Introduction
The liver, the largest internal organ in humans,
plays a vital role in the organism's physical function (1,2).
Currently, chemotherapy remains the primary treatment option for
liver cancer (3). However, the
benefits of chemotherapy are highly limited in patients with tumor
metastasis, tumor recurrence, multi-drug resistance or toxic side
effects (4).
Immunosuppressive immune cells assemble in the liver
cancer microenvironment and are associated with a poor prognosis.
Immunogenic cell death (ICD) has provoked extensive interest in the
field of cancer immunotherapy, and several clinical studies have
shown that some chemotherapy agents induce ICD (5). Doxorubicin (DOX), is a
broad-spectrum antineoplastic agent that induces ICD (6-9).
DOX has been confirmed to effectively inhibit the topoisomerase
IIα-mediated DNA replication by the intercalation into nuclear DNA
strands in cancer cells (10,11). ICD is characteristic of
danger-associated molecular patterns (DAMP), such as exposure to
calreticulin (CRT) on the cell surface, the release of
high-mobility group box 1 (HMGB1) and secretion of ATP, which
recruits innate immune cells such as dendritic cells (DCs), and
then triggers tumor specific immune responses such as cytotoxic T
lymphocytes (CTLs) to eliminate residual cancer cells (12,13). However, ICD is typically limited
by the intrinsic immunosuppressive tumor microenvironment (TME)
(14), including regulatory T
(Treg) cells, myeloid-derived suppressor cells (MDSCs) and
tumor-associated macrophages (TAMs). These immunosuppressive cells
in the TME directly or indirectly suppress effector cells by
inhibiting DCs differentiation, migration and antigen presentation
(15). The degree of functional
impairment of CTLs and other immunocompetent cells is closely
related to the prognosis of cancer (16). In addition, CTLs dysfunction
reduces the effect of ICD.
However, studies have revealed that DOX alone cannot
induce sufficient ICD to initiate a satisfactory anticancer immune
response by itself (17,18). Therefore, the combination of
Chinese herbs and DOX to enhance the effect of ICD has become a
research focus. A previous study demonstrated that the combination
of low-dose icaritin and DOX exhibited a synergistic effect on ICD
induction (19). Wu et al
(18) found that ginsenoside Rg3
nanoparticles strengthened the DOX-induced ICD effect. These
studies indicated that it is feasible to enhance the ICD effect by
combining traditional Chinese medicine with DOX.
Cucurbitacin IIa (CUIIa) is a biologically active
tetracyclic triterpenoid found in Cucurbitaceae. CUIIa has
attracted considerable attention because of its anti-inflammatory
and antiviral properties (20,21). Although the anticancer mechanisms
of several cucurbitacins have been elucidated, the anticancer
activity is rarely been reported. Studies have demonstrated that
CUIIa induces cell cycle arrest, and inhibits the proliferation and
migration of tumor cells in prostate, lung and liver cancer
(22-25). CUIIa was found to induce
caspase-3-dependent apoptosis, whereas ICD was caspase-dependent.
However, whether CUIIa regulates the ICD requires further
investigation.
In the present study, it was demonstrated that the
combination of CUIIa and DOX activated ICD biomarkers in liver
cancer, and induced an effective immune response. These findings
provided a promising approach to assist tumor chemoimmunotherapy
against liver cancer.
Materials and methods
Cells and reagents
The human liver cancer cell lines HepG2 and Hep3B
cells and the mouse liver cancer cell line H22 cells were cultured
in DMEM medium containing 10% v/v FBS (Gibco; Thermo Fisher
Scientific, Inc.), as well as 100 mg/ml of streptomycin and 100
units/ml penicillin at 37°C in a humidified environment with 5%
CO2 supply. DOX hydrochloride was obtained from Zhejiang
Hisun pharmaceutical Co., Ltd. CUIIa (cat. no. HAO62805198) was
purchased from Baoji Herbest Bio-Tech Co., Ltd.
4′,6-diamidino-2-phenylindole (DAPI) was obtained from Shanghai
Aladdin Biochemical Technology Co., Ltd. CD31 (cat. no. ab28364),
Ki-67 (cat. no. ab15580), CRT (cat. no. ab92516), Caspase-3 (cat.
no. ab184787) and HMGB1 (cat. no. ab79823) antibodies were all
obtained from Abcam.
MTT assay
The in vitro cytotoxicity of CUIIa and DOX
was determined using MTT assay. HepG2 and Hep3B cells
(1×104 per well) were seeded within 96-well plates,
respectively. Subsequently, CUIIa and DOX (concentration=0.6, 1.8,
5.4, 16.2 and 48.6 μM) was added to cells for 24-h
incubation. Cells were then added with MTT reagent (5 mg/ml in PBS)
at 37°C for 4 h, and the purple precipitate was dissolved by DMSO
(200 μl) before measurement at 570 nm. IC50 was
calculated using the GraphPad Prism software.
Colony formation
Colony formation assay was initiated by seeding
cells in 6-well plates. HepG2 and Hep3B cells (2,000 cells per
well) were seeded within 6-well plates for 6 days. The colony is
defined to consist of at least 50 cells. Then, the colony-forming
cells were treated with drugs at indicated concentration for
another 4 days. At the end, the cells were fixed with 4%
paraformaldehyde for 30 min and stained with 0.1% crystal violet
for 20 min at room temperature. The number of colonies was
quantified by ImageJ software (version 1.53f; National Institutes
of Health).
Apoptosis detection and cell cycle
analysis
HepG2 and Hep3B cells (2×105 per well)
were seeded within 6-well plates and treated with drugs at
indicated concentrations for 24 h. The apoptotic ratio was
determined by flow cytometry using Annexin V Apoptosis Detection
kit (cat. no. AT101C), which was obtained from MultiSciences
Biotech Co., Ltd. The cell cycle was determined by flow cytometry
using the cell cycle analysis kit. Experimental data were analyzed
by FlowJo and GraphPad Prism software.
Immunofluorescence staining
HepG2 cells (5×104 per well) were seeded
within 24-well plates and treated with drugs at indicated
concentrations for 24 h. Afterwards, cells were fixed with 4%
paraformaldehyde for 20 min, and washed three times in PBS. The
cells were incubated in blocking buffer [1% (w/v) BSA (Gibco;
Thermo Fisher Scientific, Inc.) in PBS] for 30 min and subsequently
incubated overnight at 4°C with primary antibodies (HMGB1 or CRT)
diluted (1:1,500) in the blocking buffer. The sample was washed
three times in PBS and incubated overnight at 4°C with secondary
antibodies in the blocking buffer. The nuclei were counterstained
with DAPI (5 μg/ml).
Hoechst 33342 staining
The apoptosis detection was evaluated by Hoechst
33342 staining assay kit (cat. No. P0133), which was obtained from
Beyotime Institute of Biotechnology. After 24 h incubation with DOX
or/and CUIIa, cells were further stained with Hoechst 33342 for 15
min at 37°C. Then the stained cells were observed under a
fluorescence microscope.
ATP release assay
After 24 h incubation with DOX or/and CUIIa,
extracellular ATP level of HepG2 cells was detected with an ATP
Assay Kit (cat. no. S0026; Beyotime Institute of Biotechnology) by
employing firefly luciferase-catalyzed oxidation of D-luciferin to
produce light in the presence of ATP. The fluorescence was detected
with the multiscan spectrum (Synergy H1; BioTek Instruments,
Inc.).
Animal study
In total, 45 specific pathogen-free male ICR mice
(age, 4 weeks-old; weight, 18-22 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Mice were
housed in a specific pathogen-free environment at a constant
temperature of 22±1°C and 55±5% humidity, and provided with
standard laboratory diet and drinking water ad libitum in a 12/12-h
dark/light cycle. The mouse liver cancer cell line H22 cells were
cultured in DMEM medium, and 1×107 cells in PBS (500
μl) were injected into the abdominal cavity of each mouse.
After seven days, the mice were sacrificed and ascites were
collected, then diluted with PBS, and subcutaneously injected into
the right flank of mice. After three days, the mice were then
randomly divided into 4 groups, including the control group (0.9%
NaCl solution) and CUIIa groups (30, 60 and 90 mg/kg). Mice in
CUIIa groups were gavaged once a day, and all the mice were
sacrificed at day 12. For the anticancer study of CUIIa and DOX
combination, mice were then randomly divided into 4 groups,
including the control group, CUIIa group (90 mg/kg), DOX group (3
mg/kg) and the combined group. DOX was injected intravenously once
every 3 days. Mice in CUIIa groups were gavaged once a day, and all
the mice were sacrificed at day 12. At experimental endpoint, all
mice were euthanized by intravenous injection of pentobarbital
sodium.
