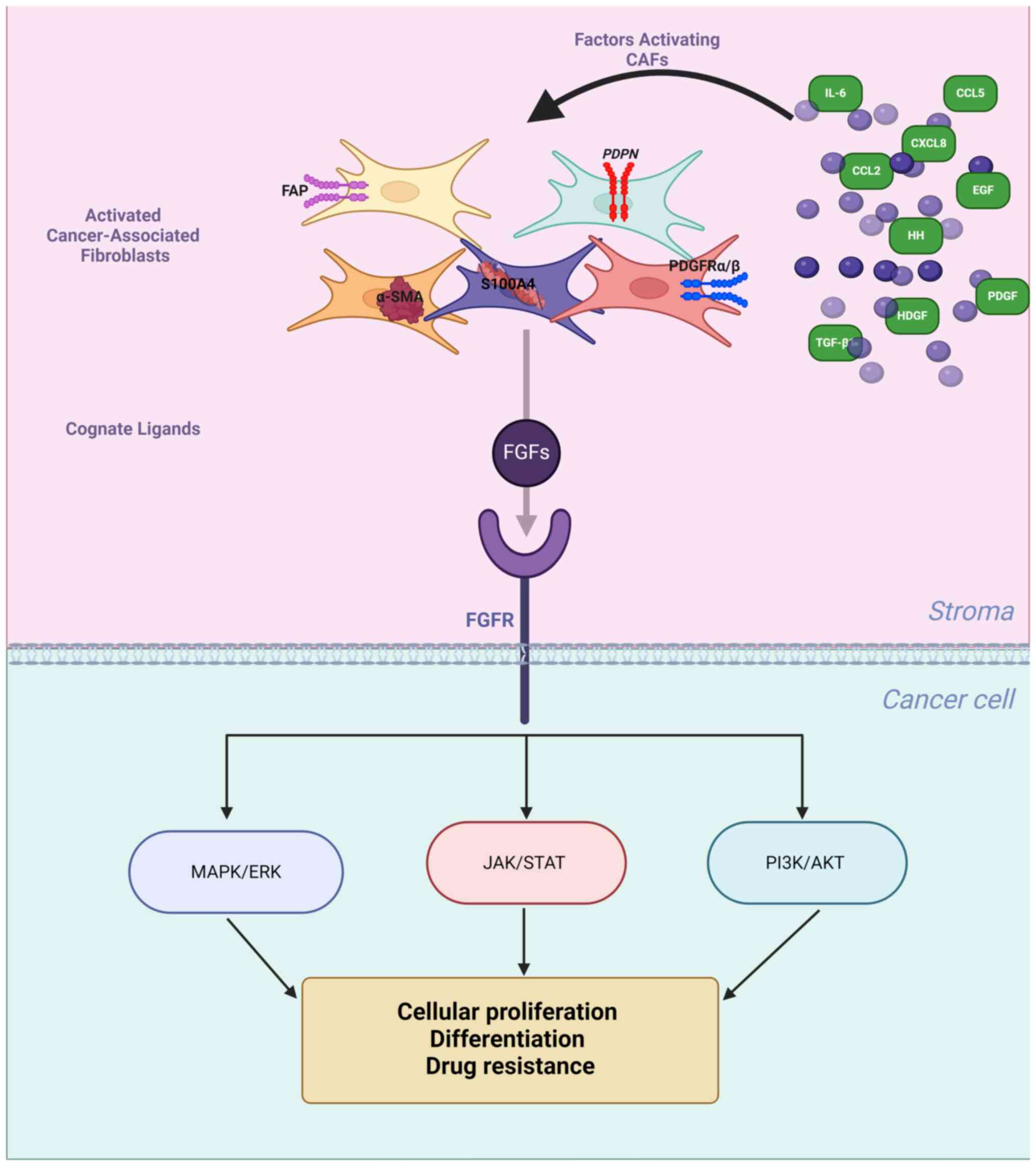
Fibroblast growth factor (FGF) receptor
(FGFR)-mediated interactions between tumour microenvironment (TME)
and breast cancer (BC) cells in progression and response to therapy
are well documented (1,2). While preclinical studies
consistently implicate FGFR signalling in BC development, clinical
evidence to support its pro-tumorigenic role is still missing
(3,4). One of the possible reasons for the
discordance between mechanistic and clinical findings as well as
disappointing results of clinical trials with FGFR inhibitors in BC
(5,6) may be an inability of in vitro
models to truly represent an in vivo setting and biological
complexity of the TME. As FGFR-pathway is regulated by TME-derived
stimuli, the clinical value of FGFR in BC ought to be analysed in
the context of the stromal component, activating or repressing its
function, in particular, cancer-associated fibroblasts (CAFs), that
either directly (cognate ligands; FGFs) or indirectly (various
factors enhancing CAFs' paracrine activity), may affect FGFR
signalling (7,8).
CAFs, the most abundant cellular component of the
TME present a highly heterogenous population, whose remarkable
phenotypic and functional diversity is due mostly to distinct
cellular origins, such as resident fibroblasts, bone marrow-derived
mesenchymal stem cells, pericytes, endothelial or cancer cell
(9). Various factors produced by
cancer cells, host immune or other stromal cells, for example
tumour growth factor β (TGF-β), platelet-derived growth factor
(PDGF), sonic hedgehog (HH), interleukin 6 (IL-6), and a wide array
of chemokines (9-18), induce differentiation and
activation of CAFs. CAFs participate in various aspects of
carcinogenesis, interacting directly and/or indirectly with tumour
cells as well as various components of TME including myeloid cells.
They have been most extensively investigated in the context tumour
immunosuppressive microenvironment and its potential clinical
significance (19,20). In BC, CAFs have been shown to
promote cancer cell proliferation, remodelling of the extracellular
matrix (ECM), angiogenesis and metastasis as well as modulate
immune responses and drug resistance (19). This suggests that the overall
impact of CAFs on tumour progression, and hence disease prognosis,
is determined by the spatio-temporal pattern of their distribution
and activation. A series of excellent recent reviews discuss
various aspects of CAFs' biology, their impact on progression and
responsiveness to therapy in several solid tumours, including
BC.
Unequivocal documentation of CAFs is notoriously
difficult, as none of the available phenotypic markers are entirely
specific or exclusive. Inherent plasticity between CAFs subtypes
further conceals their true phenotypic identity (20-23). Most commonly, CAFs are being
detected on the basis of their morphology, positivity for
mesenchymal biomarkers, for example alpha-smooth muscle actin
(αSMA), fibroblast-activating protein (FAP), fibroblast-specific
protein (FSP/S100A4) and platelet-derived growth factor receptor-β
(PDGFRβ) as well as lack of expression of lineages markers for
epithelial, endothelial or hematopoietic cells (20,21,23). However, because of their dynamic
interactions with the tumour and other TME components, not a single
marker, but a panel of phenotypically- and functionally-related
features, so-called 'a stromal signature', is better positioned to
define CAFs with regards to patients' prognosis (24). And indeed, several molecular
stromal signatures have already been shown to have a prognostic and
predictive value, complementary to that of phenotypic markers of
the BC epithelial compartment (25,26).
