Gastric cancer (GC) is a prevalent malignancy with
an incidence rate of 5.6% and a mortality rate of 7.7%, ranking 5th
in incidence and 4th in mortality globally, adversely affecting the
wellbeing and quality of life of patients (1,2).
Human epidermal growth factor receptor 2 (HER2/ERBB2)+
GC is a key GC subtype, accounting for 10-20.2% of the total
patients with GC (3).
HER2+ GC refers to GC that is detected by
immunohistochemistry (IHC) 3+ or IHC2+ simultaneous fluorescence
in situ hybridization (FISH)+ according to the
National Comprehensive Cancer Network guidelines (4). HER2, encoded by the oncogene ERBB2,
is one of the most common and well-studied areas in advanced GC
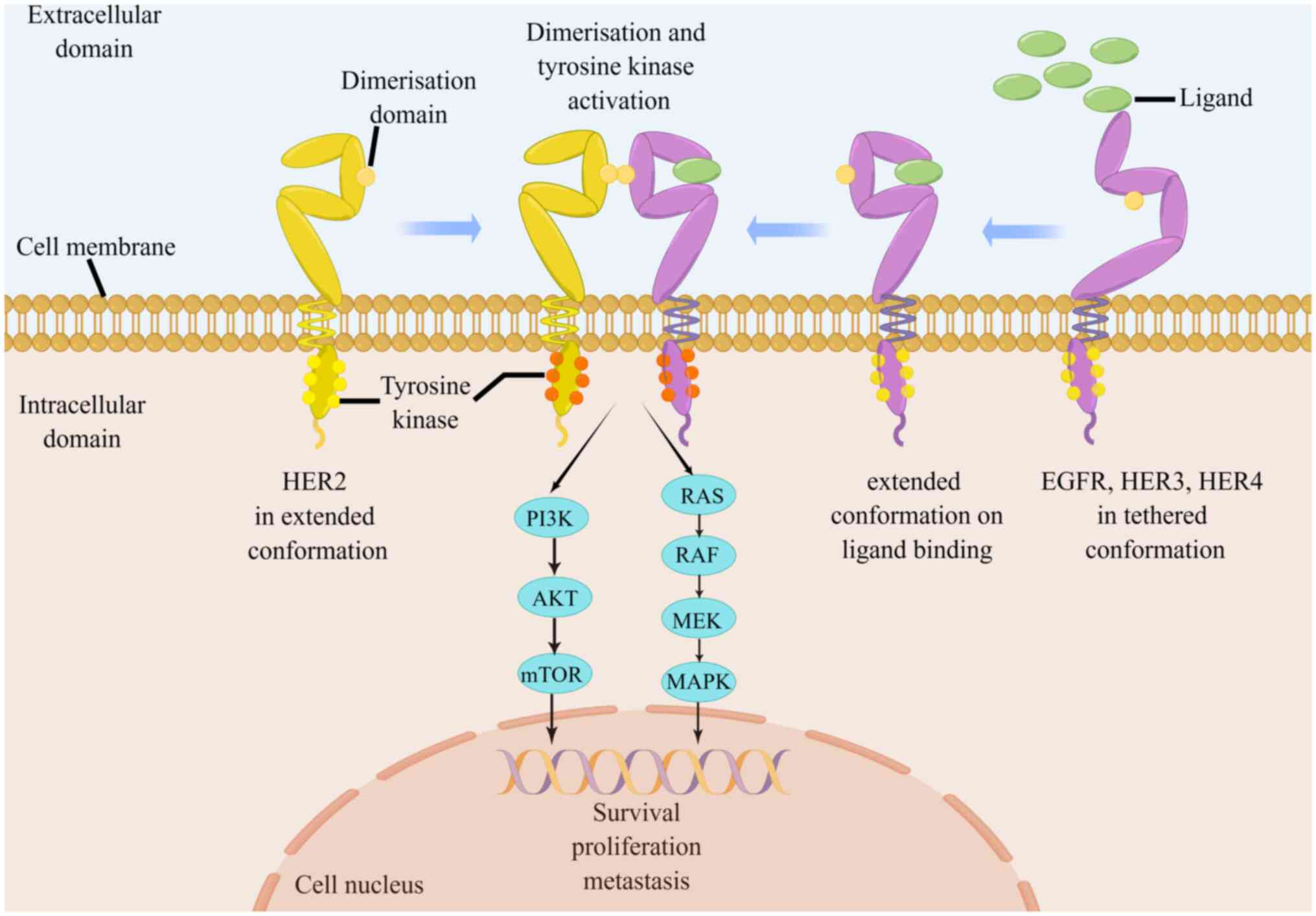
(AGC). HER2 protein forms heterodimers with other family members
including EGFR, HER3, or HER4, which promote the
autophosphorylation of intracellular tyrosine kinase domain to
enhance HER2 activation. The phosphorylated tyrosine residues
interact with several intracellular signaling molecules, leading to
the activation of downstream pathways and cross-communication with
other transmembrane signaling pathways, to regulate diverse
biological effects (Fig. 1)
(5-7). Overexpression of HER2 confers a
heightened malignant phenotype to the tumor (5). Specifically, activated HER2 promotes
GC cell proliferation and survival by regulating the expression of
cycle-related proteins such as SKP2 and p27/Cdk2 (8-10).
The overexpression of HER2 enhances vascular endothelial growth
factor (VEGF) production and angiogenesis to accelerate tumor
growth and metastasis (11,12). Furthermore, HER2 triggers
epithelial-to-mesenchymal transition by activating the
Wnt/β-catenin pathway. This activation, in turn, amplifies the
migratory and invasive capabilities of GC cells (13,14). A thorough exploration of the
features and underlying mechanisms of HER2+ AGC may
reveal valuable insights for its effective management (15).
Numerous studies have demonstrated that targeted
HER2 therapy can markedly improve the prognosis of patients with
HER2+ AGC (16-18).
Trastuzumab is a pivotal component of the initial
anti-HER2-targeted therapy for HER2+ AGC, owing to its
high efficacy (16). Currently,
primary or secondary resistance to trastuzumab has been reported in
most patients (17,19). Consequently, it is crucial to
detect drug resistance and develop strategies to improve the
sensitivity of patients to trastuzumab resistance. At present,
aside from trastuzumab, DS-8201 and RC48 have also been approved
for the posterior-line treatment of HER2+ AGC because of
their notable efficacy in pre-clinical studies (20,21). A growing number of clinical and
preclinical studies have also confirmed that anti-HER2-targeted
drugs show good anti-tumor activity in HER2+ AGC
(19-22). At present, anti-HER2-targeted
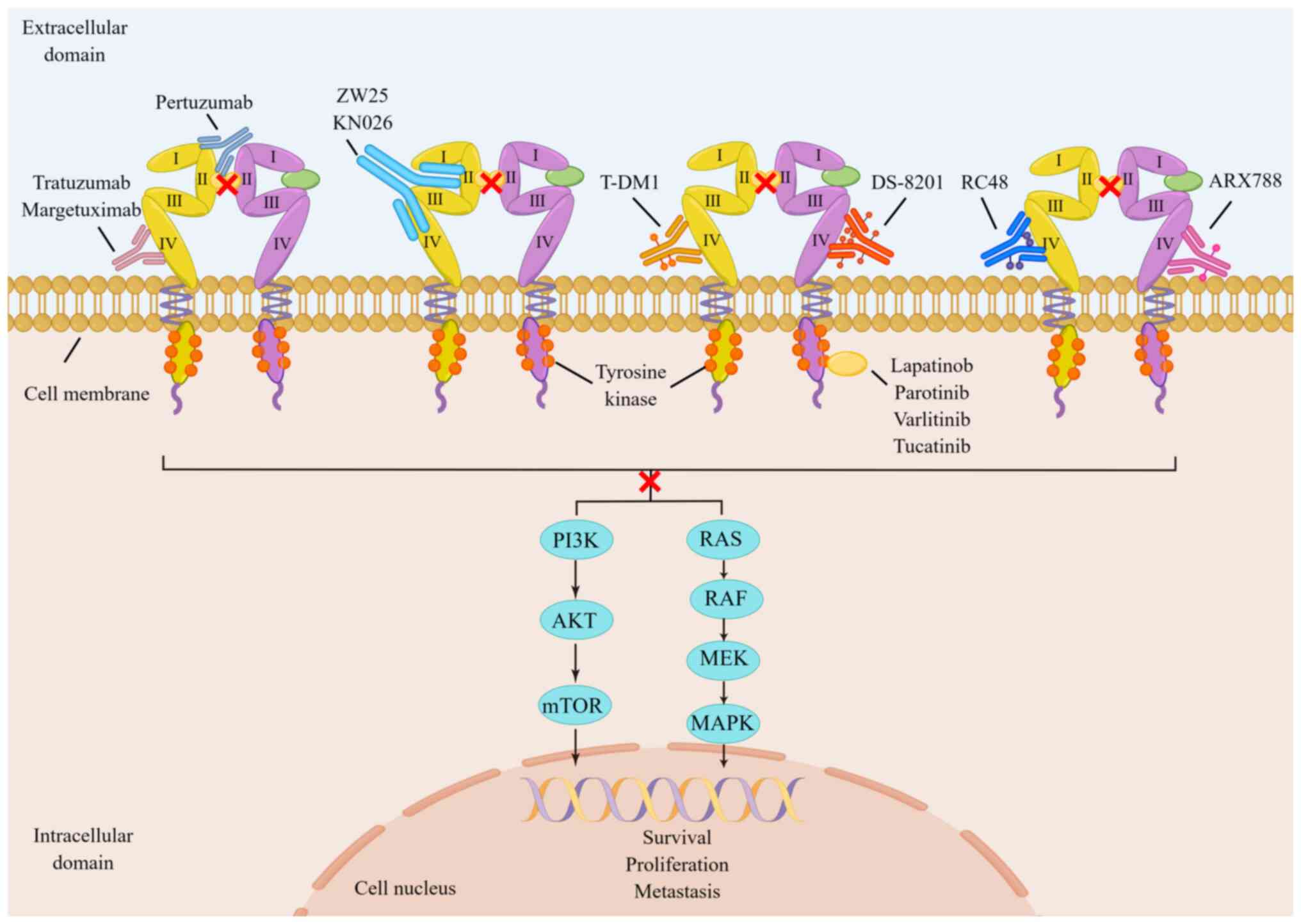
drugs are available in four categories: Anti-HER2 monoclonal
antibodies (McAbs), anti-drug conjugates (ADCs), bispecific
antibodies and tyrosine kinase inhibitors (TKIs). They possess
unique molecular structures and exert anti-HER2 targeting effects
by acting on different targets of HER2 heterodimers (Fig. 2) (23-33). Additionally, several studies
(34-36) have shown a trend to benefit the
survival of patients with HER2+ AGC treated with
immunotherapy drugs such as nivolumab and pembrolizumab in
combination with targeted therapy (Table I). Through recent optimization and
improvement, chimeric antigen receptor T-cell (CAR-T), a novel type
of tumor precision targeted immunotherapy, has achieved good
results in the treatment of clinical hematological tumors (37,38). It has also demonstrated good
tolerance in preclinical studies in solid tumors, including AGC and
has a wide range of exploration prospects (39,40). By analyzing and summarizing the
preclinical and clinical studies of HER2+ AGC, the aim
of the present review was to identify more effective therapeutic
strategies for patients with this condition.
Trastuzumab is frequently prescribed for patients
with tumors and HER2 overexpression. Its effectiveness and duration
are influenced by various factors, such as tumor cell evasion,
diminished immune cell response and potential emergence of
resistance at any stage of treatment (41-43). Timely monitoring and assessment of
drug resistance status are advocated to optimize patient treatment
plans and enhance treatment response rates in clinical practice. In
2018, Wang et al (45)
found that the method of tumor tissue biopsy IHC/FISH was
consistent (91.07%) with the detection of HER2 expression by plasma
circulating tumor DNA (ctDNA). A further indication of the
potential value of liquid biopsy in clinical practice is the fact
that the change in the copy number of the HER2 in ctDNA can
effectively feedback the efficacy of trastuzumab, and it is more
sensitive than other markers like carcinoembryonic antigen and
carbohydrate antigen 199 in predicting tumor size and progression.
In another study (44), a Chinese
team not only reached the same conclusions as Wang et al
(45), but also discovered that
in patients with acquired drug resistance, the level of HER2
somatic copy number alterations (SCNA) decreased. By contrast, the
majority of patients with congenital trastuzumab resistance
maintained high levels of HER2 SCNA in the progressive stage.
Additionally, the authors found that PIK3CA mutations were enriched
in patients with congenital drug resistance, with HER2/4 gene
mutations being the most common, accounting for 35.3% (n=6) and
29.4% (n=5) of trastuzumab resistance in baseline and progression
plasma, respectively (45).
