Introduction
Neuroblastoma (NB) is the most common extracranial
solid tumor affecting children, accounting for 7% of all tumors in
children <15 years of age and 15% of all childhood
cancer-related deaths (1). NB is
a complex tumor with unique characteristics, and its biological
heterogeneity leads to various clinical manifestations (2). Despite the aggressive multi-method
treatment in high-risk NB cases, the 5-year survival rate of
patients is <50%. At present, monoclonal GD2 specific antibodies
have significantly improved the survival rates of patients with
high-risk NB (3,4); however, the high costs associated
with the use of GD2 monoclonal antibody for immunotherapy hinders
its widespread use in patients in low- and middle-income countries.
Given the limited number of frequently mutated genes in NB, the
mode of targeted therapy against mutated oncogenes is challenging
(5). Currently, numerous scholars
have turned their attention to epigenetic drug therapy.
Histone deacetylase (HDAC) is an enzyme that
regulates gene expression by remodeling chromatin structure. The
dysregulation of HDAC expression leads to an imbalance of histone
acetylation and promotes various human tumors (6), including NB (7). HDAC inhibitors (HDACis) have been
confirmed to inhibit NB cell proliferation, and to induce
differentiation, apoptosis and cell cycle arrest (8). Additionally, the combination of
HDACis with other chemotherapeutic agents or radiation therapy has
also recently been investigated in preclinical research and
clinical trials of patients with NB (9,10).
CUDC-907 is a dual-target inhibitor of PI3K and
HDAC, which has significant potential to inhibit tumor growth and
metastasis by simultaneously destroying multiple oncogenic signal
networks (11). Previous studies
have demonstrated that CUDC-907 exerts inhibitory effects on
various tumors (12-16). Currently, CUDC-907 has been
investigated in phase I and II clinical trials for the treatment of
multiple myeloma (NCT01742988) and relapsed/refractory diffuse
large B-cell lymphoma (NCT01742988) (17,18). In the present study, we aimed to
explore the anticancer effects of CUDC-907 on NB in vivo and
in vitro, and to further investigate the mechanisms through
which CUDC-907 affects cancer-promoting pathways and the stemness
phenotype of NB cells. It is hoped that the findings presented
herein may provide a promising approach and prospects for the
treatment of NB.
Materials and methods
Study compound
CUDC-907 was purchased from TargetMol. The drug
powder was dissolved into a 10 mg/ml storage solution with an
appropriate amount of dimethyl sulfoxide (DMSO), stored at −80°C.
In vivo, CUDC-907 was diluted into suspension by normal
saline using an ultrasonic processor (QSONICA SonicatorQ700).
Cells and cell culture
All cell lines, including SK-N-SH, SK-N-BE(2), SH-SY5Y, SK-N-AS and IMR32, were
purchased from Cobioer Biosciences Co., Ltd. The SK-N-SH and IMR32
cells were cultured in minimum essential medium (MEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% 1 mM sodium
pyruvate (Gibco; Thermo Fisher Scientific, Inc.) and 1% MEM
non-essential amino acids (MEM NEAA; Gibco; Thermo Fisher
Scientific, Inc.). The SK-N-BE(2)
and SH-SY5Y cells were cultured in MEM/F12 (1:1) (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 1% 1 mM sodium
pyruvate and 1% MEM NEAA. The SK-N-AS cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS. All culture mediums
were supplemented with 1% penicillin-streptomycin solution (Gibco;
Thermo Fisher Scientific, Inc.). All cells were cultured in a 5%
CO2 and humidified incubator maintained at 37°C. All
cell lines had been authenticated by STR profiling.
Cell Counting Kit-8 (CCK-8) assay
CUDC-907 at the initial maximum concentration (2 mM)
was diluted at a gradient ratio of 1:10 in 96-well plates with
adherent cells. The blank control group received DMSO instead of
CUDC-907. In addition, each well was provided with three
vice-holes. Moreover, 10 μl CCK-8 (ApexBio) was added to
each well of the plate. The absorbance at 450 nm was measured using
a microplate reader (Tecan Spark 10M, Tecan Group, Ltd.) after
incubating at 37°C for 2 h.
Colony formation assay
The cells were prepared into a cell suspension at a
concentration of 2×103/ml. After 24 h, an appropriate
amount of PBS was added for washing. Medium containing various
concentrations (2, 4, 8 and 16 nM) of CUDC-907 was then added, and
each well was provided with three vice-holes. The cells were
incubated at 37°C in the 5% CO2 humidified incubator for
10-14 days. Following removal from the incubator, the cells were
fixed with 10% formalin (Biosharp Life Sciences) for 20 min, and
then stained with crystal violet (MilliporeSigma) for 10 min, with
both procedures conducted at room temperature.
Apoptosis analysis
The apoptosis of NB cells treated with or without
CUDC-907 was analyzed using flow cytometry. All the procedures were
performed following the manufacturer's instructions (Annexin
V-FITC/PI Kit, 4A Biotech, Co., Ltd.). Briefly, a total of
5×105 NB cells were prepared, washed in PBS twice, and
then resuspended with 500 μl binding buffer. The cells were
then incubated with 5 μl Annexin V-FITC for 15 min in the
dark at room temperature, and 5 μl propidium Iodide (PI) was
then added to each tube. The stained cells were then analyzed using
a flow cytometer (CytoFLEX, Beckman Coulter, Inc.) within 1 h.
Western blot analysis
Whole-cell lysates were generated using RIPA lysis
buffer (Thermo Fisher Scientific, Inc.), and the protein
concentration was detected using a BCA kit (Thermo Fisher
Scientific, Inc.). An equal amount of protein was separated using
10% SDS-PAGE and then transferred onto polyvinylidene fluoride
(PVDF) membranes. After blocking in 5% skim milk (EpiZyme) for 1 h
at room temperature, the PVDF membranes were incubated overnight
with primary antibodies at 4°C, followed by incubation with
secondary antibodies for 2 h at room temperature. All the
antibodies used are listed in Table
SI. Signal detection was conducted using the ECL
chemiluminescence detection system (Bio-Rad Laboratories,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cultured NB cells
using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.). RNA
was reverse transcribed using the RT001 Fast Reverse Transcription
kit (ES-RT001, ES Science). qPCR was then performed on the Bio-Rad
CFX96 (Bio-Rad Laboratories, Inc.) with SYBR-Green Master Mix
(ES-QP002, ES Science). The primers used are listed in Table SII. The amplification reactions
were conducted using the following cycling parameters 95°C for 10
min, followed by 40 cycles of 95°C for 10 sec, and 60°C for 30 sec.
The 2−ΔΔCq method was used to calculate the levels of
gene expression (19).
Matrigel invasion assay
Transwell inserts (BD Biosciences) coated with 50
μl Matrigel were used for the Matrigel invasion assay as
previously described (20).
Wound healing assay
The NB cells at a density of 105 cells/ml
were cultured in 6-well plates with scratch plug-in components. The
protocol of the wound healing assay was as previously described
(20).
Sphere formation assay
FBS-free DMEM/F12 supplemented with 2% B27
(Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml human
recombinant EGF (Invitrogen; Thermo Fisher Scientific, Inc.) and 20
ng/ml bFGF (Invitrogen; Thermo Fisher Scientific, Inc.) were used
for the sphere formation experiment. A total of 300
SK-N-BE(2) cells suspended in
tumor sphere medium were seeded into each ultra-low attachment
24-well plate for 14 days. After forming spheres, the cells were
transferred to another new 24-well plate for further culture and
different generations of sphere-forming cells were collected for
RT-qPCR assays.
Patients and specimens
A total of 55 patients included in the present study
were newly diagnosed with NB in the Sun Yat-sen University Cancer
Center between April, 2009 to June, 2016. These patients were
between 10 to 179 months old, and the median age was 45 months,
with 25 females and 30 males. The selection criteria for enrolling
patients were as follows: i) A pathological diagnosis of NB; ii) an
age <18 years; iii) tissue specimens were obtained prior to the
initiation of treatment; and iv) a complete and detailed treatment
process and follow-up data. The specimens from patients with NB
were obtained by needle biopsy or open surgery. The research
protocol was approved by the Institutional Review Board (IRB) of
the Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China;
approval no. B2021-274-01). Informed written consent was obtained
from the parents of each patient involved in the study.
