcinoma cells. Eur Rev Med
Pharmacol Sci. 22:6308–6314. 2018.
|
|
53
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
|
|
54
|
Hosseinahli N, Aghapour M, Duijf PHG and
Baradaran B: Treating cancer with microRNA replacement therapy: A
literature review. J Cell Physiol. 233:5574–5588. 2018.
|
|
55
|
Nagini S: Breast cancer: Current molecular
therapeutic targets and new players. Anticancer Agents Med Chem.
17:152–163. 2017.
|
|
56
|
Chen WY: Exogenous and endogenous hormones
and breast cancer. Best Pract Res Clin Endocrinol Metab.
22:573–585. 2008.
|
|
57
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004.
|
|
58
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320. 2012.
|
|
59
|
Li Z and Kang Y: Emerging therapeutic
targets in metastatic progression: A focus on breast cancer.
Pharmacol Ther. 161:79–96. 2016.
|
|
60
|
Cai WL, Huang WD, Li B, Chen TR, Li ZX,
Zhao CL, Li HY, Wu YM, Yan WJ and Xiao JR: microRNA-124 inhibits
bone metastasis of breast cancer by repressing Interleukin-11. Mol
Cancer. 17:92018.
|
|
61
|
Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian
K, Xie Y, Wang S, Xu N, Wang Y and Qi C: miR-124 downregulation
leads to breast cancer progression via LncRNA-MALAT1 regulation and
CDK4/E2F1 signal activation. Oncotarget. 7:16205–16216. 2016.
|
|
62
|
Yang W, Cui G, Ding M, Yang M and Dai D:
MicroRNA-124-3p.1 promotes cell proliferation through
Axin1-dependent Wnt signaling pathway and predicts a poor prognosis
of triple-negative breast cancer. J Clin Lab Anal.
34:e232662020.
|
|
63
|
Ji H, Sang M, Liu F, Ai N and Geng C:
miR-124 regulates EMT based on ZEB2 target to inhibit invasion and
metastasis in triple-negative breast cancer. Pathol Res Pract.
215:697–704. 2019.
|
|
64
|
Shi P, Chen C, Li X, Wei Z, Liu Z and Liu
Y: MicroRNA-124 suppresses cell proliferation and invasion of
triple negative breast cancer cells by targeting STAT3. Mol Med
Rep. 19:3667–3675. 2019.
|
|
65
|
Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF
and Liu X: MicroRNA-124 mediates the cholinergic anti-inflammatory
action through inhibiting the production of pro-inflammatory
cytokines. Cell Res. 23:1270–1283. 2013.
|
|
66
|
Zhang Y, Li X, Zhang J and Liang H:
Natural killer T cell cytotoxic activity in cervical cancer is
facilitated by the LINC00240/microRNA-124-3p/STAT3/MICA axis.
Cancer Lett. 474:63–73. 2020.
|
|
67
|
Liu C, Xing H, Guo C, Yang Z and Wang Y
and Wang Y: MiR-124 reversed the doxorubicin resistance of breast
cancer stem cells through STAT3/HIF-1 signaling pathways. Cell
Cycle. 18:2215–2227. 2019.
|
|
68
|
Yu X, Li Z, Zheng H, Chan MT and Wu WK:
NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif.
50:e123292017.
|
|
69
|
Jiang X, Zhou Y, Sun AJ and Xue JL: NEAT1
contributes to breast cancer progression through modulating miR-448
and ZEB1. J Cell Physiol. 233:8558–5866. 2018.
|
|
70
|
Pang Y, Wu J, Li X, Wang C, Wang M, Liu J
and Yang G: NEAT1/miR-124/STAT3 feedback loop promotes breast
cancer progression. Int J Oncol. 55:745–754. 2019.
|
|
71
|
Shi P, Liu Y, Yang H and Hu B: Breast
cancer derived exosomes promoted angiogenesis of endothelial cells
in microenvironment via circHIPK3/miR-124-3p/MTDH axis. Cell
Signal. 95:1103382022.
|
|
72
|
Wang YF, Yu L, Hu ZL, Fang YF, Shen YY,
Song MF and Chen Y: Regulation of CCL2 by EZH2 affects
tumor-associated macrophages polarization and infiltration in
breast cancer. Cell Death Dis. 13:7482022.
|
|
73
|
Guo Q, Wang H, Duan J, Luo W, Zhao R, Shen
Y, Wang B, Tao S, Sun Y, Ye Q, et al: An alternatively spliced p62
isoform confers resistance to chemotherapy in breast cancer. Cancer
Res. 82:4001–4015. 2022.
|
|
74
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.
|
|
75
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016.
|
|
76
|
Palmbos PL, Wang L, Yang H, Wang Y,
Leflein J, Ahmet ML, Wilkinson JE, Kumar-Sinha C, Ney GM, Tomlins
SA, et al: ATDC/TRIM29 drives invasive bladder cancer formation
through miRNA-mediated and epigenetic mechanisms. Cancer Res.
75:5155–5166. 2015.
|
|
77
|
Mitin T, Hunt D, Shipley WU, Kaufman DS,
Uzzo R, Wu CL, Buyyounouski MK, Sandler H and Zietman AL:
Transurethral surgery and twice-daily radiation plus
paclitaxel-cisplatin or fluorouracil-cisplatin with selective
bladder preservation and adjuvant chemotherapy for patients with
muscle invasive bladder cancer (RTOG 0233): A randomised
multicentre phase 2 trial. Lancet Oncol. 14:863–872. 2013.
|
|
78
|
Girardi DM, Ghatalia P, Singh P, Iyer G,
Sridhar SS and Apolo AB: Systemic therapy in bladder preservation.