Patients with PIK3CA/R1/C3 or HER2/4 mutations had notably lower
PFS in baseline plasma. Moreover, the study also confirmed that
neurofibromatosis type 1 gene mutation can result in trastuzumab
resistance. Trastuzumab resistance genes in HER2+ AGC
should be monitored in clinical practice using the longitudinal
ctDNA sequencing technique.
Recent studies have indicated that targeting the
circadian rhythm may be a potential approach for treating
HER2+ AGC (46,47).
Time-based therapy involving metformin has been found to disrupt
the BMAL1-CLOCK-PER1-HK2 axis and influence glycolytic
oscillations, providing a means for alleviating trastuzumab
resistance in HER2+ AGC (46). Moreover, another investigation
using metabolomic analysis showed that patients with the GC T1
subtype (HER2+/MIB+/CD3+) had
greater advantages from trastuzumab treatment compared with other
subtypes (48). Effectively
identifying specific subtypes is also considered a viable strategy
for enhancing the specificity of trastuzumab treatment.
The field of cancer treatment has been markedly
transformed by immunotherapy, which is at present an integral
component in the treatment of advanced cancer (49-51). Tumors may evade tumor immune
monitoring and inhibit antitumor immune response through several
mechanisms during the development and progression of cancer. Most
of these mechanisms are associated with the participation of the
immune checkpoint pathway. Anti-programmed cell death-1 (PD-1),
which is expressed on the surface of T cells, is one of the innate
immune checkpoints and important immunosuppressive transmembrane
proteins. However, PD-1 on the surface of immune T cells will bind
to PD-L1 abnormally expressed on the surface of tumor cells,
inhibiting the killing effect of the immune system on tumor cells
in the periphery (52,53). Immune checkpoint inhibitors (ICIs)
are essential in activating the tumor immune response and restoring
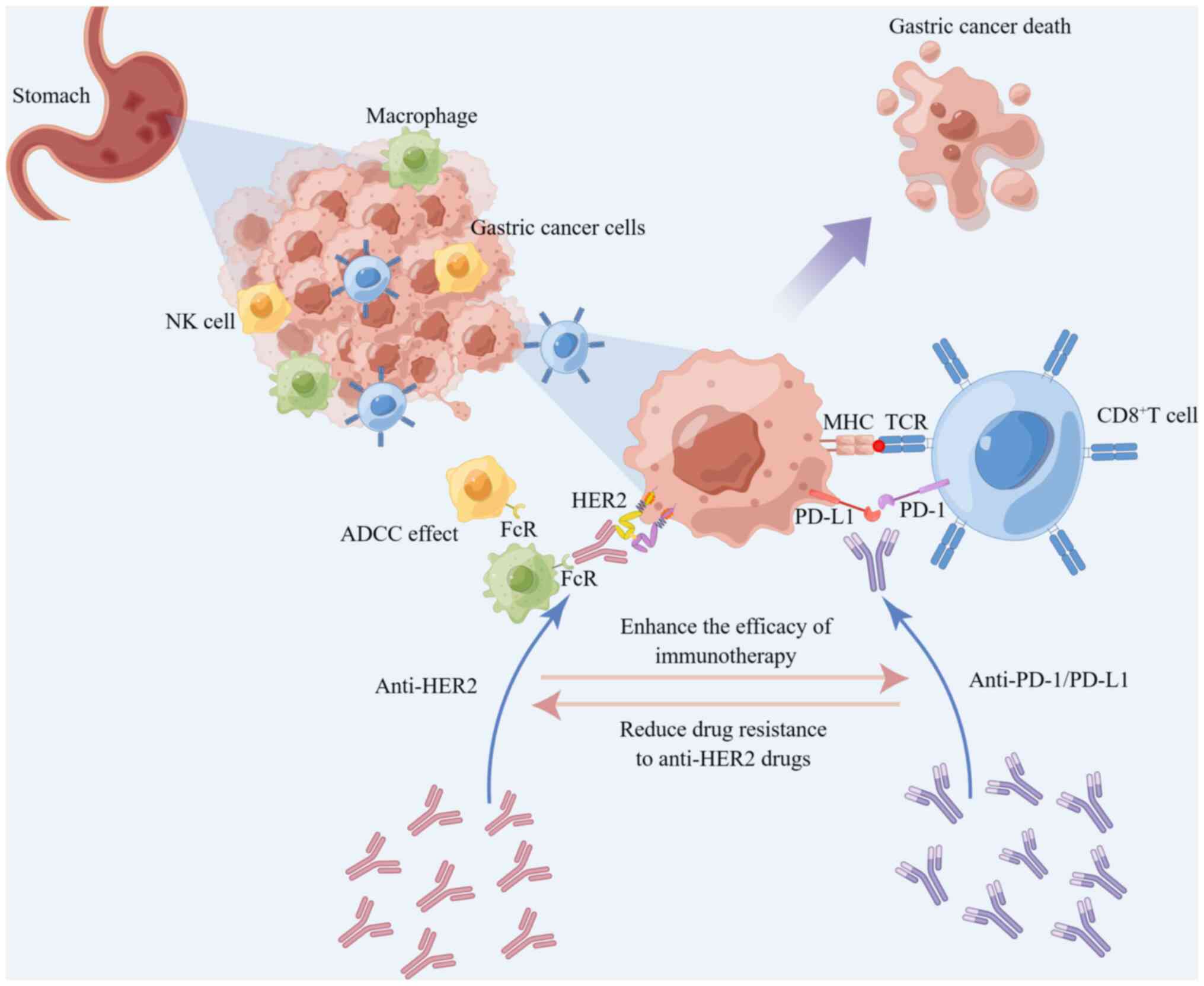
the monitoring function of immune T cells to tumor cells (54,55). It has been reported that
HER-2-targeted therapy upregulates the expression of PD-1,
cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and
tumour-associated macrophages (TAMs) within the tumor
microenvironment, enhances the antibody-dependent cell-mediated
cytotoxicity (ADCC) process, and improves the efficacy of immune
therapy (56,57). Moreover, ICIs can reduce
resistance to HER-2-targeted therapy (57,58).
Numerous studies have demonstrated that adding
PD-1/PD-L1 inhibitors to the standard treatment regimen of
HER2+ AGC markedly improves the efficacy and survival
time of patients (59-61). Combining the administration of
ICIs with trastuzumab not only enhances the HER2-specific T-cell
response, but also induces the transfer of immune cells to targeted
sites, promoting the transformation and expansion of peripheral
memory T cells (36,62,63). Trastuzumab upregulates the
expression of PD-1 and PD-L1 as well as triggers the expression of
tumor-infiltrating lymphocytes (52). Based on preclinical and clinical
data, trastuzumab increased HER2 internalization and
cross-presentation of dendritic cells, which further stimulated
HER2-specific T-cell response, suggesting HER2 and PD-1 dual
inhibition to potentially have a synergistic effect (Fig. 3) (35,64,65). Consequently, the combination of
HER2 inhibitors with PD-1/PD-L1 inhibitors may synergistically
suppress tumor cell survival, bolstering tumor-specific T cell
immune responses. This strategy has the potential to enhance
antitumor efficacy and increase the susceptibility of the tumor to
clearance by the immune system.
To assess the preliminary efficacy of trastuzumab
combined with pembrolizumab (a PD-1 inhibitor) and chemotherapy as
first-line treatment, the PANTHERA trial (NCT02901301) enrolled a
total of 43 patients with HER2+ AGC (three in phase Ib
and 40 in phase II) for exploration. The result revealed that
trastuzumab and pembrolizumab in combination with chemotherapy
(cisplatin and capecitabine) had reliable safety and promising
efficacy for treating HER2+ AGC regardless of the high
expression of PD-L1. The results showed effective disease remission
[overall response rate (ORR), 76.7%; disease control rate (DCR),
97.7%] and encouraging survival benefits [mOS, 19.3 months (95% CI,
16.5-NA) and mPFS ≤8.6 months (95% CI, 7.2-16.4)] (59). The combination of pembrolizumab,
trastuzumab and chemotherapy may also exhibit potential activity in
patients with HER2+ advanced gastroesophageal junction
(GEJ) cancer according to a phase II trial (NCT02954536) (60). This is further confirmed by
interim findings of the phase III KEYNOTE811 trial (NCT03615326)
(35). The results demonstrated
that the combination of pembrolizumab with trastuzumab and
chemotherapy had a positive response as opposed to placebo as
first-line therapy for HER2+ advanced GC and GEJ cancer
(61). The complete response (CR)
was notably improved (11 vs. 3%), and the ORR of pembrolizumab plus
trastuzumab and chemotherapy reached 74.4; 22.7% higher than that
of the placebo plus trastuzumab and chemotherapy group. Given the
aforementioned results, in May 2021, the US Food and Drug
Administration (FDA) approved pembrolizumab in combination with
trastuzumab and chemotherapy as a first-line therapy for
HER2+ AGC. This made pembrolizumab the first and only
PD-1 inhibitor for first-line treatment of this GC type. A new
treatment option for AGC is targeted therapy combined with
immunotherapy.
Aside from pembrolizumab, other PD-1 inhibitors like
nivolumab, tislelizumab as well as other ICIs including PD-L1
inhibitor avelumab and the CTLA-4 inhibitor ipilimumab, are being
investigated as first-line drugs for HER2+ GC treatment.
The combination of trastuzumab and anti-PD-1 antibody has been
shown to increase the activity of trastuzumab-induced ADCC and
demonstrate higher tumor regression in a preclinical investigation
of HER2+ breast cancer (65). Nivolumab combined with trastuzumab
and chemotherapy had reliable safety and good tolerance in patients
with HER2+ AGC (UMIN000034222) according to the
preliminary results of the Ni-HIGH study (66). Furthermore, the extended section
of that study revealed that the combination regimen had good early
efficacy in patients with HER2+ AGC. The ORR was 76.2%
(95% CI, 60.6-86.9; CR, 4.8%; PR, 71.4%), and the DCR was 97.6%
(67). A chemotherapy-free
regimen, trastuzumab coupled with nivolumab and ipilimumab is also
being tested to provide a novel treatment regimen for patients with
HER2+ GC (NCT03409848) (68). Additionally, the ORR increased
from 47 to 65% in a phase II study evaluating the efficacy of the
combination of avelumab and trastuzumab with folinic acid,
fluorouracil and oxaliplatin (FOLFOX) chemotherapy in previously
untreated advanced GC/GEJ cancer, indicating that this combination
therapy can benefit patients to some extent (69).
The WJOG71112G (T-ACT) trial revealed that the
effectiveness of continued use of trastuzumab as second-line
therapy after disease progression on trastuzumab-based first-line
therapy for HER2+ AGC is limited (70). Patients were randomly and equally
assigned to paclitaxel and paclitaxel plus trastuzumab (PT) groups.
The results showed that there was no survival benefit for patients
in the experimental group compared with those in the paclitaxel
group. The mPFS was 3.2 and 3.7 months, the mOS was 10 and 10
months and the ORR was 32 and 33% in the paclitaxel group and the
PT group, respectively. These differences were not statistically
significant, indicating that the continued use of the trastuzumab
strategy in the posterior line did not significantly benefit
patients with HER2+ AGC/GEJ cancer (70). However, a phase II clinical trial
has revealed that the combination therapy of trastuzumab with
ramucirumab, a VEGFR2 monoclonal antibody, and paclitaxel
demonstrated considerable efficacy in patients previously treated
for HER2+ GC/GEJ cancer. The mPFS and mOS were reported
at 7.1 and 13.6 months, respectively, alongside a manageable safety
profile (71). Targeting HER2
concurrently with the inhibition of VEGFR2 overexpression appears
to exert a synergistic suppressive effect, potentially enhancing
the sensitivity of patients with HER2+ AGC to
trastuzumab.
Trastuzumab, cleavable tetrapeptide junction, and
cytotoxic topoisomerase I inhibitor (camptothecin derivative DXd)
are the active ingredients in DS-8201, a second-generation ADC drug
(23). DS-8201 has more
advantageous drug payload and higher drug antibody ratio (8 vs.
3.4) than T-DM1. While T-DM1 degrades when it enters tumor cells,
DS-8201 has a better junction that is stable in plasma and spread
across the membrane to neighboring tumor cells to play the
cytotoxic bystander effect and lower the dependence of DS-8201
treatment on HER2 expression (23,72). These advantages make DS-8201
preferable in the AGC treatment with notable HER2 expression
heterogeneity. DS-8201 showed good antitumor activity in patients
with AGC patients and high as well as low HER2 expression in some
phase I clinical studies (73,74). The response rate of patients
treated with DS-8201 for severe preconditioning HER2+
AGC was ~40%, indicating good antitumor activity and safety.