Immunohistochemistry (IHC)
The NB tissue specimens were fixed in formalin,
embedded in paraffin blocks, and sectioned at a thickness of 4
μm. IHC was performed following standard protocols. The
sections were blocked with 5% goat serum (C0265, Beyotime Institute
of Biotechnology) at room temperature for 30 min. They were then
incubated with primary antibodies against Ki67 (1:500; cat. no.
ab92742, Abcam), HDAC1 (1 μg/ml; cat. no. ab19845, Abcam),
HDAC2 (1:1,000; cat. no. ab32117, Abcam), HDAC3 (1:500; cat. no.
ab32369, Abcam), CD44 (1:200; cat. no. sc-7297, Santa Cruz
Biotechnology, Inc.) at 4°C. The following day, the sections were
washed in PBS three times and then incubated with goat anti-rabbit
secondary antibody (HRP-conjugated, 1:200; cat. no. CW0103S, CWBio)
or goat anti-mouse secondary antibody (HRP-conjugated, 1:200; cat.
no. CW0102S, CWBio) for 2 h at room temperature. The results were
evaluated according to the staining intensity and the proportion of
tumor cells with an unequivocal positive reaction. The intensity
was scored as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. The frequency of positive cells was defined as follows: 0,
<5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; and 4, >75%. The
composite score was the product of the two scores. The composite
scores of 0 to 7 were considered a low expression, and 8 to 12 were
considered a high expression. A fluorescence microscope (Olympus
BX61, Olympus Corporation) was used for image acquisition and two
independent pathologists reviewed the slides and evaluated the
scores.
Luciferase assay
Human CD44 expression plasmids purchased from
Guangzhou iGene Biotechnology Co., Ltd. were transfected into
SK-N-BE(2) cells using
Lipofectamine 3000® (Thermo Fisher Scientific, Inc.) for
48 h. The cells were then treated with culture medium containing
CUDC-907 for 24 h. Subsequent procedures of assay were conducted
using the Dual-Luciferase Reporter Gene Assay kit (DL101-01, Vazyme
Biotech Co., Ltd.) following the manufacturer's protocol. A total
of 20 μl cell extract was mixed with 100 μl
luciferase assay reagent at room temperature and the reaction was
detected with a microplate reader (Tecan Spark 10M, Tecan Group,
Ltd.). The ratio of Firefly to Renilla Luciferase activity
was calculated for each hole.
Xenograft tumorigenesis in vivo
All the animal experiments were carried out in
accordance with the Animal Care and Use Committee of the Sun
Yat-sen University Cancer Center, and were approved by the Animal
Ethics Committee of Sun Yat-sen University (Approval no.
L102042020120P). A mixture of SK-N-BE(2) cell suspension (8×106
cells) and thawed Matrigel (Corning, Inc.) was injected
subcutaneously into the right inguinal of 14 female NOD/SCID mice,
aged 3-4 weeks (GemPharmatech), weighing between 15 to 20 g, in a
SPF environment (room temperature, 20-26°C; relative humidity,
40-70%; alternating time for the light/dark cycle, 12/12 h; food
and water were regularly provided by the feeders). Subcutaneous
tumor formation was palpable in 12 mice after 1 week, and the mice
were then randomly divided into a control group and a treatment
group, with 6 mice in each group. The treatment group was
administered with 100 μl CUDC-907 solution (25 mg/kg) via
gavage for 5 days, and the treatment was continued for 5 days after
an interval of 2 days. The control (vehicle) group was administered
with 100 μl normal saline via gavage at the same time.
During this period, the body weight and tumor volumes of the mice
were measured and recorded every 2 days, and tumor volumes were
calculated using the formula V=(short diameter2 × long
diameter)/2. Following a total of 10 days of treatment, the mice
were euthanized with carbon dioxide using the displacement rate at
50% volume per minute (21), and
the subcutaneous tumors were removed to measure their size and
weight. When the tumor diameter of any mice reached 2 cm, this was
regarded as the humane endpoint in this experiment.
Small interfering RNA (siRNA)
transfection and lentiviral transduction
The detailed procedures for siRNA transfection and
lentiviral transduction of the NB cells were as previously
described (20). Human siRNA
oligos targeting PTX3 were purchased from Shanghai GenePharma Co.,
Ltd, at a working concentration of 50 nM. The siRNA sequences were
as follows: siNC, UUC UCC GAA CGU GUC ACG UTT (5′-3′) and TTA AGA
GGC UUG CAC AGU GCA (3′-5′); siPTX3#1, GCA CAA AGA GGA AUC CAU ATT
(5′-3′) and UAU GGA UUC CUC UUU GUG CTT(3′-5′); siPTX3#2, GGG AUA
GUG UUC UUA GCA ATT (5′-3′) and UUG CUA AGA ACA CUA UCC CTT
(3′-5′). The PTX3 overexpression plasmids and lentiviral vectors
were purchased from GeneCopoeia.
RNA sequencing (RNA-seq) and data
analysis
The cells were treated with DMSO or CUDC-907 at 25
nM for 24 h, and total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA
library building and RNA-seq were performed using a commercially
available service (service ID F21FTSSCWLJ1037, BGI, Huada
Biotechnology). RNA-seq reads were aligned to the hg38 genome.
Differentially expressed genes (DEGs) were identified using
|log2FC| ≥1 and FDR ≤0.001.
Statistical analysis
All the experiments were performed in triplicate.
Data are expressed as the mean ± standard deviation, and
comparisons between groups were performed using an unpaired
Student's t-test or one-way ANOVA followed by Tukey's post hoc
test. Fisher's exact test was used to assess the association
between HDACs or the CD44 expression level and clinicopathological
variables. Kaplan-Meier curves were used for survival analysis
following the log-rank test. Overall survival (OS) was defined as
the endpoint of the study as the period from the date of initial
diagnosis to mortality or the last follow-up. Event-free survival
(EFS) was defined as the period from the date of the initial
diagnosis to the date of recurrence, progression, mortality, or a
second malignancy. P<0.05 was considered to indicate a
statistically significant difference. All the data was analyzed
using SPSS 25.0 software (IMB Corp.) or GraphPad Prism 8.0 software
(Graphpad Software, Inc.).
Results
CUDC-907 inhibits the proliferation,
induces the apoptosis, and suppresses the migratory ability of NB
cells
CUDC-907 is a dual target inhibitor of HDAC and
PI3K. The present study evaluated the effects of CUDC-907 on the
viability of five NB cell lines, including MYCN non-amplified NB
cell lines (SK-N-SH, SH-SY5Y and SK-N-AS) and MYCN-amplified NB
cell lines [SK-N-BE(2) and
IMR32]. The results revealed that CUDC-907 inhibited the viability
of the five NB cell lines in a concentration-dependent manner
within 72 h, and the half-maximal inhibitory concentration (IC50)
values calculated by GraphPad Prism ranged from 5.53 to 46.22 nM
(Fig. 1A and B). The colony
formation assay revealed that cell proliferation was significantly
inhibited by CUDC-907 (Fig. 1C).
Subsequently, flow cytometric analysis was performed and it was
found that CUDC-907 significantly induced apoptosis (Fig. S1A). The results of western blot
analysis demonstrated an increased expression of Bax, Bak and
cleaved caspase-3, and a decreased protein expression of Bcl-2 in
the cells treated with CUDC-907 (Fig.
1D). Furthermore, the inhibitory effects of CUDC-907 on the
migratory ability of NB cells were also demonstrated by the wound
healing assay (Fig. S1B) and the
Matrigel invasion assay (Fig.
S1C).
 | Figure 1CUDC-907 inhibits the proliferation,
induces the apoptosis, and reduces the migratory ability of NB
cells. (A and B) The indicated NB cell lines were treated with
various concentrations of CUDC-907, and the IC50 value was
calculated at 72 h. Data are presented as the mean ± SD. (C) Effect
of various concentrations of CUDC-907 on the colony formation of
SK-N-SH, SK-N-BE(2) and IMR32
cells. The histogram on the right indicates the number of clones.