Urol Oncol. 41:39–47. 2023.
|
|
79
|
Ma Y, Hu Q, Luo W, Pratt RN, Glenn ST, Liu
S, Trump DL and Johnson CS: 1α,25(OH)2D3 differentially regulates
miRNA expression in human bladder cancer cells. J Steroid Biochem
Mol Biol. 148:166–171. 2015.
|
|
80
|
Wang S, Wu G, Han Y, Song P, Chen J, Wu Y,
Yang J and Liang P: miR-124 regulates STAT3-mediated cell
proliferation, migration and apoptosis in bladder cancer. Oncol
Lett. 16:5875–5881. 2018.
|
|
81
|
Li T, Kang G, Wang T and Huang H: Tumor
angiogenesis and anti-angiogenic gene therapy for cancer. Oncol
Lett. 16:687–702. 2018.
|
|
82
|
Annese T, Tamma R, Ruggieri S and Ribatti
D: Erythropoietin in tumor angiogenesis. Exp Cell Res. 374:266–273.
2019.
|
|
83
|
Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y,
Gu X and Meng F: MiR-124 exerts tumor suppressive functions on the
cell proliferation, motility and angiogenesis of bladder cancer by
fine-tuning UHRF1. FEBS J. 282:4376–4388. 2015.
|
|
84
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86.
2015.
|
|
85
|
Xiong Y, Wang L, Li Y, Chen M, He W and Qi
L: The long non-coding RNA XIST interacted with MiR-124 to modulate
bladder cancer growth, invasion and migration by targeting androgen
receptor (AR). Cell Physiol Biochem. 43:405–418. 2017.
|
|
86
|
Lombard AP and Mudryj M: The emerging role
of the androgen receptor in bladder cancer. Endocr Relat Cancer.
22:R265–R277. 2015.
|
|
87
|
Aguiar Junior S, Oliveira MM, Silva D,
Mello CAL, Calsavara VF and Curado MP: Survival of patients with
colorectal cancer in a cancer center. Arq Gastroenterol.
57:172–177. 2020.
|
|
88
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.
|
|
89
|
Villeger R, Lopes A, Veziant J, Gagniere
J, Barnich N, Billard E, Boucher D and Bonnet M: Microbial markers
in colorectal cancer detection and/or prognosis. World J
Gastroenterol. 24:2327–2347. 2018.
|
|
90
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017.
|
|
91
|
Kass SU, Pruss D and Wolffe AP: How does
DNA methylation repress transcription? Trends Genet. 13:444–449.
1997.
|
|
92
|
Nakao M and Sasaki H: Genomic imprinting:
Significance in development and diseases and the molecular
mechanisms. J Biochem. 120:467–473. 1996.
|
|
93
|
Panning B and Jaenisch R: RNA and the
epigenetic regulation of X chromosome inactivation. Cell.
93:305–308. 1998.
|
|
94
|
Yoder JA, Walsh CP and Bestor TH: Cytosine
methylation and the ecology of intragenomic parasites. Trends
Genet. 13:335–340. 1997.
|
|
95
|
Lujambio A, Ropero S, Ballestar E, Fraga
MF, Cerrato C, Setién F, Casado S, Suarez-Gauthier A,
Sanchez-Cespedes M, Git A, et al: Genetic unmasking of an
epigenetically silenced microRNA in human cancer cells. Cancer Res.
67:1424–1429. 2007.
|
|
96
|
Chen Z, Liu S, Tian L, Wu M, Ai F, Tang W,
Zhao L, Ding J, Zhang L and Tang A: miR-124 and miR-506 inhibit
colorectal cancer progression by targeting DNMT3B and DNMT1.
Oncotarget. 6:38139–38150. 2015.
|
|
97
|
Zhu J, Zhang R, Yang D, Li J, Yan X, Jin
K, Li W, Liu X, Zhao J, Shang W and Yu T: Knockdown of Long
Non-coding RNA XIST inhibited doxorubicin resistance in colorectal
cancer by upregulation of miR-124 and downregulation of SGK1. Cell
Physiol Biochem. 51:113–128. 2018.
|
|
98
|
Zhang Y, Zheng L, Huang J, Gao F, Lin X,
He L, Li D, Li Z, Ding Y and Chen L: MiR-124 Radiosensitizes human
colorectal cancer cells by targeting PRRX1. PLoS One.
9:e939172014.
|
|
99
|
Xie XQ, Wang MJ, Li Y, Lei LP, Wang N, Lv
ZY, Chen KL, Zhou B, Ping J, Zhou ZG and Sun XF: miR-124
intensified oxaliplatin-based chemotherapy by targeting CAPN2 in
colorectal cancer. Mol Ther Oncolytics. 17:320–331. 2020.
|
|
100
|
Si M, Song Y, Wang X, Wang D, Liu X, Qu X,
Song Z and Yu X: CXCL12/CXCR7/β-arrestin1 biased signal promotes
epithelial-to-mesenchymal transition of colorectal cancer by
repressing miRNAs through YAP1 nuclear translocation. Cell Biosci.
12:1712022.
|
|
101
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.
|
|
102
|
Vaquero J, Guedj N, Claperon A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017.
|
|
103
|
Wang H, Mao J, Huang Y, Zhang J, Zhong L,
Wu Y, Huang H, Yang J, Wei Y and Tang J: Prognostic roles of
miR-124-3p and its target ANXA7 and their effects on cell migration
and invasion in hepatocellular carcinoma. Int J Clin Exp Pathol.
13:357–370. 2020.
|
|
104
|
Yue X, Cui Y, You Q, Lu Y and Zhang J:
MicroRNA-124 negatively regulates chloride intracellular channel 1
to suppress the migration and invasion of liver cancer cells. Oncol
Rep. 42:1380–1390. 2019.
|
|
105
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012.
|
|
106
|
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani
RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, et al: Repression
of E-cadherin by the polycomb group protein EZH2 in cancer.
Oncogene. 27:7274–7284. 2008.
|
|
107
|
Yu J, Cao Q, Mehra R, Laxman B, Yu J,
Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, et al:
Integrative genomics analysis reveals silencing of beta-adrenergic
signaling by polycomb in prostate cancer. Cancer Cell. 12:419–431.