Hematoxylin-eosin staining (H&E) and
immunohistochemical staining
All tumor tissues were fixed in 10% neutral formalin
for 48 h at room temperature, embedded in paraffin wax, and cut
into 4-μm thick serial sections. Tissue slices (heart,
liver, spleen, lung and kidney) were stained with H&E to assess
the toxicity of drugs. And tumor tissues were stained
immunohistochemically with primary anti-Ki-67 (1:1,000), Caspase-3
(1:1,500) and CD31 (1:2,000) antibodies according to the
manufacturers' instructions. In details, tissue sections were
deparaffinized in xylene and ethanol series (anhydrous ethanol, 95%
alcohol, 95% alcohol, and 80% alcohol) and subjected to antigen
retrieval in citrate buffer. The sections were incubated with
primary antibodies overnight at 4°C. The secondary antibody against
HRP-conjugated Goat Anti-Rabbit IgG (1:1,000) was obtained from
Abcam (cat. no. ab6721) and incubated at room temperature for 50
min. The sections were further stained with DAB and hematoxylin.
The expression of Ki67, Caspase-3 and CD31 was observed under a
light microscope (Nikon Corporation).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of TNF-α, IFN-γ, IL-12, IL-4, IL-10 and
CCL2 in the homogenate supernatant of tumor tissue were evaluated
by ELISA kits according to the manufacturer's instructions. TNF-α
(cat. no. E-MSEL-M0002), IFN-γ (cat. no. E-MSEL-M0007), IL-12 (cat.
no. E-MSEL-M0004), IL-4 (cat. no. E-MSEL-M0008), IL-10 (cat. no.
E-MSEL-M0031) and CCL2 (cat. no. E-EL-M3001) ELISA kits were
purchased from Elabscience Biotechnology Co. Ltd.
Evaluation of immune cells in mice
bearing H22 xenografts by flow cytometry
Tumor-draining lymph nodes and spleens from mice
bearing H22 tumor were harvested and processed into single cell
suspensions through a 200-mesh sterile filter. Spleen was then
treated with ACK lysis buffer to remove red blood cells. Anti-mouse
CD16/32 Fc receptor block antibody (BioLegend, Inc.) were used to
block the non-specific antibody binding. To evaluate the antigen
presentation ability of DCs cells, single cell suspensions were
stained with Fixable Viability Dye eFluor 780 (eBioscience; Thermo
Fisher Scientific, Inc.), anti-CD11c-APC, anti-CD80-PE,
anti-CD86-BV421 and anti-MHCII-FITC antibodies or an isotype IgG
control (BioLegend, Inc.) according to the manufacturers'
instruction. Samples were collected on BD FACS Verse Flow Cytometer
(BD Biosciences), and data were analyzed using FlowJo V.10.1
software (Tree Star, Inc.).
To assess the abundance of CTLs
(CD4−CD8+IFN-γ+) and TH1 cells
(CD4+CD8−IFN-γ+), single cell
suspensions were stained with Fixable Viability Dye eFluor™ 780
(eBioscience; Thermo Fisher Scientific, Inc.), anti-CD3-PE cy7,
anti-CD4-APC, anti-CD8-FITC, and anti-IFN-γ-PE antibodies
(BioLegend, Inc.). For intracellular cytokine staining,
1×106 cells were stimulated with complete RPMI-1640
containing activation cocktail and brefeldin A for 6 h (BioLegend,
Inc.). For analysis of Treg cells, the antibodies used included
APC-labeled anti-mouse CD4 antibody, PE cy7-labeled anti-mouse CD3
antibody and PE-labeled anti-mouse Foxp3 antibody (18).
For analysis of M1/M2 macrophages, the acquired
suspended cells were stained with anti-Gr1-PE, anti-F4/80-APC,
anti-MHCII-FITC and anti-CD206-PE-Cy7 antibodies (BioLegend, Inc.).
Cells were stained with anti-CD11b-APC and anti-Gr-1-PE antibodies
(BioLegend, Inc.) to examine the ratio of MDSCs. Moreover, cells
were stained with anti-CD11b-FITC, anti-Ly6G-PE, anti-Ly6C-Cy5
antibodies (BioLegend, Inc.) to detect the proportion of
M-MDSCs.
Statistical analyses
All experiments were performed at least three times.
All data were analyzed using GraphPad Prism 8.0 (Dotmatics) by
unpaired Student's t-test and one-way analysis of variance (ANOVA)
with Bonferroni correction. Data were presented as the mean ± SD.
*P<0.05 was considered to indicate a statistically
significant difference.
Results
CUIIa suppresses liver cancer tumor
growth
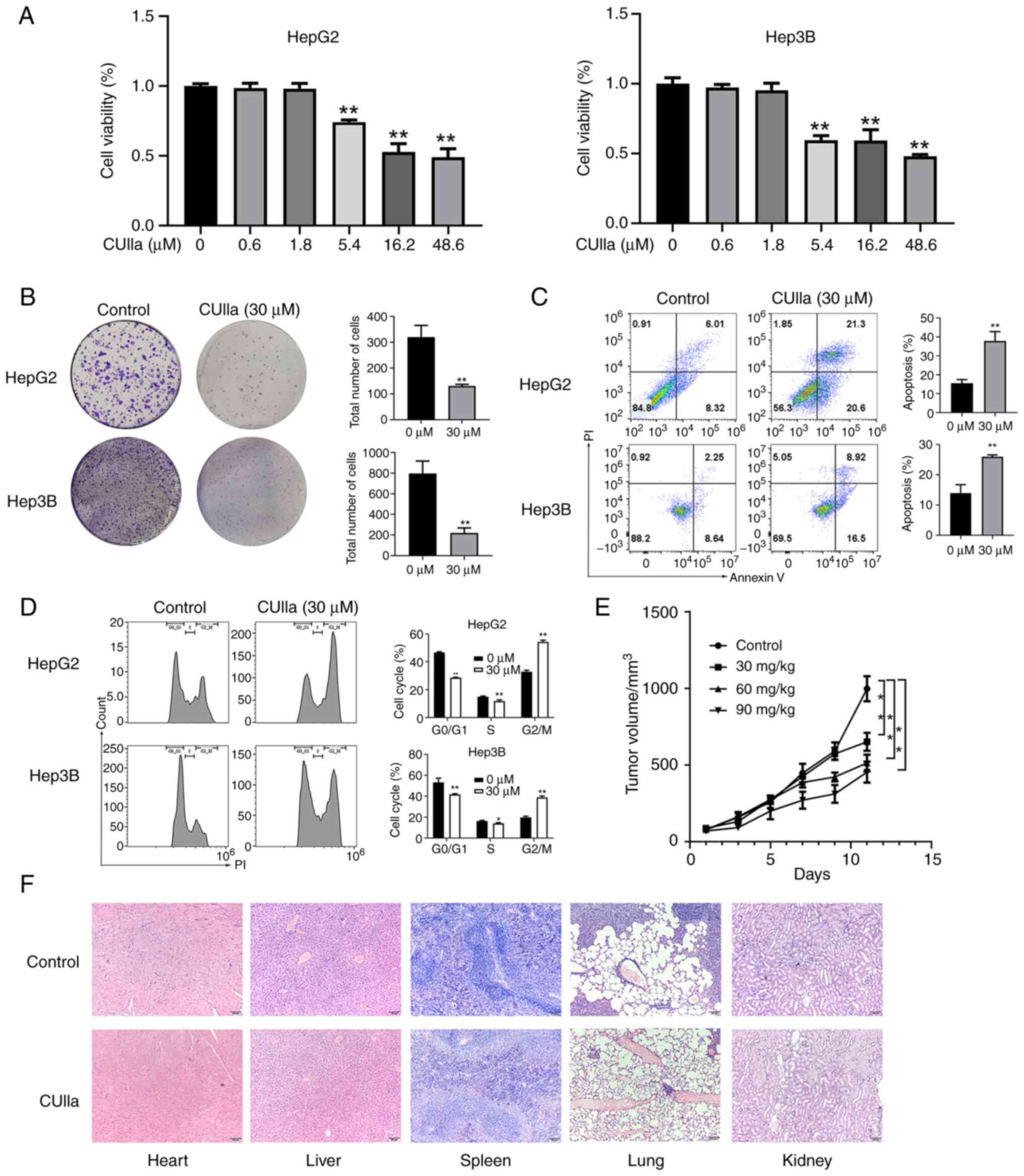
The MTT assay was used to evaluate the inhibitory
effect of CUIIa on the proliferation of liver cancer cells. The
results revealed that the inhibitory potential of CUIIa depended on
its concentration, and a more powerful effect was achieved by CUIIa
at a higher concentration. IC50 of CUIIa on HepG2 and
Hep3B cells were 31.5 and 28.1 μM, respectively (Fig. 1A). Therefore, the concentration of
30 μM was selected for subsequent experiments. CUIIa almost
blocked the colony formation in both HepG2 and Hep3B cells
(Fig. 1B). As demonstrated in
Fig. 1C, CUIIa clearly promoted
the apoptosis in both HepG2 and Hep3B cells, and the apoptotic rate
increased to 41.9 and 26.00%, respectively. Cell cycle analysis by
flow cytometry revealed that CUIIa significantly decreased the
percentage of cells in the G0/G1 phase and S phases and increased
the percentage of cells in the G2/M phase (Fig. 1D). These results suggested that
CUIIa might inhibit liver cancer cells growth by maintaining cells
in the G2/M phase.