There is growing evidence to suggest that through
their secretome, encompassing a range of biologically active
molecules such as growth factors, chemokines and cytokines, CAFs
influence the course of BC development (1,27).
In particular, being a source of FGF ligands, they act as paracrine
upstream regulators of FGFR likely to affect BC evolution and
development of therapy resistance (1,28-31).
Based on the reported data, a panel of
stroma-derived factors was selected, called henceforth an
'FGFR-related CAFs' profile', that enables identification of a
subpopulation of CAFs [phenotypic markers: αSMA,
S100A4/fibroblast-specific protein 1 (FSP-1), PDGFR, podoplanin
(PDPN), FAP (20,21)], with characteristics indicative of
their potential regulatory effect on the FGF/FGFR axis [factors
inducing CAFs paracrine activity: transforming growth factor
(TGF)-β1, HDGF, PDGF, CXCL8, C-C motif chemokine ligand (CCL) 5,
CCL2, IL-6, HH and EGF (32,33) and 3 CAFs-derived cognate FGF
ligands: FGF2, FGF5 and FGF17 (34-38)] (Table I).
In the present study, existing data on the
prognostic and, where available, predictive significance of the
individual components of the so designed 'CAFs' profile' were
summarized. To the best of our knowledge this is the first attempt
to define the traits of CAFs specifically relevant to the activity
of FGFR. Such a profile may represent a 'missing link' in the
translation between mechanistic and clinical studies, thus
supporting an evaluation of the true value of FGFR in BC
prognostication (Fig. 1 and
Table II).
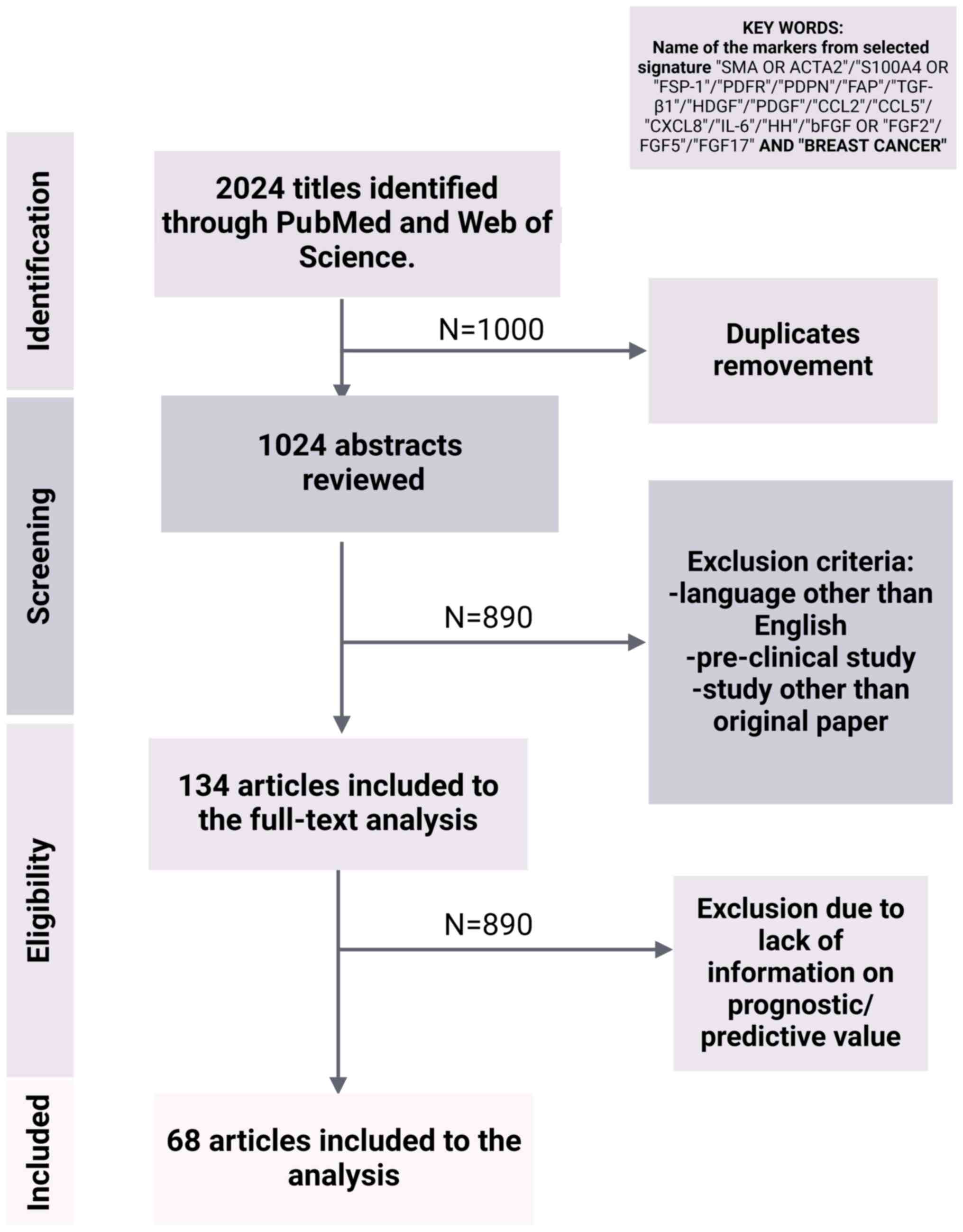
The data have been organized into distinct
paragraphs based on the tissue of origin of specific marker/s,
whether expressed in the tissue (including the cytosol) or
circulating in the blood (a substantial and easily accessible
portion of the CAFs secretome). Details regarding the literature
search, including a flowchart, are provided in Fig. 2.
αSMA, a general marker of mesenchymal cells, is the
most reliable marker of CAFs with a myofibroblast morphology
(39). Alpha SMA can exist in a
globular (G-actin) or filamentous (F-actin) form. The incorporation
of αSMA into stress fibres, associated with the transition between
G and F actin states, enhances the contractile properties of CAFs
as well as the tension of the surrounding ECM (30). Via a mechanical feedback loop,
αSMA-containing stress fibres participate in multiple cellular
functions, ranging from the maintenance of cell shape and polarity
to endosome dynamics and secretory pathways (39,40). Phenotypic transition into
myofibroblasts and acquisition of the contractile features is one
of the central traits of the activated stroma. It can be induced
through several mechanisms, including auto- and paracrine
stimulation by growth factors or cytokines (41). Activated myofibroblasts, in turn,
secrete a number of soluble modulators, which contribute to ECM
remodelling and, in cancer, promote invasiveness (39).
