Malignant tumor is a major health problem worldwide
and a leading cause of death, ranking second only to cardiovascular
disease. Immunotherapy based on the tumor microenvironment (TME)
has become a promising cancer treatment strategy (1). The TME is a complex system composed
mainly of tumor cells, mesenchymal cells, innate immune cells
[macrophages, monocytes, natural killer (NK) cells, dendritic cells
and myeloid derived suppressor cells] and adaptive immune cells (T
cells and B cells). Macrophages, also known as tumor-associated
macrophages (TAMs), among innate immune cells [macrophages,
monocytes, natural killer (NK) cells, dendritic cells and myeloid
derived suppressor cells], which are found in almost all tissues
and organs, are the first line of defense against exogenous and
endogenous injury- or pathogen-associated molecular patterns
(2). Macrophages that infiltrate
tumor tissue or congregate in the microenvironment of solid tumors
are defined as tumor-associated macrophages (TAMs). As an important
part of the TME, macrophages have a complex role in tumorigenesis
and tumor progression. They can not only inhibit tumor growth by
releasing pro-inflammatory cytokines and exerting cytotoxic
activities, but also promote tumor progression by affecting the
occurrence and growth of tumor cells, participating in tumor
angiogenesis and metastasis, and shaping an immunosuppressive
microenvironment (3). In the
present review, the heterogeneity of the origin of TAMs, the
factors affecting the polarization of TAMs and the complex role of
TAMs in tumor progression were summarized, and therapeutic
approaches targeting TAMs, such as consuming TAMs or re-educating
TAMs were discussed in order to provide a reference for colleagues
to gain insight for tumor immunotherapy.
It is generally thought that TAMs are mainly derived
from monocytes produced by hematopoietic stem cells in bone marrow
(4). However, recent evidence
suggested that certain TAMs (such as alveolar macrophages, brain
macrophages and liver macrophages) originate from pre-natal
embryonic precursors (yolk sac or fetal liver) (5). These cells were recruited into the
TME under the action of chemokines [such as C-C motif ligand 2
(CCL2), CCL3, CCL4 and CXCL12], colony-stimulating factor 1
(CSF-1), interleukin-6 (IL-6), IL-1β and vascular endothelial
growth factor (VEGF) produced by tumor cells or stromal cells to
become TAMs (6,7). As an important part of the TME,
these TAMs can affect tumorigenesis and development, tumor
angiogenesis and immune regulation through interactions with other
cell populations in the TME. Furthermore, TAMs are also associated
with poor prognosis of various tumors, such as breast cancer
(8), bladder cancer (9), head and neck neoplasm (10), glioma (11), melanoma (12) and prostate cancer (13). However, more recently, it has been
found that high macrophage infiltration is associated with better
prognosis in colorectal and gastric cancers (14). This opposite effect may be related
to the plasticity of macrophages and the resulting heterogeneity in
phenotype and function of various cancers.
Macrophages are highly plastic and their phenotype
and function are regulated by the surrounding microenvironment.
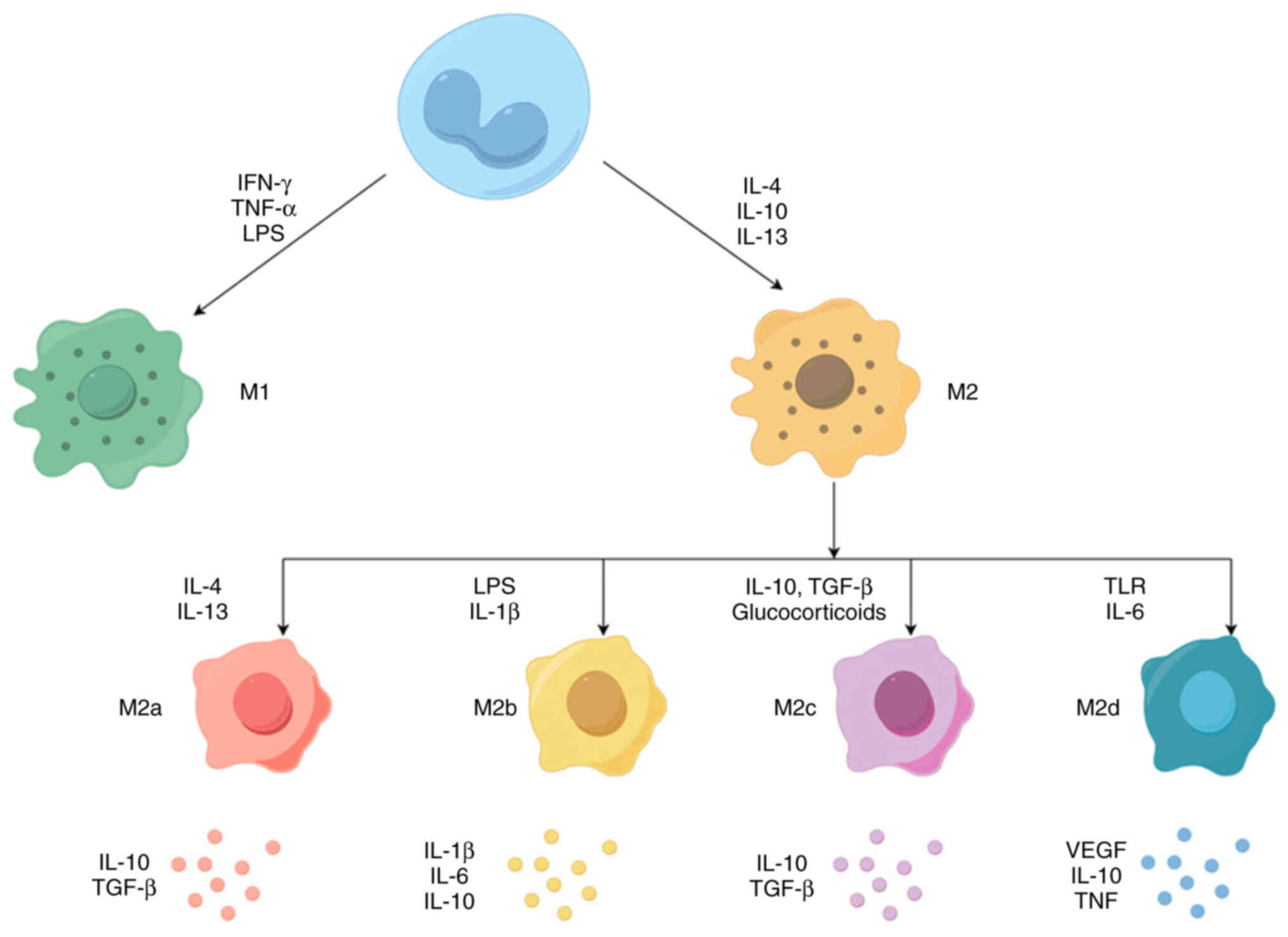
Macrophages usually exist in two distinct subsets: Classically
activated macrophages (M1) and alternately activated macrophages
(M2). M1 macrophages, normally recognized and induced by type I
T-helper cell (Th1) cytokines [e.g. IFN-γ and tumor necrosis factor
(TNF)-α] or bacterial lipopolysaccharide (LPS), secrete
pro-inflammatory cytokines such as IL-12 and TNF-α, produce high
levels of nitric oxide (NO) and reactive oxygen species (ROS), and
have powerful anti-microbial and anti-tumor activities (15). By contrast, M2 macrophages are
activated by Th2 cytokines (IL-4 and IL-13), secrete
anti-inflammatory cytokines such as IL-10, IL-13 and IL-4, and
express abundant arginase-1 (Arg-1), mannose receptor (CD206) and
scavenger receptor (CD163), which have the functions of removing
debris, promoting angiogenesis, tissue reconstruction, damage
repair, as well as promoting tumorigenesis and development
(16). However, depending on the
different activation stimuli, M2 macrophages can be further divided
into four distinct subgroups, including M2a, M2b, M2c and M2d. The
M2a subgroup is induced by IL-4 and IL-13 to produce high levels of
CD206, decoy receptor IL-1 receptor II and IL-1 receptor antagonist
(17,18). The M2b subgroup can be induced by
immune complexes combined with IL-1β or LPS, which produces
anti-inflammatory and pro-inflammatory cytokines IL-10, IL-1β, IL-6
and TNF-α (17,18). The M2c subgroup is generally
induced by glucocorticoids and IL-10, releases large amounts of
IL-10 and TGF-β and exhibits strong anti-inflammatory activity
against apoptotic cells (17,19). Finally, the M2d subgroup, induced
by Toll-like receptor (TLR) agonists via adenosine receptors,
inhibits the production of pro-inflammatory cytokines and induces
the secretion of anti-inflammatory cytokines and VEGFs (20-22). Overall, M2a and M2b macrophages
have an immunomodulatory role and promote helper T-cell responses,
while M2c macrophages are associated with immune response
suppression and tissue remodeling. M2d macrophages are involved in
angiogenesis and tumor progression (17,23) (Fig.