**P<0.01, ***P<0.001 and
****P<0.0001, vs. NC; ns, not significant. (D)
SK-N-SH, SK-N-BE(2) and IMR32
cells were collected for western blot analysis with the indicated
antibodies against apoptosis-related proteins following treatment
with various concentrations of CUDC-907 for 24 h. NB,
neuroblastoma; NC, negative control. |
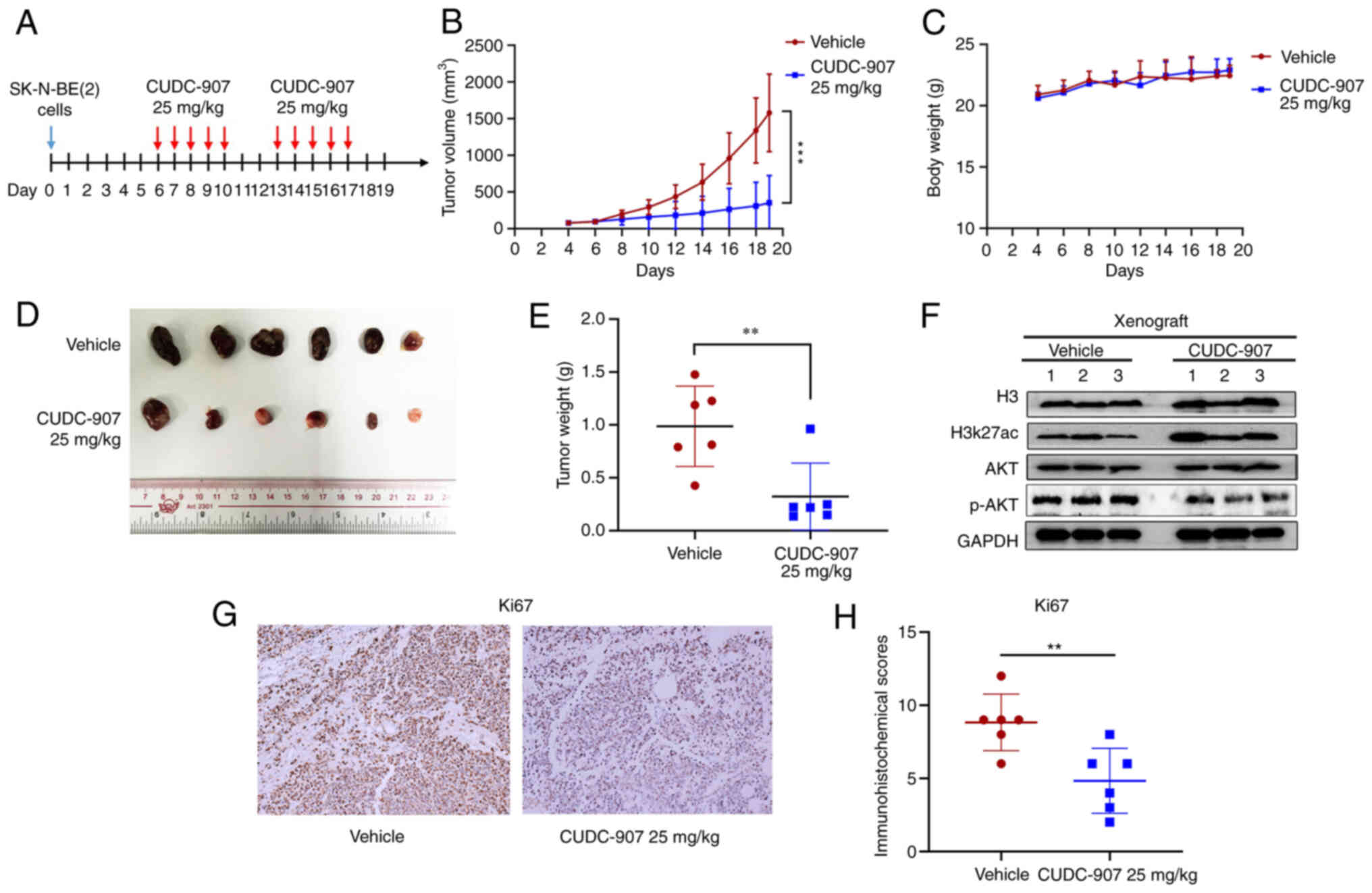
CUDC-907 inhibits the growth of NB
xenografts in vivo
The potential anti-NB effect of CUDC-907 was
explored in vivo. The detailed mode of administration is
illustrated in Fig. 2A. Following
treatment for 10 days, CUDC-907 significantly inhibited NB tumor
growth and weight compared with the vehicle control (Fig. 2B, D and E), while there was no
significant difference in the body weight of the mice in the two
groups (Fig. 2C). The results of
western blot analysis (Fig. 2F)
revealed that CUDC-907 markedly increased H3K27ac expression,
whereas it inhibited the expression of phosphorylated (p-)AKT. IHC
staining revealed that CUDC-907 downregulated the expression of the
proliferative marker, Ki67, compared to the control tissues
(Fig. 2G and H).
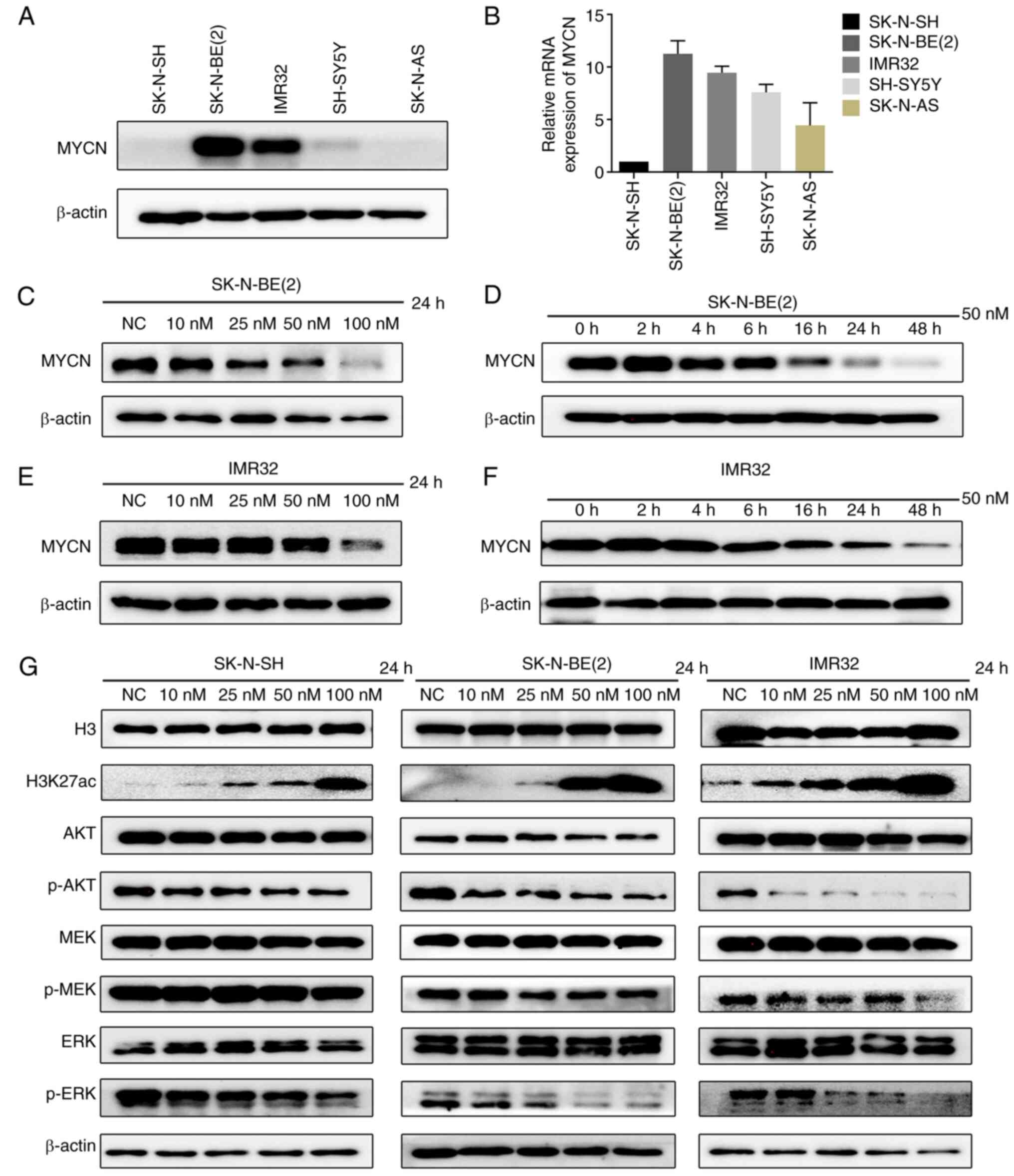
CUDC-907 downregulates MYCN expression,
and suppresses the activation of the PI3K/AKT and MAPK/ERK pathways
in NB cells
MYCN amplification is associated with the poor
prognosis of patients with NB (22); thus, the present study
investigated whether CUDC-907 downregulates the expression of MYCN.