Moreover, the phase II DESTINY-Gastric01 study (20) compared the therapeutic efficacy of
DS-8201 with standard chemotherapy in patients with
HER2+ AGC/GEJ cancer who had received ≥2 types of
treatment, including trastuzumab. The treatment with DS-8201
markedly improved the ORR of patients (51.3 vs. 14.3%) and mOS
(12.5 vs. 8.4 months) compared with standard chemotherapy. These
findings led to the approval of DS-8201 for the treatment of
patients with AGC/GEJ cancer who had previously undergone
first-line therapy that included trastuzumab. Hence, DS-8201 is the
first anti-HER2-targeted ADC drug for second-line treatment of
HER2+ AGC.
RC48 is composed of hertuzumab with improved
affinity and endocytosis, a cleavable linker and a cytotoxic drug
monomethyl auristatin E (MMAE). Its increased applicability to
patients with low expression tumor HER2 is due to its ability to
exert a similar bypass killing effect as DS-8201 (24,75). A phase I study (76) revealed that RC48 had good
tolerability and effective antitumor activity in various
HER2+ solid tumors, including AGC in HER2
IHC2+/FISH− state. The ORR, DCR, mPFS and mOS
of patients with HER2+ AGC (including HER2
IHC2+/FISH− status) who had received ≥2 types
of systematic chemotherapy was 24.4, 41.7%, 4.1 months and 7.6
months, respectively, after receiving RC48 treatment, according to
a single-arm multicenter phase II trial (21). These findings suggest that RC48 is
a possible third-line therapeutic option for patients with
HER2+ AGC, and has good efficacy and great potential.
Based on that study, National Medical Products Administration
approved RC48 as a third-line targeted therapy of patients with
HER2+ AGC/GEJ cancer who had received ≥2 types of
systematic chemotherapy, filling the gap for HER2+ AGC.
In addition, RC48 in combination with immune checkpoint PD-1
inhibitors (pembrolizumab or atezolizumab) showed good efficacy
(36) in mice, and induced
lasting immune protection in a HER2+ breast cancer mouse
model. This could be due to the increase of T-cell infiltration in
the tumor caused by MMAE, a toxin molecule present in RC48. The
combination regimen may aid in resolving drug resistance problems
in some patients with AGC after treatment with RC48.
At present, there are no other HER2-targeted
medications licensed for use in posterior-line therapy, with the
exception of DS8201 and RC48, which are approved for use in the
second and third lines of treatment of HER2+ AGC. As a
second-line treatment for HER2+ AGC, the phase III
GATSBY study examined the safety and effectiveness of the T-DM1
(ADC) in conjunction with paclitaxel compared with paclitaxel
alone; no notable results were observed (77). In a phase II trial, patients with
HER2+ AGC who had ≥1 prior round of conventional
treatment showed marked and long-lasting remission (ORR, 56%; 95%
CI, 35-76%) from the bispecific antibody KN026 (78). In addition, the results of the
phase I clinical trial on another bispecific antibody, ZW25,
suggest potential advantages for third- or fourth-line treatment in
HER2+ patients with AGC. These findings provide
encouragement for further clinical research into ZW25 and other
drug combination therapies for patients with HER2+ AGC
(79).
When immunotherapy was initially used as a
third-line treatment for AGC, it showed considerable success. The
phase II KEYNOTE-059 study (NCT0233511) shown that the study
population, which included patients with HER2+ or
HER2− AGC and had previously received trastuzumab
treatment, could benefit from it independently of their PD-L1
status (80). However, later
phase II/III KEYNOTE-061 trial investigations indicated that
compared with paclitaxel, pembrolizumab monotherapy did not
markedly enhance OS in patients with AGC (81). The CP-MGAH22-05 study demonstrated
a synergistic antitumor effect when combining pembrolizumab, an
anti-PD-1 checkpoint inhibitor, with margetuximab, a fragment
crystallizable (Fc) optimized anti-HER2 drug. Among the 92
evaluable patients with HER2+ AGC who had previously
received ≥1 conventional trastuzumab-containing therapy, a
favorable ORR was observed in 17 patients (18.48%; 95% CI,
11.15-27.93) (82). Consequently,
the combination regimens of ICIs and anti-HER2-targeted drugs may
become a promising option for post-treatment of patients with
HER2+ AGC and trastuzumab resistance.
Pertuzumab is a recombinant human McAb that
primarily disrupts the dimerization of HER2 and other family
members to play a targeted role different from the binding site of
trastuzumab (25). The
combination of pertuzumab and trastuzumab has a stronger inhibitory
effect on HER2 overexpression, as well as better therapeutic effect
than trastuzumab alone. This dual-targeted therapy markedly
improves colorectal cancer treatment outcomes and prolongs the
survival of patients of breast cancer for 10 months (83). Pertuzumab combined with
trastuzumab had greater antitumor activity than single-targeted
therapy in the xenotransplantation model of HER2+ AGC
(84). The JACOB phase III
clinical trial (85) examined the
effectiveness of pertuzumab in combination with trastuzumab and
chemotherapy in patients with HER2+ metastatic GC/GEJ
cancer. Although there were no statistically significant results in
terms of the OS between the pertuzumab group and the control group,
the pertuzumab group showed a 3.3-month increase in the mOS
compared with that in the control group (17.5 vs. 14.2 months; HR,
0.84; 95% CI, 0.71-1.00; P=0.057). According to the Chinese
subgroup analysis, the mOS for the pertuzumab group and control
group was 18.7 and 16.1 months (HR, 0.75; 95% CI, 0.49-1.14),
respectively, and the mPFS was 10.5 and 8.6 months (HR, 0.85; 95%
CI, 0.60-1.21), respectively (86). The therapeutic effect of the
Chinese population (86) was
comparable with that of the global intention-to-treat analysis
population, with the HR of the mOS and the mPFS being lower than
those of the control group. Pertuzumab combination with trastuzumab
and chemotherapy as a first-line treatment could prolong the
survival time of Chinese patients with HER2+ AGC.
Furthermore, the treatment regimen was well-tolerated in the
patients. Pertuzumab plus trastuzumab and chemotherapy also
increased the ORR of the perioperative patients with
HER2+ AGC from 25 to 45% according to the INNOVATION
trial (87). Tumor heterogeneity
and population differences affect the efficacy of trastuzumab
combined with pertuzumab. Therefore, it is necessary to further
improve the trial design and selection criteria to identify
patients who are more likely to benefit from dual
anti-HER2-targeted therapy.
Margetuximab and trastuzumab have similar HER2
binding as well as antitumor proliferation effects. Compared with
trastuzumab, margetuximab has greater antitumor activity in
vitro due to its improved Fc domain, which also improves
immunological mechanisms such as ADCC (26,88). A phase I study (89), showed that margetuximab has good
tolerance and antitumor effectiveness when used as a monotherapy
against a variety of solid tumors with HER2 overexpression,
including AGC. After the successful III phase SOPHIA trial, FDA
approved margetuximab in combination with chemotherapy as the
first-line treatment for previously treated metastatic
HER2+ breast cancer (90). Catenacci et al (82,91-94) conducted multiple preliminary
studies on the efficacy of combining margetuximab and pembrolizumab
in treating HER2+ AGC. One of these studies (82), CP-MGAH22-05, is a single-arm,
phase IB/II trial to evaluate the safety and tolerance of
margetuximab combined with pembrolizumab in the treatment of
patients with HER2+ GEJ cancer. There was no
statistically significant dose-limiting toxicity in the
dose-increasing phase, with the combined therapy having acceptable
safety and tolerance. Furthermore, the phase II/III MAHOGANY
clinical trial (95), currently
being conducted by Catenacci et al is an exploratory attempt
to integrate margetuximab with other new immunotherapeutic drugs
including retifanlimab and tebotelimab as a novel method for
HER2+ AGC treatment. Previous studies suggest that
double inhibition of PD-1 and lymphocyte-activation gene 3 (LAG-3)
targeting could enhance the efficacy of margetuximab by
strengthening the innate and adaptive immune response to tumor
cells overexpressing HER2 (96-98). According to researchers, targeting
HER2 and PD-1 using margetuximab plus retifanlimab, or HER2 and
PD-1 plus LAG-3 by combining margetuximab plus tebotelimab presents
a chance to augment antineoplastic response in patients with
HER2+ AGC compared with single drug treatment.
The combination of trastuzumab and mitotic inhibitor
emtansine forms a complex T-DM1 (29). Numerous pivotal phase III studies
have shown that T-DM1 markedly prolonged the OS and PFS of patients
with metastatic HER2+ breast cancer compared with
traditional chemotherapy (99-101). By contrast, the multicenter
randomized controlled phase II/III Gatsby study (77) revealed that patients with
HER2+ AGC treated with T-DM1 lacked survival advantages
compared with those treated with paclitaxel or docetaxel. Patients
who received T-DM1 had a mOS of 7.9 months, whereas those who
received taxane had a mOS of 8.6 months. There was no statistically
significant difference between the two treatment groups (HR, 1.15;
95% CI, 0.87-1.51; P=0.86). These findings suggest that T-DM1 may
not have an additional therapeutic advantage in this patient
population. This could be because AGC has notable heterogeneity,
and some patients have hereditary or acquired drug resistance to
T-DM1. A study demonstrated that the upregulation of recombinant
matrix metalloproteinase 7 (MMP7) has a strong relationship with
the drug resistance of AGC cells to T-DM1 (102). The activation of MMP7 during
this process has been found to be influenced by DKK1 and
Wnt/β-catenin.
A humanized McAb that specifically targets the HER2
protein on cancer cells and a cytotoxic tubulin inhibitor known as
AS269 that is conjugated to the McAb const ARX788, a site-specific
anti-HER2 antibody-drug combination (30,103) In preclinical studies, ARX788
demonstrated effective anticancer activity in breast cancer and AGC
with minimal expression of HER2 and T-DM1 resistance in addition to
good safety and antitumor activity in AGC and breast cancer with
overexpression of HER2 (104,105). The results of a multicenter
dose-expanded phase I clinical trial (106) showed that ARX788 had good
tolerance and antitumor efficacy in patients with HER2+
AGC/GEJ cancer who were non-responsive to trastuzumab. The patients
had an ORR of 37.9% and a DCR of 55.2%. With a median follow-up of
10 months, the mPFS and mOS were 4.1 and 10.7 months, respectively
(CTR20190639). At present, other clinical trials of ARX788 for AGC
treatment are ongoing (NCT03255070 and CTR20201708).
ZW25 is an example of a HER2-targeted bispecific
antibody, which can simultaneously bind two non-overlapping
epitopes of HER2, the trastuzumab-binding domain ECD4 and the
pertuzumab-binding domain ECD2 (27). Preclinical studies (107,108) have indicated that ZW25 exhibits
good antitumor activity and can silence HER2 signaling in xenograft
mouse models expressing HER2, including GC. Meric-Bernstam et
al (109) confirmed the
safety and efficacy of ZW25 in patients with advanced solid tumors
expressing HER2 in this phase I study. A phase I basket trial
(108) showed that patients,
including 10 patients with GEJ cancer, five patients with
colorectal cancer and nine patients with other malignant tumors
except breast cancer, treated with ZW25 had a high ORR (41%) with
mild side effects most of which were rated as grade 1 or 2. At the
network meeting of the American Society of Clinical
Oncology-Gastrointestinal Cancers Symposium (ASCO-GI) in 2021, the
phase I clinical trial (79) of
ZW25 on third-line and fourth-line treatment of patients with
HER2+ AGC showed that ORR and DCR of ZW25 monotherapy
were 33 and 61% respectively, while the combined chemotherapy
showed better efficacy, with ORR of 54% and DCR of 79%
(NCT02892123). This result revealed the great potential of ZW25 as
a posterior line therapy. The safety and antitumor efficacy of ZW25
in combination with other drugs for the treatment of
HER2+ AGC (NCT04276493, NCT03929666 and NCT05027139) is
currently being investigated in several phase I and II clinical
trials.