Next, the anticancer effect of CUIIa on H22
cells-bearing mice was examined. Most mice in the control group
were in poor health, and became lethargic, listless and indulged in
sleep. Fur withered as tumor size increased. The tumor growth
curves of H22 xenografts are depicted in Fig. 1E. The average volume was 651.53
mm3 in CUIIa low-dose group and 513.66 mm3 in
CUIIa medium-dose group. The average tumor volume in the CUIIa
high-dose group was 453.76 mm3, significantly smaller
than that of the control group (998.02 mm3) at day 11.
In addition, no pathological changes were observed in the major
organs (heart, liver, spleen, lungs and kidneys) of the control and
high-dose CUIIa groups by H&E staining (Fig. 1F).
CUIIa promotes the anticancer effect of
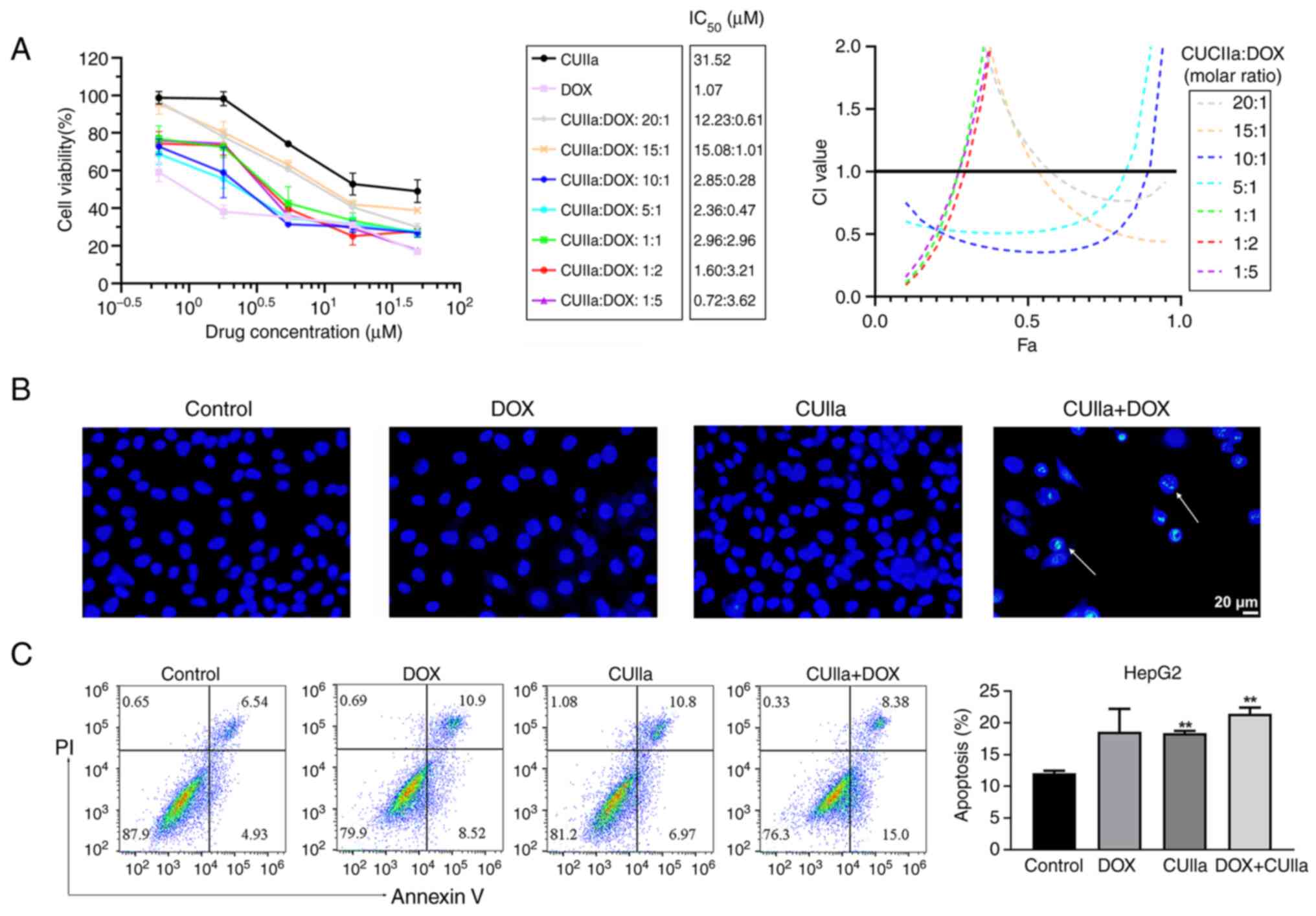
DOX in vitro
Dox is widely used in liver cancer chemotherapy
treatment. However, DOX-induced cardiotoxicity greatly limits its
clinical therapeutic utility (26). In the present study, it was
examined whether CUIIa strengthened the anticancer effect of DOX
and lowered cardiotoxicity. As revealed in Fig. 2A, CUIIa promoted DOX cytotoxicity
in HepG2 cells. The combination index (CI) was used to evaluate the
combined effect of DOX plus CUIIa (27), namely, synergistic (CI<1),
additive (CI=1) or antagonistic (CI>1) effects (28). The IC50 of DOX
monotherapy was 1.1 μM for HepG2 cells, while the
IC50 of CUIIa monotherapy was 31.5 μM. When used
in combination, the inhibition rate reached 50% in CUIIa (2.85
μM) plus DOX (0.28 μM) group (10:1 ratio), and
meanwhile the calculated CI was equal to 0.36 (Fig. 2A). Low doses with a pleasant
treatment efficacy shall reduce the side effects of DOX. Therefore,
the concentration of CUIIa (2.86 μM) plus DOX (0.29
μM) was chosen for the following in vitro study.
Hoechst staining and flow cytometry verified that
this combination induced apoptosis in HepG2 cells. As demonstrated
in Fig. 2B, the majority of
nuclei in the control, CUIIa and DOX groups exhibited round and
uniform light blue fluorescence, whereas apoptotic nuclear staining
in the combination group was enhanced, and the fluorescence was
brighter. The structures were either condensed or clumpy. Moreover,
the number of cells in the field of vision was significantly
reduced in the combination group. Accordingly, FITC-annexin V-PI
flow cytometry identified a significantly higher rate of apoptosis
in the combination group compared with the control group (Fig. 2C).