The two MYCN-amplified cell lines, SK-N-BE(2) and IMR32, exhibited higher MYCN mRNA
and protein levels compared to the MYCN non-amplified cell lines,
SK-N-SH, SH-SY5Y and SK-N-AS (Fig. 3A
and B). The SK-N-BE(2) or
IMR32 cells were treated with CUDC-907 at the indicated
concentrations (0, 10, 25, 50 and 100 nM) for 24 h, or with a fixed
concentration of 50 nM for various periods of time (0, 2, 4, 6, 16,
24 and 48 h) to observe the changes in MYCN protein levels. The
results revealed that CUDC-907 downregulated MYCN expression in a
concentration- and time-dependent manner in the MYCN-amplified NB
cell lines (Fig. 3C-F).
Furthermore, the downstream targets directly
regulated by CUDC-907 were verified. The results revealed that
H3K27ac expression was markedly increased in the NB cell lines,
SK-N-SH, SK-N-BE(2) and IMR32,
following exposure to CUDC-907 for 24 h. Moreover, the expression
of p-AKT, which is downstream of activated PI3K, was also obviously
decreased (Fig. 3G). Activating
mutations in the RAS-MAPK-ERK pathway are known to occur at a high
frequency in relapsed NB (23,24). Thus, the present study examined
the changes in the downstream proteins of MAPK, p-MEK/MEK and
p-ERK/ERK, and found that the p-ERK expression level was markedly
decreased by CUDC-907 (Fig.
3G).
CUDC-907 inhibits the stem-like
properties of NB by inhibiting PTX3
Cancer stem cells (CSCs) lead to post-transplant
relapse and are associated with poor survival outcomes of patients
with high-risk NB (25,26). Herein, to explore whether CUDC-907
affects the stem cell-like properties of NB, a sphere formation
assay was performed using the SK-N-BE(2) cells exposed to the indicated
concentrations (0, 2, 4, 8 and 16 nM) of CUDC-907. Following
treatment of the SK-N-BE(2) cells
with 4, 8 or 16 nM CUDC-907, clonogenic formation exhibited a
marked decrease compared to the controls in concentration-dependent
manner (Fig. 4A and B).
Furthermore, both the mRNA and protein levels of several stem cell
markers, including CD44, SOX2, OCT4 and BMI-1, were significantly
downregulated by CUDC-907 (Fig. 4C
and D).
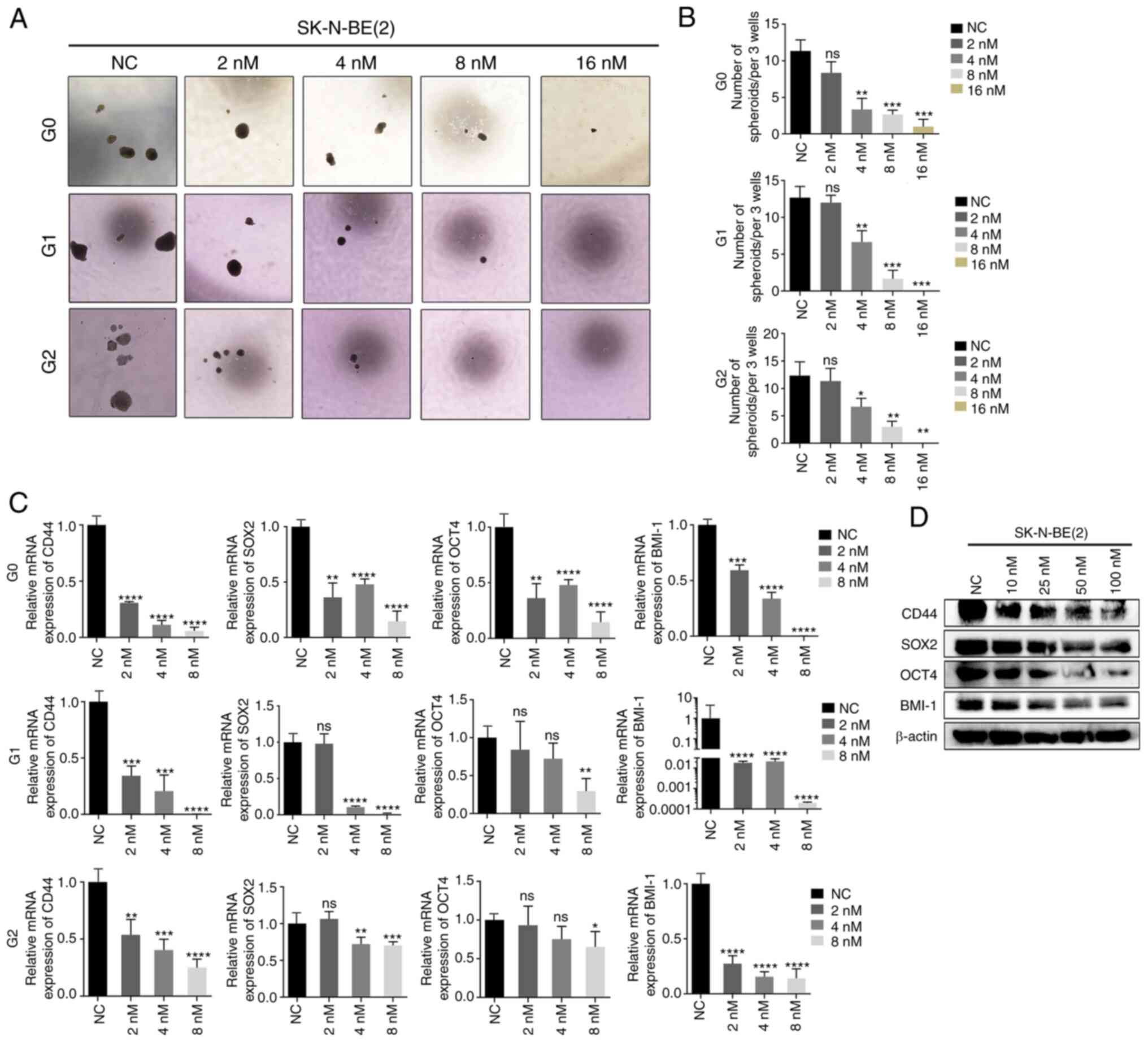 | Figure 4NB cells treated with CUDC-907
exhibit weaker CSC-like properties. (A) Sphere images of the first,
second and third generation of SK-N-BE(2) cells treated with various
concentrations of CUDC-907. (B) The sphere numbers of the first,
second and third generation of SK-N-BE(2) cells treated with various
concentrations of CUDC-907. *P<0.05,
**P<0.01 and ***P<0.001; ns, not
significant. (C) Reverse transcription-quantitative PCR of the
expression of the stem cell markers, CD44, SOX2, OCT4 and BMI-1, in
the first, second and third generation of SK-N-BE(2) cells treated with the indicated
concentrations of CUDC-907. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001, vs. NC group; ns, not significant. (D)
Following CUDC-907 treatment, western blot analyses were performed
on the indicated stem cell markers in SK-N-BE(2) cells at the protein level. NB,
neuroblastoma; SOX2, sex determining region Y-box 2; OCT4,
octamer-binding transcription factor 4; BMI-1, B-cell-specific
Moloney murine leukemia virus integration site 1. |
To further explore the mechanisms through which
CUDC-907 affects the stem-like properties of NB cells, RNA-seq
analysis was performed using two NB cell lines [SK-N-BE(2) and SK-N-SH]. According to the DEGs
painted into a heatmap, 20 DEGs were identified (Fig. S1D), of which PTX3 was the only
downregulated gene. CUDC-907 significantly inhibited the expression
of PTX3 at the mRNA level in NB cells (Fig. 5A). PTX3 is a ligand and upstream
protein of CD44, which has been identified as a CSC marker in
several types of cancer, including NB (27-29). PTX3 has also been reported to
promote the stemness of breast cancer cells by activating the
downstream ERK1/2, AKT and NF-κB pathways (30); however, its role in NB has not
been reported to date, at least to the best of our knowledge. In
the present study, PTX3 siRNA was transfected into SK-N-BE(2) cells to knockdown PTX3 expression. It
was observed that PTX3 knockdown significantly decreased the
expression of CD44 and that of a series of other
stemness-associated genes, as well as tumor sphere forming ability
of the cells (Fig. 5B-E). Even
though previous research has indicated that PTX3 activates the AKT
pathway (30), the present study
revealed that the level of p-AKT did not exhibit any obvious change
following the knockdown of PTX3. This discrepancy may be attributed
to the different functions of PTX3 in various cell types; thus,
these findings need to be verified in future studies. In addition,
it was found that the reduction of sphere formation and CD44
mRNA/protein expression by CUDC-907 was reversed by exogenous PTX3
overexpression (Fig. 5F-I). These
findings indicated that CUDC-907 inhibited the stem-like properties
of NB cells and CD44 expression by inhibiting PTX3.