KN026 can target two non-overlapping epitopes on
HER2 derived from trastuzumab (domain IV) and pertuzumab (domain
II) simultaneously, to achieve the combination effect of
trastuzumab and pertuzumab (110). In addition, KN026 had an
inhibitory effect on some tumor cells with low HER2 expression and
trastuzumab-resistant cell lines (78,110). The safety and pharmacokinetics
of KN026 as a monotherapy, even in patients with more severe
preconditioning was recently evaluated in a first phase I study in
patients with HER2+ metastatic breast cancer (111). That study showed that patients
who benefit more from treatment with KN026 may be defined by
co-amplification of HER2/CDK12. Additionally, KN026 has
demonstrated promising efficacy as an initial treatment for
patients with AGC/GEJ cancer with HER2 expression (112). The findings of an ongoing phase
II study on KN026, which was presented at the ASCO-GI annual
meeting in 2021, indicated that KN026 is effective in treating
HER2+ AGC/GEJ cancer, regardless of prior treatment with
trastuzumab. According to the study, the ORR was 55.6% and the DCR
was 72.2% in patients with high HER2 expression. The ORR and the
DCR in patients who previously received trastuzumab were 44.4 and
66.7%, respectively. A phase II study of KN026 was conducted on
patients with HER2+ AGC who had undergone ≥1 round of
standard chemotherapy. That study indicated an ORR of 56% for the
HER2 overexpression group with an acceptable safety (NCT03925974)
(78). There are several phase
I/II clinical trials (NCT03925974; NCT03619681; NCT03847168)
ongoing. The CDE has officially approved the phase III clinical
trial application of KN026 combined with chemotherapy for AGC/GEJ
with disease progression after receiving standard first-line
chemotherapy, implying that KN026 has the potential as a
HER2-targeted drug. Additionally, several studies have demonstrated
that KN026 combined with anti-PD-L1/CTLA-4 bispecific antibody
KN046 has favorable safety, tolerability and efficacy in patients
with HER2+ gastrointestinal tumors as well as other
HER2-abnormal solid tumors (113-115). Therefore, combining KN026 with
other targeted drugs may provide a better treatment option for
patients with HER2+ AGC.
IBI315 is the first anti-HER2/PD-1 bispecific
antibody in the world. It can simultaneously bind PD-1 molecules on
T cells and HER2 molecules on tumor cells, and act as an immune
activator and tumor cell inhibitor. The remarkable therapeutic
effects of IBI315 were observed in preclinical models, including
organoids. Furthermore, IBI315 demonstrated the capacity to
regulate gasdermin B expression in tumor cells, specifically in
HER2+ AGC, inducing cell pyroptosis and promoting T cell
activation. Activated T cells release IFNγ, further enhancing
gasdermin B expression, creating a positive feedback loop that
efficiently eradicates HER2+ GC cells (116). CIBI315A101 is the first clinical
trial (NCT04162327) to assess the safety, tolerance and efficacy of
IBI315 monotherapy and combination chemotherapy for patients with
advanced solid malignant tumors expressing HER2. The preliminary
results of dose escalation of this trial presented orally at the
Chinese Society of Clinical Oncology in 2021 demonstrated good
safety and efficacy.
Pyrotinib is a novel pan-HER small molecule TKI
which irreversibly binds to the ATP binding sites of HER1, HER2 and
HER4 intracellular kinases (32).
Consequently, it blocks the activation of downstream signal
pathways, thereby inhibiting the growth of tumor cells (122,123). Some studies have shown that
pyrotinib can effectively treat HER2+ advanced solid
tumors (124,125) without notable toxicity.
Additionally, the combination of pyrotinib and SHR6390, a CDK4/6
inhibitor, showed good efficacy and safety in treating patients
with refractory HER2+ AGC and solid tumors (126). In one case report (127), a 72-year-old male patient with
HER2+ AGC developed resistance to trastuzumab and
camrelizumab (a PD-1 inhibitor) combined with apatinib. When the
patient was treated with capecitabine combined with pyrotinib, the
tumor size was markedly reduced, which prolonged the patient's
survival. The patient has survived for >30 months after
postoperative recurrence and is currently receiving combination
therapy with pyrotinib and capecitabine, with a PFS >8.5 months.
Furthermore, several analogous cases have shown a favorable outcome
following pyrotinib administration as a later-line treatment
(128-130). Pyrotinib may be another
promising targeted drug for the treatment of HER2+
AGC.
Varlitinib is a reversible pan-HER TKI. In a
patient-derived xenograft model of GC expressing HER2, it was found
that varlitinib induced antitumor activity (33). A phase II trial of varlitinib as
the first-line treatment of patients with AGC co-expressing
HER1/HER2 (NCT03130790) showed that varlitinib did not reach the
primary endpoint of marked tumor size reduction after 12 weeks of
treatment. Another phase IB clinical trial (KCT0003583) for
varlitinib in combination with paclitaxel as a second-line therapy
for HER1/HER2 co-expressing AGC indicated that the combination
treatment had a favorable safety profile (131). Currently, the multi-site phase
II clinical trial are under way (KCT0003583) to test the efficacy
of this treatment (131).
Tucatinib, a highly selective small molecule
HER2-targeted TKI, has been shown to markedly improve the OS of
patients with breast cancer (132). Additionally, it is currently
being investigated as a potential treatment for advanced colorectal
cancer and other gastrointestinal tumors, including AGC. In GC/GEJ
cancer xenotransplantation models, dual targeting HER2 of tucatinib
and trastuzumab showed superior antitumor activity compared with
any single drug treatment (133). These data support the clinical
application potential of tucatinib. The mid-term results of
MOUNTAINEER trial (NCT03043313) showed that tucatinib and
trastuzumab had marked antitumor activity in HER2+
colorectal cancer (134). The
MOUNTAINEER-02 trial, a phase II/III study (NCT04499924), is
currently been conducted (135,136) to evaluate the effectiveness of
tucatinib, ramucirumab, and paclitaxel in treating HER2+
AGC/GEJ cancer. The trial aims to determine the effectiveness of
tucatinib compared with that of ramucirumab and paclitaxel as
single drug treatments for this type of cancer.
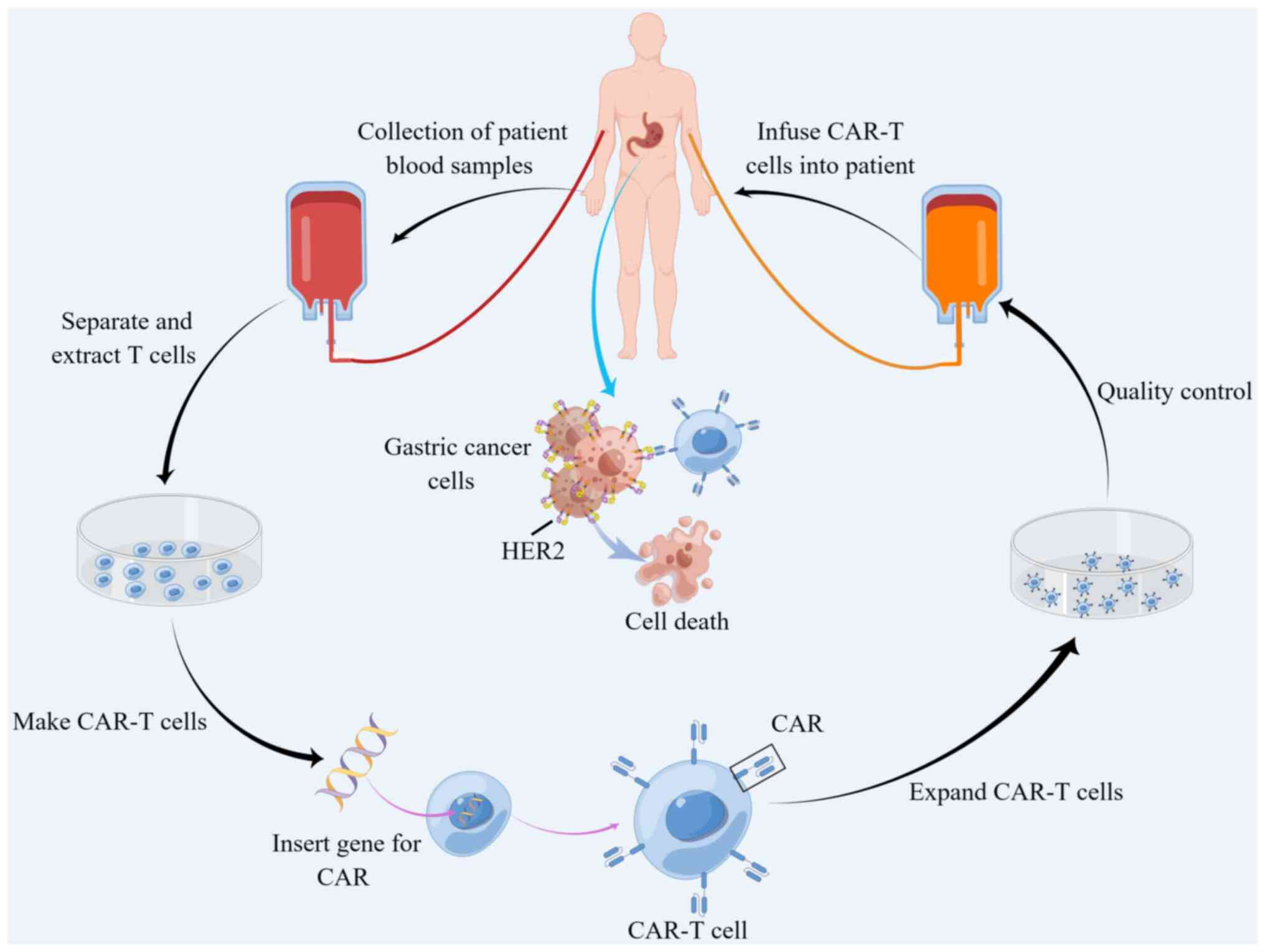
CAR-T cell therapy is an innovative technique for
treating cancer that involves genetic engineering of T lymphocytes
removed from patients, with the goal of directing them towards
cancer cells (137,138). Initially, CAR-T cell therapy was
used to treat hematological malignancies with satisfactory outcomes
(139,140). Therefore, it has become the
preferred therapy for patients showing resistance to conventional
chemotherapy or radiotherapy (141). In recent years, studies found
that CAR-T elicit good response from various solid tumors (39,142). HER-2-targeting CAR-T cells for
AGC are currently been investigated to develop strategies to reduce
drug resistance and improve therapeutic effect (Fig. 4) (143). In another study, a novel type of
genetically modified human CAR-T cells targeting the GC cell
antigen HER2 was developed. These cells embody the CD137 and CD3ζ
moieties (40). The activated
CAR-T cells, expanded in number, effectively target HER2 antigens
in an major histocompatibility complex-independent manner, and
demonstrate notable efficacy in eliminating HER2+ GC
cells derived from patients. Furthermore, CAR-T cells exhibited
markedly enhanced tumor inhibition and targeting in
HER2+ xenograft tumors compared with controls (40). A preclinical study (144) reported that humanized chimeric
antigen 21 (ChA21) single chain antibody fragment-based CAR-T cell
therapy can treat AGC with overexpression of HER2. The important
constituent of this specific CAR-T cell, 4-1BBz, is constructed
from the intracellular domain of 4-1BB (TNFRSF9 or CD137) and the
CD3z signaling domain. ChA21-4-1BBz CAR-T cells showed specific
helper T cell 1 skewed cytokine response and efficient cytolysis of
human GC cells with high expression of HER2 in vitro, and
its effect was mediated by HER2 expression on the surface of tumor
cells. In the established models of subcutaneous
xenotransplantation and peritoneal metastasis, chA21-4-1BBz CAR-T
cells markedly promoted the regression of tumors with HER2
overexpression, prolonged the survival time of mice bearing
HER2+ tumors, and enhanced the circulatory ability of
CAR-T cells, as well as their specific fate and accumulation at the
tumor site (144).
In terms of the clinical application of CAR-T in
other solid tumors, there is a need to balance between its side
effects and curative effects. A phase I clinical trial
(NCT01935843) (145), evaluating
the safety, feasibility and activity of HER2 immunotherapy for
patients with advanced biliary tract cancer and pancreatic cancer,
showed that 1/11 selected patients developed partial response (PR)
for 4.5 months, and five patients were in stable disease (SD) and
their mPFS was 4.8 months, indicating that the immunotherapy had
good clinical efficacy. Nevertheless, two patients experienced
severe upper gastrointestinal bleeding, suggesting a potential
threat of HER2-targeted CART cells in anti-tumor process. The CAR-T
cell therapy targeting other gastrointestinal cancer markers, such
as claudin 18.2 and 6, has also shown promising efficacy in early
clinical trials (146-148). Therefore, by optimizing the
design of CAR-T cell therapy involving regulation of the tumor
load, and antigen expression and distribution, its clinical
application in patients with HER2+ AGC may be
feasible.