1).
The factors influencing TAM polarization are diverse
and regulated by a variety of signals in tumor cells and stromal
cells in the TME, mainly including the following factors,
immunogenic signals, hypoxia (tumor-derived metabolic signals) and
extracellular matrix (ECM) components.
Immunogenic signaling mainly refers to the
cytokines, chemokines and growth factors released by tumor cells,
stromal cells and other infiltrating cells in the microenvironment,
which are key determinants of TAM polarization. Among these
factors, CCL2 and CSF-1 are the most well-studied stimulators.
Studies have shown that tumor-derived chemokine CCL2 binds to C-C
chemokine receptor 2 (CCR2) expressed on the surface of macrophages
to polarize macrophages towards the pro-tumor phenotype. Blocking
the CCL2-CCR2 interaction by gene ablation or antibodies was
observed to significantly inhibit tumor metastasis, prolong the
survival of tumor-bearing mice and reduce the expression of
pro-tumor cytokines (24-26). Later, it was proved that, in
addition to the influence of CCL2 derived from tumor cells on the
polarization of macrophages, the chemotactic signal of CCL2
expressed by tumor-associated fibroblasts could also recruit
macrophages to the tumor site and drive the polarization of
macrophages towards the pro-tumor phenotype to increase the
aggressiveness of cancer cells and the occurrence of breast tumors
(27). CSF-1, another important
factor affecting the polarization of macrophages, is generally
widely overexpressed at the invasive margins of various tumors and
is associated with tumor progression (15). A study on follicular lymphoma
found that CSF-1 derived from tumor cells promoted the polarization
of macrophages toward pro-tumor M2-like phenotypes and the use of
CSF-1R inhibitor (PLX3397) resulted in the repolarization of
macrophages toward the M1-type, which showed synergistic anti-tumor
effects when combined with anti-CD20 rituximab (28). In addition to the above factors,
there are other factors involved in the induction of macrophage
polarization, such as VEGF-A, epidermal growth factor (EGF) and
prostaglandin E2 (29-31).
Hypoxia, as one of the key drivers of macrophage
recruitment and polarization in the TME, is caused by tumor cells
with vigorous metabolism and rapid growth but poor vascular
organization, which is a common feature of most solid tumors
(32). Hypoxia can regulate the
phenotype of TAMs through various factors, particularly through
lactic acid produced by glycolysis affecting macrophage
polarization (33). For instance,
a study on gastric cancer showed that lactic acid produced by
glycolysis can induce changes in the macrophage phenotype through
monocarboxylate channel transporter-hypoxia-inducible factor 1α
signaling, polarizing macrophages towards an M2-like state
(34). In addition, the hypoxic
TME can stimulate the secretion of tumor-derived exosomes, regulate
the macrophage phenotype and promote tumor development. For
instance, exosomes of hypoxic tumor cells are enriched with
immunomodulatory proteins and chemokines, including CSF-1, CCL2,
ferritin heavy chain, ferritin light chain and TGF-β, which
influence macrophage recruitment and promote macrophage
polarization to the M2 phenotype (35). Therefore, the hypoxic
microenvironment can shape a specific macrophage phenotype, which
promotes immune escape and metastasis of tumor cells.
Last but not least, the ECM component of the TME
also has a regulatory effect on macrophage polarization. The ECM is
a highly dynamic and complex macromolecular network consisting of a
variety of fibrins, proteoglycans and stromal cell-associated
proteins (36). The ECM molecule,
elastin microfibril interfacer 2 (EMILIN-2), which belongs to the
EDEN protein family, exerts a tumor-suppressive effect by affecting
the polarization state of macrophages in a variety of tumors
(37,38). In a study on colorectal cancer,
EMILIN-2 was found to promote M1 polarization through activation of
the TLR-4/myeloid differentiation factor-88/NF-κB signaling
pathway. EMILIN-2 deficiency is associated with increased M2
macrophage infiltration (39).
This suggests that certain components of the ECM are key regulators
of the tumor-associated inflammatory environment and may represent
promising prognostic biomarkers for tumor patients. In summary, the
polarization of TAMs is regulated by complex biological networks,
which is closely related to cancer development. Understanding the
mechanisms of macrophage polarization may enable researchers to
manipulate the macrophage polarization status to stimulate their
anti-tumor potential for therapeutic purposes.
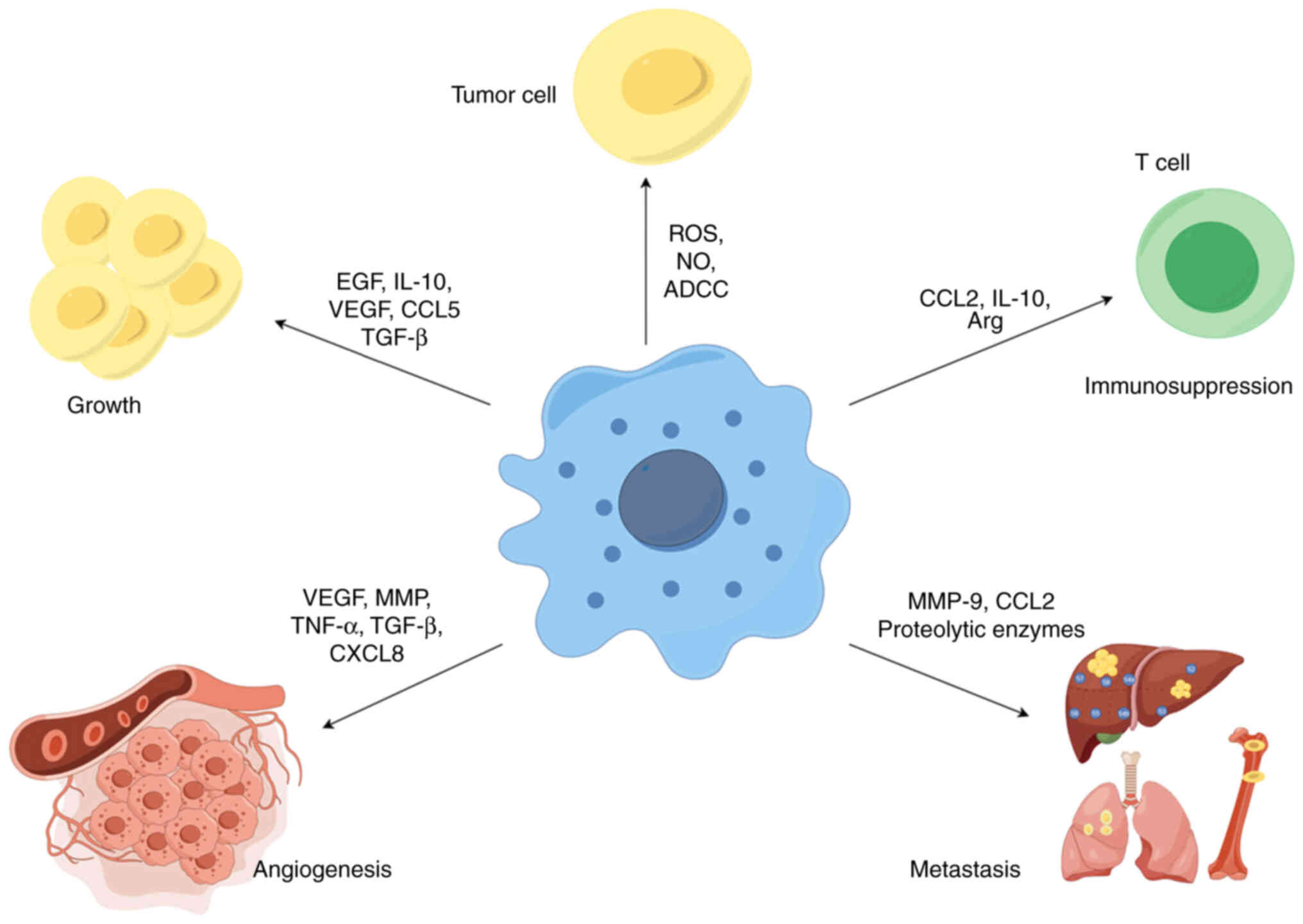
TAM is a key player in the interaction between
cancer cells and their microenvironment and has a dual potential in
tumorigenesis and development. As a tumor suppressor, M1-type
macrophages have high cytotoxicity and immunostimulatory effects
against tumor cells, and can kill tumor cells through two different
mechanisms. One is that M1-type macrophages directly mediate the
cytotoxic effect of killing tumor cells, i.e., macrophages directly
target infected cells or tumor cells by releasing lysosomal enzymes
or cytotoxic molecules (such as ROS and NO), which is a slow
process (40). Another way to
kill tumors is antibody-dependent cell-mediated cytotoxicity, which
usually requires the involvement of anti-tumor antibodies to kill
tumor cells in a short period of time (41). Therefore, M1-type macrophages are
considered anti-tumor or 'good' macrophages (Fig. 2).