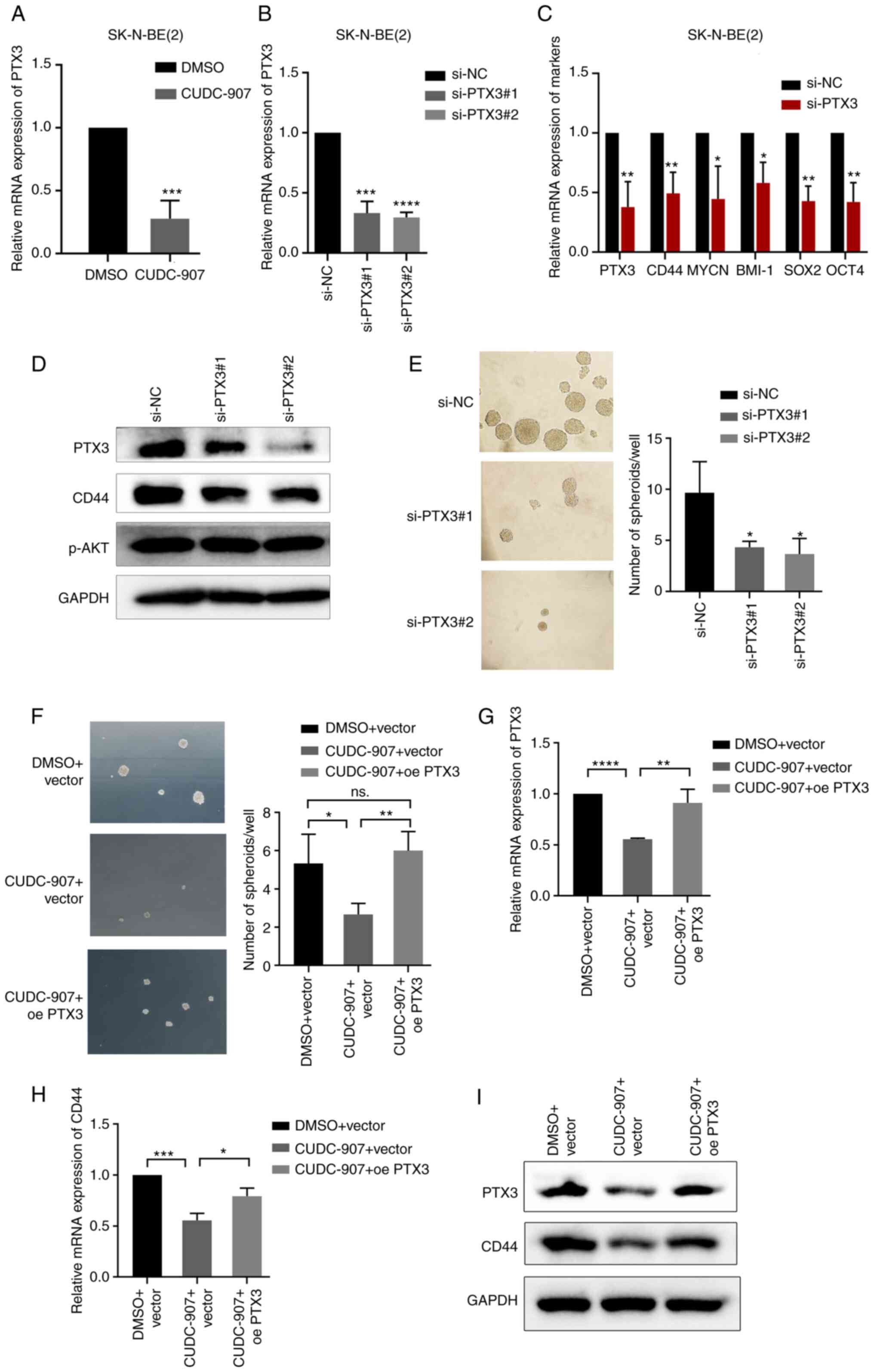 | Figure 5CUDC-907 inhibits the stem-like
properties of NB by suppressing PTX3. (A) mRNA levels of PTX3 in
cells treated with 50 nM CUDC-907 for 24 h.
***P<0.001, vs. DMSO control. (B) mRNA levels of PTX3
in cells transfected with PTX3 siRNA. ***P<0.001 and
****P<0.0001, vs. negative control. (C) mRNA levels
of PTX3, CD44, MYCN, BMI-1, SOX2 and OCT4 expression in cells
transfected with negative control or PTX3 siRNA.
*P<0.05 and **P<0.01, vs. negative
control. (D) PTX3, CD44 and p-AKT protein expression levels in
cells transfected with PTX3 siRNA. (E) Sphere formation of NB cells
transfected with PTX3 siRNA. *P<0.05, vs. negative
control. (F) Sphere formation of empty vector-transfected cells
exposed to DMSO, empty vector-transfected cells exposed to 50 nM
CUDC-907 and PTX3-overexpressing cells exposed to 50 nM CUDC-907.
*P<0.05 and **P<0.01; ns, not
significant. (G-I) The mRNA/protein expression levels of PTX3 and
CD44 in empty vector-transfected cells exposed to DMSO, empty
vector-transfected cells exposed to 50 nM CUDC-907 and
PTX3-overexpressing cells exposed to 50 nM CUDC-907.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. NB,
neuroblastoma; PTX3, pentraxin 3; SOX2, sex determining region
Y-box 2; OCT4, octamer-binding transcription factor 4; BMI-1,
B-cell-specific Moloney murine leukemia virus integration site
1. |
High expression of HDAC1, 2, 3 and CD44
is associated with the poor prognosis of patients with NB
To observe whether the target proteins of CUDC-907
are associated with the prognosis of patients with NB, IHC of
HDAC1, 2, 3 and CD44 was performed on paraffin-embedded sections of
55 patients with NB from Sun Yat-sen University Cancer Center
(Fig. 6A). The median follow-up
time was 38.9 months (range, 9.6 to 124.6 months). Up to the final
follow-up date, 17 patients (30.9%) succumbed and 33 patients
(60.0%) suffered disease progression or recurrence.
The association between HDAC1, 2, 3 expression and
the clinical characteristics of patients with NB was analyzed
(Tables SIII-SV). The results revealed that the
expression level of HDAC3 was related to MYCN amplification (OR,
15.789; P=0.002), the International Neuroblastoma Staging System
(INSS) staging system (OR, 6.400; P=0.043) and the Children's
Oncology Group (COG) risk group (OR, 7.243; P=0.017) (Table SV). Kaplan-Meier survival
analysis indicated that the upregulated mRNA levels of HDAC1, HDAC2
and HDAC3 were significantly associated with a poor OS (HDAC1,
P=0.0209; HDAC2, P=0.0143; HDAC3, P=0.0063) and EFS (HDAC1,
P=0.0023; HDAC2, P=0.0026; HDAC3, P=0.0309) (Fig. 6B-G). It was also found that the
high expression of CD44 was significantly associated with a poor OS
(P=0.0239) and EFS (P=0.0477) of patients with NB (Fig. 6H and I). However, the high
expression of CD44 was found not to be significantly associated
with the clinical characteristics of patients with NB (Table SVI).