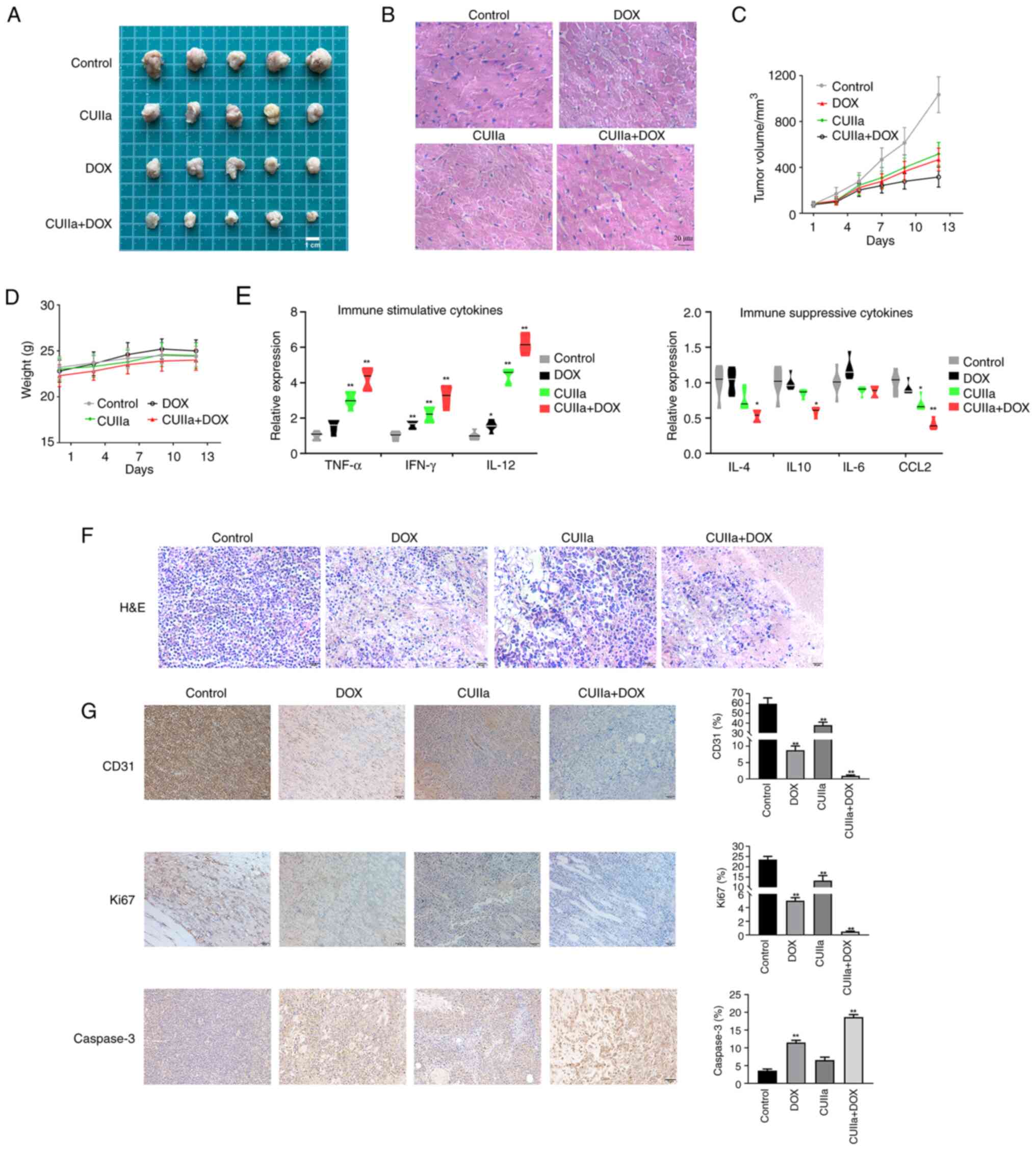
Combination of CUIIa and DOX displays
potent inhibitory effect on H22 tumor growth
After confirming the anticancer effect of the
combination in vitro, it was further examined the anticancer
effect in vivo. A significant reduction in the tumor volume
was observed after the application of combination therapy to mice
with subcutaneous tumors in vivo (Fig. 3A and C). H&E staining revealed
necrosis, vacuolar cardiomyocyte and inflammatory cells in
DOX-treated tumor-bearing mice, consistent with a previous study
(29). After the combinatory
treatment, the pathological status of heart was improved obviously
(Fig. 3B). Therefore, CUIIa
ameliorated DOX-induced myocardial toxicity. No body weight loss
was observed in any of the treated groups (Fig. 3D). The combination therapy
significantly promoted the level of immuno-stimulatory cytokines
(TNF-α, IFN-γ and IL-12) and inhibited the secretion of the
immunosuppressive cytokines (IL-4, IL-10 and CCL2) in tumor tissues
(Fig. 3E). H&E staining was
also used to observe the morphology of the tumor tissue in each
group and evaluate the therapeutic effect of the combinatory
treatment (Fig. 3F). The tumor
cells in the control group were densely arranged with a high
nuclear-to-cytoplasmic ratio. In both DOX group and CUIIa group,
cells and nuclei were more irregular in shape with a low nuclear to
cytoplasmic ratio. The combination group had the lowest
nuclear-to-cytoplasmic ratio among the four groups. The cells were
thinly arranged and the cytoplasm was broken and dissolved. To
further investigate the proliferation and apoptosis of tumor cells,
the expression of the proliferation marker Ki67, the apoptosis
marker Caspase-3 and the endovascular epithelial marker CD31 in
xenograft tumors was evaluated by immunohistochemistry (Fig. 3G). The results revealed that
combination therapy inhibited tumor growth as well as the
expression of Ki67 and CD31 in H22 mice, and meanwhile promoted the
expression of Caspase-3.
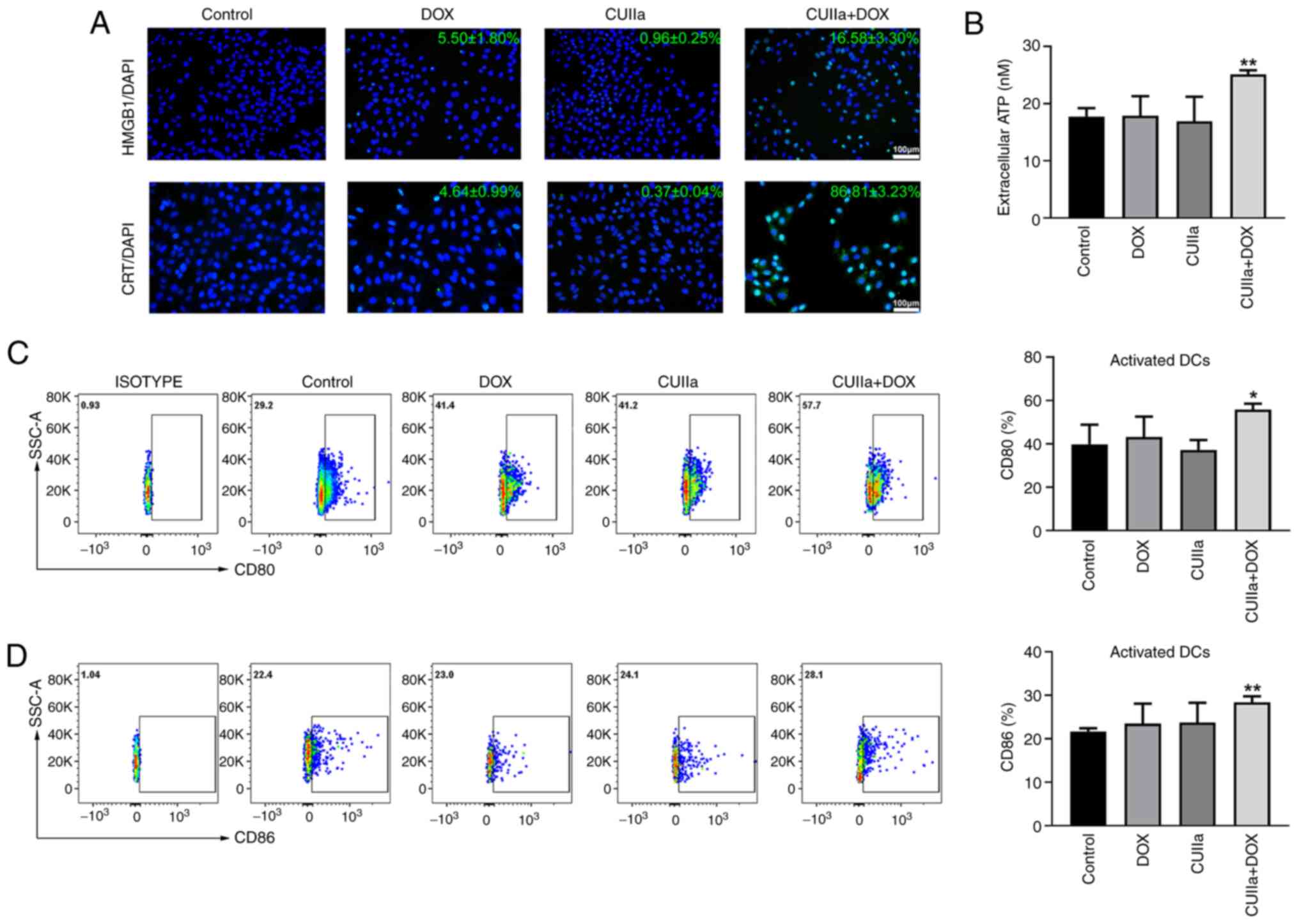
CUIIa strengthens the ICD induced by
DOX
Exposure of CRT on the cell membrane as well as the
release of ATP and HMGB1 into the extracellular compartment occur
during ICD (30).