2007.
|
|
108
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454.
2002.
|
|
109
|
Chen G, Shi Y, Liu M and Sun J: circHIPK3
regulates cell proliferation and migration by sponging miR-124 and
regulating AQP3 expression in hepatocellular carcinoma. Cell Death
Dis. 9:1752018.
|
|
110
|
Agre P: The aquaporin water channels. Proc
Am Thorac Soc. 3:5–13. 2006.
|
|
111
|
Magni F, Sarto C, Ticozzi D, Soldi M,
Bosso N, Mocarelli P and Kienle MG: Proteomic knowledge of human
aquaporins. Proteomics. 6:5637–5649. 2006.
|
|
112
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006.
|
|
113
|
Jablonski EM, Mattocks MA, Sokolov E,
Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH and McKillop IH:
Decreased aquaporin expression leads to increased resistance to
apoptosis in hepatocellular carcinoma. Cancer Lett. 250:36–46.
2007.
|
|
114
|
Chen XF, Li CF, Lu L and Mei ZC:
Expression and clinical significance of aquaglyceroporins in human
hepatocellular carcinoma. Mol Med Rep. 13:5283–5289. 2016.
|
|
115
|
Yu Q, Chen W, Li Y, He J, Wang Y, Yang S
and Zhou J: The novel circular RNA HIPK3 accelerates the
proliferation and invasion of hepatocellular carcinoma cells by
sponging the micro RNA-124 or micro RNA-506/pyruvate dehydrogenase
kinase 2 axis. Bioengineered. 13:4717–4729. 2022.
|
|
116
|
Liang T, Wang Y, Jiao Y, Cong S, Jiang X,
Dong L, Zhang G and Xiao D: LncRNA MALAT1 accelerates cervical
carcinoma proliferation by suppressing miR-124 expression in
cervical tumor cells. J Oncol. 2021:88360782021.
|
|
117
|
Wang Z, Li S and Zhang G: LncRNA DSCAM-AS1
negatively interacts with miR-124 to promote hepatocellular
carcinoma proliferation. Crit Rev Eukaryot Gene Expr. 32:1–8.
2022.
|
|
118
|
Li J, Feng Q, Wei X and Yu Y: MicroRNA-490
regulates lung cancer metastasis by targeting poly r(C)-binding
protein 1. Tumour Biol. 37:15221–15228. 2016.
|
|
119
|
Tabchi S, Kassouf E, Rassy EE, Kourie HR,
Martin J, Campeau MP, Tehfe M and Blais N: Management of stage III
non-small cell lung cancer. Semin Oncol. 44:163–177. 2017.
|
|
120
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
|
|
121
|
Ramnath N, Dilling TJ, Harris LJ, Kim AW,
Michaud GC, Balekian AA, Diekemper R, Detterbeck FC and Arenberg
DA: Treatment of stage III non-small cell lung cancer: Diagnosis
and management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest.
143(5 Suppl): e314S–e40S. 2013.
|
|
122
|
Kaplan JA, Liu R, Freedman JD, Padera R,
Schwartz J, Colson YL and Grinstaff MW: Prevention of lung cancer
recurrence using cisplatin-loaded superhydrophobic nanofiber
meshes. Biomaterials. 76:273–281. 2016.
|
|
123
|
Deng XF, Jiang L, Liu QX, Zhou D, Hou B,
Cui K, Min JX and Dai JG: Lymph node micrometastases are associated
with disease recurrence and poor survival for early-stage non-small
cell lung cancer patients: A meta-analysis. J Cardiothorac Surg.
11:282016.
|
|
124
|
Yang Q, Wan L, Xiao C, Hu H, Wang L, Zhao
J, Lei Z and Zhang HT: Inhibition of LHX2 by miR-124 suppresses
cellular migration and invasion in non-small cell lung cancer.
Oncol Lett. 14:3429–3436. 2017.
|
|
125
|
Kim BN, Ahn DH, Kang N, Yeo CD, Kim YK,
Lee KY, Kim TJ, Lee SH, Park MS, Yim HW, et al: TGF-β induced EMT
and stemness characteristics are associated with epigenetic
regulation in lung cancer. Sci Rep. 10:105972020.
|
|
126
|
Zu L, Xue Y and Wang J, Fu Y, Wang X, Xiao
G, Hao M, Sun X, Wang Y, Fu G and Wang J: The feedback loop between
miR-124 and TGF-β pathway plays a significant role in non-small
cell lung cancer metastasis. Carcinogenesis. 37:333–343. 2016.
|
|
127
|
Wang P, Chen L, Zhang J, Chen H, Fan J,
Wang K, Luo J, Chen Z, Meng Z and Liu L: Methylation-mediated
silencing of the miR-124 genes facilitates pancreatic cancer
progression and metastasis by targeting Rac1. Oncogene. 33:514–524.
2014.
|
|
128
|
Wilting SM, van Boerdonk RA, Henken FE,
Meijer CJ, Diosdado B, Meijer GA, le Sage C, Agami R, Snijders PJ
and Steenbergen RD: Methylation-mediated silencing and tumour
suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer.
9:1672010.
|
|
129
|
Qi MM, Ge F, Chen XJ, Tang C and Ma J:
MiR-124 changes the sensitivity of lung cancer cells to cisplatin
through targeting STAT3. Eur Rev Med Pharmacol Sci. 23:5242–5250.
2019.
|
|
130
|
Yu H, Chen Y and Jiang P: Circular RNA
HIPK3 exerts oncogenic properties through suppression of miR-124 in
lung cancer. Biochem Biophys Res Commun. 506:455–462. 2018.
|
|
131
|
Wu J, Weng Y, He F, Liang D and Cai L:
LncRNA MALAT-1 competitively regulates miR-124 to promote EMT and
development of non-small-cell lung cancer. Anticancer Drugs.