In BC, overexpression of αSMA in the stroma is
consistently shown to be associated with unfavourable prognosis-an
increased risk of metastases, shorter overall survival (OS) and
disease-free survival (DFS) (28,42-47). Upregulation of αSMA was found to
correlate with tumour high grade and positive nodal status
(44,47,48). In human epidermal growth factor
receptor 2 (HER2)-enriched BC, co-expression of αSMA, FGF5 and
FGFR2, associated with upregulated c-Src, correlated with poor
response to treatment (28),
implicating the CAF (αSMA+)-FGF5-FGFR2-c-Src axis in
development of resistance to HER2-targeted therapies. This was
further supported by a demonstration of a link between αSMA
overexpression in the stromal compartment and resistance to
trastuzumab in patients with BC (45).
S100A4 protein or FSP-1, localized in the cytoplasm
and/or nucleus, is involved in the regulation of a number of
cellular processes including cell cycle progression and
differentiation. S100A4 is a polypeptide with two calcium-binding
motifs, known to regulate, in a calcium-dependent manner, various
cytoplasmic proteins, including the cytoskeletal components. The
structural conformation of S100A4 upon calcium binding facilitates
its interaction with RAGE on fibroblasts, activating intracellular
signalling cascades such as the MAPK/ERK pathway (49). Furthermore, being secreted to the
TME by activated stromal cells such as fibroblasts and immune
cells, S100A4 supports growth factors release and angiogenesis,
thus promoting tumour progression and metastasis (50). In macrophages, S100A4 binds to
several intracellular proteins, which through the changes in
cytoskeletal dynamics, promotes their recruitment to the tumour
vicinity. In addition, S100A4 contributes to the pro-tumour
macrophage polarization (51).
In patients with BC, S100A4 is an unfavourable
prognostic factor and its upregulation correlates with tumour high
grade and shorter OS (47,54-58).
S100A4 expression varies between BC molecular subtypes and
histologic features of the stroma (59). Upregulation of S100A4 in stromal
cells was found to be associated with estrogen receptor
(ER)-negativity and nodal metastases (54,56).
In BC, stromal PDGRFβ overexpression is associated
with unfavourable clinicopathological characteristics such as high
histological grade, larger tumour size (T), ER-negativity,
upregulated HER2, as well as shorter DFS, recurrence-free survival
(RFS) and OS (64-66). Expression of stromal PDGRβ was
found prognostically significant particularly in tumours from
premenopausal patients (67).
Furthermore, randomized clinical studies of two BC cohorts
identified stromal PDGFRβ as a marker of a therapeutic benefit of
tamoxifen in early BC (65).
PDPN, a mucin-type glycoprotein, promotes cancer
cell migration and invasiveness and is expressed on fibroblasts,
macrophages and tumour cells (20). PDPN is a transmembrane mucin-type
glycoprotein with an extracellular domain rich in O-glycosylation
sites. The single transmembrane helix anchors PDPN in the cell
membrane and plays a role in transmitting signals from the
extracellular environment to the intracellular activation of the
RhoA/ROCK signalling pathway, which is involved in cytoskeletal
dynamics, cell contractility and motility (68). Several studies demonstrated a
positive association between increased levels of stromal PDPN,
tumour grade, size (T), nodal status, ER- and progesterone receptor
(PR)-negativity as well as shorter DFS and OS (47,52,69-73). Friedman et al (52) showed that, together with S100A4,
PDPN may be instrumental in identification of CAF subpopulations,
the ratio of which, having clinical implications across BC
subtypes, is particularly correlated with BRCA mutations in
triple-negative BC (TNBC). It was also demonstrated that the
phenotypic composition of CAF population tends to fluctuate over
time of cancer development. This supports the concept of CAFs'
plasticity, as a key trait of a dynamic TME, co-evolving with the
tumour, to nurture and provide a permissive microenvironment for
its continuous growth (52).
FAP is a transmembrane serine protease with both
dipeptidyl peptidase and endopeptidase activities. FAP's
proteolytic activity allows it to degrade components of the ECM,
such as gelatin, collagen and fibronectin. By ECM lysis, FAP
generates bioactive fragments that can activate pro-tumorigenic
signalling pathways in CAFs (74). FAP is a surface marker of
activated fibroblasts in >90% of cancers (21). FAP-expressing CAFs are involved in
various cancer-related processes, for example ECM-remodelling, but
their key pro-tumorigenic role is ascribed to an impact on immune
cell polarization and development of immunosuppressive TME
(21). A number of
FAP+ CAF-derived soluble effector molecules, such as
stromal-derived factor 1 and CCL2, have been implicated in creation
of an environment facilitating tumour development (75). In particular, FAP+
CAF-mediated activation of the uPAR-FAK-c-Src-JAK2-STAT3-CCL2
cascade enables recruitment of circulating myeloid-derived stem
cells expressing CCR2, a cognate CCL2 receptor (21). Lo et al (76) demonstrated a correlation between
FAP overexpression in CAFs and regulatory T cell-dependent
immunosuppression. Accordingly, in BC, increased FAP+
CAFs were associated with features of poor prognosis, such as
distant metastases and decreased RFS (77-79). By contrast, Ariga et al
(78) has found high density of
FAP+ CAFs prognostic of a longer OS and DFS (78), suggesting that FAP overexpression
may also be related to extensive tissue remodelling and ECM
turnover.
The TGF-β, a large family of structurally related
cytokines and growth factors, are involved in a vast number of
cellular processes in development and homoeostasis of most human
tissues. TGF-β1, in particular, a key regulator of the synthesis
and expression of collagen, elastin and MMPs, acts through the
canonical Smad-dependent and non-Smad pathways, that involve a
number of receptors and interacting networks, and is strongly
implicated in the pathogenesis of fibrosis (80,81). The major targets of TGF-β1 are
fibroblasts, but other cell types, including macrophages,
epithelial and vascular cells are also affected. In tumours,
expressed at high level, TGF-β1 mediates EMT, promotes
angiogenesis, and together with IL-1β, induces expression of FAP in
fibroblasts (20). It is widely
acknowledged, that an overall outcome of TGF-β1 activity depends on
a type of a cell and its microenvironment. TGF-β1 was demonstrated
to significantly affect both the fibroblast-myofibroblast
transition and the rate of invasion (82). Koumoundourou et al
(83) has also shown that
downregulation of TGF-β1 in BC tissue was a marker of poor
prognosis and recurrence. Using immunohistochemistry for TGF-β1,
pSmad2/3 and Smad4, the authors demonstrated an inverse association
between TGF-β1 and PR, as well as between Smad4 and ER, but not
with any other clinicopathological features. Interestingly,
although neither TGF-β1 nor pSmad2/3 were related to ER, loss of
pSmad2/3 expression was prognostic of a shorter DFS in all
patients, including those with ER-positive BC (83). Tissue expression of TGF-β1 and
survivin was evaluated in BC samples by Liu et al (84), who found that, although none of
them, separately, had an independent prognostic value, increased
TGF-β1/survivin co-expression was associated with shorter OS and
PFS in patients with TNBC (84).