Immunofluorescence staining demonstrated that the combination of
CUIIa and DOX induced a strong ICD response in HepG2 cells, as
evidenced by enhanced CRT exposure and HMGB1 release from tumor
cells, which are key biomarkers of ICD (Fig. 4A). In addition, the combination of
CUIIa and DOX significantly promoted the secretion of ATP into the
extracellular compartment of HepG2 cells (Fig. 4B).
DCs play a key role in initiating immune responses
and maintaining immune tolerance (31). Activation of DCs is an important
part of ICD. Flow cytometry showed a significant increase in the
levels of the costimulatory molecules CD80 and CD86 on the surface
of DCs in the spleen and lymph nodes of H22 mice subjected to the
combined treatment (Fig. 4C and
D). These results suggested that the combinatory treatment
stimulated the maturation of DCs.
The combination of CUIIa and DOX remolds
the immune microenvironment
To further explore whether the combination
facilitated an immunoregulatory effect, the ratio of immune cells
in the spleen and draining lymph nodes was measured by flow
cytometry. Research has shown that CD8+ T cells can be
specifically activated to become tumor-specific cytotoxic
lymphocytes that generate an antitumor response (32). T helper 1 cells (TH1) are the
subpopulation of CD4+ T helper cells that improve the
immune response and enhance antitumor effects and other roles
primarily through the secretion of cytokines (33,34). Although no statistical
significance was observed, the percentage of splenic TH1
(IFN-γ+CD4+ T) cells was increased in the
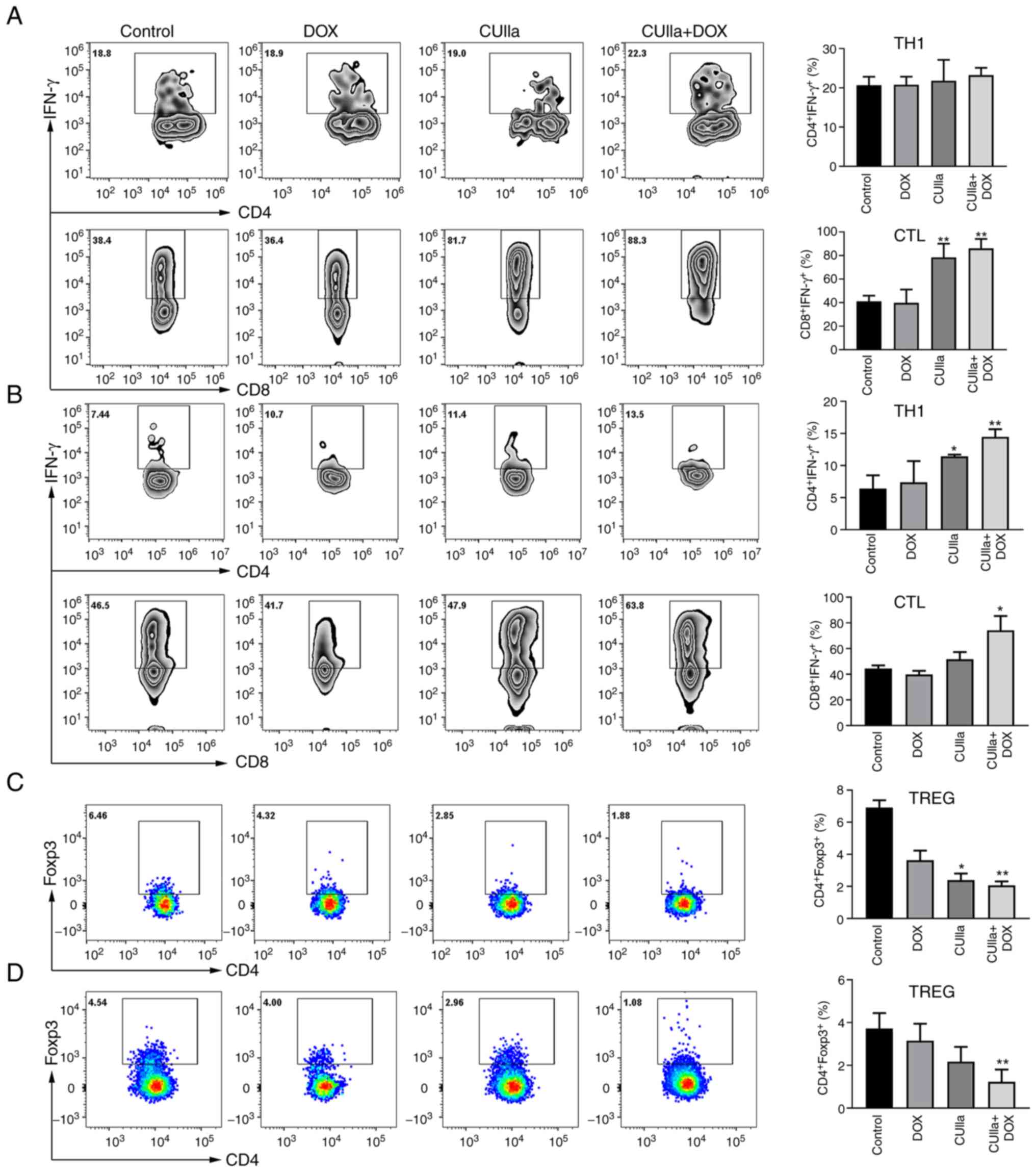
combination group, and exhibited the highest proportion (Fig. 5A). Both CUIIa and DOX monotherapy
presented partial effect on proportion of TH1 and CTLs cells. The
proportion of CTLs (IFN-γ+CD8+ T) cells in
the spleen was significantly increased in the combined group
compared with the control group. The combination treatment also
increased the numbers of TH1 and CTLs cells in the draining lymph
nodes (Fig. 5B). Furthermore, the
combination therapy also considerably decreased the frequency of
Treg cells in the spleen (Fig.
5C) and draining lymph nodes (Fig. 5D) of H22 mice.