In contrast, in most formed tumors, macrophages
contribute to cancer initiation, progression and metastasis through
a variety of mechanisms, including promoting cancer cell survival
and proliferation, angiogenesis and suppression of immune responses
(Fig. 2).
TAMs not only provide structural support within the
tumor stroma but also promote tumorigenesis and tumor cell growth
by producing growth factors, cytokines and chemokines such as EGF,
platelet-derived growth factor, TGF-β, hepatocyte growth factor,
basic fibroblast growth factor, IL-10 and other cytokines, e.g.
CXCL, CCL and VEGF (42). For
example, M2-type macrophages have a promoting role in the
proliferation and invasion of oral squamous cell carcinoma through
the production of EGF and the number of CD206+ TAMs is
positively correlated with a poorer clinical prognosis in oral
squamous cell carcinoma (43).
Furthermore, in a study on clear cell renal cell carcinoma (ccRCC),
it was found that TAM-derived chemokine CCL5 can promote tumor cell
proliferation and the formation of an immunosuppressive TME, which
is closely related to the poor prognosis for patients with ccRCC
(44). In addition to supporting
tumor cell growth, TAM has also been found to have a role in
supporting the growth of cancer stem cells (CSCs). In a recent
study on breast cancer, TAM-derived IL-6 was found to regulate the
enrichment of CSCs in breast cancer through the STAT-3 pathway and
lead to tumor growth (45)
(Fig. 2).
Angiogenesis is essential for tumor growth and
metastasis, which is considered a 'hallmark' of cancer. An
increasing amount of evidence indicates that TAMs are closely
related to angiogenesis in tumors (46). On the one hand, TAMs participate
in angiogenesis by secreting pro-angiogenic factors, including
VEGF-A, matrix metalloproteinase (MMP), EGF, TGF-β, TNF-α, CCL2,
CXCL8 and CXCL12 (47). For
example, in a study on bladder cancer, TAM-derived CXCL8 was found
to be highly associated with tumor migration, invasion and
angiogenesis (48). In addition,
the release of thymine phosphorylase (TP) and urine-stimulated
plasminogen activator by TAMs can stimulate the migration of
endothelial cells, increase ECM degradation and indirectly promote
tumor angiogenesis (49). Studies
have found that macrophage-derived TP is significantly associated
with tumor angiogenesis and poor prognosis in gastric cancer
(50). In addition, TAMs also
promote tumor angiogenesis by secreting inflammatory mediators such
as IL-1 and IL-6. A study on breast cancer found that TAM-derived
IL-6 affected breast cancer cell migration and angiogenesis and
induced CSC populations, leading to tumor growth (51,52). In summary, these studies suggest
that TAMs promote tumor vascularization in different ways and are
closely related to tumor progression (Fig. 2).
Macrophages have an important role in every step of
the metastasis process, including preparation for pre-metastatic
niche formation, intravasation, survival of circulating tumor
cells, extravasation and invasion. In terms of forming a
pre-metastatic niche, macrophages are recruited to the
pre-metastatic site in response to various factors secreted by
tumor cells, which provide a roadmap for the homing of circulating
tumor cells to the pre-metastatic niche through enhanced expression
of chemokines and secretion of molecules such as MMPs and integrins
(53-55). In terms of intravasation,
macrophages can decompose the surrounding matrix by secreting
various proteolytic enzymes to promote the intravasation of tumor
cells. For instance, Gocheva et al (56) found that IL-4 induces cathepsin
activity in TAMs, promoting tumor growth and invasion. In terms of
circulating tumor cell survival, macrophages secrete chemokines or
cytokines to promote the successful survival of numerous tumor
cells at metastatic sites. A study on breast cancer found that
macrophages bind to vascular cell adhesion molecule-1 on the
surface of tumor cells via α4 integrin, triggering the PI3K/Akt
survival signaling pathway in cancer cells and protecting tumor
cells from pro-apoptotic cytokines (57). In terms of extravasation, when
tumor cells settle in the capillaries of the target organ, they
attempt to attach and extrude through the vessel wall with the
assistance of macrophages, and the extravasation rate decreases
significantly after the loss of macrophages, indicating that
macrophages have an important role in promoting the extravasation
of circulating tumor cells (58,59). In terms of invasion, TAM
contributes to tumor invasion and metastasis mainly through
epithelial-mesenchymal transformation (EMT). A recent study found
that CCL2, secreted by TAMs, promotes EMT in triple-negative breast
cancer (TNBC) cells through activating the AKT/β-catenin signaling
pathway, which may provide a new strategy for the diagnosis and
treatment of TNBC (60) (Fig. 2).
In addition to their tumor-killing role, TAMs can
also mediate immunosuppression, reshape the tumor immune
microenvironment and promote tumor development. On the one hand,
TAMs can express ligands of inhibitory receptors [such as
programmed cell death protein-1 (PD-1) ligand 1, CD80/CD86 and
death receptor ligands Fas ligand or tumour necrosis factor
(TNF)-related apoptosis-inducing ligand (TRAIL)], which bind to the
immune cell surface receptors PD-1, cytotoxic T-lymphocyte
antigen-4, FaS and TRAIL-RI/-RII, thereby inhibiting the anti-tumor
effects of immune cells (such as T cells and NK cells) (61,62). On the other hand, TAMs can also
form an immunosuppressive microenvironment by producing chemokines,
cytokines and enzymes. For instance, a study in ovarian cancer
found that the TAM-secreted chemokine CCL22 recruited CCR4 +
T-regulatory cells (Tregs) to promote an immunosuppressive
microenvironment (63). It has
also been observed in a mouse model of colorectal cancer that
CCL20, a TAM-derived chemokine, recruited CCR6(+) Treg cells to the
tumor mass, creating an immunosuppressive microenvironment that
promoted tumor development (64).
In addition, Xu et al (9)
found that TAM-derived cytokine IL-10 was associated with the
depletion of CD8+ T cells and dysfunction of NK cells in the tumor
immune microenvironment, which led to poor prognosis in patients
with bladder cancer. Recently, a study on pancreatic cancer found
that TAM-derived Arg-1 drives immunosuppression by depleting
arginine and inhibiting T-cell activation (65,66). In conclusion, these findings
support the immunomodulatory role of TAM in promoting tumor
progression by shaping the immunosuppressive microenvironment
(Fig. 2).
TAMs are abundant in the TME of most cancer types
and are commonly associated with poor clinical prognosis for cancer
patients. TAMs are becoming a key target for immunotherapy and the
different approaches targeting TAMs that have been explored may be
broadly divided into three main categories: i) Eliminating TAMs
already present in the TME; ii) inhibiting monocyte recruitment;
iii) reprogramming of TAMs (67,68). These strategies have been
investigated in preclinical models and some of them have been
translated into clinical studies as adjuncts to immunotherapy
(69). In the present review,
some of the current approaches to targeting macrophages and
clinical trials were outlined and the potential advantages and
disadvantages of these approaches were discussed (Table I).
Selective elimination of TAMs has been used in
cancer treatment. An attractive strategy for depleting TAMs in the
TME is to trigger its apoptosis and restore local immune
surveillance in the TME, which can effectively inhibit tumor
growth. Several compounds have been shown to induce apoptosis in
macrophages, mainly including bisphosphonates and trabectedin.
Bisphosphonates are a class of anti-bone resorption
drugs, which can be divided into two categories according to their
structural characteristics: Non-nitrogenous bisphosphonates and
nitrogenous bisphosphonates (70). Bisphosphonates exhibit direct or
indirect antitumor properties. They can inhibit cancer cell
proliferation, induce tumor cell apoptosis, block angiogenesis and
interfere with immune surveillance. At the same time,
bisphosphonates can also inhibit the proliferation, migration and
invasion of macrophages, leading to the apoptosis of macrophages
(71,72). For instance, in earlier studies,
dichloromethylenediphosphonates (also known as clodronates) from
the non-nitrogenous bisphosphonate family were often used to
consume macrophages in the liver and spleen when loaded with
liposomes (73); Zoledronate, the
third generation of nitrogenous bisphosphonates, is selectively
cytotoxic to TAMs expressing MMP9 and inhibits the progression of
cervical cancer (74). In
addition, a study of non-small cell lung cancer found that calcium
zoledronate nanoparticles modified with biotin and mannose
preferentially targeted biotin-expressing tumor cells and
mannose-expressing TAMs, ultimately suppressing tumor growth and
survival (75). A study about
thyroid cancer found that zoledronic acid inhibits thyroid cancer
stemness and metastasis by repressing M2-like TAM polarization and
the Wnt/β-catenin pathway, reducing the tumor burden (76). Furthermore, a prospective phase II
clinical trial found that zoledronic acid combined with
radiotherapy reduced bone pain and improved quality of life in
patients with bone metastases from gastrointestinal tumors
(77). Trabectedin is a
tetrahydroisoquinoline alkaloid that directly kills tumor cells by
interfering with multiple transcription factors, DNA-binding
proteins and DNA repair pathways (78). Furthermore, it also selectively
consumes monocytes and macrophages in the TME by activating caspase
8 through a TNF-related apoptosis-inducing ligand-dependent
mechanism (79). In a study on
fibrosarcoma, Trabectedin selectively reduced macrophages in the
TME and enhanced the anti-tumor response to anti-PD-1 therapy
(80). In several clinical
trials, Trabectedin was found to show good safety and efficacy in
the treatment of soft tissue sarcoma and ovarian cancer (81,82). However, disappointing results were
obtained in malignant pleural mesothelioma and pancreatic cancer
tumors (83,84).