Tumor vaccines have become research hotspots in
recent years. Various forms of tumor antigens, such as
tumor-related proteins, peptides and genes expressing tumor
antigens have been introduced to patients to overcome
immunosuppression caused by the tumors, enhance immunogenicity,
stimulate the patient's immune system, and induce cellular and
humoral immune responses to control or eradicate the tumors
(149,150). At present, diverse vaccine
types, including DNA, mRNA, polypeptides and dendritic cell
vaccines have been formulated to stimulate the body's immune
response against cancer cells. These vaccines are specifically
designed based on the biological characteristics of HER2 expression
in tumor cells (151,152). There have been marked
developments in research on HER2-targeted vaccines and their
application in the treatment of advanced breast cancer (152-155). This suggests that other solid
tumors with similarly high HER2 expression may benefit from such
therapies. A research team from Austria developed a therapeutic B
cell epitope vaccine (IMU-131/HER-Vaxx), composed of three fused B
cell epitopes from the HER2 extracellular domain coupled to CRM197
with Montanide as an adjuvant (156). The team further conducted a
phase IB clinical trial (NCT02795988) and evaluated the
immunogenicity and optimal dose (detection dose, 10, 30 or 50
μg) in patients with HER2+ AGC based on the HER2
specific antibody and cellular response. The results showed that
none of the 11 patients experienced major adverse reactions, and
one patient had a complete response, five patients had partial
response, and four patients had stable disease, indicating that
IMU-131 had good tolerability, safety and therapeutic potential
(156). The induced
HER2-specific antibody and cell response showed a dose-dependent
relationship and it was markedly associated with clinical response.
Therefore, the recommended maximum dose (50 μg) has been
applied in phase II clinical trials to further evaluate clinical
response.
Taken together, trastuzumab is still the first-line
treatment for patients with HER2+ AGC targeting HER2.
However, its efficacy is limited by the development of drug
resistance. The expression of HER2 should be detected using ctDNA
to monitor the efficacy of trastuzumab, and liquid biopsy may also
help to predict tumor progression. In addition, anti-HER2-targeted
therapy combined with immunotherapy has become a novel approach for
improving the prognosis of patients with HER2+ AGC.
Moreover, investigating new therapy strategies, such as therapy
based on biological rhythms or identifying specific subtypes
sensitive to HER2 targeting, would be an attractive strategy for
increasing the efficacy of trastuzumab in the treatment of
HER2+ AGC (46,48).
Meanwhile, ADC drugs such as DS-8201 and RC48, have been approved
as second-line and third-line treatments for patients with
HER2+ AGC, respectively (20,21). Anti-HER2 bispecific antibodies
such as MGAH22 and ZW25 have good efficacy. However, given their
widespread use in the treatment of breast cancer, anti-HER2-TKIs
have not been approved for GC treatment. This may be explained by
the high heterogeneity of GC (157,158).
The advent of CAR-T cell therapy has revitalized
prospects for patients with HER2+ AGC. While CAR-T cell
therapy for solid tumors poses challenges distinct from
hematological tumors, the approach of targeting various antigens
through CAR-T cell therapy can still yield positive outcomes for
specific patient groups. Simultaneously employing ICIs to
counteract immunosuppressive factors within the tumor
microenvironment holds promise for enhancing the effectiveness of
CAR-T cells in HER2+ AGC (148,159,160). HER2-targeted cancer vaccines can
activate the patient's own immune system to kill cancer cells,
providing a more durable therapeutic effect. However, it is
noteworthy that HER2 cancer vaccines are still in the early stages
of development. Immunologic escape and immune tolerance remain
major challenges that limit their progress (156,161). Further research is required to
identify optimal conditions for their use and potential side
effects. Moreover, there is a need to develop strategies to
identify patients who may benefit from targeted therapy and
immunotherapy, while also monitoring drug efficacy. This will lead
to the development of novel drugs and strategies for the management
of HER2+ AGC.
Not applicable.
HHH was responsible for writing the majority of the
manuscript and preparing the figures and tables. SQW revised the
manuscript. HCZ and ZSC supervised the process and participated in
writing the manuscript. XBC and XJS conceived the study and
provided oversight throughout the process. All authors have read
and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The authors would like to thank for the financial support
provided by the National Natural Science Foundation of China (grant
nos. 82103560 and 82103996), the Young and Middle-Aged Health
Science and Technology Innovation Talent Training Project of Henan
Province (grant nos. YXKC2022048 and YXKC2020008), the Science and
Technique Foundation of Henan Province (grant no. 202102310413) and
the Natural Science Foundation of Henan Province (grant nos.
232300421119, 212300410270 and 212300410253).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram L, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
|
|
2
|
Joshi SS and Badgwell BD: Current
treatment and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021.
|
|
3
|
Marano L and Roviello F: The distinctive
nature of HER2-positive gastric cancers. Eur J Surg Oncol.
41:271–273. 2015.
|
|
4
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das R Enzinger PC, Enzler T, Fanta R, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022.
|
|
5
|
Moasser MM: The oncogene HER2: Its
signaling and transforming functions and its role in human cancer
pathogenesis. Oncogene. 26:6469–6487. 2007.
|
|
6
|
Palle J, Rochand A, Pernot S, Gallois C,
Taieb J and Zaanan A: Human epidermal growth factor receptor 2
(HER2) in advanced gastric cancer: Current knowledge and future
perspectives. Drugs. 80:401–415. 2020.
|
|
7
|
Yang HY, Zhou BP, Hung MC and Lee MH:
Oncogenic signals of HER-2/neu in regulating the stability of the
cyclin-dependent kinase inhibitor p27. J Biol Chem.
275:24735–24739. 2000.
|
|
8
|
Yang ZY, Zhao YW, Xue JR, Guo R, Zhao Z,
Liu HD, Ren ZG and Shi M: Thioridazine reverses trastuzumab
resistance in gastric cancer by inhibiting S-phase kinase
associated protein 2-mediated aerobic glycolysis. World J
Gastroentero. 29:5974–5987. 2023.
|
|
9
|
Lane HA, Motoyama AB, Beuvink I and Hynes
NE: Modulation of p27/Cdk2 complex formation through 4D5-mediated
inhibition of HER2 receptor signaling. Ann Oncol. 12(Suppl 1):
S21–S22. 2001.
|
|
10
|
Kwon HJ, Park Y, Nam SK, Kang E, Kim KK,
Jeong I, Kwak Y, Yoon J, Kim TY, Lee KW, et al: Genetic and immune
microenvironment characterization of HER2-positive gastric cancer:
Their association with response to trastuzumab-based treatment.
Cancer Med. 12:10371–10384. 2023.
|
|
11
|
Yao X, He Z, Qin C, Zhang P, Sui C, Deng
X, Fang Y, Li G and Shi J: Inhibition of PFKFB3 in HER2-positive
gastric cancer improves sensitivity to trastuzumab by inducing
tumour vessel normalisation. Brit J Cancer. 127:811–823. 2022.
|
|
12
|
Kim BJ, Jee HJ, Rha SY, Han HS, Ryu MH,
Park SH, Kim JG, Bae WK, Lee KW, Oh DY, et al: Ramucirumab plus
paclitaxel as a second-line treatment in HER2-positive gastric
cancer: Subgroup analysis of a nationwide, real-world study in
Korea (KCSG-ST19-16). Gastric Cancer. 25:609–618. 2022.
|
|
13
|
Kim Y, Bae YJ, Kim JH, Kim H, Shin SJ,
Jung DH and Park H: Wnt/β-catenin pathway is a key signaling
pathway to trastuzumab resistance in gastric cancer cells. BMC
Cancer. 23:9222023.
|
|
14
|
De Re V, Alessandrini L, Brisotto G,
Caggiari L, De Zorzi M, Casarotto M, Miolo G, Puglisi F, Garattini
SK, Lonardi S, et al: HER2-CDH1 interaction via Wnt/B-catenin is
associated with Patients' survival in HER2-positive metastatic
gastric adenocarcinoma. Cancers (Basel). 14:12662022.
|
|
15
|
Kumagai S, Koyama S and Nishikawa H:
Antitumour immunity regulated by aberrant ERBB family signalling.
Nat Rev Cancer. 21:181–197. 2021.
|
|
16
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697.
2010.
|
|
17
|
Piro G, Carbone C, Cataldo I, Di
Nicolantonio F, Giacopuzzi S, Aprile G, Simionato F, Boschi F,
Zanotto M, Mina MM, et al: An FGFR3, Autocrine loop sustains
acquired resistance to trastuzumab in gastric cancer patients. Clin
Cancer Res. 22:6164–6175. 2016.
|
|
18
|
Xu Q, Xu X, Tang H, Yan J, Li J, Bao H, Wu
X, Shao Y, Luo C, Wen H, et al: Exploring potential molecular
resistance and clonal evolution in advanced HER2-positive gastric
cancer under trastuzumab therapy. Oncogenesis. 12:212023.
|
|
19
|
Zhu Y, Zhu X, Wei X, Tang C and Zhang W:
HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev
Cancer. 1876:1885492021.
|
|
20
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab Deruxtecan in previously treated HER2-positive gastric
cancer. New Engl J Med. 382:2419–2430. 2020.
|
|
21
|
Peng Z, Liu T, Wei J, Wang A, He Y, Yang
L, Zhang X, Fan N, Luo S, Gong J, et al: A phase II study of
efficacy and safety of RC48-ADC in patients with locally advanced
or metastatic HER2-overexpressing gastric or gastroesophageal
junction cancers. J Clin Oncol. 38(Suppl 15): S45602020.
|
|
22
|
Lin W, Zhang Y, Yang Y, Lin B, Zhu M, Xu
J, Chen Y, Wu W, Chen B, Chen X, et al: Anti-PD-1/Her2 Bispecific
antibody IBI315 enhances the treatment effect of Her2-positive
gastric cancer through Gasdermin B-cleavage induced Pyroptosis. Adv
Sci. 10:e23039082023.
|
|
23
|
Ogitani Y, Aida T, Hagihara K, Yamaguchi
J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et
al: DS-8201a, A novel HER2-targeting ADC with a Novel DNA
Topoisomerase I inhibitor, demonstrates a promising antitumor
efficacy with differentiation from T-DM1. Clin Cancer Res.
22:5097–5108. 2016.
|
|
24
|
Li L, Xu MZ, Wang L, Jiang J, Dong LH,
Chen F, Dong K and Song HF: Conjugating MMAE to a novel anti-HER2
antibody for selective targeted delivery. Eur Rev Med Pharmaco.
24:12929–12937. 2020.
|
|
25
|
Agus DB, Akita RW, Fox WD, Lewis GD,
Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K,
et al: Targeting ligand-activated ErbB2 signaling inhibits breast
and prostate tumor growth. Cancer Cell. 2:127–137. 2002.
|
|
26
|
Nordstrom JL, Muth J, Erskine CL, Sanders
C, Yusko EC, Emerson RO, Lee M, Lee S, Trepel JB, Im S, et al: High
frequency of HER2-specific immunity observed in patients (pts) with
HER2+cancers treated with margetuximab (M), an Fc-enhanced
anti-HER2 monoclonal antibody (mAb). J Clin Oncol. 37(Suppl 15):
S10302019.
|
|
27
|
Yu S, Liu Q, Han X, Qin S, Zhao W, Li A
and Wu K: Development and clinical application of anti-HER2
monoclonal and bispecific antibodies for cancer treatment. Exp
Hematol Oncol. 6:312017.
|
|
28
|
Labrijn AF, Janmaat ML, Reichert JM and
Parren P: Bispecific antibodies: A mechanistic review of the
pipeline. Nat Rev Drug Discov. 18:585–608. 2019.
|
|
29
|
Lewis PG, Li G, Dugger DL, Crocker LM,
Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, et
al: Targeting HER2-positive breast cancer with trastuzumab-DM1, an
anti-body-cytotoxic drug conjugate. Cancer Res. 68:9280–9290.
2008.
|
|
30
|
Humphreys RC, Kirtely J, Hewit A, Biroc S,
Knudsen N, Skidmore L and Wahl A: Abstract 639: Site specific
conjugation of ARX-788, an antibody drug conjugate (ADC) targeting
HER2, generates a potent and stable targeted therapeutic for
multiple cancers. Cancer Res. 75:6392015.
|
|
31
|
Johnston SRD and Leary A: Lapatinib: A
novel EGFR/HER2 tyrosine kinase inhibitor for cancer. Drug Today.
42:441–453. 2006.
|
|
32
|
Zhu Y, Li L, Zhang G, Wan H, Yang C, Diao
X, Chen X, Zhang L and Zhong D: Metabolic characterization of
pyrotinib in humans by ultra-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry. J
Chromatogr B Analyt Technol Biomed Life Sci. 1033-1034:117–127.