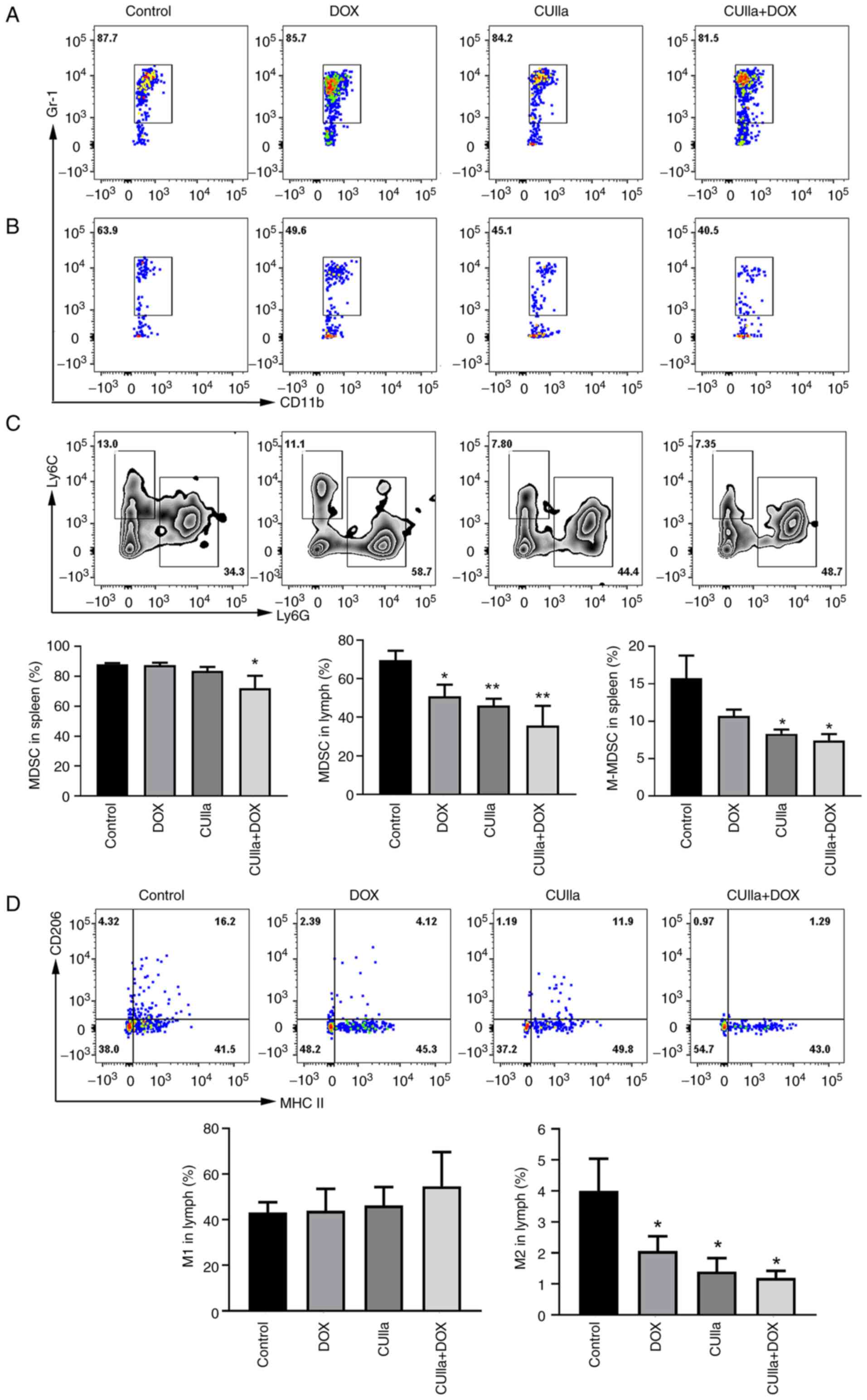
MDSCs and M2-polarized macrophages play a central
role in tumor immune evasion and tumor metastasis. Moreover,
increased numbers of MDSCs and M2 macrophages are positively
associated with poor prognosis and reduced survival in cancer
patients (35,36). The combination therapy
significantly decreased the frequencies of MDSCs in both the spleen
and lymph nodes (Fig. 6A and B).
In detail, there was no significant change for M-MDSCs ratio
between the DOX group and the control group in the spleen; however,
M-MDSCs decreased significantly after the combination treatment
(Fig. 6C). In the lymph nodes,
single CUIIa treatment, as well as in combination with DOX, all
increased the proportion of M1 macrophages and decreased the
proportion of M2 polarized macrophages (Fig. 6D).
Discussion
The ICD in tumor cells is expected to provide new
opportunities for immunotherapy. These ICD-inducing chemotherapy
drugs can function both via the chemotherapy role and via
ICD-triggered cell-eliminating immune responses, thus attaining
more pleasant curative effects (3,10).
However, the immunosuppressive TME and feeble antigen presentation
capacity has greatly limited DOX-stimulated immune responses. In
the present study, it was investigated whether CUIIa and DOX
combination therapy would provoke a stronger ICD effect and reshape
the immune microenvironment in liver cancer. Insights were also
provided into the anticancer effects of the combination on liver
cancer growth and the underlying mechanisms were explored.
The induction of apoptosis and autophagy, and cell
cycle arrest have been reported to be involved in the anticancer
mechanism of CUIIa (20,23,24). Consistently, CUIIa significantly
inhibited the viability and colony formation of liver cancer cells
in the present study. CUIIa also promoted the apoptosis of HepG2
and Hep3B cells and induced cell cycle arrest at the G2/M phase.
Moreover, CUIIa considerably repressed tumor growth in H22 mice,
without causing obvious damage to the major organs. Thus, CUIIa is
a promising drug for the treatment of liver cancer. It is
noteworthy that cucurbitacins displayed unique advantages when
combined with chemotherapeutic drugs. For instance, the synergistic
antitumor effects of Cucurbitacin E and Dox have been demonstrated
on gastric cancer both in vitro and in vivo (37). Cucurbitacin B enhanced the
inhibition ability of cisplatin on resistant ovarian cancer cells,
and played an important role in eliciting antitumor immunity
(38). In the present study, as
expected, the IC50 of DOX on HepG2 cells was
significantly decreased after the co-administration with CUIIa.
Particularly, the strongest antitumor effect was attained when the
molar ratio of CUIIa to DOX was 10:1. Hoechst staining and flow
cytometric analysis also verified that CUIIa promoted DOX-induced
apoptosis in HepG2 cells. Mice bearing H22 subcutaneous xenograft
were used to evaluate the combined effect of CUIIa and DOX in
vivo. The combination group significantly inhibited tumor
growth and the expression of Ki67 and CD31 in mice. More
importantly, DOX-induced myocardial toxicity was alleviated after
combinatory treatment. Therefore, the combined administration can
bring down the dosage of DOX chemotherapy while simultaneously
ensuring an anticancer effect at the same time, inferring that
CUIIa might potentially function as a chemotherapy adjuvant in
treating liver cancer.
Notably, DOX can also stimulate ICD and thus
triggering an immune response, although the immunogenicity induced
by DOX is not strong enough to eliminate cancer cells. It was found
that CUIIa promoted DOX-induced apoptosis and that ICD was
caspase-dependent. The upregulation of various DAMPs can serve as
markers of ICD occurrence. Therefore, expression of DAMPs was next
detected to examine whether the combination of CUIIa and DOX could
reinforce ICD. During the ICD process, CRT is exposed on the
membrane of dying cells, which is considered as an 'Eat Me' signal,
attracting and activating DCs (39). HMGB1 is released from the nucleus
during the late stages of apoptosis, promoting chemotaxis of DCs
and antigen presentation to T cells (40,41). ATP, which acts as a find-me
signal, induces migration of DCs to tumor cells (42). In the present study, the
combination of CUIIa and DOX induced ICD with the upregulation of
various DAMPs, indicating that when combined with CUIIa, DOX
provokes a satisfactory ICD effect, even at a low dose.
In the liver cancer microenvironment, the
inflammatory cell infiltrate is unbalanced towards an
immunosuppressive phenotype, with a prevalence of Tregs, regulatory
B cells (Bregs), M2 macrophages and MDSCs, over M1 macrophages,
DCs, TH1 and CTLs. DAMPs excreted by dying cells can initiate an
immune response, followed by the maturation of the
antigen-presenting DCs. However, the immunosuppressive TME hinders
the anticancer immune response triggered by DCs (43). MDSCs, Tregs and tumor cells
secrete suppressive cytokines, that can inhibit CTLs (44,45). Bregs were shown to facilitate
liver cancer progression by promoting IL-10 and TGF-β secretion.
Bregs cells also inhibit T cell antitumor immune response by
converting naive CD4+ T cells to Treg cells in the TME
(46). Accumulation of monocytic
MDSCs (M-MDSCs) in fibrotic livers is meaningfully associated with
abridged tumor-infiltrating lymphocytes and amplified
tumorigenicity in mice (47).
Hormone divergences play an important part in modifying the TME. In
the future precision therapies, gender-specific medicine will
become a significant modality. As male-female differences might
affect the TME (48), only male
mice were chosen for the present study. It was found that CUIIa
combined with DOX improved the immune microenvironment of mice
bearing H22 tumor. Immuno-surveillance cells including TH1, CTLs,
M1 macrophages and activated DCs, increased, whereas the number of
immunosuppressive cells, including M2 macrophages, Tregs, MDSCs and
M-MDSCs, declined. Concerning the important role of Bregs in liver
cancer progression, the role of Bregs in DOX-induced ICD will be
evaluated in the authors' future studies.
CUIIa has been reported to increase levels of LC3-II
conjugates and formation of LC3 puncta of RAW 264.7 cells,
suggesting that autophagy can be triggered by CUIIa (49). Autophagy was regarded as an
inducer of ICD. DAMPs released by dying cells including autophagic
cells bind to the receptor on phagocytic cells and subsequently
trigger an immune response (19).