Macrophages have an important regulatory role in
maintaining host defenses and tissue homeostasis, and a major
problem with the depletion of macrophages is the inability to avoid
the side effects that arise from non-selective macrophage
depletion. Therefore, the key to minimizing potential toxic side
effects is to develop drugs that preferentially target M2-like
macrophages. A recent study designed M2-targeting nanoliposomes,
which effectively depleted M2-type TAMs, remodeled the TME and
effectively inhibited tumor growth (85). Consequently, targeted elimination
of M2-like TAMs is a promising approach for cancer
immunotherapy.
As mentioned earlier, most TAMs originate from the
production of bone marrow monocytes. TAMs are recruited to tumor
sites in response to tumor-derived chemokines. Therefore, the
application of monoclonal antibodies or small molecule inhibitors
to interfere with chemokine signaling may be an effective way to
prevent the accumulation of TAMs in the TME.
The expansion of TAMs in tumors is usually mediated
by monocyte recruitment on the CCL2-CCR2 axis. Monocytes expressing
the CCR-2 receptor are recruited to the tumor site by CCL-2
released by tumor cells, macrophages and stromal cells within the
TME, where they further mature into TAMs (86). Therefore, reducing macrophage
recruitment and infiltration into the TME by blocking the CCL2/CCR2
axis may be a promising therapeutic anti-tumor strategy. A study on
esophageal cancer found that blocking the CCL2-CCR2 axis
significantly reduced the tumor incidence by preventing TAM
recruitment and also enhanced the anti-tumor effects of CD8+ T
cells in the TME (87). Several
CCL-2 antibodies are undergoing clinical trials. The two main drugs
tested so far are the anti-CCL2 monoclonal antibody Carlumab (CNTO
888) and a targeted small molecule inhibitor (PF04136309), which
have shown a certain benefit in tumor control (88,89).
In addition, BMS-813160, a CCR2/5 inhibitor, has
been selected as a clinical candidate for its ability to inhibit
the migration of inflammatory monocytes and macrophages. Clinical
trials of BMS-813160 are ongoing in non-small cell carcinoma, liver
cancer and pancreatic cancer (90) (Table
I).
CSF-1 controls the proliferation, differentiation,
recruitment, survival and function of mononuclear phagocytes (e.g.,
macrophages, monocytes) (91).
Therefore, targeting the CSF1/CSF1R signaling pathway is considered
to be another important and effective strategy for the treatment of
malignant tumors. Ries et al (92) found that the use of CSF1R
monoclonal antibody, RG7155, in patients with advanced diffuse
giant cell tumors significantly reduced CSF1R+CD163+ macrophages in
the TME. In a study of advanced solid tumors, RG7155 specifically
depleted immunosuppressed M2-like macrophages and was used in
combination with paclitaxel to enhance anti-tumor responses
(93). In addition, in a study on
endometrial cancer, it was found to promote TAM recruitment in the
TME and tumor cell proliferation, which was significantly
diminished by a CSF1R blocker (PLX3397) (94). A phase I clinical trial found
PLX3397 to have favorable safety and tolerability in Asian patients
with advanced solid tumors (95).
In a rare tumor called tendonsynovial giant cell tumor,
vimseltinib, a small molecule inhibitor targeting CSF1R, was found
to persistently inhibit CSF1R activity in vitro and in
vivo, depleting macrophages and other CSF1R-dependent cells,
and inhibiting tumor growth and bone degradation in a mouse model
of cancer (96).
Although CSF1/CSF1R and CCL2/CCR2 blockade are the
most widely studied axes of TAM depletion, other cytokines have
also been shown to have a role in this process. In a mouse model of
malignant lobular tumors, monocytes are recruited into tumors
through the interaction between CCL5 and CCR5. Blockade of the
CCL5-CCR5 axis by CCR5 inhibitors resulted in markedly attenuated
monocyte recruitment into tumors and inhibition of tumor growth
(97). Therefore, actively
exploring the factors that interfere with macrophage recruitment
will provide a new idea for targeted macrophage therapy.
As mentioned above, it is widely acknowledged that
M2 and M1 macrophages have opposite roles in tumor growth and
metastasis. Therefore, therapeutic strategies to re-educate the
tumor-promoting M2 phenotype into the tumor-killing M1 phenotype
have been proposed. Reprogramming macrophages involves the
following two aspects: Restoration of phagocytosis and promotion of
the polarization phenotype.
CD24 is a highly glycosylated
glycosylphosphatidylinositol-anchored surface protein that acts as
a 'don't eat me' signal. It regulates phagocytosis in macrophages
through interaction with sialic-binding IG-10 (Siglec-10), an
inhibitory receptor on TAMs (98). CD24 is commonly overexpressed in
cancers and its overexpression is associated with poor prognosis in
various cancers (99). It has
been found in ovarian cancer and TNBC that CD24 expressed by tumor
cells can interact with Siglec-10 on TAMs, blocking phagocytosis of
macrophages. Blockade of CD24 or Siglec-10 enhanced the
phagocytosis of macrophages on tumors and inhibited tumor growth
(100). In addition, a recent
study in mantle cell lymphoma and follicular lymphoma found that
high expression of CD24 was associated with poor prognosis of
patients. Treatment with CD24 monoclonal antibody significantly
enhanced phagocytosis by macrophages and inhibited tumor
progression (101). These
studies demonstrate the therapeutic potential of CD24 blockade as a
cancer immunotherapy.
CD47, a transmembrane glycoprotein widely expressed
on cancer cells, can block macrophage phagocytosis by binding to
the signal-regulatory protein (SIRP)α on the surface of
macrophages, enabling cancer cells to escape immune surveillance
(102). Based on this
characteristic, targeting the CD47-SIRPα axis is an effective
modality. In a study of thyroid cancer, it was found that the
degree of infiltration of TAMs in xenografted mice treated with
anti-CD47 antibody was significantly increased and phagocytosis of
tumor cells by macrophages was enhanced, which inhibited tumor
growth (103). Furthermore, in
small cell lung cancer (SCLC), macrophages showed increased
phagocytosis and enhanced anti-tumor effects on GFP-expressing SCLC
cells in mice treated with radiotherapy combined with CD47 block,
compared with CD47 block alone, a finding that is particularly
important for cancer patients suffering from metastatic disease
(104). In addition to
increasing phagocytosis of tumor cells, anti-CD47 therapy has also
been shown to modulate TAM phenotypic changes. For instance, a
glioblastoma study found that anti-CD47 treatment not only enhanced
macrophage phagocytosis of tumor cells but also shifted the
phenotype of macrophages towards the M1 subtype (105). Hence, preclinical studies of
CD47-SIRPα blockade suggest its potential for clinical efficacy. To
date, clinical studies have indicated that CD47 inhibitors
(Hu5F9-G4) in combination with rituximab showed good anti-tumor
activity in patients with non-Hodgkin lymphoma, while CD47
inhibitors (CC90002) have provided disappointing results in
patients with acute myeloid leukemia (106,107). Clinical trials targeting SIRP
molecules are still ongoing (Table
I).
As a member of the class 1B family, PI3Kγ is usually
associated with G-protein-coupled receptor signaling and is
abundantly expressed on a variety of immune cells, including
macrophages and neutrophils, and the PI3Kγ pathway is related to
the phenotypic transformation of TAMs as well as immunosuppressive
states (108,109). For instance, in a mouse model
carrying breast cancer, blocking PI3Kγ was also found to reduce the
number of M2-like macrophages and enhance the role of cytotoxic T
lymphocytes (CTLs), which significantly prevented tumor progression
and prolonged survival (110).
In addition, Carnevalli et al (111) found that AZD3458, a PI3Kγ
inhibitor, did not alter tumor macrophage polarization, but instead
promoted antigen-presenting macrophage and cytotoxic macrophage
activation, which activated CD8 T cell-mediated antitumor activity
of associated immune checkpoint inhibitors. A phase I clinical
study found that a highly selective PI3Kγ inhibitor (IPI-549)
demonstrated significant anti-tumor activity in patients with
advanced solid disease (112).