Discussion
NB is one of the most common extracranial solid
tumors affecting children. More than half of patients with NB are
initially diagnosed with high-risk disease and have a poor
prognosis. Although immunotherapy with GD2 monoclonal antibody is
available, 30-40% of high-risk patients still ultimately succumb
due to tumor progression (31).
The literature demonstrates that HDACs are abnormally highly
expressed in NB tissues, and pan-HDACis exert potent antitumor
effects on NB cells in vitro (7). Moreover, the pathological activation
of AKT frequently occurs in NB and is associated with a poor
prognosis (32), and upstream
PI3K signaling play a crucial role in NB cell growth/survival
(33,34). Thus far, either HDACis or PI3K
inhibitors as monotherapy have not been particularly successful in
clinical trials (35-37). CUDC-907, as a dual-target
inhibitor of HDAC and PI3K, synergistically enhances the antitumor
activity in lymphomas (12,17), acute myeloid leukemia (13), chronic lymphocytic leukemia
(14), pancreatic cancer
(15) and high-grade glioma
(16), indicating its promising
role in the treatment of human tumors. However, its specific effect
and the underlying regulatory mechanisms in NB remain unclear.
Although Chilamakuri et al (38) evaluated the antitumor effects of
CUDC-907 on NB cells in vitro, the present study found that
CUDC-907 significantly inhibited the stem-like properties of NB
cells, which has not been reported to date, at least to the best of
our knowledge. The present study further explored the effects of
CUDC-907 on NB in vitro and in vivo, as well as its
potential mechanisms, to discover promising clinical drugs for
targeted therapy.
In the present study, it was verified that CUDC-907
significantly increased histone H3 acetylation and inhibited the
phosphorylation of AKT in NB cells. Using CCK-8, colony formation,
wound healing and Matrigel invasion assays, the inhibitory effects
of CUDC-907 on the proliferation and migration of NB cells were
illustrated. Through western blot analysis, it was verified that
CUDC-907 promoted apoptosis in a concentration-dependent manner by
regulating the Bcl-2 family and activating caspase-3.
In NB xenografts, it was confirmed that CUDC-907
significantly inhibited tumor growth, which was consistent with the
reported studies revealing the antitumor effect of CUDC-907 in
vivo (11-15). CUDC-907 has been shown to
effectively inhibit the proliferation of a variety of tumor cells
with an IC50 value of 0.7-120 nM, and the dosage used in mice is
25-300 mg/kg for oral administration. Qian et al (11) and Mondello et al (39) found that CUDC-907 (25, 50 and 100
mg/kg p.o.) significantly delayed the growth of transplanted tumors
without significant toxicity. Based on these findings, the present
study used a dose of 25 mg/kg for oral administration. Further
studies with increased dosage groups and a positive control are
expected in future studies.
In terms of the drug distribution after CUDC-907
enters the body, even though this was not explored in the present
study, the experimental results using mice illustrated the
effectiveness of CUDC-907 in NB animal models. Furthermore, there
are already several clinical trials (NCT03002623, NCT02307240,
NCT02674750, NCT02909777 and NCT01742988) evaluating the safety,
tolerability and pharmacokinetics of CUDC-907 in human tumors,
including NB (ClinicalTrials.gov), which will help to verify the
safety and effective dose of CUDC-907 in human tumors.
MYCN amplification and gene mutations in the
RASMAPK-ERK pathway are two of the most common genetic alterations
related to recurrent NB. MYCN amplification has been shown to be
associated with the poor prognosis of patients with NB, occurring
in 25-30% of patients (25,40). The present study demonstrated that
CUDC-907 significantly downregulated MYCN expression in a
concentration- and time-dependent manner in MYCN-amplified NB
cells. Previous studies have indicated that HDACis downregulate
MYCN mRNA expression (41,42),
while the PI3K/AKT/mTOR axis contributes to MYCN protein
stabilization (43,44). Therefore, HDAC and PI3K
antagonists may cooperate to inhibit the expression of MYCN in NB.
It has been reported that 78% of mutations detected in relapsed NB
are associated with the activation of the RAS-MAPK pathway
(21,45). The aberrant regulation of the
RAS-MAPK-ERK signaling pathway is critical for maintaining the
self-renewal ability of CSCs, which promotes the proliferation,
angiogenesis, metastasis and therapeutic resistance of NB cells,
and also predicts the poor prognosis of patients with NB (46-48). In the present study, it was
observed that the exposure of NB cells to CUDC-907 resulted in a
decrease in p-ERK levels and in the suppression of the CSC
phenotype in NB cells.
Based on the results of RNA sequencing, it was found
that CUDC-907 inhibited the expression of PTX3 in SK-N-BE(2) and SK-N-SH NB cells. The secreted
protein, PTX3, has been reported to be a partner of CD44 and
promote the stem cell performance of tumor cells (30,49). CD44 is a transmembrane
glycoprotein involved in cell-cell interactions and has been
identified as a CSC and poor prognostic marker in various adult
cancers (50-56) and pediatric cancers (57-60), including NB. However, the function
of PTX3 and its association with CD44 in NB remains unknown.
Herein, it was found that the knockdown of PTX3 using siRNA or its
suppression using CUDC-907 weakened the sphere-forming ability and
CD44 expression of the cells, both of which were reversed by the
exogenous overexpression of PTX3. This indicated that CUDC-907 may
reduce the stem like properties and CD44 stem cell marker
expression via the inhibition of PTX3.
Finally, using the IHC of NB tissues, it was found
that the high expression of HDAC1, HDAC2, HDAC3 and CD44 was
associated with the poor prognosis of patients with NB, which
indicated that HDACs or CD44 may be used as tumor biomarkers and
potential therapeutic targets. However, a limitation of the present
study was that the association between the expression levels of
HDACs and CD44 was not explored. This needs to be investigated in
future studies.
In conclusion, the present study demonstrates that
CUDC-907 exerts a significant antitumor effect on NB, and may thus
be worthy of further clinical development. Further studies are
required to explore the role of CUDC-907 in NB and to provide more
specific and accurate guidance for its translation into clinical
practice.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FS, YQ, YZ, ML and YHu conceived and designed the
study. ML, YHu, YX and YHo conducted the experiments and collected
the data. JW, LZ, SL, ZZ and JH analyzed and interpreted the data.
YHu, ML, JW and FS were involved in the writing and preparation of
the original draft. JZ and QL were involved in data curation and
validation and were also involved in data analysis. ML, JW, YQ and
YZ were involved in the reviewing, editing of the manuscript. YQ,
FS and YZ were involved in funding acquisition. YHu and ML confirm
the authenticity of all the raw data. All authors have read and
agreed to the published version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of the Sun Yat-sen University Cancer Center
(SYSUCC; Guangzhou, China; approval no. B2021-274-01). Informed
written consent was obtained from the parents of each patient
involved in the study. All the animal experiments were carried out
in accordance with the Animal Care and Use Committee of the Sun
Yat-sen University Cancer Center, and were approved by the Animal
Ethics Committee of Sun Yat-sen University (Approval no.
L102042020120P).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Key Technology Research
Project of Guangzhou Science, Technology and Innovation Committee
(grant no. 201902020001), the Guangzhou Science and Technology
project (grant no. 201905010004), the National Scientific
Foundation of China (grant no. 82002835) and the National Key
Research and Development Program of China (grant no.
2022YFC2705005).
References
|
1
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007.
|
|
2
|
Whittle SB, Smith V, Doherty E, Zhao S,
McCarty S and Zage PE: Overview and recent advances in the
treatment of neuroblastoma. Expert Rev Anticancer Ther. 17:369–386.