29:628–636. 2018.
|
|
132
|
Li H, Guo X, Li Q, Ran P, Xiang X, Yuan Y,
Dong T, Zhu B, Wang L, Li F, et al: Long non-coding RNA 1308
promotes cell invasion by regulating the miR-124/ADAM 15 axis in
non-small-cell lung cancer cells. Cancer Manag Res. 10:6599–6609.
2018.
|
|
133
|
Liu Y, Li L and Song XL: Exosomal circPVT1
derived from lung cancer promotes the progression of lung cancer by
targeting miR-124-3p/EZH2 axis and regulating macrophage
polarization. Cell Cycle. 21:514–530. 2022.
|
|
134
|
Kim K, Zang R, Choi SC, Ryu SY and Kim JW:
Current status of gynecological cancer in China. J Gynecol Oncol.
20:72–76. 2009.
|
|
135
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95(Suppl
1): S161–S192. 2006.
|
|
136
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019.
|
|
137
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012.
|
|
138
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007.
|
|
139
|
Zhang L, Volinia S, Bonome T, Calin GA,
Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K,
et al: Genomic and epigenetic alterations deregulate microRNA
expression in human epithelial ovarian cancer. Proc Natl Acad Sci
USA. 105:7004–7009. 2008.
|
|
140
|
Mezzanzanica D, Bagnoli M, De Cecco L,
Valeri B and Canevari S: Role of microRNAs in ovarian cancer
pathogenesis and potential clinical implications. Int J Biochem
Cell Biol. 42:1262–1272. 2010.
|
|
141
|
Kunej T, Godnic I, Ferdin J, Horvat S,
Dovc P and Calin GA: Epigenetic regulation of microRNAs in cancer:
An integrated review of literature. Mutat Res. 717:77–84. 2011.
|
|
142
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013.
|
|
143
|
Deng X, Chen Y, Liu Z and Xu J:
MiR-124-3p.1 sensitizes ovarian cancer cells to mitochondrial
apoptosis induced by carboplatin. Onco Targets Ther. 13:5375–5386.
2020.
|
|
144
|
Chen L and Kong C: LINC00173 regulates
polycystic ovarian syndrome progression by promoting apoptosis and
repressing proliferation in ovarian granulosa cells via the
microRNA-124-3p (miR-124-3p)/jagged canonical Notch ligand 1 (JAG1)
pathway. Bioengineered. 13:10373–10385. 2022.
|
|
145
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics 2020. CA Cancer J Clin. 70:7–30. 2020.
|
|
146
|
Gomella LG, Petrylak DP and Shayegan B:
Current management of advanced and castration resistant prostate
cancer. Can J Urol. 21(2 Suppl 1): S1–S6. 2014.
|
|
147
|
Scher HI, Buchanan G, Gerald W, Butler LM
and Tilley WD: Targeting the androgen receptor: Improving outcomes
for castration-resistant prostate cancer. Endocr Relat Cancer.
11:459–476. 2004.
|
|
148
|
Salinas CA, Tsodikov A, Ishak-Howard M and
Cooney KA: Prostate cancer in young men: An important clinical
entity. Nat Rev Urol. 11:317–323. 2014.
|
|
149
|
Han M, Partin AW, Pound CR, Epstein JI and
Walsh PC: Long-term biochemical disease-free and cancer-specific
survival following anatomic radical retropubic prostatectomy. The
15-year Johns Hopkins experience. Urol Clin North Am. 28:555–565.
2001.
|
|
150
|
Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX
and Wang WC: MiR-124 suppresses cell motility and adhesion by
targeting Talin 1 in prostate cancer cells. Cancer Cell Int.
15:492015.
|
|
151
|
Shi XB, Ma AH, Xue L, Li M, Nguyen HG,
Yang JC, Tepper CG, Gandour-Edwards R, Evans CP, Kung HJ and deVere
White RW: miR-124 and androgen receptor signaling inhibitors
repress prostate cancer growth by downregulating androgen receptor
splice variants, EZH2, and Src. Cancer Res. 75:5309–5317. 2015.
|
|
152
|
Yan K, Hou L, Liu T, Jiao W, Ma Q, Fang Z,
Zhang S, Song D, Liu J, Gao X and Fan Y: lncRNA OGFRP1 functions as
a ceRNA to promote the progression of prostate cancer by regulating
SARM1 level via miR-124-3p. Aging (Albany NY). 12:8880–8892.
2020.
|
|
153
|
Shield KD, Ferlay J, Jemal A,
Sankaranarayanan R, Chaturvedi AK, Bray F and Soerjomataram I: The
global incidence of lip, oral cavity, and pharyngeal cancers by
subsite in 2012. CA Cancer J Clin. 67:51–64. 2017.
|
|
154
|
Solomon B, Young RJ and Rischin D: Head
and neck squamous cell carcinoma: Genomics and emerging biomarkers
for immunomodulatory cancer treatments. Semin Cancer Biol.
52:228–240. 2018.
|
|
155
|
Alsahafi E, Begg K, Amelio I, Raulf N,
Lucarelli P, Sauter T and Tavassoli M: Clinical update on head and
neck cancer: Molecular biology and ongoing challenges. Cell Death
Dis. 10:5402019.
|
|
156
|
Salazar C, Nagadia R, Pandit P,
Cooper-White J, Banerjee N, Dimitrova N, Coman WB and Punyadeera C:
A novel saliva-based microRNA biomarker panel to detect head and
neck cancers. Cell Oncol (Dordr). 37:331–338. 2014.
|
|
157
|
Yoshizawa JM and Wong DT: Salivary
microRNAs and oral cancer detection. Methods Mol Biol. 936:313–324.
2013.
|
|
158
|
Lopez-Cortes A, Guerrero S, Redal MA,
Alvarado AT and Quinones LA: State of art of cancer
pharmacogenomics in Latin American Populations. Int J Mol Sci.