Therefore, the above studies have demonstrated the important role
of PI3Kγ in TAMs reprogramming and recruitment of immune cells that
inhibit tumor growth, suggesting that PI3Kγ may be a promising
target for tumor therapy.
TLRs are innate immune pattern recognition receptors
that have an important role in the activation of innate immune
responses (113). In the
reprogramming of macrophages, the phenotype of macrophages can
change and they may be polarized in a pro-inflammatory direction.
Numerous therapies aim at targeting TLR to repolarize macrophages
from an M2-like activated state to an M1-like activated state and
to enhance the immune response to cancer cells. For instance, local
in situ inoculation of melanoma and neck tumor models with
the tumor antigen (protein and peptide) adjuvant nanoemulsion
loaded with TLR7/8 agonists induced recruitment and activation of
innate immune cells, infiltration of lymphocytes and polarization
of tumor-associated M2 macrophages, resulting in inhibition of
tumor growth and prolonged tumor survival (114). Furthermore, oxaliplatin combined
with TLR agonist R848 was found to reverse the polarization of
macrophages and enhance the anti-tumor effects of oxaliplatin in
colorectal cancer resistant to oxaliplatin (115). In addition to TLR7 and TLR8, it
was found that TLR3 and TLR4 agonists also have the effect of
altering the macrophage polarization status (116,117). These findings suggest the
clinical relevance of TLR signaling and its potential application
for cancer treatment by targeting TAMs. To date, several targeting
TLRs have been tested in clinical trials with promising results
(118-120).
CD40 is a member of the TNF receptor superfamily and
is highly expressed on antigen-presenting cells, such as
macrophages. The ligand of CD40 is CD40L, which is mainly expressed
by activated T cells, B cells and platelets (121). The CD40-CD40L interaction
promotes the polarization of macrophages towards pro-inflammatory
macrophages. For instance, in a mouse model of melanoma, it was
found that myeloid-derived suppressor cells induce macrophage
reprogramming by inhibiting the expression of CD40 on the surface
of macrophages, thereby promoting melanoma progression (122). In addition, a study on
pancreatic cancer found that CD40 agonists could drive the
transformation of the macrophage phenotype towards M1, remodeling
the pancreatic cancer microenvironment and inhibiting the
development of pancreatic cancer (123). More recently, Frankish et
al (124) found that
HERA-CD40L, a novel molecule targeting CD40-mediated signal
transduction, activates the signaling transduction mechanisms in
dendritic cells, leading to an increase in intratumoral T cells and
manipulating TME phenotypic changes to repolarize M2 macrophages to
M1, thereby enhancing tumor control. In addition, a clinical trial
in pancreatic ductal adenocarcinoma showed that selicrelumab, an
agonist CD40 antibody, induced changes in the TME in patients with
resectable pancreatic cancer, a reduction in M2-like TAMs and a
significant reduction in tumor burden (125). Based on the above observations,
reprogramming TAMs to anti-tumor phenotypes using CD40, rather than
targeting and ablating TAMs, may be the preferred therapeutic
paradigm for cancer treatment.
TAMs are one of the most important innate immune
cell types in TME and have a complex regulatory role in tumor
therapy. Therefore, it is crucial to reveal the exact regulatory
mechanisms and specific targets of macrophages on tumors in order
to optimize the effectiveness of current tumor therapies. In the
present review, the heterogeneity of the origin of TAMs, the
relevant regulators of recruitment and polarization and the complex
roles of TAMs in tumorigenesis, progression and metastasis were
discussed. Certain therapeutic approaches targeting TAMs, such as
consumption of TAMs or re-education of TAMs were also outlined to
provide insight for tumor immunotherapy. Specifically, targeting
TAMs is a promising immunotherapy strategy. However, the clinical
application of current therapeutic strategies is still very
limited. For instance, the efficacy is restricted to certain
patients, the anti-tumor spectrum is narrow, the adverse reactions
are more frequent and drug resistance may easily occur. These
defects limit the clinical application of targeting macrophages in
tumor therapy. In addition, numerous questions remain regarding the
nature and function of macrophages in the TME; many unknown
molecular mechanisms have a crucial role in regulating tumor growth
and development, and various potential targets require more
research and attention. Therefore, it is necessary to further
investigate the dialogue between macrophages and tumor cells. With
a greater understanding of macrophage diversity through single-cell
sequencing and other techniques, TAM-targeted therapies will be an
important complement to cancer immunotherapy.
Not applicable.
CL, YL and LM conceived the article. CL wrote the
first version of the manuscript with critical input from all
authors. XK, HL, HR and XZ performed the literature search. BZ and
XN edited the manuscript for important intellectual content. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors have no competing interests to
declare.
Not applicable.
This work was supported by XC, the host of the Capacity
Construction Project of Major Clinical (specialized) Departments of
Traditional Chinese Medicine of Liaoning Province (grant no.
LNZYXZK201909 to XC) and the host of the Distinguished Professor
Program of Liaoning Province (grant no. 203296 to XC). It was also
supported by YL, the host of the Hospital Research Fund by
Affiliated Zhongshan Hospital of Dalian University (grant no.
20230320 to YL).
|
1
|
Yan S and Wan G: Tumor-associated
macrophages in immunotherapy. FEBS J. 288:6174–6186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Song Y, Du W, Gong L, Chang H and
Zou Z: Tumor-associated macrophages: An accomplice in solid tumor
progression. J Biomed Sci. 26:782019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pathria P, Louis TL and Varner JA:
Targeting tumor-associated macrophages in cancer. Trends Immunol.
40:310–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Okreglicka KM, Pohlmeier LM,
Schneider C and Kopf M: Fetal monocytes possess increased metabolic
capacity and replace primitive macrophages in tissue macrophage
development. EMBO J. 39:e1032052020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar
|
|
7
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang WJ, Wang XH, Gao ST, Chen C, Xu XY,
Sun Q, Zhou ZH, Wu GZ, Yu Q, Xu G, et al: Tumor-associated
macrophages correlate with phenomenon of epithelial-mesenchymal
transition and contribute to poor prognosis in triple-negative
breast cancer patients. J Surg Res. 222:93–101. 2018. View Article : Google Scholar
|
|
9
|
Xu Y, Zeng H, Jin K, Liu Z, Zhu Y, Xu L,
Wang Z, Chang Y and Xu J: Immunosuppressive tumor-associated
macrophages expressing interlukin-10 conferred poor prognosis and
therapeutic vulnerability in patients with muscle-invasive bladder
cancer. J Immunother Cancer. 10:e0034162022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar AT, Knops A, Swendseid B,
Martinez-Outschoom U, Harshyne L, Philp N, Rodeck U, Luginbuhl A,
Cognetti D, Johnson J and Curry J: Prognostic significance of
tumor-associated macrophage content in head and neck squamous cell
carcinoma: A meta-analysis. Front Oncol. 9:6562019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Luo YB, Wu W, Zhang L, Wang Z,
Dai Z, Feng S, Cao H, Cheng Q and Liu Z: The molecular feature of
macrophages in tumor immune microenvironment of glioma patients.
Comput Struct Biotechnol J. 19:4603–4618. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Z, Lei K, Li H, He J and Shi E:
Transcriptome-based network analysis related to M2-like
tumor-associated macrophage infiltration identified VARS1 as a
potential target for improving melanoma immunotherapy efficacy. J
Transl Med. 20:4892022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuri P, Shigemura K, Kitagawa K, Hadibrata
E, Risan M, Zulfiqqar A, Soeroharjo I, Hendri AZ, Danarto R, Ishii
A, et al: Increased tumor-associated macrophages in the prostate
cancer microenvironment predicted patients' survival and responses
to androgen deprivation therapies in Indonesian patients cohort.
Prostate Int. 8:62–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cortese N, Carriero R, Laghi L, Mantovani
A and Marchesi F: Prognostic significance of tumor-associated
macrophages: Past, present and future. Semin Immunol.
48:1014082020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Movahedi K, Laoui D, Gysemans C, Baeten M,
Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De
Baetselier P and Van Ginderachter JA: Different tumor
microenvironments contain functionally distinct subsets of
macrophages derived from Ly6C(high) monocytes. Cancer Res.
70:5728–5739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar
|
|
19
|
Zizzo G, Hilliard BA, Monestier M and
Cohen PL: Efficient clearance of early apoptotic cells by human
macrophages requires M2c polarization and MerTK Induction. J
Immunol. 189:3508–3520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrante AW Jr: Macrophages, fat, and the
emergence of immunometabolism. J Clin Invest. 123:4992–4993. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haskó G, Pacher P, Deitch EA and Vizi ES:
Shaping of monocyte and macrophage function by adenosine receptors.