2017.
|
|
3
|
Yu AL, Gilman AL, Ozkaynak MF, London WB,
Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay
KK, et al: Anti-GD2 antibody with GM-CSF, interleukin-2, and
isotretinoin for neuroblastoma. N Engl J Med. 363:1324–1334.
2010.
|
|
4
|
McGinty L and Kolesar J: Dinutuximab for
maintenance therapy in pediatric neuroblastoma. Am J Health Syst
Pharm. 74:563–567. 2017.
|
|
5
|
Schramm A, Köster J, Assenov Y, Althoff K,
Peifer M, Mahlow E, Odersky A, Beisser D, Ernst C, Henssen AG, et
al: Mutational dynamics between primary and relapse neuroblastomas.
Nat Genet. 47:872–877. 2015.
|
|
6
|
Mohammad HP, Barbash O and Creasy CL:
Targeting epigenetic modifications in cancer therapy: Erasing the
roadmap to cancer. Nat Med. 25:403–418. 2019.
|
|
7
|
Witt O, Deubzer HE, Lodrini M, Milde T and
Oehme I: Targeting histone deacetylases in neuroblastoma. Curr
Pharm Des. 15:436–447. 2009.
|
|
8
|
Robey RW, Chakraborty AR, Basseville A,
Luchenko V, Bahr J, Zhan Z and Bates SE: Histone deacetylase
inhibitors: Emerging mechanisms of resistance. Mol Pharm.
8:2021–2031. 2011.
|
|
9
|
Yin L, Liu Y, Peng Y, Peng Y, Yu X, Gao Y,
Yuan B, Zhu Q, Cao T, He L, et al: PARP inhibitor veliparib and
HDAC inhibitor SAHA synergistically co-target the UHRF1/BRCA1 DNA
damage repair complex in prostate cancer cells. J Exp Clin Cancer
Res. 37:1532018.
|
|
10
|
McClure JJ, Li X and Chou CJ: Advances and
challenges of HDAC inhibitors in cancer therapeutics. Adv Cancer
Res. 138:183–211. 2018.
|
|
11
|
Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu
GX, Atoyan R, Qu H, Yin L, Samson M, et al: Cancer network
disruption by a single molecule inhibitor targeting both histone
deacetylase activity and phosphatidylinositol 3-kinase signaling.
Clin Cancer Res. 18:4104–4113. 2012.
|
|
12
|
Guo H, Zeng D, Zhang H, Bell T, Yao J, Liu
Y, Huang S, Li CJ, Lorence E, Zhou S, et al: Dual inhibition of
PI3K signaling and histone deacetylation halts proliferation and
induces lethality in mantle cell lymphoma. Oncogene. 38:1802–1814.
2019.
|
|
13
|
Li X, Su Y, Madlambayan G, Edwards H,
Polin L, Kushner J, Dzinic SH, White K, Ma J, Knight T, et al:
Antileukemic activity and mechanism of action of the novel PI3K and
histone deacetylase dual inhibitor CUDC-907 in acute myeloid
leukemia. Haematologica. 104:2225–2240. 2019.
|
|
14
|
Chen Y, Peubez C, Smith V, Xiong S,
Kocsis-Fodor G, Kennedy B, Wagner S, Balotis C, Jayne S, Dyer MJ
and Macip S: CUDC-907 blocks multiple pro-survival signals and
abrogates microenvironment protection in CLL. J Cell Mol Med.
23:340–348. 2019.
|
|
15
|
Fu XH, Zhang X, Yang H, Xu XW, Hu ZL, Yan
J, Zheng XL, Wei RR, Zhang ZQ, Tang SR, et al: CUDC-907 displays
potent antitumor activity against human pancreatic adenocarcinoma
in vitro and in vivo through inhibition of HDAC6 to downregulate
c-Myc expression. Acta Pharmacol Sin. 40:677–688. 2019.
|
|
16
|
Pal S, Kozono D, Yang X, Fendler W, Fitts
W, Ni J, Alberta JA, Zhao J, Liu KX, Bian J, et al: Dual HDAC and
PI3K inhibition abrogates NF kappa B- and FOXM1-mediated DNA damage
response to radiosensitize pediatric high-grade gliomas. Cancer
Res. 78:4007–4021. 2018.
|
|
17
|
Younes A, Berdeja JG, Patel MR, Flinn I,
Gerecitano JF, Neelapu SS, Kelly KR, Copeland AR, Akins A, Clancy
MS, et al: Safety, tolerability, and preliminary activity of
CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K,
in patients with relapsed or refractory lymphoma or multiple
myeloma: An open-label, dose-escalation, phase 1 trial. Lancet
Oncol. 17:622–631. 2016.
|
|
18
|
Oki Y, Kelly KR, Flinn I, Patel MR,
Gharavi R, Ma A, Parker J, Hafeez A, Tuck D and Younes A: CUDC-907
in relapsed/refractory diffuse large B-cell lymphoma, including
patients with MYC-alterations: Results from an expanded phase I
trial. Haematologica. 102:1923–1930. 2017.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
20
|
Li M, Sun C, Bu X, Que Y, Zhang L, Zhang
Y, Zhang L, Lu S, Huang J, Zhu J, et al: ISL1 promoted
tumorigenesis and EMT via Aurora kinase A-induced activation of
PI3K/AKT signaling pathway in neuroblastoma. Cell Death Dis.
12:6202021.
|
|
21
|
Hickman DL: Minimal exposure times for
irreversible euthanasia with carbon dioxide in mice and rats. J Am
Assoc Lab Anim Sci. 61:283–286. 2022.
|
|
22
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Primers. 2:160782016.
|
|
23
|
Eleveld TF, Oldridge DA, Bernard V, Koster
J, Colmet DL, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard
M, et al: Relapsed neuroblastomas show frequent RAS-MAPK pathway
mutations. Nat Genet. 47:864–871. 2015.
|
|
24
|
Mlakar V, Morel E, Mlakar SJ, Ansari M and
Gumy-Pause F: A review of the biological and clinical implications
of RAS-MAPK pathway alterations in neuroblastoma. J Exp Clin Cancer
Res. 40:1892021.
|
|
25
|
Veschi V, Verona F and Thiele CJ: Cancer
stem cells and neuroblastoma: Characteristics and therapeutic
targeting options. Front Endocrinol (Lausanne). 10:7822019.
|
|
26
|
Aravindan N, Somasundaram DB, Herman TS
and Aravindan S: Significance of hematopoietic surface antigen CD34
in neuroblastoma prognosis and the genetic landscape of
CD34-expressing neuroblastoma CSCs. Cell Biol Toxicol. 37:461–478.
2021.
|
|
27
|
Mehrazma M, Madjd Z, Kalantari E, Panahi
M, Hendi A and Shariftabrizi A: Expression of stem cell markers,
CD133 and CD44, in pediatric solid tumors: a study using tissue
microarray. Fetal Pediatr Pathol. 32:192–204. 2013.
|
|
28
|
Mesrati MH, Syafruddin SE, Mohtar MA and
Syahir A: CD44: A multifunctional mediator of cancer progression.
Biomolecules. 11:18502021.
|
|
29
|
Gomez KE, Wu F, Keysar SB, Morton JJ,
Miller B, Chimed TS, Le PN, Nieto C, Chowdhury FN, Tyagi A, et al:
Cancer cell CD44 mediates macrophage/monocyte-driven regulation of
head and neck cancer stem cells. Cancer Res. 80:4185–4198.
2020.
|
|
30
|
Hsiao YW, Chi JY, Li CF, Chen LY, Chen YT,
Liang HY, Lo YC, Hong JY, Chuu CP, Hung LY, et al: Disruption of
the pentraxin 3/CD44 interaction as an efficient therapy for
triple-negative breast cancers. Clin Transl Med. 12:e7242022.
|
|
31
|
Zafar A, Wang W, Liu G, Wang X, Xian W,
McKeon F, Foster J, Zhou J and Zhang R: Molecular targeting
therapies for neuroblastoma: Progress and challenges. Med Res Rev.
41:961–1021. 2021.
|
|
32
|
Westhoff MA, Karpel-Massler G, Brühl O,
Enzenmuller S, La Ferla-Bruhl K, Siegelin MD, Nonnenmacher L and
Debatin KM: A critical evaluation of PI3K inhibition in
glioblastoma and neuroblastoma therapy. Mol Cell Ther.