18:6392017.
|
|
159
|
Salazar-Ruales C, Arguello JV,
Lopez-Cortes A, Cabrera-Andrade A, Garcia-Cardenas JM,
Guevara-Ramirez P, Peralta P, Leone PE and Paz-Y-Miño C: Salivary
MicroRNAs for Early detection of head and neck squamous cell
carcinoma: A case-control study in the high altitude mestizo
Ecuadorian population. Biomed Res Int. 2018:97927302018.
|
|
160
|
Zhao Y, Ling Z, Hao Y, Pang X, Han X,
Califano JA, Shan L and Gu X: MiR-124 acts as a tumor suppressor by
inhibiting the expression of sphingosine kinase 1 and its
downstream signaling in head and neck squamous cell carcinoma.
Oncotarget. 8:25005–25020. 2017.
|
|
161
|
Yang S, Yuan ZJ, Zhu YH, Chen X and Wang
W: lncRNA PVT1 promotes cetuximab resistance of head and neck
squamous cell carcinoma cells by inhibiting miR-124-3p. Head Neck.
43:2712–2723. 2021.
|
|
162
|
Chen W, Yang J, Fang H, Li L and Sun J:
Relevance function of Linc-ROR in the pathogenesis of cancer. Front
Cell Dev Biol. 8:6962020.
|
|
163
|
Zare A, Ahadi A, Larki P, Omrani MD, Zali
MR, Alamdari NM and Ghaedi H: The clinical significance of miR-335,
miR-124, miR-218 and miR-484 downregulation in gastric cancer. Mol
Biol Rep. 45:1587–1595. 2018.
|
|
164
|
Wu Q, Zhong H, Jiao L, Wen Y, Zhou Y, Zhou
J, Lu X, Song X and Ying B: MiR-124-3p inhibits the migration and
invasion of Gastric cancer by targeting ITGB3. Pathol Res Pract.
216:1527622020.
|
|
165
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006.
|
|
166
|
Clevers H and Nusse R: Wnt/beta-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
167
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004.
|
|
168
|
Dai J, Qian C, Su M, Chen M and Chen J:
Gastrokine-2 suppresses epithelial mesenchymal transition through
PI3K/AKT/GSK3β signaling in gastric cancer. Tumour Biol.
37:12403–12410. 2016.
|
|
169
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012.
|
|
170
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012.
|
|
171
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005.
|
|
172
|
Parsons R: Phosphatidylinositol 3-kinase
inhibitors are a triple threat to ovarian cancer. Clin Cancer Res.
11:7965–7966. 2005.
|
|
173
|
Hoesel B and Schmid JA: The complexity of
NF-kappaB signaling in inflammation and cancer. Mol Cancer.
12:862013.
|
|
174
|
Romagnoli M, Belguise K, Yu Z, Wang X,
Landesman-Bollag E, Seldin DC, Chalbos D, Barillé-Nion S, Jézéquel
P, Seldin ML and Sonenshein GE: Epithelial-to-mesenchymal
transition induced by TGF-β1 is mediated by Blimp-1-dependent
repression of BMP-5. Cancer Res. 72:6268–6278. 2012.
|
|
175
|
Tobar N, Villar V and Santibanez JF:
ROS-NFkappaB mediates TGF-beta1-induced expression of
urokinase-type plasminogen activator, matrix metalloproteinase-9
and cell invasion. Mol Cell Biochem. 340:195–202. 2010.
|
|
176
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
|
|
177
|
Rudan I, Sidhu S, Papana A, Meng SJ,
Xin-Wei Y, Wang W, Campbell-Page RM, Demaio AR, Nair H, Sridhar D,
et al: Prevalence of rheumatoid arthritis in low- and middle-income
countries: A systematic review and analysis. J Glob Health.
5:0104092015.
|
|
178
|
Yar Saglam AS, Alp E, Elmazoglu Z and
Menevse S: Treatment with cucurbitacin B alone and in combination
with gefitinib induces cell cycle inhibition and apoptosis via EGFR
and JAK/STAT pathway in human colorectal cancer cell lines. Hum Exp
Toxicol. 35:526–543. 2016.
|
|
179
|
Saravanan S, Islam VI, Babu NP, Pandikumar
P, Thirugnanasambantham K, Chellappandian M, Raj CS, Paulraj MG and
Ignacimuthu S: Swertiamarin attenuates inflammation mediators via
modulating NF-κB/IκB and JAK2/STAT3 transcription factors in
adjuvant induced arthritis. Eur J Pharm Sci. 56:70–86. 2014.
|
|
180
|
Wang Z, Li Y, Kong D, Ahmad A, Banerjee S
and Sarkar FH: Cross-talk between miRNA and Notch signaling
pathways in tumor development and progression. Cancer Lett.
292:141–148. 2010.
|
|
181
|
Kume T: Novel insights into the
differential functions of Notch ligands in vascular formation. J
Angiogenes Res. 1:82009.
|
|
182
|
Lu ML, Zhang Y, Li J, Fu Y, Li WH, Zhao
GF, Li XH, Wei L, Liu GB and Huang H: MicroRNA-124 inhibits
colorectal cancer cell proliferation and suppresses tumor growth by
interacting with PLCB1 and regulating Wnt/β-catenin signaling
pathway. Eur Rev Med Pharmacol Sci. 23:121–136. 2019.
|
|
183
|
Lemieux E, Cagnol S, Beaudry K, Carrier J
and Rivard N: Oncogenic KRAS signalling promotes the Wnt/β-catenin
pathway through LRP6 in colorectal cancer. Oncogene. 34:4914–4927.