2016.
|
|
33
|
Doi AGL, Lindmark BE, McHale M, Hung HT
and Ong R: Abstract 4719: Varlitinib demonstrates potent antitumor
efficacy in patient-derived gastric cancer xenograft models. Cancer
Res. 76:47192016.
|
|
34
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos BA, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
|
|
35
|
Janjigian YY, Kawazoe A, Yanez P, Li N,
Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al: The
KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive
gastric cancer. Nature. 600:727–730. 2021.
|
|
36
|
Huang L, Wang R, Xie K, Zhang J, Tao F, Pi
C, Feng Y, Gu H and Fang J: A HER2 target antibody drug conjugate
combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in
hPD-1 transgenic mouse model and contributes immune memory
formation. Breast Cancer Res Treat. 191:51–61. 2022.
|
|
37
|
Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge
W, Cui Q, Mu Q, Chen G, Han L, et al: Genetically modified
CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy
for relapsed/refractory CD7-positive hematological malignancies: A
phase I clinical study. Cell Res. 32:995–1007. 2022.
|
|
38
|
Lu J and Jiang G: The journey of CAR-T
therapy in hematological malignancies. Mol Cancer. 21:1942022.
|
|
39
|
Hou AJ, Chen LC and Chen YY: Navigating
CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug
Discov. 20:531–550. 2021.
|
|
40
|
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo
Y, Zhao X, Wang Y, Wang Z, Han W, et al: Effective and persistent
antitumor activity of HER2-directed CAR-T cells against gastric
cancer cells in vitro and xenotransplanted tumors in vivo. Protein
Cell. 9:867–878. 2018.
|
|
41
|
Vivekanandhan S and Knutson KL: Resistance
to trastuzumab. Cancers (Basel). 14:51152022.
|
|
42
|
Hino K, Nishina T, Kajiwara T, Bando H,
Nakamura M, Kadowaki S, Minashi K, Yuki S, Ohta T, Hara H, et al:
Association of ERBB2 copy number and gene coalterations with
trastuzumab efficacy and resistance in human epidermal growth
factor receptor 2-Positive Esophagogastric and gastric cancer. JCO
Precis Oncol. 6:e22001352022.
|
|
43
|
Du R, Zhang X, Lu X, Ma X, Guo X, Shi C,
Ren X, Ma X, He Y, Gao Y and Liu Y: PDPN positive CAFs contribute
to HER2 positive breast cancer resistance to trastuzumab by
inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug
Resist Updat. 68:1009472023.
|
|
44
|
Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng
ZL, Liu Z, Zhao Q, He CY, Lu JH, et al: Liquid biopsies to track
trastuzumab resistance in metastatic HER2-positive gastric cancer.
Gut. 68:1152–1161. 2019.
|
|
45
|
Wang H, Li B, Liu Z, Gong J, Shao L, Ren
J, Niu Y, Bo S, Li Z, Lai Y, et al: HER2 copy number of circulating
tumour DNA functions as a biomarker to predict and monitor
trastuzumab efficacy in advanced gastric cancer. Eur J Cancer.
88:92–100. 2018.
|
|
46
|
Wang S, Khan S, Nabi G and Li HY:
Circadian rhythm as a key player in cancer progression as well as a
therapeutic target in HER2-positive advanced gastric cancer
treatment. Front Oncol. 13:12406762023.
|
|
47
|
Wang J, Huang Q, Hu X, Zhang S, Jiang Y,
Yao G, Hu K, Xu X, Liang B, Wu Q, et al: Disrupting circadian
rhythm via the PER1-HK2 axis reverses trastuzumab resistance in
gastric cancer. Cancer Res. 82:1503–1517. 2022.
|
|
48
|
Wang J, Kunzke T, Prade VM, Shen J, Buck
A, Feuchtinger A, Haffner I, Luber B, Liu D, Langer R, et al:
Spatial metabolomics identifies distinct tumor-specific subtypes in
gastric cancer patients. Clin Cancer Res. 28:2865–2877. 2022.
|
|
49
|
Wang J, Tu S, Chavda VP, Chen ZS and Chen
X: Successes and failures of immunotherapy for gastric cancer. Drug
Discov Today. 27:1033432022.
|
|
50
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015.
|
|
51
|
Riley RS, June CH, Langer R and Mitchell
MJ: Delivery technologies for cancer immunotherapy. Nat Rev Drug
Discov. 18:175–196. 2019.
|
|
52
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000.
|
|
53
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020.
|
|
54
|
Goel G and Sun W: Cancer immunotherapy in
clinical practice-the past, present, and future. Chin J Cancer.
33:445–457. 2014.
|
|
55
|
Pang K, Shi ZD, Wei LY, Dong Y, Ma YY,
Wang W, Wang GY, Cao MY, Dong JJ, Chen YA, et al: Research progress
of therapeutic effects and drug resistance of immunotherapy based
on PD-1/PD-L1 blockade. Drug Resist Updat. 66:1009072023.
|
|
56
|
Stein A, Paschold L, Tintelnot J, Goekkurt
E, Henkes SS, Simnica D, Schultheiss C, Willscher E, Bauer M,
Wickenhauser C, et al: Efficacy of ipilimumab vs FOLFOX in
combination with nivolumab and trastuzumab in patients with
previously untreated ERBB2-positive Esophagogastric adenocarcinoma:
The AIO INTEGA randomized clinical trial. JAMA Oncol. 8:1150–1158.
2022.
|
|
57
|
Aisa A, Weng S, Li X, Zhang D and Yuan Y:
Immune checkpoint inhibitors combined with HER-2 targeted therapy
in HER-2 positive gastroesophageal cancer. Crit Rev Oncol Hemat.
180:1038642022.
|
|
58
|
Agostinetto E, Montemurro F, Puglisi F,
Criscitiello C, Bianchini G, Del ML, Introna M, Tondini C, Santoro
A and Zambelli A: Immunotherapy for HER2-positive breast cancer:
Clinical evidence and future perspectives. Cancers (Basel).
14:21362022.
|
|
59
|
Rha SY, Lee C, Kim HS, Kang B, Jung M,
Kwon WS, Bae WK, Koo D, Shin S, Jeung H, et al: A
multi-institutional phase Ib/II trial of first-line triplet regimen
(Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive
advanced gastric and gastroesophageal junction cancer (PANTHERA
Trial): Molecular profiling and clinical update. J Clin Oncol.
39(Suppl 3): S2182021.
|
|
60
|
Janjigian YY, Maron SB, Chatila WK,
Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ,
Momtaz P, et al: First-line pembrolizumab and trastuzumab in
HER2-positive oesophageal, gastric, or gastro-oesophageal junction
cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol.
21:821–831. 2020.
|
|
61
|
Chung HC, Bang Y, S Fuchs C, Qin SK, Satoh
T, Shitara K, Tabernero J, Cutsem EV, Alsina M, Cao ZA, et al:
First-line pembrolizumab/placebo plus trastuzumab and chemotherapy
in HER2-positive advanced gastric cancer: KEYNOTE-811. Future
Oncol. 17:491–501. 2021.
|
|
62
|
Killock D: Pembrolizumab for
HER2+ gastric cancer. Nat Rev Clin Oncol.
19:1502022.
|
|
63
|
Kuznetsova M, Lopatnikova J, Shevchenko J,
Silkov A, Maksyutov A and Sennikov S: Cytotoxic activity and memory
T cell subset distribution of in vitro-Stimulated CD8+ T
cells specific for HER2/neu Epitopes. Front Immunol.
10:10172019.
|
|
64
|
Müller P, Kreuzaler M, Khan T, Thommen DS,
Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al:
Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly
susceptible to CTLA-4/PD-1 blockade. Sci Transl Med.
7:315ra1882015.
|
|
65
|
Stagg J, Loi S, Divisekera U, Ngiow SF,
Duret H, Yagita H, Teng MW and Smyth MJ: Anti-ErbB-2 mAb therapy
requires type I and II interferons and synergizes with anti-PD-1 or
anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 108:7142–7147.
2011.
|
|
66
|
Takahari D, Wakatsuki T, Ishizuka N,
Fukuda N, Shoji H, Hara H, Minashi K, Boku N and Yamaguchi K: A
phase Ib study of nivolumab plus trastuzumab with S-1/capecitabine
plus oxaliplatin for HER2-positive advanced gastric cancer (Ni-HIGH
study): Safety evaluation. J Clin Oncol. 38:45252020.
|
|
67
|
Takahari D, Shoji H, Minashi K, Hara H,
Chin K, Oki A, Ogura M, Nakayama I, Kato K, Iwasa S, et al: Safety
and early efficacy results of a phase Ib study of nivolumab plus
trastuzumab with S-1/capecitabine plus oxaliplatin for
HER2-positive advanced gastric cancer (Ni-HIGH study). J Clin
Oncol. 40:2762022.
|
|
68
|
Tintelnot J, Goekkurt E, Binder M,
Thuss-Patience P, Lorenzen S, Knorrenschild JR, Kretzschmar A,
Ettrich T, Lindig U, Jacobasch L, et al: Ipilimumab or FOLFOX with
Nivolumab and Trastuzumab in previously untreated HER2-positive
locally advanced or metastatic EsophagoGastric Adenocarcinoma-the
randomized phase 2 INTEGA trial (AIO STO 0217). BMC Cancer.
20:5032020.
|
|
69
|
Lee MS, Chao J, Mulcahy MF, Kasi PM,
Alistar AT, Mukherjee S, Akce M, Moore DT, Carlson CA and Mcree AJ:
Abstract CT174: Phase II study of avelumab and trastuzumab with
FOLFOX chemotherapy in previously untreated HER2-amplified
metastatic gastroesophageal adenocarcinoma. Cancer Res.
81:CT1742021.
|
|
70
|
Makiyama A, Sukawa Y, Kashiwada T, Kawada
J, Hosokawa A, Horie Y, Tsuji A, Moriwaki T, Tanioka H, Shinozaki
K, et al: Randomized, Phase II study of trastuzumab beyond
progression in patients with HER2-positive advanced gastric or
gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin
Oncol. 38:1919–1927. 2020.
|
|
71
|
Kim CG, Jung M, Kim HS, Lee CK, Jeung HC,
Koo DH, Bae WK, Zang DY, Kim BJ, Kim H, et al: Trastuzumab combined
with ramucirumab and paclitaxel in patients with previously treated
human epidermal growth factor receptor 2-positive advanced gastric
or gastroesophageal junction cancer. J Clin Oncol. 41:4394–4405.
2023.
|
|
72
|
Ogitani Y, Hagihara K, Oitate M, Naito H
and Agatsuma T: Bystander killing effect of DS-8201a, a novel
anti-human epidermal growth factor receptor 2 antibody-drug
conjugate, in tumors with human epidermal growth factor receptor 2
heterogeneity. Cancer Sci. 107:1039–1046. 2016.
|
|
73
|
Doi T, Shitara K, Naito Y, Shimomura A,
Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N,
et al: Safety, pharmacokinetics, and antitumour activity of
trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug
conjugate, in patients with advanced breast and gastric or
gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet
Oncol. 18:1512–1522. 2017.
|
|
74
|
Shitara K, Iwata H, Takahashi S, Tamura K,
Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, et
al: Trastuzumab deruxtecan (DS-8201a) in patients with advanced
HER2-positive gastric cancer: A dose-expansion, phase 1 study.
Lancet Oncol. 20:827–836. 2019.
|
|
75
|
Staudacher AH and Brown MP: Antibody drug
conjugates and bystander killing: Is antigen-dependent
internalisation required? Brit J Cancer. 117:1736–1742. 2017.
|
|
76
|
Xu Y, Wang Y, Gong J, Zhang X, Peng Z,
Sheng X, Mao C, Fan Q, Bai Y, Ba Y, et al: Phase I study of the
recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate
RC48-ADC in patients with HER2-positive advanced solid tumors.
Gastric Cancer. 24:913–925. 2021.
|
|
77
|
Thuss-Patience PC, Shah MA, Ohtsu A, Van
Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G,
Shitara K, et al: Trastuzumab emtansine versus taxane use for
previously treated HER2-positive locally advanced or metastatic
gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An
international randomised, open-label, adaptive, phase 2/3 study.
Lancet Oncol. 18:640–653. 2017.
|
|
78
|
Xu J, Ying J, Liu R, Wu J, Ye F, Xu N,
Zhang Y, Zhao R, Xiang X, Wang J, et al: KN026 (anti-HER2
bispecific antibody) in patients with previously treated, advanced
HER2-expressing gastric or gastroesophageal junction cancer. Eur J
Cancer. 178:1–12. 2023.
|
|
79
|
Meric-Bernstam F, Hamilton EP, Beeram M,
Hanna DL, El-Khoueiry AB, Kang Y, Lee KW, Lee J, Rha SY, Chaves JM,
et al: Zanidatamab (ZW25) in HER2-expressing gastroesophageal
adenocarcinoma (GEA): Results from a phase I study. J Clin Oncol.
39(Suppl 3): S1642021.
|
|
80
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges J, et al: Safety and
efficacy of Pembrolizumab Monotherapy in patients with previously
treated advanced gastric and Gastroesophageal junction cancer. JAMA
Oncol. 4:e1800132018.
|
|
81
|
Shitara K, Özguroglu M, Bang YJ, Di
Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNO TE-061): A randomised, open-label, controlled, phase 3
trial. Lancet. 392:123–133. 2018.
|
|
82
|
Catenacci D, Kang YK, Park H, Uronis HE,
Lee KW, Ng M, Enzinger PC, Park SH, Gold PJ, Lacy J, et al:
Margetuximab plus pembrolizumab in patients with previously
treated, HER2-positive gastro-oesophageal adenocarcinoma
(CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol.