Heparin binding growth factor or hepatoma-derived
growth factor (HDGF) shares homology with the high mobility group 1
protein and was first purified from a human hepatoma-derived cell
line (85). Widely expressed in
normal tissues, HDGF promotes proliferation of various cells,
including fibroblasts, as both a DNA-binding nuclear factor and a
secreted protein via a receptor-mediated pathway (86). In BC, Chen et al (87) demonstrated that strong expression
of nuclear HDGF was associated with high tumour grade, high stage,
high proliferation index (Ki-67 index >20%), lymph node
metastases and shorter RFS. This was confirmed by Qiu et al
(88), who reported a negative
correlation between high expression of HDGF and DFS.
PDGF is a growth factor that is secreted by cancer
cells and induces activation of fibroblasts (89). There are five PDGF isoforms (A-D)
but only PDGF-A and -B can form functional heterodimers, that
stimulate their cognate receptors (90). In most tumours, populations of
PDGF-expressing cancer cells are markedly denser than those
positive for PDGFR, which indicates that PDFG plays a role as a
mediator of paracrine activity of cancer cells towards the
neighbouring stroma (90). The
primary effect of PDGF on fibroblasts is their recruitment and
stimulation of proliferation with, unlike TGF-β, no influence on
the phenotypic switch into myofibroblasts (90). In BC, high expression of PDGF (AA
and BB) was found to be correlated with high grade, high Ki67,
young age (<50 years), tumour size, triple negativity and
shorter DFS (91). Moreover,
confirmed to be associated with poor prognosis in stage IV BC, PDGF
(AA, BB, CC) was also shown to be predictive of a low response rate
to chemotherapy (92).
The role of CAFs in orchestration of inflammation in
immune TME is well recognized (62). Both target and source of a number
of immune-modulatory and chemo-attractive mediators such as
Chemokine (C-X-C motif) ligand 8 (CXCL8), CCL5, CCL2 and IL-6, CAFs
participate in the recruitment of suppressive myeloid and
regulatory T cells, polarization of M2 macrophages, suppression of
cytotoxic lymphocytes and dendritic cells, that contribute to the
modulation of TME towards tumour-promoting immunosuppressive
environment (62,93).
CXCL8, CCL2 and CCL5, essential components of the
tumour-stroma-inflammatory network, are associated with aggressive
BC phenotype and increased risk of recurrence. Produced mostly by
macrophages, these pro-inflammatory chemokines attract and activate
resident immune cells (93),
induce EMT (94), promote tumour
metastasis (95) as well as
modify tumour response to therapy (96). Higher levels of these three
chemokines were shown to be associated predominantly with basal BC
(93). Several studies have
demonstrated an association between high expression of CCL2, high
grade, lymph node metastases and HER2-negativity as well as shorter
DFS and RFS (97,98). CCL5 was analysed by Yamaguchi
et al (99), who found
that stromal CCL5 was negatively associated with tumour size, as
well as ER and PR expression. CCL5 levels significantly correlated
with the aggressive phenotype and this was noted particularly in
the CCR3-positive tumours (99).
Moreover, in another study, expression of CCL5 was prognostic of
shortened RFS, suggesting that CCL5 promotes BC progression and
contributes to the worse disease outcome (100).
The HH-signalling pathway plays a fundamental role
in embryonic development of various organs and its dysregulation
has been associated with several malignancies. In mammary gland,
overactivation of HH-signalling has been suggested to stimulate
self-renewal of normal and tumorigenic stem cells, thus promoting
BC formation (101). However,
prognostic and predictive value of HH pathway in BC still remains
largely understudied. In a single study comprising 36 patients with
TNBC, activation of HH combined with Wnt pathway identified
patients at risk for early recurrence (102).
FGFs are a family of signalling proteins, which in
humans comprises 23 members, with paracrine (FGF1-10, FGF 16-18,
FGF 20 and FGF22) or endocrine (FGF19, FGF21 and FGF23) mode of
action. FGFs bind with different affinity to one or several of the
four transmembrane FGF receptors (FGFR1-4) (103), activation of which, through the
Ras-dependent MAPK, PI3K/AKT or STATs-dependent pathways,
influences cell proliferation, differentiation and survival
(Fig. 1) (104). Cellular responses induced by
FGF/FGFR vary between biological contexts, which are determined by
a number of factors, including cell type-specific adaptor
molecules, signal transduction enhancers, transcription factors and
co-activators, as well as interacting other signalling networks
(34). Whereas translational
significance of FGFRs' alterations in human cancer is being
analysed by numerous research groups, albeit with conflicting
results, available data on the clinical value of their ligands
(FGFs) in BC are scarce, often inconclusive, and restricted only
to: FGF2, FGF5 and FGF17. For example, while downregulation of FGF2
was found a marker of poor prognosis (105), its upregulation was associated
with both shorter and longer RFS and OS (34,106-108). In a study by Colomer et
al (105), downregulation of
FGF2 was associated with longer OS and RFS; whereas Granato et
al (109) reported that an
association with survival parameters was not significant. Moreover,
although upregulation of FGF2 correlated with small tumour size and
decreased incidence of nodal metastases, it was inconsistently
(both low and high) linked to tumour grade (106,107,110,111). Predictive value of FGF2 was
reported by Shee et al (34), who demonstrated that its
upregulation in ER+ BC was significantly predictive of
anti-estrogen resistance and shorter RFS and OS. Upregulation of
FGF5 was found to be associated with shorter OS and RFS (28). The only existing study of a
prognostic value of FGF17 in BC demonstrated that its upregulation
inversely correlated with tumour grade and nodal status (112).
By contrast, for TGF-b1 and IL-6, several studies
documented their association with a good disease outcome. For
example, Panis et al (121), showed that an early presentation
of TNBC (<45 years of age) was associated with high levels of
circulating TGF-β1, while in metastatic BC, TGF-β1 plasma
concentration was lower than in non-metastatic disease. In a
40-month follow-up, women with low TGF-β1 levels (<20 pg/ml)
were shown to have a tendency for a reduced OS and doxorubicin
induced decrease in TGF-β1 concentration, promptly after drug
infusion. Interestingly, levels of plasma TGF-β1 were not affected
by surgical removal of the primary tumour and did not differ
between patients with responsive and resistant disease (121). Milovanović et al
(122) has found serum
concentration of IL-6 as the independent prognostic factor of a
good disease outcome.
The discrepancies between the studies are, at least
partially, due to the well documented pleiotropy of both cytokines
that, via either proor anti-inflammatory activity, may differently
affect tumour progression.
Low level of serum CXCL8 was associated with higher
pathological complete response of patients with TNBC in response to
neoadjuvant chemotherapy (123).
In an attempt to identify the cluster/s that would
support the prognostic significance of longitudinal serum cytokine
analysis, Paccagnella et al (124) have recently shown in patients
with BC treated with eribulin that, after four courses of therapy,
low levels of TGF-β, IL-4, IL-6, IL-8, IL-10, CCL-2 and CCL-4 (out
of 18 cytokines evaluated) were associated with the best patient
survival. This novel approach to design a kind of a prognostic
'serum signature', if validated, may prove very valuable in
decision making related to the type and time course of applied
therapy (124).