2015.
|
|
184
|
Shahmohamadnejad S, Nouri Ghonbalani Z,
Tahbazlahafi B, Panahi G, Meshkani R, Emami Razavi A, Shokri Afra H
and Khalili E: Aberrant methylation of miR-124 upregulates DNMT3B
in colorectal cancer to accelerate invasion and migration. Arch
Physiol Biochem. 128:1503–1509. 2022.
|
|
185
|
Hernandez-Caballero ME, Sierra - Ramirez
JA, Villalobos-Valencia R and Sesena-Mendez E: Potential of
Kalanchoe pinnata as a cancer treatment adjuvant and an
epigenetic regulator. Molecules. 27:64252022.
|
|
186
|
Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek
TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM and Costantini
F: The mouse Fused locus encodes Axin, an inhibitor of the Wnt
signaling pathway that regulates embryonic axis formation. Cell.
90:181–192. 1997.
|
|
187
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016.
|
|
188
|
Lang Q and Ling C: MiR-124 suppresses cell
proliferation in hepatocellular carcinoma by targeting PIK3CA.
Biochem Biophys Res Commun. 426:247–252. 2012.
|
|
189
|
Napetschnig J and Wu H: Molecular basis of
NF-kappaB signaling. Annu Rev Biophys. 42:443–468. 2013.
|
|
190
|
Hinz M, Stilmann M, Arslan SC, Khanna KK,
Dittmar G and Scheidereit C: A cytoplasmic ATM-TRAF6-cIAP1 module
links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB
activation. Mol Cell. 40:63–74. 2010.
|
|
191
|
Xie C, Zhang LZ, Chen ZL, Zhong WJ, Fang
JH, Zhu Y, Xiao MH, Guo ZW, Zhao N, He X and Zhuang SM: A
hMTR4-PDIA3P1-miR-125/124-TRAF6 regulatory axis and its function in
NF kappa B signaling and chemoresistance. Hepatology. 71:1660–1677.
2020.
|
|
192
|
Lv Y, Chen S, Wu J, Lin R, Zhou L, Chen G,
Chen H and Ke Y: Upregulation of long non-coding RNA OGFRP1
facilitates endometrial cancer by regulating miR-124-3p/SIRT1 axis
and by activating PI3K/AKT/GSK-3β pathway. Artif Cells Nanomed
Biotechnol. 47:2083–2090. 2019.
|
|
193
|
Li Y, Zhang Z, Liu X, Huang T, He W, Shen
Y, Liu X, Hong K and Cao Q: miR-124 functions as a tumor suppressor
in the endometrial carcinoma cell line HEC-1B partly by suppressing
STAT3. Mol Cell Biochem. 388:219–231. 2014.
|
|
194
|
Yuan D, Zhu D, Yin B, Ge H, Zhao Y, Huang
A, Wang X, Cao X, Xia N and Qian H: Expression of lncRNA NEAT1 in
endometriosis and its biological functions in ectopic endometrial
cells as mediated via miR-124-3p. Genes Genomics. 44:527–537.
2022.
|
|
195
|
Hu YZ, Hu ZL, Liao TY, Li Y and Pan YL:
LncRNA SND1-IT1 facilitates TGF-beta1-induced
epithelial-to-mesenchymal transition via miR-124/COL4A1 axis in
gastric cancer. Cell Death Discov. 8:732022.
|
|
196
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016.
|
|
197
|
Xiao HJ, Ji Q, Yang L, Li RT, Zhang C and
Hou JM: In vivo and in vitro effects of microRNA-124 on human
gastric cancer by targeting JAG1 through the Notch signaling
pathway. J Cell Biochem. 119:2520–2534. 2018.
|
|
198
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015.
|
|
199
|
Wu Z, Huang W, Chen B, Bai PD, Wang XG and
Xing JC: Up-regulation of miR-124 inhibits invasion and
proliferation of prostate cancer cells through mediating JAK-STAT3
signaling pathway. Eur Rev Med Pharmacol Sci. 21:2338–2345.
2017.
|
|
200
|
Qin Z and Liu X: miR-124, a potential
therapeutic target in colorectal cancer. Onco Targets Ther.
12:749–751. 2019.
|
|
201
|
Zhou Z, Lv J, Wang J, Yu H, Lu H, Yuan B,
Han J, Zhou R, Zhang X, Yang X, et al: Role of MicroRNA-124 as a
prognostic factor in multiple neoplasms: A meta-analysis. Dis
Markers. 2019:16547802019.
|
|
202
|
Jiao D, Li Z, Zhu M, Wang Y, Wu G and Han
X: LncRNA MALAT1 promotes tumor growth and metastasis by targeting
miR-124/foxq1 in bladder transitional cell carcinoma (BTCC). Am J
Cancer Res. 8:748–760. 2018.
|
|
203
|
Gao XR, Wang HP, Zhang SL, Wang MX and Zhu
ZS: Pri-miR-124 rs531564 polymorphism and colorectal cancer risk.
Sci Rep. 5:148182015.
|
|
204
|
Long HD, Ma YS, Yang HQ, Xue SB, Liu JB,
Yu F, Lv ZW, Li JY, Xie RT, Chang ZY, et al: Reduced hsa-miR-124-3p
levels are associated with the poor survival of patients with
hepatocellular carcinoma. Mol Biol Rep. 45:2615–2623. 2018.
|
|
205
|
Cong C, Wang W, Tian J, Gao T, Zheng W and
Zhou C: Identification of serum miR-124 as a biomarker for
diagnosis and prognosis in osteosarcoma. Cancer Biomark.
21:449–454. 2018.
|
|
206
|
Davis FG and McCarthy BJ: Current
epidemiological trends and surveillance issues in brain tumors.
Expert Rev Anticancer Ther. 1:395–401. 2001.
|
|
207
|
Chen T, Wang XY, Li C and Xu SJ:
Downregulation of microRNA-124 predicts poor prognosis in glioma
patients. Neurol Sci. 36:131–135. 2015.
|
|
208
|
Romero-Lopez MJ, Jimenez-Wences H, Cruz-De
la Rosa MI, Roman-Fernandez IV and Fernandez-Tilapa G: miR-23b-3p,
miR-124-3p and miR-218-5p synergistic or additive effects on
cellular processes that modulate cervical cancer progression? A
molecular balance that needs attention. Int J Mol Sci.