Pharmacol Ther. 113:264–275. 2007. View Article : Google Scholar
|
|
22
|
Pinhal-Enfield G, Ramanathan M, Hasko G,
Vogel SN, Salzman AL, Boons GJ and Leibovich SJ: An angiogenic
switch in macrophages involving synergy between Toll-like receptors
2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol.
163:711–721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang YH, Cai K, Xu PP, Wang L, Huang CX,
Fang Y, Cheng S, Sun XJ, Liu F, Huang JY, et al: CREBBP/EP300
mutations promoted tumor progression in diffuse large B-cell
lymphoma through altering tumor-associated macrophage polarization
via FBXW7-NOTCH-CCL2/CSF1 axis. Signal Transduct Target Ther.
6:102021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sierra-Filardi E, Nieto C, Domínguez-Soto
Á, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven
J, Ardavín C, Rodríguez-Fernández JL, et al CCL2 Shapes Macrophage
Polarization by GM-CSF and M-CSF: Identification of
CCL2/CCR2-dependent gene expression profile. J Immunol.
192:3858–3867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Archer M, Bernhardt SM, Hodson LJ,
Woolford L, Van der Hoek M, Dasari P, Evdokiou A and Ingman WV:
CCL2-Mediated stromal interactions drive macrophage polarization to
increase breast tumorigenesis. Int J Mol Sci. 24:73852023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valero JG, Matas-Céspedes A, Arenas F,
Rodriguez V, Carreras J, Serrat N, Guerrero-Hernández M, Yahiaoui
A, Balagué O, Martin S, et al: The receptor of the
colony-stimulating factor-1 (CSF-1R) is a novel prognostic factor
and therapeutic target in follicular lymphoma. Leukemia.
35:2635–2649. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mu G, Zhu Y, Dong Z, Shi L, Deng Y and Li
H: Calmodulin 2 facilitates angiogenesis and metastasis of gastric
cancer via STAT3/HIF-1A/VEGF-A mediated macrophage polarization.
Front Oncol. 11:7273062021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lian G, Chen S, Ouyang M, Li F, Chen L and
Yang J: Colon cancer cell secretes EGF to Promote M2 Polarization
of TAM Through EGFR/PI3K/AKT/mTOR pathway. Technol Cancer Res
Treat. 18:15330338198490682019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazzoni M, Mauro G, Erreni M, Romeo P,
Minna E, Vizioli MG, Belgiovine C, Rizzetti MG, Pagliardini S,
Avigni R, et al: Senescent thyrocytes and thyroid tumor cells
induce M2-like macrophage polarization of human monocytes via a
PGE2-dependent mechanism. J Exp Clin Cancer Res. 38:2082019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9(Suppl 5): S4–S9. 2004. View Article : Google Scholar
|
|
33
|
Zhou HC, Xin-Yan Yan, Yu WW, Liang XQ, Du
XY, Liu ZC, Long JP, Zhao GH and Liu HB: Lactic acid in macrophage
polarization: The significant role in inflammation and cancer.
Inter Rev Immunol. 41:4–18. 2021. View Article : Google Scholar
|
|
34
|
Zhang L and Li S: Lactic acid promotes
macrophage polarization through MCT-HIF1α signaling in gastric
cancer. Exp Cell Res. 388:1118462020. View Article : Google Scholar
|
|
35
|
Park JE, Dutta B, Tse SW, Gupta N, Tan CF,
Low JK, Yeoh KW, Kon OL, Tam JP and Sze SK: Hypoxia-induced tumor
exosomes promote M2-like macrophage polarization of infiltrating
myeloid cells and microRNA-mediated metabolic shift. Oncogene.
38:5158–5173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hynes RO: The extracellular matrix: Not
just pretty fibrils. Science. 326:1216–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colombatti A, Spessotto P, Doliana R,
Mongiat M, Bressan GM and Esposito G: The EMILIN/Multimerin family.
Front Immunol. 2:932012. View Article : Google Scholar :
|
|
38
|
Mongiat M, Marastoni S, Ligresti G,
Lorenzon E, Schiappacassi M, Perris R, Frustaci S and Colombatti A:
The extracellular matrix glycoprotein elastin microfibril interface
located protein 2: A dual role in the tumor microenvironment.
Neoplasia. 12:294–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Andreuzzi E, Fejza A, Polano M, Poletto E,
Camicia L, Carobolante G, Tarticchio G, Todaro F, Di Carlo E,
Scarpa M, et al: Colorectal cancer development is affected by the
ECM molecule EMILIN-2 hinging on macrophage polarization via the
TLR-4/MyD88 pathway. J Exp Clin Cancer Res. 41:602022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bernsmeier C, van der Merwe S and Périanin
A: Innate immune cells in cirrhosis. J Hepatol. 73:186–201. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bruns H, Büttner M, Fabri M, Mougiakakos
D, Bittenbring JT, Hoffmann MH, Beier F, Pasemann S, Jitschin R,
Hofmann AD, et al: Vitamin D-dependent induction of cathelicidin in
human macrophages results in cytotoxicity against high-grade B cell
lymphoma. Sci Transl Med. 7:282ra472015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-Associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haque ASMR, Moriyama M, Kubota K, Ishiguro
N, Sakamoto M, Chinju A, Mochizuki K, Sakamoto T, Kaneko N,
Munemura R, et al: CD206+tumor-associated macrophages promote
proliferation and invasion in oral squamous cell carcinoma via EGF
production. Sci Rep. 9:146112019. View Article : Google Scholar
|
|
44
|
Xu W, Wu Y, Liu W, Anwaier A, Tian X, Su
J, Huang H, Wei G, Qu Y, Zhang H and Ye D: Tumor-associated
macrophage-derived chemokine CCL5 facilitates the progression and
immunosuppressive tumor microenvironment of clear cell renal cell
carcinoma. Int J Biol Sci. 18:4884–4900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Radharani NNV, Yadav AS, Nimma R, Kumar
TVS, Bulbule A, Chanukuppa V, Kumar D, Patnaik S, Rapole S and
Kundu GC: Tumor-associated macrophage derived IL-6 enriches cancer
stem cell population and promotes breast tumor progression via
Stat-3 pathway. Cancer Cell Int. 22:1222022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Valković T, Dobrila F, Melato M, Sasso F,
Rizzardi C and Jonjić N: Correlation between vascular endothelial
growth factor, angiogenesis, and tumor-associated macrophages in
invasive ductal breast carcinoma. Virchows Arch. 440:583–588. 2002.
View Article : Google Scholar
|
|
47
|
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY and
Mou XZ: The roles of tumor-associated macrophages in tumor
angiogenesis and metastasis. Cell Immunol. 353:1041192020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu H, Zhang X, Han D, Cao J and Tian J:
Tumour-associated macrophages mediate the invasion and metastasis
of bladder cancer cells through CXCL8. PeerJ. 8:e87212020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawahara A, Hattori S, Akiba J, Nakashima
K, Taira T, Watari K, Hosoi F, Uba M, Basaki Y, Koufuji K, et al:
Infiltration of thymidine phosphorylase-positive macrophages is
closely associated with tumor angiogenesis and survival in
intestinal type gastric cancer. Oncol Rep. 24:405–415. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hori T, Sasayama T, Tanaka K, Koma YI,
Nishihara M, Tanaka H, Nakamizo S, Nagashima H, Maeyama M, Fujita
Y, et al: Tumor-associated macrophage related interleukin-6 in
cerebrospinal fluid as a prognostic marker for glioblastoma. J Clin
Neurosci. 68:281–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou M, Na R, Lai S, Guo Y, Shi J, Nie J,
Zhang S, Wang Y and Zheng T: The present roles and future
perspectives of Interleukin-6 in biliary tract cancer. Cytokine.
169:1562712023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu X and Kang Y: Organotropism of breast
cancer metastasis. J Mammary Gland Biol Neoplasia. 12:153–162.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen Q, Zhang XH and Massagué J:
Macrophage binding to receptor VCAM-1 transmits survival signals in
breast cancer cells that invade the lungs. Cancer Cell. 20:538–549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y,
Li J, Lang RA and Pollard JW: A distinct macrophage population
mediates metastatic breast cancer cell extravasation, establishment
and growth. PLoS One. 4:e65622009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Genna A, Duran CL, Entenberg D, Condeelis
JS and Cox D: Macrophages Promote tumor cell extravasation across
an endothelial barrier through thin membranous connections. Cancers
(Basel). 15:20922023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen X, Yang M, Yin J, Li P, Zeng S, Zheng
G, He Z, Liu H, Wang Q, Zhang F and Chen D: Tumor-associated
macrophages promote epithelial-mesenchymal transition and the
cancer stem cell properties in triple-negative breast cancer
through CCL2/AKT/β-catenin signaling. Cell Commun Signal.
20:922022. View Article : Google Scholar
|
|
61
|
Li X, Shao C, Shi Y and Han W: Lessons
learned from the blockade of immune checkpoints in cancer
immunotherapy. J Hematol Oncol. 11:312018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
DeNardo DG and Ruffell B: Macrophages as
regulators of tumour immunity and immunotherapy. Nat Rev Immunol.