Known as the main ligand of the epidermal growth
factor (EGF) receptor, a dominant oncogenic driver in numerous
cancers, EGF plays also an important role in fibroblast
differentiation and activation. Signalling from EGF downregulates
Rho-GTP levels, which gives permissive signals for Rac1 activation
and fibroblast polarization (125). This leads to fibroblast
transformation into myofibroblasts which, together with
growth-promoting action of TGF-β1, reciprocally promotes cancer
cell invasion (125). However,
at the clinical level, there is no evidence to demonstrate any
translation value of stromal EGF in BC. Results of the only
existing study evaluating EGF plasma level have demonstrated no
significant associations with clinicopathological features or
disease prognosis (126).
The data on FGF2 showed that in a parallel
evaluation in the serum and tissue, there was no correlation
between its tumour expression and the corresponding serum level
(105).
In summary, although determination of biomarkers
obtainable by liquid biopsy appears to be very attractive for
minimally invasive and inexpensive diagnosis and prognostication,
the level of a secreted and/or shredded protein in the plasma,
being not cell- or tissue-specific, does not faithfully reflect the
expression in the analysed organ, and hence its role in the
mechanisms of disease development. This implies that a more complex
approach combining evaluation of a panel of both serum and
histological biomarkers with imaging-based metrics may provide a
more efficient tool for clinical practice.
Available evidence clearly demonstrates that CAFs
contain a valuable prognostic information in BC. While presented
studies of single markers (for example αSMA, S100A4, PDGFR or PDPN)
identify promising candidates, analyses combining several stromal,
either CAFs-specific or CAF-derived features (for example
S100A4/PDPN (52), αSMA/FGF5
(28), or PDGFR/FGF2/FGF7
(63) reveal the traits of
clinically more significant implications. This indicates that
comprehensive analyses of CAFs in relation to other components of
the stroma might improve an assessment of CAFs involvement in the
tumorigenic process and their value as prognostic and predictive
biomarkers.
CAFs are not currently used in routine
histopathological practice. The challenge is to design biologically
meaningful signatures that would capture essential molecular
profiles and could be exploited in prognostication. Given CAFs'
heterogeneity, plasticity and an intricate cross-talk with other
TEM components, it appears that this could be achieved by profiling
the chosen (for example micro-dissected) area of the stroma towards
carefully selected panel of makers relevant to specific aspects of
BC biology. In particular, the proposed 'FGFR-related stromal
profile of CAFs' might provide (after a necessary further clinical
validation) the context for an assessment of the true FGFR's
clinical value as well as the criteria for further classification
of patients with BC for the FGFR-targeted therapy.
Not applicable.
JS designed the framework, performed the literature
search, prepared the figure and the tables, and wrote the
manuscript. MB supervised the research and selection of the
literature. RS provided substantive support and critical review.
HMR provided the concept and edited the manuscript. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Science Centre
(grant no. UMO-2020/39/B/NZ4/02696).
|
1
|
Santolla MF and Maggiolini M: The FGF/FGFR
system in breast cancer: Oncogenic features and therapeutic
perspectives. Cancers (Basel). 12:30292020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Servetto A, Formisano L and Arteaga CL:
FGFR signaling and endocrine resistance in breast cancer:
Challenges for the clinical development of FGFR inhibitors. Biochim
Biophys Acta Rev Cancer. 1876:1885952021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Braun M, Piasecka D, Tomasik B,
Mieczkowski K, Stawiski K, Zielinska A, Kopczynski J, Nejc D,
Kordek R, Sadej R and Romanska HM: Hormonal receptor status
determines prognostic significance of FGFR2 in invasive breast
carcinoma. Cancers (Basel). 12:27132020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mieczkowski K, Kitowska K, Braun M,
Galikowska-Bogut B, Gorska-Arcisz M, Piasecka D, Stawiski K, Zaczek
AJ, Nejc D, Kordek R, et al: FGF7/FGFR2-JunB signalling counteracts
the effect of progesterone in luminal breast cancer. Mol Oncol.
16:2823–2842. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meric-Bernstam F, Bahleda R, Hierro C,
Sanson M, Bridgewater J, Arkenau HT, Tran B, Kelley RK, Park JO,
Javle M, et al: Futibatinib, an irreversible FGFR1-4 inhibitor, in
patients with advanced solid tumors harboring FGF/FGFR aberrations:
A phase I dose-expansion study. Cancer Discov. 12:402–415. 2022.
View Article : Google Scholar
|
|
6
|
Coombes RC, Badman PD, Lozano-Kuehne JP,
Liu X, Macpherson IR, Zubairi I, Baird RD, Rosenfeld N,
Garcia-Corbacho J, Cresti N, et al: Results of the phase IIa
RADICAL trial of the FGFR inhibitor AZD4547 in endocrine resistant
breast cancer. Nat Commun. 13:32462022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Luca A, Frezzetti D, Gallo M and
Normanno N: FGFR-targeted therapeutics for the treatment of breast
cancer. Expert Opin Investig Drugs. 26:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chew NJ, Lim Kam Sian TCC, Nguyen EV, Shin
SY, Yang J, Hui MN, Deng N, McLean CA, Welm AL, Lim E, et al:
Evaluation of FGFR targeting in breast cancer through interrogation
of patient-derived models. Breast Cancer Res. 23:822021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ronnov-Jessen L, Petersen OW, Koteliansky
VE and Bissell MJ: The origin of the myofibroblasts in breast
cancer. Recapitulation of tumor environment in culture unravels
diversity and implicates converted fibroblasts and recruited smooth
muscle cells. J Clin Invest. 95:859–873. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tejada ML, Yu L, Dong J, Jung K, Meng G,
Peale FV, Frantz GD, Hall L, Liang X, Gerber HP and Ferrara N:
Tumor-driven paracrine platelet-derived growth factor receptor
alpha signaling is a key determinant of stromal cell recruitment in
a model of human lung carcinoma. Clin Cancer Res. 12:2676–2688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian H, Callahan CA, DuPree KJ, Darbonne
WC, Ahn CP, Scales SJ and de Sauvage FJ: Hedgehog signaling is
restricted to the stromal compartment during pancreatic
carcinogenesis. Proc Natl Acad Sci USA. 106:4254–4259. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aggarwal V, Tuli HS, Varol A, Thakral F,
Yerer MB, Sak K, Varol M, Jain A, Khan MA and Sethi G: Role of
reactive oxygen species in cancer progression: Molecular mechanisms
and recent advancements. Biomolecules. 9:7352019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Direkze NC, Hodivala-Dilke K, Jeffery R,
Hunt T, Poulsom R, Oukrif D, Alison MR and Wright NA: Bone marrow
contribution to tumor-associated myofibroblasts and fibroblasts.
Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kidd S, Spaeth E, Watson K, Burks J, Lu H,
Klopp A, Andreeff M and Marini FC: Origins of the tumor
microenvironment: Quantitative assessment of adipose-derived and
bone marrow-derived stroma. PLoS One. 7:e305632012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abe R, Donnelly SC, Peng T, Bucala R and
Metz CN: Peripheral blood fibrocytes: Differentiation pathway and
migration to wound sites. J Immunol. 166:7556–7562. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Timperi E, Gueguen P, Molgora M, Magagna
I, Kieffer Y, Lopez-Lastra S, Sirven P, Baudrin LG, Baulande S,
Nicolas A, et al: Lipid-associated macrophages are induced by
cancer-associated fibroblasts and mediate immune suppression in
breast cancer. Cancer Res. 82:3291–3306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nurmik M, Ullmann P, Rodriguez F, Haan S
and Letellier E: In search of definitions: Cancer-associated
fibroblasts and their markers. Int J Cancer. 146:895–905. 2020.
View Article : Google Scholar
|
|
21
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar
|
|
22
|
Costa A, Kieffer Y, Scholer-Dahirel A,
Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L,
Bernard C, et al: Fibroblast heterogeneity and immunosuppressive
environment in human breast cancer. Cancer Cell. 33:463–479.e410.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glabman RA, Choyke PL and Sato N:
Cancer-associated fibroblasts: Tumorigenicity and targeting for
cancer therapy. Cancers (Basel). 14:39062022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paulsson J and Micke P: Prognostic
relevance of cancer-associated fibroblasts in human cancer. Semin
Cancer Biol. 25:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marchini C, Montani M, Konstantinidou G,
Orrù R, Mannucci S, Ramadori G, Gabrielli F, Baruzzi A, Berton G,
Merigo F, et al: Mesenchymal/stromal gene expression signature
relates to basal-like breast cancers, identifies bone metastasis
and predicts resistance to therapies. PLoS One. 5:e141312010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frings O, Augsten M, Tobin NP, Carlson J,
Paulsson J, Pena C, Olsson E, Veerla S, Bergh J, Ostman A and
Sonnhammer EL: Prognostic significance in breast cancer of a gene
signature capturing stromal PDGF signaling. Am J Pathol.
182:2037–2047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lappano R, Rigiracciolo DC, Belfiore A,
Maggiolini M and De Francesco EM: Cancer associated fibroblasts:
Role in breast cancer and potential as therapeutic targets. Expert
Opin Ther Targets. 24:559–572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernández-Nogueira P, Mancino M, Fuster G,
López-Plana A, Jauregui P, Almendro V, Enreig E, Menéndez S, Rojo
F, Noguera-Castells A, et al: Tumor-associated fibroblasts promote
HER2-targeted therapy resistance through FGFR2 activation. Clin
Cancer Res. 26:1432–1448. 2020. View Article : Google Scholar
|
|
29
|
Palmieri C, Roberts-Clark D, Assadi-Sabet
A, Coope RC, O'Hare M, Sunters A, Hanby A, Slade MJ, Gomm JJ, Lam
EW and Coombes RC: Fibroblast growth factor 7, secreted by breast
fibroblasts, is an interleukin-1beta-induced paracrine growth
factor for human breast cells. J Endocrinol. 177:65–81. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cerliani JP, Guillardoy T, Giulianelli S,
Vaque JP, Gutkind JS, Vanzulli SI, Martins R, Zeitlin E, Lamb CA
and Lanari C: Interaction between FGFR-2, STAT5, and progesterone
receptors in breast cancer. Cancer Res. 71:3720–3731. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar
|
|
33
|
Louault K, Li RR and DeClerck YA:
Cancer-associated fibroblasts: Understanding their heterogeneity.
Cancers (Basel). 12:31082020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shee K, Yang W, Hinds JW, Hampsch RA, Varn
FS, Traphagen NA, Patel K, Cheng C, Jenkins NP, Kettenbach AN, et
al: Therapeutically targeting tumor microenvironment-mediated drug
resistance in estrogen receptor-positive breast cancer. J Exp Med.
215:895–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clayton NS, Wilson AS, Laurent EP, Grose
RP and Carter EP: Fibroblast growth factor-mediated crosstalk in
cancer etiology and treatment. Dev Dyn. 246:493–501. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Z, Wu B, Tang X, Ke R and Zou Q:
Comprehensive analysis of fibroblast growth factor receptor (FGFR)
family genes in breast cancer by integrating online databases and
bioinformatics. Med Sci Monit. 26:e9235172020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suh J, Kim DH, Lee YH, Jang JH and Surh
YJ: Fibroblast growth factor-2, derived from cancer-associated
fibroblasts, stimulates growth and progression of human breast
cancer cells via FGFR1 signaling. Mol Carcinog. 59:1028–1040. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin
M, Ni Z, Zhang B, Zhang D, Luo F, et al: FGF/FGFR signaling in
health and disease. Signal Transduct Target Ther. 5:1812020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Otranto M, Sarrazy V, Bonte F, Hinz B,
Gabbiani G and Desmouliere A: The role of the myofibroblast in
tumor stroma remodeling. Cell Adh Migr. 6:203–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chakrabarti R, Lee M and Higgs HN:
Multiple roles for actin in secretory and endocytic pathways. Curr
Biol. 31:R603–R618. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ao Z, Shah SH, Machlin LM, Parajuli R,
Miller PC, Rawal S, Williams AJ, Cote RJ, Lippman ME, Datar RH and
El-Ashry D: Identification of cancer-associated fibroblasts in
circulating blood from patients with metastatic breast cancer.
Cancer Res. 75:4681–4687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim S, You D, Jeong Y, Yu J, Kim SW, Nam
SJ and Lee JE: TP53 upregulates α-smooth muscle actin expression in
tamoxifen-resistant breast cancer cells. Oncol Rep. 41:1075–1082.
2019.
|
|
43
|
Wang T, Srivastava S, Hartman M, Buhari
SA, Chan CW, Iau P, Khin LW, Wong A, Tan SH, Goh BC and Lee SC:
High expression of intratumoral stromal proteins is associated with
chemotherapy resistance in breast cancer. Oncotarget.
7:55155–55168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamashita M, Ogawa T, Zhang X, Hanamura N,
Kashikura Y, Takamura M, Yoneda M and Shiraishi T: Role of stromal
myofibroblasts in invasive breast cancer: Stromal expression of
alpha-smooth muscle actin correlates with worse clinical outcome.
Breast Cancer. 19:170–176. 2012. View Article : Google Scholar
|
|
45
|
Vathiotis IA, Moutafi MK, Divakar P, Aung
TN, Qing T, Fernandez A, Yaghoobi V, El-Abed S, Wang Y, Guillaume
S, et al: Alpha-smooth muscle actin expression in the stroma
predicts resistance to trastuzumab in patients with early-stage
HER2-positive breast cancer. Clin Cancer Res. 27:6156–6163. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Busch S, Rydén L, Stål O, Jirström K and
Landberg G: Low ERK phosphorylation in cancer-associated
fibroblasts is associated with tamoxifen resistance in
pre-menopausal breast cancer. PLoS One. 7:e456692012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu G, Wang S, Xu F, Ding Q, Chen W, Zhong
K, Huang L and Xu Q: Tumor-infiltrating podoplanin+ fibroblasts
predict worse outcome in solid tumors. Cell Physiol Biochem.