It was assumed that CUIIa enhanced DOX-induced ICD via triggering
autophagy, and it was found that CUIIa promoted LC3 expression in
HCC cells (data not shown). To verify the hypothesis and explore
the specific mechanism by which CUIIa enhances DOX-induced ICD,
more profound work needs be performed in the future such as
colocalization of autophagosome and mitochondria, and expression of
mitophagy biomarkers. Moreover, Bafilomycin A1, an autophagy
inhibitor, will be used to verify the association of autophagy and
ATP release.
In conclusion, the present findings demonstrated the
capability of CUIIa to potentiate the anticancer effect of DOX in
liver cancer, probably by inducing apoptosis and ICD, as well as by
reprogramming the immune microenvironment. This suggested the
feasibility and safety of using CUIIa as an adjuvant drug for DOX
in liver cancer therapy to improve therapy responsiveness, reduce
unwanted cardiotoxicity, and overcome the adverse effects of the
TME.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJL and SW carried out the experiment and wrote the
first draft of the manuscript. SJL, GLW and SW designed the
experiment. LXL, JS, APZ and GLW analyzed the data. ZJF interpreted
the data and revised the manuscript. ZJF and GLW gave the final
approval and supervised the project. All authors read and approved
the final manuscript. SJL and GLW confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
All mice experiments were approved by the
Institutional Animal Care and Use Committee of the Jiangsu Academy
of Chinese Medicine (approval no. AEWC-20220505-203; Nanjing,
China). All animal experiments complied with the ARRIVE guidelines,
the U.K. Animals (Scientific Procedures) Act, 1986, as well as the
National Research Council's Guide for the Care and Use of
Laboratory Animals. Great effort was made to decrease the pain and
number of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CI
|
combination index
|
|
CRT
|
calreticulin
|
|
CTLs
|
cytotoxic T lymphocytes
|
|
CUIIa
|
cucurbitacin IIa
|
|
DCs
|
dendritic cells
|
|
DOX
|
doxorubicin
|
|
HMGB1
|
high-mobility group box 1
|
|
ICD
|
immunogenic cell death
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
TAM
|
tumor-associated macrophages
|
|
TH1
|
T helper 1 cells
|
|
Treg
|
regulatory T cells
|
|
TME
|
tumor microenvironment
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81873055), the Jiangsu Clinical
Innovation Center of Digestive Cancer of Traditional Chinese
Medicine (grant no. 2021.6), the Jiangsu Traditional Chinese
Medicine Development Plan Project (grant no. MS2023033) and the
Jiangsu Provincial Association for Maternal and Child Health
Studies (grant no. JSFY202202).
References
|
1
|
Huang J, Hao P, Zhang YL, Deng FX, Deng Q,
Hong Y, Wang XW, Wang Y, Li TT, Zhang XG, et al: Discovering
multiple transcripts of human hepatocytes using massively parallel
signature sequencing (MPSS). BMC Genomics. 8:2072007.
|
|
2
|
Plaz Torres MC, Bodini G, Furnari M,
Marabotto E, Zentilin P, Strazzabosco M and Giannini EG:
Surveillance for hepatocellular carcinoma in patients with
non-alcoholic fatty liver disease: universal or selective? Cancers
(Basel). 12:14222020.
|
|
3
|
He T, Wang L, Gou S, Lu L, Liu G, Wang K,
Yang Y, Duan Q, Geng W, Zhao P, et al: Enhanced immunogenic cell
death and antigen presentation via engineered bifidobacterium
bifidum to boost chemo-immunotherapy. ACS Nano. 17:9953–9971.
2023.
|
|
4
|
Lu Q, Huang H, Wang X, Luo L, Xia H, Zhang
L, Xu J, Huang Y, Luo X and Luo J: Echinatin inhibits the growth
and metastasis of human osteosarcoma cells through Wnt/β-catenin
and p38 signaling pathways. Pharmacol Res. 191:1067602023.
|
|
5
|
Vanmeerbeek I, Sprooten J, De Ruysscher D,
Tejpar S, Vandenberghe P, Fucikova J, Spisek R, Zitvogel L, Kroemer
G, Galluzzi L, et al: Trial watch: Chemotherapy-induced immunogenic
cell death in immuno-oncology. Oncoimmunology. 9:17034492020.
|
|
6
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007.
|
|
7
|
Green DR, Ferguson T, Zitvogel L and
Kroemer G: Immunogenic and tolerogenic cell death. Nat Rev Immunol.
9:353–363. 2009.
|
|
8
|
Casares N, Pequignot MO, Tesniere A,
Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs
S, Obeid M, et al: Caspase-dependent immunogenicity of
doxorubicin-induced tumor cell death. J Exp Med. 202:1691–1701.
2005.
|
|
9
|
Birmpilis AI, Paschalis A, Mourkakis A,
Christodoulou P, Kostopoulos IV, Antimissari E, Terzoudi G,
Georgakilas AG, Armpilia C, Papageorgis P, et al: Immunogenic cell
death, DAMPs and prothymosin α as a putative anticancer immune
response biomarker. Cells. 11:14152022.
|
|
10
|
Zhai J, Gu X, Liu Y, Hu Y, Jiang Y and
Zhang Z: Chemotherapeutic and targeted drugs-induced immunogenic
cell death in cancer models and antitumor therapy: An update
review. Front Pharmacol. 14:11529342023.
|
|
11
|
Shi Y, Hou X, Yu S, Pan X, Yang M, Hu J
and Wang X: Targeted delivery of doxorubicin into tumor cells to
decrease the in vivo toxicity of glutathione-sensitive
prodrug-poloxamer188-b-polycaprolactone nanoparticles and improve
their anti-tumor activities. Colloids Surf B Biointerfaces.
220:1128742022.
|
|
12
|
Wu PJ, Chiou HL, Hsieh YH, Lin CL, Lee HL,
Liu IC and Ying TH: Induction of immunogenic cell death effect of
licoricidin in cervical cancer cells by enhancing endoplasmic
reticulum stress-mediated high mobility group box 1 expression.
Environ Toxicol. 38:1641–1650. 2023.
|
|
13
|
Aria H and Rezaei M: Immunogenic cell
death inducer peptides: A new approach for cancer therapy, current
status and future perspectives. Biomed Pharmacother.
161:1145032023.
|
|
14
|
Lu Y, Sun W, Du J, Fan J and Peng X:
Immuno-photodynamic therapy (IPDT): Organic photosensitizers and
their application in cancer ablation. JACS Au. 3:682–699. 2023.
|
|
15
|
Shimabukuro-Vornhagen A, Draube A, Liebig
TM, Rothe A, Kochanek M and von Bergwelt-Baildon MS: The
immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the
antigen-presenting function of CD40-activated B cells. J Exp Clin
Cancer Res. 31:472012.
|
|
16
|
Fan X, Jin J, Yan L, Liu L, Li Q and Xu Y:
The impaired anti-tumoral effect of immune surveillance cells in
the immune microenvironment of gastric cancer. Clin Immunol.
219:1085512020.
|
|
17
|
Dai Z, Tang J, Gu Z, Wang Y, Yang Y, Yang
Y and Yu C: Eliciting immunogenic cell death via a unitized
nanoinducer. Nano Lett. 20:6246–6254. 2020.
|
|
18
|
Wu H, Wei G, Luo L, Li L, Gao Y, Tan X,
Wang S, Chang H, Liu Y, Wei Y, et al: Ginsenoside Rg3 nanoparticles
with permeation enhancing based chitosan derivatives were
encapsulated with doxorubicin by thermosensitive hydrogel and
anti-cancer evaluation of peritumoral hydrogel injection combined
with PD-L1 antibody. Biomater Res. 26:772022.
|
|
19
|
Yu Z, Guo J, Hu M, Gao Y and Huang L:
Icaritin exacerbates mitophagy and synergizes with doxorubicin to
induce immunogenic cell death in hepatocellular carcinoma. ACS
Nano. 14:4816–4828. 2020.
|
|
20
|
Zeng Y, Wang J, Huang Q, Ren Y, Li T,
Zhang X, Yao R and Sun J: Cucurbitacin IIa: A review of
phytochemistry and pharmacology. Phytother Res. 35:4155–4170.