19:369–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng
Q and Wang H, Chen J and Wang H: Tumor-associated macrophages
recruit CCR6+ regulatory T cells and promote the development of
colorectal cancer via enhancing CCL20 production in mice. PLoS One.
6:e194952011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arlauckas SP, Garren SB, Garris CS, Kohler
RH, Oh J, Pittet MJ and Weissleder R: Arg1 expression defines
immunosuppressive subsets of tumor-associated macrophages.
Theranostics. 8:5842–5854. 2018. View Article : Google Scholar
|
|
66
|
Menjivar RE, Nwosu ZC, Du W, Donahue KL,
Hong HS, Espinoza C, Brown K, Velez-Delgado A, Yan W, Lima F, et
al: Arginase 1 is a key driver of immune suppression in pancreatic
cancer. Elife. 12:e807212023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
van der Heide D, Weiskirchen R and Bansal
R: Therapeutic targeting of hepatic macrophages for the treatment
of liver diseases. Front Immunol. 10:28522019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Roelofs AJ, Thompson K, Gordon S and
Rogers MJ: Molecular mechanisms of action of bisphosphonates:
Current status. Clin Cancer Res. 12(20 Pt 2): 6222s–6230s. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Van Acker HH, Anguille S, Willemen Y,
Smits EL and Van Tendeloo VF: Bisphosphonates for cancer treatment:
Mechanisms of action and lessons from clinical trials. Pharmacol
Ther. 158:24–40. 2016. View Article : Google Scholar
|
|
72
|
Rogers TL and Holen I: Tumour macrophages
as potential targets of bisphosphonates. J Transl Med. 9:1772011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Van Rooijen N, Kors N, vd Ende M and
Dijkstra CD: Depletion and repopulation of macrophages in spleen
and liver of rat after intravenous treatment with
liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue
Res. 260:215–222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Giraudo E, Inoue M and Hanahan D: An
amino-bisphosphonate targets MMP-9-expressing macrophages and
angiogenesis to impair cervical carcinogenesis. J Clin Invest.
114:623–633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zang X, Zhou J, Zhang X, Chen D, Han Y and
Chen X: Dual-targeting tumor cells and tumor associated macrophages
with lipid coated calcium zoledronate for enhanced lung cancer
chemoimmunotherapy. Int J Pharm. 594:1201742021. View Article : Google Scholar
|
|
76
|
Lv J, Chen FK, Liu C, Liu PJ, Feng ZP, Jia
L, Yang ZX, Hou F and Deng ZY: Zoledronic acid inhibits thyroid
cancer stemness and metastasis by repressing M2-like
tumor-associated macrophages induced Wnt/β-catenin pathway. Life
Sci. 256:1179252020. View Article : Google Scholar
|
|
77
|
Choi J, Lee EJ, Yang SH, Im YR and Seong
J: A prospective phase II study for the efficacy of radiotherapy in
combination with zoledronic acid in treating painful bone
metastases from gastrointestinal cancers. J Radiat Res. 60:242–248.
2019. View Article : Google Scholar :
|
|
78
|
D'Incalci M and Galmarini CM: A review of
trabectedin (ET-743): A unique mechanism of action. Mol Cancer
Ther. 9:2157–2163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Belgiovine C, Frapolli R, Liguori M,
Digifico E, Colombo FS, Meroni M, Allavena P and D'Incalci M:
Inhibition of tumor-associated macrophages by trabectedin improves
the antitumor adaptive immunity in response to anti-PD-1 therapy.
Eur J Immunol. 51:2677–2686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
de Sande González LM, Martin-Broto J,
Kasper B, Blay JY and Le Cesne A: Real-world evidence of the
efficacy and tolerability of trabectedin in patients with advanced
soft-tissue sarcoma. Expert Rev Anticancer Ther. 20:957–963. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Romero I, López-Guerrero JA and Pignata S:
Real-world experience with trabectedin for the treatment of
recurrent ovarian cancer. Expert Rev Anticancer Ther. 21:1089–1095.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cortinovis D, Grosso F, Carlucci L, Zucali
PA, Pasello G, Tiseo M, Sperandi F, Hollander L, Galli F, Torri V,
et al: Trabectedin in malignant pleural mesothelioma: Results from
the multicentre, single arm, phase II ATREUS study. Clin Lung
Cancer. 22:361–370.e3. 2021. View Article : Google Scholar
|
|
84
|
Belli C, Piemonti L, D'Incalci M,
Zucchetti M, Porcu L, Cappio S, Doglioni C, Allavena P, Ceraulo D,
Maggiora P, et al: Phase II trial of salvage therapy with
trabectedin in metastatic pancreatic adenocarcinoma. Cancer
Chemother Pharmacol. 77:477–484. 2016. View Article : Google Scholar
|
|
85
|
Cao Y, Qiao B, Chen Q, Xie Z, Dou X, Xu L,
Ran H, Zhang L and Wang Z: Tumor microenvironment remodeling via
targeted depletion of M2-like tumor-associated macrophages for
cancer immunotherapy. Acta Biomater. 160:239–251. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kalbasi A, Komar C, Tooker GM, Liu M, Lee
JW, Gladney WL, Ben-Josef E and Beatty GL: Tumor-Derived CCL2
mediates resistance to radiotherapy in pancreatic ductal
adenocarcinoma. Clin Cancer Res. 23:137–148. 2017. View Article : Google Scholar
|
|
87
|
Yang H, Zhang Q, Xu M, Wang L, Chen X,
Feng Y, Li Y, Zhang X, Cui W and Jia X: CCL2-CCR2 axis recruits
tumor associated macrophages to induce immune evasion through PD-1
signaling in esophageal carcinogenesis. Mol Cancer. 19:412020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Noel M, O'Reilly EM, Wolpin BM, Ryan DP,
Bullock AJ, Britten CD, Linehan DC, Belt BA, Gamelin EC, Ganguly B,
et al: Phase 1b study of a small molecule antagonist of human
chemokine (C-C motif) receptor 2 (PF-04136309) in combination with
nab-paclitaxel/gemcitabine in first-line treatment of metastatic
pancreatic ductal adenocarcinoma. Invest New Drugs. 38:800–811.
2020. View Article : Google Scholar :
|
|
89
|
Brana I, Calles A, LoRusso PM, Yee LK,
Puchalski TA, Seetharam S, Zhong B, de Boer CJ, Tabernero J and
Calvo E: Carlumab, an anti-C-C chemokine ligand 2 monoclonal
antibody, in combination with four chemotherapy regimens for the
treatment of patients with solid tumors: An open-label, multicenter
phase 1b study. Target Onco. 10:111–123. 2015. View Article : Google Scholar
|
|
90
|
Cherney RJ, Anjanappa P, Selvakumar K,
Batt DG, Brown GD, Rose AV, Vuppugalla R, Chen J, Pang J, Xu S, et
al: BMS-813160: A Potent CCR2 and CCR5 dual antagonist selected as
a clinical candidate. ACS Med Chem Lett. 12:1753–1758. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lelios I, Cansever D, Utz SG, Mildenberger
W, Stifter SA and Greter M: Emerging roles of IL-34 in health and
disease. J Exp Med. 217:e201902902020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ries CH, Cannarile MA, Hoves S, Benz J,
Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I,
et al: Targeting tumor-associated macrophages with anti-CSF-1R
antibody reveals a strategy for cancer therapy. Cancer Cell.
25:846–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gomez-Roca CA, Italiano A, Le Tourneau C,
Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat
D, Cannarile MA, et al: Phase I study of emactuzumab single agent
or in combination with paclitaxel in patients with
advanced/metastatic solid tumors reveals depletion of
immunosuppressive M2-like macrophages. Ann Oncol. 30:1381–1392.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hua F, Tian Y, Gao Y, Li C and Liu X:
Colony-stimulating factor 1 receptor inhibition blocks macrophage
infiltration and endometrial cancer cell proliferation. Mol Med
Rep. 19:3139–3147. 2019.PubMed/NCBI
|
|
95
|
Lee JH, Chen TW, Hsu CH, Yen YH, Yang JC,
Cheng AL, Sasaki SI, Chiu LL, Sugihara M, Ishizuka T, et al: A
phase I study of pexidartinib, a colony-stimulating factor 1
receptor inhibitor, in Asian patients with advanced solid tumors.
Invest New Drugs. 38:99–110. 2020. View Article : Google Scholar :
|
|
96
|
Smith BD, Kaufman MD, Wise SC, Ahn YM,
Caldwell TM, Leary CB, Lu WP, Tan G, Vogeti L, Vogeti S, et al:
Vimseltinib: A Precision CSF1R therapy for tenosynovial giant cell
tumors and diseases promoted by macrophages. Mol Cancer Ther.