21:1066–1076. 2020.
|
|
83
|
Swain SM, Miles D, Kim SB, Im YH, Im SA,
Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al:
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic
breast cancer (CLEOPATRA): End-of-study results from a
double-blind, randomised, placebo-controlled, phase 3 study. Lancet
Oncol. 21:519–530. 2020.
|
|
84
|
Yamashita-Kashima Y, Iijima S, Yorozu K,
Furugaki K, Kurasawa M, Ohta M and Fujimoto-Ouchi K: Pertuzumab in
combination with trastuzumab shows significantly enhanced antitumor
activity in HER2-positive human gastric cancer xenograft models.
Clin Cancer Res. 17:5060–5070. 2011.
|
|
85
|
Tabernero J, Hoff PM, Shen L, Ohtsu A,
Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, et al:
Pertuzumab plus trastuzumab and chemotherapy for HER2-positive
metastatic gastric or gastro-oesophageal junction cancer (JACOB).
Final analysis of a double-blind, randomised, placebo-controlled
phase 3 study. Lancet Oncol. 19:1372–1384. 2018.
|
|
86
|
Liu T, Qin Y, Li J, Xu R, Xu J, Yang S,
Qin S, Bai Y, Wu C, Mao Y, et al: Pertuzumab in combination with
trastuzumab and chemotherapy for Chinese patients with
HER2-positive metastatic gastric or gastroesophageal junction
cancer. A subpopulation analysis of the JACOB trial. Cancer Commun
(Lond). 39:382019.
|
|
87
|
Wagner AD, Grabsch HI, Mauer M, Marreaud
S, Caballero C, Thuss-Patience P, Mueller L, Elme A, Moehler MH,
Martens U, et al: EORTC-1203-GITCG-the 'INNOVATION'-trial: Effect
of chemotherapy alone versus chemotherapy plus trastuzumab, versus
chemotherapy plus trastuzumab plus pertuzumab, in the perioperative
treatment of HER2 positive, gastric and gastroesophageal junction
adenocarcinoma on pathologic response rate: A randomized phase
II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer
Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group.
BMC Cancer. 19:4942019.
|
|
88
|
Nordstrom JL, Gorlatov S, Zhang W, Yang Y,
Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, et al:
Anti-tumor activity and toxicokinetics analysis of MGAH22, an
anti-HER2 monoclonal antibody with enhanced Fcgamma receptor
binding properties. Breast Cancer Res. 13:R1232011.
|
|
89
|
Bang YJ, Giaccone G, Im SA, Oh DY, Bauer
TM, Nordstrom JL, Li H, Chichili GR, Moore PA, Hong S, et al:
First-in-human phase 1 study of margetuximab (MGAH22), an
Fc-modified chimeric monoclonal antibody, in patients with
HER2-positive advanced solid tumors. Ann Oncol. 28:855–861.
2017.
|
|
90
|
Rugo HS, Im SA, Cardoso F, Cortes J,
Curigliano G, Musolino A, Pegram MD, Wright GS, Saura C,
Escriva-de-Romani S, et al: Efficacy of Margetuximab vs trastuzumab
in patients with pretreated ERBB2-positive advanced breast cancer.
A phase 3 randomized clinical trial. JAMA Oncol. 7:573–584.
2021.
|
|
91
|
Catenacci DVT, Kim SS, Gold PJ, Philip AP,
Enzinger PC, Coffie J, Schmidt EV, Baldwin M, Nordstrom JL, Bonvini
E, et al: A phase 1b/2, open label, dose-escalation study of
margetuximab (M) in combination with pembrolizumab (P) in patients
with relapsed/refractory advanced HER2+ gastroesophageal (GEJ)
junction or gastric (G) cancer. J Clin Oncol. 35(Suppl 4):
TPS2192017.
|
|
92
|
Catenacci DVT, Park H, Lockhart AC, Gold
PJ, Enzinger PC, Nordstrom JL, Hong S, Hochster HS, Kelly RJ,
Uronis HE, et al: Phase 1b/2 study of margetuximab (M) plus
pembrolizumab (P) in advanced HER2+ gastroesophageal junction (GEJ)
or gastric (G) adenocarcinoma (GEA). J Clin Oncol. 36(Suppl 4):
S1402018.
|
|
93
|
Catenacci DVT, Park H, Uronis HE, Kang Y,
Lacy J, Enzinger PC, Park SH, Lee KW, Ng MCH, Gold PJ, et al:
Margetuximab (M) plus pembrolizumab (P) in ERBB2-amplified
PD-L1+gastroesophageal adenocarcinoma (GEA) post trastuzumab (T). J
Clin Oncol. 36(Suppl 15): S40302018.
|
|
94
|
Catenacci DVT, Lim KH, Uronis HE, Kang Y,
Ng MCH, Gold PJ, Enzinger PC, Lee KW, Lacy J, Park SH, et al:
Antitumor activity of margetuximab (M) plus pembrolizumab (P) in
patients (pts) with advanced HER2+(IHC3+) gastric carcinoma (GC). J
Clin Oncol. 37(Suppl 4): S652019.
|
|
95
|
Catenacci DVT, Rosales M, Chung HC, Yoon
HH, Shen L, Moehler M and Kang Y: MAHOGANY: margetuximab
combination in HER2(+) unresectable/metastatic
gastric/gastroesophageal junction adenocarcinoma. Future Oncol.
17:1155–1164. 2021.
|
|
96
|
Luke JJ, Patel MR, Blumenschein GR,
Hamilton E, Chmielowski B, Ulahannan SV, Connolly RM, Santa-Maria
CA, Wang J, Bahadur SW, et al: The PD-1- and LAG-3-targeting
bispecific molecule tebotelimab in solid tumors and hematologic
cancers. A phase 1 trial. Nat Med. 29:2814–2824. 2023.
|
|
97
|
Catenacci DV, Rosales M, Chung HC, H YH,
Shen L, Moehler M and Kang YK: MAHOGANY: Margetuximab combination
in HER2+ unresectable/metastatic Gastric/Gastroesophageal junction
adenocarcinoma. Future Oncol. 17:1155–1164. 2021.
|
|
98
|
Catenacci D, Kang YK, Yoon HH, Shim BY,
Kim ST, Oh DY, Spira AI, Ulahannan SV, Avery EJ, Boland PM, et al:
Margetuximab with retifanlimab as first-line therapy in
HER2+/PD-L1+ unresectable or metastatic gastroesophageal
adenocarcinoma: MAHOGANY cohort A. Esmo Open. 7:1005632022.
|
|
99
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. New
Engl J Med. 367:1783–1791. 2012.
|
|
100
|
Krop IE, Kim SB, Gonzalez-Martin A,
LoRusso PM, Ferrero JM, Smitt M, Yu R, Leung AC and Wildiers H:
Trastuzumab emtansine versus treatment of physician's choice for
pretreated HER2-positive advanced breast cancer (TH3RESA). A
randomised, open-label, phase 3 trial. Lancet Oncol. 15:689–699.
2014.
|
|
101
|
Dieras V, Miles D, Verma S, Pegram M,
Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, et al:
Trastuzumab emtansine versus capecitabine plus lapatinib in
patients with previously treated HER2-positive advanced breast
cancer (EMILIA): A descriptive analysis of final overall survival
results from a randomised, open-label, phase 3 trial. Lancet Oncol.
18:732–742. 2017.
|
|
102
|
Li H, Xu X, Liu Y, Li S, Zhang D, Meng X,
Lu L and Li Y: MMP7 induces T-DM1 resistance and leads to the poor
prognosis of gastric adenocarcinoma via a DKK1-dependent manner.
Anticancer Agents Med Chem. 18:2010–2016. 2018.
|
|
103
|
Nagaraja SP, Zhu J, Skidmore L, Liang X,
Ji Y, Gu Y, Tian F, Yao S and Xia G: Nonclinical development of
Next-generation Site-specific HER2-targeting Antibody-drug
Conjugate (ARX788) for breast cancer treatment. Mol Cancer Ther.
19:1822–1832. 2020.
|
|
104
|
Skidmore L, Sakamuri S, Knudsen NA, Hewet
AG, Milutinovic S, Barkho W, Biroc SL, Kirtley J, Marsden R, Storey
K, et al: ARX788, a Site-specific Anti-HER2 Antibody-drug
conjugate, demonstrates potent and selective activity in HER2-low
and T-DM1-resistant breast and gastric cancers. Mol Cancer Ther.
19:1833–1843. 2020.
|
|
105
|
Barok M, Le Joncour V, Martins A, Isola J,
Salmikangas M, Laakkonen P and Joensuu H: ARX788, a novel anti-HER2
antibody-drug conjugate, shows anti-tumor effects in preclinical
models of trastuzumab emtansine-resistant HER2-positive breast
cancer and gastric cancer. Cancer Lett. 473:156–163. 2020.
|
|
106
|
Zhang Y, Qiu MZ, Wang JF, Zhang YQ, Shen
A, Yuan XL, Zhang T, Wei XL, Zhao HY, Wang DS, et al: Phase 1
multicenter, dose-expansion study of ARX788 as monotherapy in
HER2-positive advanced gastric and gastroesophageal junction
adenocarcinoma. Cell Rep Med. 3:1008142022.
|
|
107
|
Weisser N, Wickman G, Davies R and Rowse
G: Preclinical development of a novel biparatopic HER2 antibody
with activity in low to high HER2 expressing cancers. Cancer Res.
77(Suppl 13): S172017.
|
|
108
|
ZW25 Effective in HER2-positive cancers.
Cancer Discov. 9:82019.
|
|
109
|
Meric-Bernstam F, Beeram M, Mayordomo JI,
Hanna DL, Ajani JA, Murphy MAB, Murthy RK, Piha-Paul SA, Bauer TM,
Bendell JC, et al: Single agent activity of ZW25, a HER2-targeted
bispecific antibody, in heavily pretreated HER2-expressing cancers.
J Clin Oncol. 36(15 Suppl): S25002018.
|
|
110
|
Wei H, Cai H, Jin Y, Wang P, Zhang Q, Lin
Y, Wang W, Cheng J, Zeng N, Xu T and Zhou A: Structural basis of a
novel heterodimeric Fc for bispecific antibody production.
Oncotarget. 8:51037–51049. 2017.
|
|
111
|
Zhang J, Ji D, Cai L, Yao H, Yan M, Wang
X, Shen W, Du Y, Pang H, Lai X, et al: First-in-human HER2-targeted
Bispecific Antibody KN026 for the treatment of patients with
HER2-positive metastatic breast cancer: Results from a Phase I
study. Clin Cancer Res. 28:618–628. 2022.
|
|
112
|
Xu J, Zhang Y, Wu J, Xu N, Ying J, Xiang
X, Zhang Y, Wang J, Zhao R, Ye F, et al: The preliminary efficacy
of KN026 (Anti-HER2 BsAb) in advanced gastric and gastroesophageal
junction cancer patients with HER2 expression. J Clin Oncol.
39(Suppl 15): e160052021.
|
|
113
|
Gong J, Shen L, Dong Z, Liu D, Xu J, Yang
J, Yang Y, Qi Y, Men J, Kong P, et al: Preliminary safety,
tolerability and efficacy results of KN026 in combination with
KN046 in patients with HER2 aberrated solid tumors. J Immunother
Cancer. 83:A485–A486. 2020.
|
|
114
|
Gong J, Dong Z, Liu D, Xu J, Yang J, Yang
Y, Qi Y, Men J, Kong P, Xu T, et al: Preliminary safety,
tolerability and efficacy results of KN026 (a HER2-targeted
bispecific antibody) in combination with KN046 (an
anti-PD-Ll/CTLA-4 bispecific antibody) in patients (pts) with HER2
aberrated solid tumors. J Immunother Cancer. 83:A2072020.
|
|
115
|
Shen L, Gong J, Niu Z, Zhao R, Chen L, Liu
L, Deng T, Lu L, Zhang Y, Li Z, et al: 1210P the preliminary
efficacy and safety of KN026 combined with KN046 treatment in
HER2-positive locally advanced unresectable or metastatic
gastric/gastroesophageal junction cancer without prior systemic
treatment in a phase II study. Ann Oncol. 33(Suppl 7):
S11022022.
|
|
116
|
Lin W, Zhang Y, Yang Y, Lin B, Zhu M, Xu
J, Chen Y, Wu W, Chen B, Chen X, et al: Anti-PD-1/Her2 bispecific
antibody IBI315 enhances the treatment effect of Her2-positive
gastric cancer through Gasdermin B-cleavage induced pyroptosis. Adv
Sci (Weinh). 10:e23039082023.
|
|
117
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
human epidermal growth factor receptor 2-positive advanced or
metastatic gastric esophageal, or gastroesophageal adenocarcinoma:
TRIO-013/LOGiC-A randomized phase III trial. J Clin Oncol.