2021.
|
|
21
|
Peng Y, Chen T, Luo L, Li L, Cao W, Xu X,
Zhang Y, Yue P, Dai X, Ji Z, et al: Isoforskolin and cucurbitacin
IIa promote the expression of anti-inflammatory regulatory factor
SIGIRR in human macrophages stimulated with Borrelia burgdorferi
basic membrane protein A. Int Immunopharmacol. 88:1069142020.
|
|
22
|
Singh N, Krishnakumar S, Kanwar RK, Cheung
CH and Kanwar JR: Clinical aspects for survivin: A crucial molecule
for targeting drug-resistant cancers. Drug Discov Today.
20:578–587. 2015.
|
|
23
|
Zhang J, Song Y, Liang Y, Zou H, Zuo P,
Yan M, Jing S, Li T, Wang Y, Li D, et al: Cucurbitacin IIa
interferes with EGFR-MAPK signaling pathway leads to proliferation
inhibition in A549 cells. Food Chem Toxicol. 132:1106542019.
|
|
24
|
Boykin C, Zhang G, Chen YH, Zhang RW, Fan
XE, Yang WM and Lu Q: Cucurbitacin IIa: a novel class of
anti-cancer drug inducing non-reversible actin aggregation and
inhibiting survivin independent of JAK2/STAT3 phosphorylation. Br J
Cancer. 104:781–789. 2011.
|
|
25
|
Yu K, Yang X, Li Y, Cui X, Liu B and Yao
Q: Synthesis of cucurbitacin IIa derivatives with
apoptosis-inducing capabilities in human cancer cells. RSC Adv.
10:3872–3881. 2020.
|
|
26
|
Kuang Z, Wu J, Tan Y, Zhu G, Li J and Wu
M: MicroRNA in the diagnosis and treatment of doxorubicin-induced
cardiotoxicity. Biomolecules. 13:5682023.
|
|
27
|
Yu S, Cai X, Wu C, Liu Y, Zhang J, Gong X,
Wang X, Wu X, Zhu T, Mo L, et al: Targeting HSP90-HDAC6 regulating
network implicates precision treatment of breast cancer. Int J Biol
Sci. 13:505–517. 2017.
|
|
28
|
O'Donohue TJ, Ibáñez G, Coutinho DF,
Mauguen A, Siddiquee A, Rosales N, Calder P, Ndengu A, You D, Long
M, et al: Translational strategies for repotrectinib in
neuroblastoma. Mol Cancer Ther. 20:2189–2197. 2021.
|
|
29
|
Bhagat A and Kleinerman ES:
Anthracycline-induced cardiotoxicity: Causes, mechanisms, and
prevention. Adv Exp Med Biol. 1257:181–192. 2020.
|
|
30
|
Ma X, Yang S, Zhang T, Wang S, Yang Q,
Xiao Y, Shi X, Xue P, Kang Y, Liu G, et al: Bioresponsive
immune-booster-based prodrug nanogel for cancer immunotherapy. Acta
Pharm Sin B. 12:451–466. 2022.
|
|
31
|
Bao L, Hao C, Wang J, Wang D, Zhao Y, Li Y
and Yao W: High-dose cyclophosphamide administration orchestrates
phenotypic and functional alterations of immature dendritic cells
and regulates Th cell polarization. Front Pharmacol.
11:7752020.
|
|
32
|
Shan Z, Wang H, Zhang Y and Min W: The
role of tumor-derived exosomes in the abscopal effect and
immunotherapy. Life (Basel). 11:3812021.
|
|
33
|
Deng Z, Zhang M, Zhu T, Zhili N, Liu Z,
Xiang R, Zhang W and Xu Y: Dynamic changes in peripheral blood
lymphocyte subsets in adult patients with COVID-19. Int J Infect
Dis. 98:353–358. 2020.
|
|
34
|
Yu H, Zou W, Mi C, Wang Q, Dai G, Zhang T,
Zhang G, Xie K, Wang J and Shi H: Research Note: Expression of T
cell-related cytokines in chicken cecal and spleen tissues
following Eimeria tenella infection in vivo. Poult Sci.
100:1011612021.
|
|
35
|
Sasidharan Nair V, Saleh R, Toor SM, Taha
RZ, Ahmed AA, Kurer MA, Murshed K, Alajez NM, Abu Nada M and Elkord
E: Transcriptomic profiling disclosed the role of DNA methylation
and histone modifications in tumor-infiltrating myeloid-derived
suppressor cell subsets in colorectal cancer. Clin Epigenetics.
12:132020.
|
|
36
|
Wellenstein MD and de Visser KE:
Cancer-cell-intrinsic mechanisms shaping the tumor immune
landscape. Immunity. 48:399–416. 2018.
|
|
37
|
Si W, Lyu J, Liu Z, Wang C, Huang J, Jiang
L and Ma T: Cucurbitacin E inhibits cellular proliferation and
enhances the chemo-response in gastric cancer by suppressing AKt
activation. J Cancer. 10:5843–5851. 2019.
|
|
38
|
Yin S, Mai Z, Liu C, Xu L and Xia C:
Label-free-based quantitative proteomic analysis of the inhibition
of cisplatin-resistant ovarian cancer cell proliferation by
cucurbitacin B. Phytomedicine. 111:1546692023.
|
|
39
|
Ni K, Lan G, Guo N, Culbert A, Luo T, Wu
T, Weichselbaum RR and Lin W: Nanoscale metal-organic frameworks
for x-ray activated in situ cancer vaccination. Sci Adv.
6:eabb52232020.
|
|
40
|
Sun D, Zou Y, Song L, Han S, Yang H, Chu
D, Dai Y, Ma J, O'Driscoll CM, Yu Z and Guo J: A cyclodextrin-based
nanoformulation achieves co-delivery of ginsenoside Rg3 and
quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm
Sin B. 12:378–393. 2022.
|
|
41
|
Yang Q, Shi G, Chen X, Lin Y, Cheng L,
Jiang Q, Yan X, Jiang M, Li Y, Zhang H, et al: Nanomicelle protects
the immune activation effects of Paclitaxel and sensitizes tumors
to anti-PD-1 immunotherapy. Theranostics. 10:8382–8399. 2020.
|
|
42
|
Wu Q, Li B, Li J and Sun S, Yuan J and Sun
S: Cancer-associated adipocytes as immunomodulators in cancer.
Biomark Res. 9:22021.
|
|
43
|
Szczygieł A, Węgierek-Ciura K, Wróblewska
A, Mierzejewska J, Rossowska J, Szermer-Olearnik B, Świtalska M,
Anger-Góra N, Goszczyński TM and Pajtasz-Piasecka E: Combined
therapy with methotrexate nanoconjugate and dendritic cells with
downregulated IL-10R expression modulates the tumor
microenvironment and enhances the systemic anti-tumor immune
response in MC38 murine colon carcinoma. Front Immunol.
14:11553772023.
|
|
44
|
Evgin L and Vile RG: Parking CAR T cells
in tumours: Oncolytic viruses as valets or vandals? Cancers
(Basel). 13:11062021.
|
|
45
|
Li J, Zhao M, Liang W, Wu S, Wang Z and
Wang D: Codelivery of Shikonin and siTGF-β for enhanced triple
negative breast cancer chemo-immunotherapy. J Control Release.
342:308–320. 2022.
|
|
46
|
Shao Y, Lo CM, Ling CC, Liu XB, Ng KTP,
Chu ACY, Ma YY, Li CX, Fan ST and Man K: Regulatory B cells
accelerate hepatocellular carcinoma progression via CD40/CD154
signaling pathway. Cancer Lett. 355:264–272. 2014.
|
|
47
|
Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu
F, Cheung OKW, Sun H, Zeng X, Tang W, et al: Targeting
monocyte-intrinsic enhancer reprogramming improves immunotherapy
efficacy in hepatocellular carcinoma. Gut. 69:365–379. 2020.
|
|
48
|
He F, Furones AR, Landegren N, Fuxe J and
Sarhan D: Sex dimorphism in the tumor microenvironment-from bench
to bedside and back. Semin Cancer Biol. 86:166–179. 2022.
|
|
49
|
He J, Wang Y, Xu LH, Qiao J, Ouyang DY and
He XH: Cucurbitacin IIa induces caspase-3-dependent apoptosis and
enhances autophagy in lipopolysaccharide-stimulated RAW 264.7
macrophages. Int Immunopharmacol. 16:27–34. 2013.
|