20:2098–2109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nie Y, Huang H, Guo M, Chen J, Wu W, Li W,
Xu X, Lin X, Fu W, Yao Y, et al: Breast Phyllodes Tumors Recruit
and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to
promote malignant progression, which can be inhibited by CCR5
inhibition therapy. Clin Cancer Res. 25:3873–3886. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Altevogt P, Sammar M, Hüser L and
Kristiansen G: Novel insights into the function of CD24: A driving
force in cancer. Int J Cancer. 148:546–559. 2021. View Article : Google Scholar
|
|
99
|
Tarhriz V, Bandehpour M, Dastmalchi S,
Ouladsahebmadarek E, Zarredar H and Eyvazi S: Overview of CD24 as a
new molecular marker in ovarian cancer. J Cell Physiol.
234:2134–2142. 2019. View Article : Google Scholar
|
|
100
|
Barkal AA, Brewer RE, Markovic M, Kowarsky
M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal
LJ and Weissman IL: CD24 signalling through macrophage Siglec-10 is
a target for cancer immunotherapy. Nature. 572:392–396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Freile JÁ, Ustyanovska Avtenyuk N,
Corrales MG, Lourens HJ, Huls G, van Meerten T, Cendrowicz E and
Bremer E: CD24 Is a Potential Immunotherapeutic Target for Mantle
Cell Lymphoma. Biomedicines. 10:11752022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Maute R, Xu J and Weissman IL:
CD47-SIRPα-targeted therapeutics: Status and prospects. Immunooncol
Technol. 13:1000702022. View Article : Google Scholar
|
|
103
|
Schürch CM, Roelli MA, Forster S, Wasmer
MH, Brühl F, Maire RS, Di Pancrazio S, Ruepp MD, Giger R, Perren A,
et al: Targeting CD47 in anaplastic thyroid carcinoma enhances
tumor phagocytosis by macrophages and is a promising therapeutic
strategy. Thyroid. 29:979–992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nishiga Y, Drainas AP, Baron M,
Bhattacharya D, Barkal AA, Ahrari Y, Mancusi R, Ross JB, Takahashi
N, Thomas A, et al: Radiotherapy in combination with CD47 blockade
elicits a macrophage-mediated abscopal effect. Nat Cancer.
3:1351–1366. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang M, Hutter G, Kahn SA, Azad TD,
Gholamin S, Xu CY, Liu J, Achrol AS, Richard C, Sommerkamp P, et
al: Anti-CD47 treatment stimulates phagocytosis of glioblastoma by
M1 and M2 polarized macrophages and promotes M1 polarized
macrophages in vivo. PLoS One. 11:e01535502016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's
Lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zeidan AM, DeAngelo DJ, Palmer J, Seet CS,
Tallman MS, Wei X, Raymon H, Sriraman P, Kopytek S, Bewersdorf JP,
et al: Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in
patients with relapsed/refractory acute myeloid leukemia and
high-risk myelodysplastic syndromes. Ann Hematol. 101:557–569.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Solinas G and Becattini B: The role of
PI3Kγ in metabolism and macrophage activation. Oncotarget.
8:106145–106146. 2017. View Article : Google Scholar :
|
|
109
|
Qiu X, Tian Y, Liang Z, Sun Y, Li Z and
Bian J: Recent discovery of phosphoinositide 3-kinase γ inhibitors
for the treatment of immune diseases and cancers. Future Med Chem.
11:2151–2169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Qin H, Yu H, Sheng J, Zhang D, Shen N, Liu
L, Tang Z and Chen X: PI3Kgamma inhibitor attenuates
immunosuppressive effect of Poly(l-Glutamic Acid)-Combretastatin A4
conjugate in metastatic breast cancer. Adv Sci (Weinh).
6:19003272019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Carnevalli LS, Taylor MA, King M,
Coenen-Stass AML, Hughes AM, Bell S, Proia TA, Wang Y,
Ramos-Montoya A, Wali N, et al: Macrophage activation status rather
than repolarization is associated with enhanced checkpoint activity
in combination with PI3Kγ Inhibition. Mol Cancer Ther.
20:1080–1091. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hong DS, Postow M, Chmielowski B, Sullivan
R, Patnaik A, Cohen EEW, Shapiro G, Steuer C, Gutierrez M,
Yeckes-Rodin H, et al: Eganelisib a first-in-class PI3Kγ inhibitor,
in patients with advanced solid tumors: Results of the phase 1/1b
MARIO-1 trial. Clin Cancer Res. 29:2210–2219. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Brennan JJ and Gilmore TD: Evolutionary
Origins of Toll-like Receptor Signaling. Mol Biol Evol.
35:1576–1587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim SY, Kim S, Kim JE, Lee SN, Shin IW,
Shin HS, Jin SM, Noh YW, Kang YJ, Kim YS, et al: Lyophilizable and
multifaceted toll-like receptor 7/8 agonist-loaded nanoemulsion for
the reprogramming of tumor microenvironments and enhanced cancer
immunotherapy. ACS Nano. 13:12671–12686. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu
Z, Mao H, Yu M and Wang X: TLR 7/8 agonist reverses oxaliplatin
resistance in colorectal cancer via directing the myeloid-derived
suppressor cells to tumoricidal M1-macrophages. Cancer Lett.
469:173–185. 2020. View Article : Google Scholar
|
|
116
|
Vidyarthi A, Khan N, Agnihotri T, Negi S,
Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK and Agrewala
JN: TLR-3 Stimulation Skews M2 Macrophages to M1 Through IFN-αβ
signaling and restricts tumor progression. Front Immunol.
9:16502018. View Article : Google Scholar
|
|
117
|
Sun L, Kees T, Almeida AS, Liu B, He XY,
Ng D, Han X, Spector DL, McNeish IA, Gimotty P, et al: Activating a
collaborative innate-adaptive immune response to control
metastasis. Cancer Cell. 39:1361–1374.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chow LQM, Morishima C, Eaton KD, Baik CS,
Goulart BH, Anderson LN, Manjarrez KL, Dietsch GN, Bryan JK,
Hershberg RM, et al: Phase Ib trial of the toll-like receptor 8
agonist, motolimod (VTX-2337), combined with cetuximab in patients
with recurrent or metastatic SCCHN. Clin Cancer Res. 23:2442–2450.
2017. View Article : Google Scholar
|
|
119
|
Shayan G, Kansy BA, Gibson SP, Srivastava
RM, Bryan JK, Bauman JE, Ohr J, Kim S, Duvvuri U, Clump DA, et al:
Phase Ib study of immune biomarker modulation with neoadjuvant
cetuximab and TLR8 stimulation in head and neck cancer to overcome
suppressive myeloid signals. Clin Cancer Res. 24:62–72. 2018.
View Article : Google Scholar :
|
|
120
|
Trutnovsky G, Reich O, Joura EA, Holter M,
Ciresa-König A, Widschwendter A, Schauer C, Bogner G, Jan Z, Boandl
A, et al: Topical imiquimod versus surgery for vulvar
intraepithelial neoplasia: A multicentre, randomised, phase 3,
non-inferiority trial. Lancet. 399:1790–1798. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Elgueta R, Benson MJ, de Vries VC, Wasiuk
A, Guo Y and Noelle RJ: Molecular mechanism and function of
CD40/CD40L engagement in the immune system. Immunol Rev.
229:152–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Valencia JC, Erwin-Cohen RA, Clavijo PE,
Allen C, Sanford ME, Day CP, Hess MM, Johnson M, Yin J, Fenimore
JM, et al: Myeloid-Derived suppressive cell expansion promotes
melanoma growth and autoimmunity by inhibiting CD40/IL27 regulation
in macrophages. Cancer Res. 81:5977–5990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lim CY, Chang JH, Lee WS, Kim J and Park
IY: CD40 agonists alter the pancreatic cancer microenvironment by
shifting the macrophage phenotype toward M1 and suppress human
pancreatic cancer in organotypic slice cultures. Gut Liver.
16:645–659. 2022. View Article : Google Scholar :
|
|
124
|
Frankish J, Mukherjee D, Romano E,
Billian-Frey K, Schröder M, Heinonen K, Merz C, Redondo Müller M,
Gieffers C, Hill O, et al: The CD40 agonist HERA-CD40L results in
enhanced activation of antigen presenting cells, promoting an
anti-tumor effect alone and in combination with radiotherapy. Front
Immunol. 14:11601162023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Byrne KT, Betts CB, Mick R, Sivagnanam S,
Bajor DL, Laheru DA, Chiorean EG, O'Hara MH, Liudahl SM, Newcomb C,
et al: Neoadjuvant selicrelumab, an agonist CD40 antibody, induces
changes in the tumor microenvironment in patients with resectable
pancreatic cancer. Clin Cancer Res. 27:4574–4586. 2021. View Article : Google Scholar : PubMed/NCBI
|