2:322014.
|
|
33
|
Li Z and Thiele CJ: Targeting Akt to
increase the sensitivity of neuroblastoma to chemotherapy: Lessons
learned from the brain-derived neurotrophic factor/TrkB signal
transduction pathway. Expert Opin Ther Targets. 11:1611–1621.
2007.
|
|
34
|
Boller D, Schramm A, Doepfner KT, Shalaby
T, von Bueren AO, Eggert A, Grotzer MA and Arcaro A: Targeting the
phosphoinositide 3-kinase isoform p110delta impairs growth and
survival in neuroblastoma cells. Clin Cancer Res. 14:1172–1181.
2008.
|
|
35
|
Iwamoto M, Friedman EJ, Sandhu P, Agrawal
NG, Rubin EH and Wagner JA: Clinical pharmacology profile of
vorinostat, a histone deacetylase inhibitor. Cancer Chemother
Pharmacol. 72:493–508. 2013.
|
|
36
|
Zorzi AP, Bernstein M, Samson Y, Wall DA,
Desai S, Nicksy D, Nancy W, Elizabeth E and Sylvain B: A phase I
study of histone deacetylase inhibitor, pracinostat (SB939), in
pediatric patients with refractory solid tumors: IND203 a trial of
the NCIC IND pro-gram/C17 pediatric phase I consortium. Pediatr
Blood Cancer. 60:1868–1874. 2013.
|
|
37
|
Yang J, Nie J, Ma X, Wei Y, Peng Y and Wei
X: Targeting PI3K in cancer: Mechanisms and advances in clinical
trials. Mol Cancer. 18:262019.
|
|
38
|
Chilamakuri R and Agarwal S: Dual
targeting of PI3K and HDAC by CUDC-907 inhibits pediatric
neuroblastoma growth. Cancers (Basel). 14:10672022.
|
|
39
|
Mondello P, Derenzini E, Asgari Z, Philip
J, Brea EJ, Seshan V, Hendrickson RC, de Stanchina E, Scheinberg DA
and Younes A: Dual inhibition of histone deacetylases and
phosphoinositide 3-kinase enhances therapeutic activity against B
cell lymphoma. Oncotarget. 8:14017–14028. 2017.
|
|
40
|
Vega FM, Colmenero-Repiso A, Gomez-Munoz
MA, Rodriguez-Prieto I, Aguilar-Morante D, Ramirez G, Marquez C,
Cabello R and Pardal R: CD44-high neural crest stem-like cells are
associated with tumour aggressiveness and poor survival in
neuroblastoma tumours. EBioMedicine. 49:82–95. 2019.
|
|
41
|
Fabian J, Lodrini M, Oehme I, Schier MC,
Thole TM, Hielscher T, Kopp-Schneider A, Opitz L, Capper D, von
Deimling A, et al: GRHL1 acts as tumor suppressor in neuroblastoma
and is negatively regulated by MYCN and HDAC3. Cancer Res.
74:2604–2616. 2014.
|
|
42
|
Fabian J, Opitz D, Althoff K, Lodrini M,
Hero B, Volland R, Beckers A, de Preter K, Decock A, Patil N, et
al: MYCN and HDAC5 transcriptionally repress CD9 to trigger
invasion and metastasis in neuroblastoma. Oncotarget.
7:66344–66359. 2016.
|
|
43
|
Chesler L, Schlieve C, Goldenberg DD,
Kenney A, Kim G, McMillan A, Matthay KK, Rowitch D and Weiss WA:
Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn
protein and blocks malignant progression in neuroblastoma. Cancer
Res. 66:8139–8146. 2006.
|
|
44
|
Smith JR, Moreno L, Heaton SP, Chesler L,
Pearson AD and Garrett MD: Novel pharmacodynamic biomarkers for
MYCN protein and PI3K/AKT/mTOR pathwaysignaling in children with
neuroblastoma. Mol Oncol. 10:538–552. 2016.
|
|
45
|
Valencia-Sama I, Ladumor Y, Kee L,
Adderley T, Christopher G, Robinson CM, Kano Y, Ohh M and Irwin MS:
NRAS status determines sensitivity to SHP2 inhibitor combination
therapies targeting the RAS-MAPK pathway in neuroblastoma. Cancer
Res. 80:3413–3423. 2020.
|
|
46
|
Chakrabarti L, Abou-Antoun T, Vukmanovic S
and Sandler AD: Reversible adaptive plasticity: A mechanism for
neuroblastoma cell heterogeneity and chemo-resistance. Front Oncol.
2:822012.
|
|
47
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016.
|
|
48
|
Ross RA, Walton JD, Han D, Guo HF and
Cheung NK: A distinct gene expression signature characterizes human
neuroblastoma cancer stem cells. Stem Cell Res. 15:419–426.
2015.
|
|
49
|
Dong W, Xu X, Luo Y, Yang C, He Y, Dong X
and Wang J: PTX3 promotes osteogenic differentiation by triggering
HA/CD44/FAK/AKT positive feedback loop in an inflammatory
environment. Bone. 154:1162312022.
|
|
50
|
Zhang H, Brown RL, Wei Y, Zhao P, Liu S,
Liu X, Deng Y, Hu X, Zhang J, Gao XD, et al: CD44 splice isoform
switching determines breast cancer stem cell state. Genes Dev.
33:166–179. 2019.
|
|
51
|
Louhichi T, Ziadi S, Saad H, Dhiab MB,
Mestiri S and Trimeche M: Clinicopathological significance of
cancer stem cell markers CD44 and ALDH1 expression in breast
cancer. Breast Cancer. 25:698–705. 2018.
|
|
52
|
Elkashty OA, Elghanam GA, Su X, Liu Y,
Chauvin PJ and Tran SD: Cancer stem cells enrichment with surface
markers CD271 and CD44 in human head and neck squamous cell
carcinomas. Carcinogenesis. 41:458–466. 2020.
|
|
53
|
Chen F, Chen X, Ren Y, Weng G, Keng PC,
Chen Y and Lee SO: Radiation-induced glucocorticoid receptor
promotes CD44+ prostate cancer stem cell growth through activation
of SGK1-Wnt/beta-catenin signaling. J Mol Med (Berl). 97:1169–1182.
2019.
|
|
54
|
Tomizawa F, Jang MK, Mashima T and Seimiya
H: c-KIT regulates stability of cancer stemness in CD44-positive
colorectal cancer cells. Biochem Biophys Res Commun. 527:1014–1020.
2020.
|
|
55
|
Sadeghi A, Roudi R, Mirzaei A, Zare MA,
Madjd Z and Abolhasani M: CD44 epithelial isoform inversely
associates with invasive characteristics of colorectal cancer.
Biomark Med. 13:419–426. 2019.
|
|
56
|
Kumazoe M, Takai M, Bae J, Hiroi S, Huang
Y, Takamatsu K, Won Y, Yamashita M, Hidaka S, Yamashita S, et al:
FOXO3 is essential for CD44 expression in pancreatic cancer cells.
Oncogene. 36:2643–2654. 2017.
|
|
57
|
Cai HY, Yu B, Feng ZC, Qi X and Wei XJ:
Clinical significance of CD44 expression in children with
hepatoblastoma. Genet Mol Res. 14:13203–13207. 2015.
|
|
58
|
Ghanem MA, Van Steenbrugge GJ, Van Der
Kwast TH, Sudaryo MK, Noordzij MA and Nijman RJ: Expression and
prognostic value Of CD44 isoforms in nephroblastoma (Wilms tumor).
J Urol. 168:681–686. 2002.
|
|
59
|
Amirghofran Z, Asiaee E and Kamazani FM:
Soluble CD44 and CD44v6 and prognosis in children with B-cell acute
lymphoblastic leukemia. Asia Pac J Clin Oncol. 12:e375–e382.
2016.
|
|
60
|
Legras S, Gunthert U, Stauder R, Curt F,
Oliferenko S, Kluin-Nelemans HC, Marie JP, Proctor S, Jasmin C and
Smadja-Joffe F: A strong expression of CD44-6v correlates with
shorter survival of patients with acute myeloid leukemia. Blood.
91:3401–3413. 1998.
|