51:1041–1050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yazhou C, Wenlv S, Weidong Z and Licun W:
Clinicopathological significance of stromal myofibroblasts in
invasive ductal carcinoma of the breast. Tumour Biol. 25:290–295.
2004. View Article : Google Scholar
|
|
49
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
D'Ambrosi N, Milani M and Apolloni S:
S100A4 in the physiology and pathology of the central and
peripheral nervous system. Cells. 10:7982021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu S, Zhang H, Li Y, Zhang Y, Bian Y,
Zeng Y, Yao X, Wan J, Chen X, Li J, et al: S100A4 enhances protumor
macrophage polarization by control of PPAR-γ-dependent induction of
fatty acid oxidation. J Immunother Cancer. 9:e0025482021.
View Article : Google Scholar
|
|
52
|
Friedman G, Levi-Galibov O, David E,
Bornstein C, Giladi A, Dadiani M, Mayo A, Halperin C,
Pevsner-Fischer M, Lavon H, et al: Cancer-associated fibroblast
compositions change with breast cancer progression linking the
ratio of S100A4+ and PDPN+ CAFs clinical
outcome. Nat Cancer. 1:692–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Grum-Schwensen B, Klingelhofer J, Berg CH,
El-Naaman C, Grigorian M, Lukanidin E and Ambartsumian N:
Suppression of tumor development and metastasis formation in mice
lacking the S100A4(mts1) gene. Cancer Res. 65:3772–3780. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park CK, Jung WH and Koo JS: Expression of
cancer-associated fibroblast-related proteins differs between
invasive lobular carcinoma and invasive ductal carcinoma. Breast
Cancer Res Treat. 159:55–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
de Silva Rudland S, Martin L, Roshanlall
C, Winstanley J, Leinster S, Platt-Higgins A, Carroll J, West C,
Barraclough R and Rudland P: Association of S100A4 and osteopontin
with specific prognostic factors and survival of patients with
minimally invasive breast cancer. Clin Cancer Res. 12:1192–1200.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pedersen KB, Nesland JM, Fodstad O and
Maelandsmo GM: Expression of S100A4, E-cadherin, alphaand
beta-catenin in breast cancer biopsies. Br J Cancer. 87:1281–1286.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li WL, Zhang Y, Liu BG, Du Q, Zhou CX and
Tian XS: Correlation between the expression of S100A4 and the
efficacy of TAC neoadjuvant chemotherapy in breast cancer. Exp Ther
Med. 10:1983–1989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McKiernan E, McDermott EW, Evoy D, Crown J
and Duffy MJ: The role of S100 genes in breast cancer progression.
Tumour Biol. 32:441–450. 2011. View Article : Google Scholar
|
|
59
|
Park SY, Kim HM and Koo JS: Differential
expression of cancer-associated fibroblast-related proteins
according to molecular subtype and stromal histology in breast
cancer. Breast Cancer Res Treat. 149:727–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Donovan J, Shiwen X, Norman J and Abraham
D: Platelet-derived growth factor alpha and beta receptors have
overlapping functional activities towards fibroblasts. Fibrogenesis
Tissue Repair. 6:102013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Claesson-Welsh L, Ronnstrand L and Heldin
CH: Biosynthesis and intracellular transport of the receptor for
platelet-derived growth factor. Proc Natl Acad Sci USA.
84:8796–8800. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lavie D, Ben-Shmuel A, Erez N and
Scherz-Shouval R: Cancer-associated fibroblasts in the single-cell
era. Nat Cancer. 3:793–807. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pietras K, Pahler J, Bergers G and Hanahan
D: Functions of paracrine PDGF signaling in the proangiogenic tumor
stroma revealed by pharmacological targeting. PLoS Med. 5:e192008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Paulsson J, Sjöblom T, Micke P, Pontén F,
Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirström K and Ostman
A: Prognostic significance of stromal platelet-derived growth
factor beta-receptor expression in human breast cancer. Am J
Pathol. 175:334–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Paulsson J, Rydén L, Strell C, Frings O,
Tobin NP, Fornander T, Bergh J, Landberg G, Stål O and Östman A:
High expression of stromal PDGFRβ is associated with reduced
benefit of tamoxifen in breast cancer. J Pathol Clin Res. 3:38–43.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hu G, Huang L, Zhong K, Meng L, Xu F, Wang
S and Zhang T: PDGFR-β+ fibroblasts deteriorate survival
in human solid tumors: a meta-analysis. Aging (Albany NY).
13:13693–13707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yam C, Murthy RK, Rauch GM, Murray JL,
Walters RS, Valero V, Brewster AM, Bast RC Jr, Booser DJ, Giordano
SH, et al: A phase II study of imatinib mesylate and letrozole in
patients with hormone receptor-positive metastatic breast cancer
expressing c-kit or PDGFR-b. Invest New Drugs. 36:1103–1109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Niemiec J, Adamczyk A, Harazin-Lechowska
A, Ambicka A, Grela-Wojewoda A, Majchrzyk K, Kruczak A,
Sas-Korczyńska B and Ryś J: Podoplanin-positive cancer-associated
stromal fibroblasts in primary tumor and synchronous lymph node
metastases of HER2-overexpressing breast carcinomas. Anticancer
Res. 38:1957–1965. 2018.PubMed/NCBI
|
|
70
|
Schoppmann SF, Berghoff A, Dinhof C,
Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H and Birner P:
Podoplanin-expressing cancer-associated fibroblasts are associated
with poor prognosis in invasive breast cancer. Breast Cancer Res
Treat. 134:237–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tanaka Y, Ohno T, Kadonaga T, Kidokoro Y,
Wakahara M, Nosaka K, Sakabe T, Suzuki Y, Nakamura H and Umekita Y:
Podoplanin expression in cancer-associated fibroblasts predicts
unfavorable prognosis in node-negative breast cancer patients with
hormone receptor-positive/HER2-negative subtype. Breast Cancer.
28:822–828. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pula B, Jethon A, Piotrowska A,
Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J
and Ugorski M, et al: Podoplanin expression by cancer-associated
fibroblasts predicts poor outcome in invasive ductal breast
carcinoma. Histopathology. 59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pula B, Wojnar A, Werynska B, Ambicka A,
Kruczak A, Witkiewicz W, Ugorski M, Podhorska-Okolow M and Dziegiel
P: Impact of different tumour stroma assessment methods regarding
podoplanin expression on clinical outcome in patients with invasive
ductal breast carcinoma. Anticancer Res. 33:1447–1455.