23:135512022.
|
|
209
|
Ruan F, Wang YF and Chai Y: Diagnostic
values of miR-21, miR-124, and M-CSF in patients with early
cervical cancer. Technol Cancer Res Treat.
19:15330338209149832020.
|
|
210
|
Liang F, Zhang H, Qiu Y, Xu Q, Jian K,
Jiang L, Wang F and Lu X: MiR-124-5p inhibits the progression of
gastric cancer by targeting MIEN1. Technol Cancer Res Treat.
19:15330338209791992020.
|
|
211
|
Liu F, Hu H, Zhao J, Zhang Z, Ai X, Tang L
and Xie L: miR-124-3p acts as a potential marker and suppresses
tumor growth in gastric cancer. Biomed Rep. 9:147–155. 2018.
|
|
212
|
Wu DH, Liang H, Lu SN, Wang H, Su ZL,
Zhang L, Ma JQ, Guo M, Tai S and Yu S: miR-124 suppresses
pancreatic ductal adenocarcinoma growth by regulating
monocarboxylate transporter 1-mediated cancer lactate metabolism.
Cell Physiol Biochem. 50:924–935. 2018.
|
|
213
|
Zhang YH, Wang QQ, Li H, Ye T, Gao F and
Liu YC: miR-124 radiosensitizes human esophageal cancer cell TE-1
by targeting CDK4. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
214
|
Gao C, Xu YJ, Qi L, Bao YF, Zhang L and
Zheng L: CircRNA VIM silence synergizes with sevoflurane to inhibit
immune escape and multiple oncogenic activities of esophageal
cancer by simultaneously regulating miR-124/PD-L1 axis. Cell Biol
Toxicol. 38:825–845. 2022.
|
|
215
|
Hu D, Li M, Su J, Miao K and Qiu X:
Dual-targeting of miR-124-3p and ABCC4 promotes sensitivity to
Adriamycin in breast cancer cells. Genet Test Mol Biomarkers.
23:156–165. 2019.
|
|
216
|
Du J, He Y, Wu W, Li P, Chen Y, Hu Z and
Han Y: Targeting EphA2 with miR-124 mediates Erlotinib resistance
in K-RAS mutated pancreatic cancer. J Pharm Pharmacol. 71:196–205.
2019.
|
|
217
|
Wang L, Wu M and Zhou X: Long non-coding
RNA UCA1 promotes retinoblastoma progression by modulating the
miR-124/c-myc axis. Am J Transl Res. 14:1592–1605. 2022.
|
|
218
|
Qian C and Wang Y, Ji Y, Chen D, Wang C,
Zhang G and Wang Y: Neural stem cell-derived exosomes transfer
miR-124-3p into cells to inhibit glioma growth by targeting FLOT2.
Int J Oncol. 61:1152022.
|
|
219
|
Gupta D, Zickler AM and El Andaloussi S:
Dosing extracellular vesicles. Adv Drug Deliv Rev.
178:1139612021.
|
|
220
|
Lasser C, Eldh M and Lotvall J: Isolation
and characterization of RNA-containing exosomes. J Vis Exp.
9:e30372012.
|
|
221
|
Barile L and Vassalli G: Exosomes: Therapy
delivery tools and biomarkers of diseases. Pharmacol Ther.
174:63–78. 2017.
|
|
222
|
Isaac R, Reis FCG, Ying W and Olefsky JM:
Exosomes as mediators of intercellular crosstalk in metabolism.
Cell Metab. 33:1744–1762. 2021.
|
|
223
|
Groot M and Lee H: Sorting mechanisms for
MicroRNAs into extracellular vesicles and their associated
diseases. Cells. 9:10442020.
|
|
224
|
Shuang O, Zhou J, Cai Z, Liao L, Wang Y,
Wang W and Xu M: EBF1-mediated up-regulation of lncRNA FGD5-AS1
facilitates osteosarcoma progression by regulating miR-124-3p/G3BP2
axis as a ceRNA. J Orthop Surg Res. 17:3322022.
|
|
225
|
Bronisz A, Godlewski J and Chiocca EA:
Extracellular vesicles and MicroRNAs: Their role in tumorigenicity
and therapy for brain tumors. Cell Mol Neurobiol. 36:361–376.
2016.
|
|
226
|
Ma Y, Shen N, Wicha MS and Luo M: The
roles of the Let-7 family of MicroRNAs in the regulation of cancer
stemness. Cells. 10:24152021.
|
|
227
|
Masliah-Planchon J, Garinet S and Pasmant
E: RAS-MAPK pathway epigenetic activation in cancer: miRNAs in
action. Oncotarget. 7:38892–38907. 2016.
|
|
228
|
Kanlikilicer P, Rashed MH, Bayraktar R,
Mitra R, Ivan C, Aslan B, Zhang X, Filant J, Silva AM,
Rodriguez-Aguayo C, et al: Ubiquitous release of Exosomal tumor
suppressor miR-6126 from ovarian cancer cells. Cancer Res.