34:443–451. 2016.
|
|
118
|
Press MF, Ellis CE, Gagnon RC, Grob TJ,
Buyse M, Villalobos I, Liang Z, Wu S, Bang YJ, Qin SK, et al: HER2
status in advanced or metastatic gastric, esophageal, or
gastroesophageal adenocarcinoma for entry to the TRIO-013/LOGiC
trial of Lapatinib. Mol Cancer Ther. 16:228–238. 2017.
|
|
119
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 32:2039–2049.
2014.
|
|
120
|
Chen CT, Kim H, Liska D, Gao S,
Christensen JG and Weiser MR: MET activation mediates resistance to
lapatinib inhibition of HER2-amplified gastric cancer cells. Mol
Cancer Ther. 11:660–669. 2012.
|
|
121
|
Ning G, Zhu Q, Kang W, Lee H, Maher L, Suh
Y, Michaud M, Silva M, Kwon JY, Zhang C, et al: A novel treatment
strategy for lapatinib resistance in a subset of HER2-amplified
gastric cancer. BMC Cancer. 21:9232021.
|
|
122
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: phase I study and biomarker
analysis of pyrotinib, a novel irreversible Pan-ErbB receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017.
|
|
123
|
Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P,
Fan Y, Wang J, Luo Y, Yuan P, et al: Safety efficacy, and biomarker
analysis of pyrotinib in combination with Capecitabine in
HER2-positive metastatic breast cancer patients: A phase I clinical
trial. Clin Cancer Res. 25:5212–5220. 2019.
|
|
124
|
Yin Y, Yang H, Liu Z, Tan J, Zhu C, Chen
M, Zhou R, Wang L and Qian J: Studies on the safety and efficacy of
pyrotinib in the treatment of HER2-positive advanced solid tumors
excluding breast cancer. Cancer Manag Res. 12:13479–13487.
2020.
|
|
125
|
Fan S, Jiang D, Lv Y, Cui Y and Hou R:
Studies on the efficacy of pyrotinib in the treatment of HER-2
positive advanced solid tumors. J Clin Oncol. 38:e156392020.
|
|
126
|
Liu D, Xu YY, Gong JF, Yang F, Shen L and
Li HJ: Pyrotinib combined with SHR6390 in the treatment of
refractory advanced HER2 positive gastric cancer or solid tumors:
Safety and efficacy results from a phase Id trial. J Clin Oncol.
39(Suppl 15): e160092021.
|
|
127
|
Li X, Gu X, Xu J, Chen L, Li H, Meng D,
Bai H, Yang J and Qian J: Sustained clinical benefit of pyrotinib
combined with capecitabine rescue therapy after trastuzumab
resistance in HER2-positive advanced gastric cancer: A case report.
Oncotargets Ther. 14:3983–3989. 2021.
|
|
128
|
Wu J, Li L, Qin J, Yan Z, Chen S, Jin T
and Xu J: Case report: Durable clinical response to third-line
pyrotinib after resistance to trastuzumab in a gastric cancer
patient. Front Oncol. 11:7805772021.
|
|
129
|
Chen Z, Xu Y, Gong J, Kou F, Zhang M, Tian
T, Zhang X, Zhang C, Li J, Li Z, et al: Pyrotinib combined with
CDK4/6 inhibitor in HER2-positive metastatic gastric cancer: A
promising strategy from AVATAR mouse to patients. Clin Transl Med.
10:e1482020.
|
|
130
|
Huang LT, Ma JT, Zhang SL, Li XH, Sun L,
Jing W, Zhao JZ, Wang YR and Han CB: Durable clinical response to
pyrotinib after resistance to prior Anti-HER2 therapy for
HER2-positive advanced gastric cancer: A case report. Front Oncol.
9:14532019.
|
|
131
|
Sang Y, Jung M, Kim HS, Kang B, Lee C,
Chung HC and Rha SY: Phase Ib trial of varlitinib plus weekly
paclitaxel in EGFR/HER2 co-expressing advanced gastric cancer
(AGC). J Clin Oncol. 39(Suppl 3): S2272021.
|
|
132
|
Murthy RK, Loi S, Okines A, Paplomata E,
Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et
al: Tucatinib, trastuzumab, and Capecitabine for HER2-positive
metastatic breast cancer. New Engl J Med. 382:597–609. 2020.
|
|
133
|
Peterson S, de Vries P, Piasecki J and
Rosler R: Tucatinib, a HER2 selective kinase inhibitor, is active
in patient derived xenograft (PDX) models of HER2-amplified
colorectal, esophageal and gastric cancers. Ann Oncol. 28(Suppl 5):
V5762017.
|
|
134
|
Strickler JH, Cercek A, Siena S, Andre T,
Ng K, Van Cutsem E, Wu C, Paulson AS, Hubbard JM, Coveler AL, et
al: Tucatinib plus trastuzumab for chemotherapy-refractory,
HER2-positive, RAS wild-type unresectable or metastatic colorectal
cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study.
Lancet Oncol. 24:496–508. 2023.
|
|
135
|
Strickler JH, Nakamura Y, Yoshino T,
Catenacci DVT, Janjigian YY, Barzi A, Bekaii-Saab TS, Lenz H, Lee
J, Van Cutsem E, et al: MOUNTAINEER-02: Phase II/III study of
tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously
treated HER2+ gastric or gastroesophageal junction
adenocarcinoma-trial in progress. J Clin Oncol. 39(Suppl 5):
S1071–S1072. 2021.
|
|
136
|
Tabernero J, Strickler J, Nakamura Y,
Shitara K, Janjigian Y, Barzi A, Bekaii-Saab T, Lenz H, Yoshino T,
Siena S, et al: MOUNTAINEER-02: Phase 2/3 study of tucatinib,
trastuzumab, ramucirumab, and paclitaxel in previously treated
HER2+ gastric or gastroesophageal junction adenocarcinoma: Trial in
progress. Ann Oncol. 33(Suppl): S305–S306. 2022.
|
|
137
|
Siddiqui RS and Sardar M: A systematic
review of the role of chimeric antigen receptor T (CAR-T) cell
therapy in the treatment of solid tumors. Cureus J Med Science.
13:e144942021.
|
|
138
|
Liu D, Zhao J and Song Y: Engineering
switchable and programmable universal CARs for CAR T therapy. J
Hematol Oncol. 12:692019.
|
|
139
|
Holstein SA and Lunning MA: CAR T-cell
therapy in hematologic malignancies: A voyage in progress. Clin
Pharmacol Ther. 107:112–122. 2020.
|
|
140
|
Munshi NC, Anderson LJ, Shah N, Madduri D,
Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, et al:
Idecabtagene Vicleucel in relapsed and refractory multiple myeloma.
New Engl J Med. 384:705–716. 2021.
|
|
141
|
Miao YR, Liu W, Zhong Z, You Y, Tang Y, Li
W, Zhu X and Guo AY: Case Report: Multi-Omics analysis and CAR-T
treatment of a chronic myeloid leukemia blast crisis case 5 years
after the discontinuation of TKI. Front Oncol. 11:7398712021.
|
|
142
|
Dana H, Chalbatani GM, Jalali SA, Mirzaei
HR, Grupp SA, Suarez ER, Raposo C and Webster TJ: CAR-T cells:
Early successes in blood cancer and challenges in solid tumors.
Acta Pharm Sin B. 11:1129–1147. 2021.
|
|
143
|
Sun J, Li X, Chen P and Gao Y: From
Anti-HER-2 to Anti-HER-2-CAR-T cells: An evolutionary immunotherapy
approach for gastric cancer. J Inflamm Res. 15:4061–4085. 2022.
|
|
144
|
Han Y, Liu C, Li G, Li J, Lv X, Shi H, Liu
J, Liu S, Yan P, Wang S, et al: Antitumor effects and persistence
of a novel HER2 CAR T cells directed to gastric cancer in
preclinical models. Am J Cancer Res. 8:106–119. 2018.
|
|
145
|
Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H,
Yang Q, Wang Y and Han W: Phase I study of chimeric antigen
receptor modified T cells in treating HER2-positive advanced
biliary tract cancers and pancreatic cancers. Protein Cell.
9:838–847. 2018.
|
|
146
|
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S,
Zhang M, Peng Z, Zhou J, Cao Y, et al: Claudin18.2-specific CAR T
cells in gastrointestinal cancers: Phase 1 trial interim results.
Nat Med. 28:1189–1198. 2022.
|
|
147
|
Simon AG, Lyu SI, Laible M, Woell S,
Tuereci O, Sahin U, Alakus H, Fahrig L, Zander T, Buettner R, et
al: The tight junction protein claudin 6 is a potential target for
patient-individualized treatment in esophageal and gastric
adenocarcinoma and is associated with poor prognosis. J Transl Med.
21:5522023.
|
|
148
|
Entezam M, Sanaei M, Mirzaei Y, Mer AH,
Abdollahpour-Alitappeh M, Azadegan-Dehkordi F and Bagheri N:
Current progress and challenges of immunotherapy in gastric cancer:
A focus on CAR-T cells therapeutic approach. Life Sci.
318:1214592023.
|
|
149
|
Clark RE, Basabrain AA, Austin GM,
Holcroft AK, Loaiza S, Apperley JF, Law C, Scott L, Parry AD,
Bonnett L, et al: Validation of CIP2A as a biomarker of subsequent
disease progression and treatment failure in chronic myeloid
leukaemia. Cancers (Basel). 13:21552021.
|
|
150
|
Morse MA, Gwin WR and Mitchell DA: Vaccine
therapies for cancer: Then and now. Target Oncol. 16:121–152.
2021.
|
|
151
|
Pallerla S, Abdul A, Comeau J and Jois S:
Cancer vaccines, treatment of the future: With emphasis on
HER2-positive breast cancer. Int J Mol Sci. 22:7792021.
|
|
152
|
Costa R, Soliman H and Czerniecki BJ: The
clinical development of vaccines for HER2+ breast cancer: Current
landscape and future perspectives. Cancer Treat Rev. 61:107–115.
2017.
|
|
153
|
Al-Awadhi A, Lee MJ and Ibrahim NK:
Developing anti-HER2 vaccines: Breast cancer experience. Int J
Cancer. 143:2126–2132. 2018.
|
|
154
|
Mittendorf EA, Lu B, Melisko M, Price HJ,
Bondarenko I, Brunt AM, Sergii G, Petrakova K and Peoples GE:
Efficacy and safety analysis of Nelipepimut-S vaccine to prevent
breast cancer recurrence: A randomized, multicenter, phase III
clinical trial. Clin Cancer Res. 25:4248–4254. 2019.
|
|
155
|
Disis M, Guthrie KA, Liu Y, Coveler AL,
Higgins DM, Childs JS, Dang Y and Salazar LG: Safety and outcomes
of a plasmid DNA vaccine encoding the ERBB2 intracellular domain in
patients with advanced-stage ERBB2-positive breast cancer: A phase
1 nonrandomized clinical trial. JAMA Oncol. 9:71–78. 2023.
|
|
156
|
Wiedermann U, Garner-Spitzer E, Chao Y,
Maglakelidze M, Bulat I, Dechaphunkul A, Arpornwirat W, Charoentum
C, Yen CJ, Yau TC, et al: Clinical and Immunologic responses to a
B-cell epitope vaccine in patients with HER2/neu-overexpressing
advanced gastric cancer-results from phase Ib trial IMU. ACS.001.
Clin Cancer Res. 27:3649–3660. 2021.
|
|
157
|
Bang K, Cheon J, Park YS, Kim HD, Ryu MH,
Park Y, Moon M, Lee H and Kang YK: Association between HER2
heterogeneity and clinical outcomes of HER2-positive gastric cancer
patients treated with trastuzumab. Gastric Cancer. 25:794–803.
2022.
|
|
158
|
Paterson AL, Shannon NB, Lao-Sirieix P,
Ong CA, Peters CJ, O'Donovan M and Fitzgerald RC: A systematic
approach to therapeutic target selection in oesophago-gastric
cancer. Gut. 62:1415–1424. 2013.
|
|
159
|
Kronig M, Wehrli M, Salas-Benito D and
Maus MV: Hurdles race for CAR T-cell therapy in digestive tract
cancer. Immunol Rev. 320:100–119. 2023.
|
|
160
|
Najafi S and Mortezaee K: Modifying CAR-T
cells with anti-checkpoints in cancer immunotherapy: A focus on
anti PD-1/PD-L1 antibodies. Life Sci. 338:1223872024.
|
|
161
|
Kaczmarek M, Poznanska J, Fechner F,
Michalska N, Paszkowska S, Napierala A and Mackiewicz A: Cancer
vaccine therapeutics: Limitations and effectiveness-a literature
review. Cells-Basel. 12:21592023.
|