76:7194–7207. 2016.
|
|
229
|
Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G,
Qin Y, Chen Y, Cui K, Zhou L, et al: Colorectal Cancer-derived
small extracellular vesicles promote tumor immune evasion by
upregulating PD-L1 expression in tumor-associated macrophages. Adv
Sci (Weinh). 9:21026202022.
|
|
230
|
Urabe F, Kosaka N, Sawa Y, Yamamoto Y, Ito
K, Yamamoto T, Kimura T, Egawa S and Ochiya T: miR-26a regulates
extracellular vesicle secretion from prostate cancer cells via
targeting SHC4, PFDN4, and CHORDC1. Sci Adv. 6:eaay30512020.
|
|
231
|
Jiang F and Doudna JA: CRISPR-Cas9
Structures and Mechanisms. Annu Rev Biophys. 46:505–529. 2017.
|
|
232
|
Wang SW, Gao C, Zheng YM, Yi L, Lu JC,
Huang XY, Cai JB, Zhang PF, Cui YH and Ke AW: Current applications
and future perspective of CRISPR/Cas9 gene editing in cancer. Mol
Cancer. 21:572022.
|
|
233
|
Horodecka K and Duchler M: CRISPR/Cas9:
Principle, applications, and delivery through extracellular
vesicles. Int J Mol Sci. 22:60722021.
|
|
234
|
Bao A, Burritt DJ, Chen H, Zhou X, Cao D
and Tran LP: The CRISPR/Cas9 system and its applications in crop
genome editing. Crit Rev Biotechnol. 39:321–336. 2019.
|
|
235
|
Chen M, Mao A, Xu M, Weng Q, Mao J and Ji
J: CRISPR-Cas9 for cancer therapy: Opportunities and challenges.
Cancer Lett. 447:48–55. 2019.
|
|
236
|
Li W, Cho MY, Lee S, Jang M, Park J and
Park R: CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer
invasion through reduced epithelial-mesenchymal transition. PLoS
One. 14:e02208602019.
|
|
237
|
Zhou SJ, Deng YL, Liang HF, Jaoude JC and
Liu FY: Hepatitis B virus X protein promotes CREB-mediated
activation of miR-3188 and Notch signaling in hepatocellular
carcinoma. Cell Death Differ. 24:1577–1587. 2017.
|
|
238
|
Liu C, Ding X, Wei C, Pei Y, Meng F, Zhong
Y and Liu Y: LncRNA LNCOC1 is Upregulated in melanoma and serves as
a potential regulatory target of miR-124 to suppress cancer cell
invasion and migration. Clin Cosmet Investig Dermatol. 15:751–762.
2022.
|
|
239
|
Gupta D, Bhattacharjee O, Mandal D, Sen
MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, et
al: CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life
Sci. 232:1166362019.
|
|
240
|
Huo W, Zhao G, Yin J, Ouyang X, Wang Y,
Yang C, Wang B, Dong P, Wang Z, Watari H, et al: Lentiviral
CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the
epithelial to mesenchymal transition in ovarian cancer cells. J
Cancer. 8:57–64. 2017.
|
|
241
|
Kunej T, Godnic I, Horvat S, Zorc M and
Calin GA: Cross talk between microRNA and coding cancer genes.
Cancer J. 18:223–231. 2012.
|
|
242
|
Hu S, Zang R, Wang Y, Liang Y, Mu J, Zhang
Y and Ma J: Highly expressed microRNA-124 inhibits migration and
promotes apoptosis of esophageal cancer cells by degrading PDCD6. J
BUON. 24:805–812. 2019.
|
|
243
|
Zhao X, He W, Li J, Huang S, Wan X, Luo H
and Wu D: MiRNA-125b inhibits proliferation and migration by
targeting SphK1 in bladder cancer. Am J Transl Res. 7:2346–2354.
2015.
|
|
244
|
Liu Z, Guo S, Sun H, Bai Y, Song Z and Liu
X: Circular RNA CircHIPK3 elevates CCND2 expression and promotes
cell proliferation and invasion through miR-124 in Glioma. Front
Genet. 11:10132020.
|
|
245
|
Sanuki R and Yamamura T: Tumor suppressive
effects of miR-124 and its function in neuronal development. Int J
Mol Sci. 22:59192021.
|
|
246
|
Yong T, Sun A, Henry MD, Meyers S and
Davis JN: Down regulation of CSL activity inhibits cell
proliferation in prostate and breast cancer cells. J Cell Biochem.
112:2340–2351. 2011.
|
|
247
|
Gao Y, Yang M, Wei L, Liang X, Wu F, Huang
Y and Yang T: miR-34a-5p inhibits cell proliferation, migration and
invasion through targeting JAG1/Notch1 pathway in HPV-infected
human epidermal keratinocytes. Pathol Oncol Res. 26:1851–1859.
2020.
|
|
248
|
Cheng Z, Li X, Ye X, Yu R and Deng Y:
Purpurogallin reverses neuronal apoptosis and enhances 'M2'
polarization of microglia under ischemia via mediating the
miR-124-3p/TRAF6/NF-kappaB axis. Neurochem Res. 48:375–392.
2023.
|
|
249
|
Wu Z, Huang W, Chen B, Bai PD, Wang XG and
Xing JC: Up-regulation of miR-124 inhibits invasion and
proliferation of prostate cancer cells through mediating JAK-STAT3
signaling pathway. Eur Rev Med Pharmacol Sci. 24:75462020.
|
|
250
|
Gao S, Yu J, Shan Z, Zuo L, Huang W, Gan L
and Tian H: IncRNA XIST targets miR-124/JAG1 via CeRNA mechanism to
facilitate the migration and proliferation of tongue squamous cell
carcinoma. Clin Lab. 68:2022. View Article : Google Scholar
|
|
251
|
Guo W, Liu GM, Guan JY, Chen YJ, Zhao YZ,
Wang K and Bai O: Epigenetic regulation of cutaneous T-cell
lymphoma is mediated by dysregulated lncRNA MALAT1 through
modulation of tumor microenvironment. Front Oncol.
12:9772662022.
|
|
252
|
Zhang WL, Zhao YN, Shi ZZ, Gu GY, Cong D,
Wei C and Bai YS: HOXA11-AS promotes the migration and invasion of
hepatocellular carcinoma cells by inhibiting miR-124 expression by
binding to EZH2. Hum Cell. 32:504–514. 2019.
|
|
253
|
Ghafouri-Fard S, Shoorei H, Bahroudi Z,
Abak A, Majidpoor J and Taheri M: An update on the role of miR-124
in the pathogenesis of human disorders. Biomed Pharmacother.
135:1111982021.
|