2013.PubMed/NCBI
|
|
74
|
Liu R, Li H, Liu L, Yu J and Ren X:
Fibroblast activation protein: A potential therapeutic target in
cancer. Cancer Biol Ther. 13:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sarkar M, Nguyen T, Gundre E, Ogunlusi O,
El-Sobky M, Giri B and Sarkar TR: Cancer-associated fibroblasts:
The chief architect in the tumor microenvironment. Front Cell Dev
Biol. 11:10890682023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lo A, Wang LCS, Scholler J, Monslow J,
Avery D, Newick K, O'Brien S, Evans RA, Bajor DJ, Clendenin C, et
al: Tumor-promoting desmoplasia is disrupted by depleting
FAP-expressing stromal cells. Cancer Res. 75:2800–2810. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tashireva LA, Denisov EV, Gerashchenko TS,
Pautova DN, Bulda kov MA, Zavyalova MV, Kzhysh kowska J,
Cherdyntseva NV and Perelmuter VM: Intratumoral heterogeneity of
macrophages and fibroblasts in breast cancer is associated with the
morphological diversity of tumor cells and contributes to lymph
node metastasis. Immunobiology. 222:631–640. 2017. View Article : Google Scholar
|
|
78
|
Ariga N, Sato E, Ohuchi N, Nagura H and
Ohtani H: Stromal expression of fibroblast activation
protein/seprase, a cell membrane serine proteinase and gelatinase,
is associated with longer survival in patients with invasive ductal
carcinoma of breast. Int J Cancer. 95:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bonneau C, Eliès A, Kieffer Y, Bourachot
B, Ladoire S, Pelon F, Hequet D, Guinebretière JM, Blanchet C,
Vincent-Salomon A, et al: A subset of activated fibroblasts is
associated with distant relapse in early luminal breast cancer.
Breast Cancer Res. 22:762020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shi X, Young CD, Zhou H and Wang X:
Transforming growth factor-β signaling in fibrotic diseases and
cancer-associated fibroblasts. Biomolecules. 10:16662020.
View Article : Google Scholar
|
|
82
|
Casey TM, Eneman J, Crocker A, White J,
Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H and
Plaut K: Cancer associated fibroblasts stimulated by transforming
growth factor beta1 (TGF-beta 1) increase invasion rate of tumor
cells: A population study. Breast Cancer Res Treat. 110:39–49.
2008. View Article : Google Scholar
|
|
83
|
Koumoundourou D, Kassimatis T, Zolota V,
Tzorakoeleftherakis E, Ravazoula P, Vassiliou V, Kardamakis D and
Varakis J: Prognostic significance of TGFbeta-1 and pSmad2/3 in
breast cancer patients with T1-2,N0 tumours. Anticancer Res.
27:2613–2620. 2007.PubMed/NCBI
|
|
84
|
Liu N, Qi D, Jiang J, Zhang J and Yu C:
Significance of combined TGF-β1 and survivin expression on the
prognosis of patients with triple-negative breast cancer. Oncol
Lett. 23:1932022. View Article : Google Scholar
|
|
85
|
Nakamura H, Kambe H, Egawa T, Kimura Y,
Ito H, Hayashi E, Yamamoto H, Sato J and Kishimoto S: Partial
purification and characterization of human hepatoma-derived growth
factor. Clin Chim Acta. 183:273–284. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Enomoto H, Nakamura H, Liu W and
Nishiguchi S: Hepatoma-derived growth factor: Its possible
involvement in the progression of hepatocellular carcinoma. Int J
Mol Sci. 16:14086–14097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen X, Yun J, Fei F, Yi J, Tian R, Li S
and Gan X: Prognostic value of nuclear hepatoma-derived growth
factor (HDGF) localization in patients with breast cancer. Pathol
Res Pract. 208:437–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qiu L, Ma Y, Chen X, Zhou L, Zhang H,
Zhong G, Zhang L and Tang J: Heparin-binding growth factor (HDGF)
drives radioresistance in breast cancer by activating the STAT3
signaling pathway. J Transl Med. 19:3442021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Anderberg C and Pietras K: On the origin
of cancer-associated fibroblasts. Cell Cycle. 8:1461–1462. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kuzet SE and Gaggioli C: Fibroblast
activation in cancer: When seed fertilizes soil. Cell Tissue Res.
365:607–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jansson S, Aaltonen K, Bendahl PO, Falck
AK, Karlsson M, Pietras K and Rydén L: The PDGF pathway in breast
cancer is linked to tumour aggressiveness, triple-negative subtype
and early recurrence. Breast Cancer Res Treat. 169:231–241. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Seymour L, Dajee D and Bezwoda WR: Tissue
platelet derived-growth factor (PDGF) predicts for shortened
survival and treatment failure in advanced breast cancer. Breast
Cancer Res Treat. 26:247–252. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liubomirski Y, Lerrer S, Meshel T,
Rubinstein-Achiasaf L, Morein D, Wiemann S, Körner C and Ben-Baruch
A: Tumor-stroma-inflammation networks promote pro-metastatic
chemokines and aggressiveness characteristics in triple-negative
breast cancer. Front Immunol. 10:7572019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li
H, Zhang L, Yu J and Yuan S: Lactate-activated macrophages induced
aerobic glycolysis and epithelial-mesenchymal transition in breast
cancer by regulation of CCL5-CCR5 axis: A positive metabolic
feedback loop. Oncotarget. 8:110426–110443. 2017. View Article : Google Scholar
|
|
95
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhou B, Sun C, Li N, Shan W, Lu H, Guo L,
Guo E, Xia M, Weng D, Meng L, et al: Cisplatin-induced CCL5
secretion from CAFs promotes cisplatin-resistance in ovarian cancer
via regulation of the STAT3 and PI3K/Akt signaling pathways. Int J
Oncol. 48:2087–2097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yao M, Yu E, Staggs V, Fan F and Cheng N:
Elevated expression of chemokine C-C ligand 2 in stroma is
associated with recurrent basal-like breast cancers. Mod Pathol.
29:810–823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Heiskala M, Leidenius M, Joensuu K and
Heikkilä P: High expression of CCL2 in tumor cells and abundant
infiltration with CD14 positive macrophages predict early relapse
in breast cancer. Virchows Arch. 474:3–12. 2019. View Article : Google Scholar
|
|
99
|
Yamaguchi M, Takagi K, Narita K, Miki Y,
Onodera Y, Miyashita M, Sasano H and Suzuki T: Stromal CCL5
promotes breast cancer progression by interacting with CCR3 in
tumor cells. Int J Mol Sci. 22:19182021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yaal-Hahoshen N, Shina S, Leider-Trejo L,
Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I and
Ben-Baruch A: The chemokine CCL5 as a potential prognostic factor
predicting disease progression in stage II breast cancer patients.
Clin Cancer Res. 12:4474–4480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Arnold KM, Pohlig RT and Sims-Mourtada J:
Co-activation of Hedgehog and Wnt signaling pathways is associated
with poor outcomes in triple negative breast cancer. Oncol Lett.
14:5285–5292. 2017.PubMed/NCBI
|
|
103
|
Beenken A and Mohammadi M: The FGF family:
Biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Babina IS and Turner NC: Advances and
challenges in targeting FGFR signalling in cancer. Nat Rev Cancer.
17:318–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Colomer R, Aparicio J, Montero S, Guzmán
C, Larrodera L and Cortés-Funes H: Low levels of basic fibroblast
growth factor (bFGF) are associated with a poor prognosis in human
breast carcinoma. Br J Cancer. 76:1215–1220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Linderholm BK, Lindh B, Beckman L,
Erlanson M, Edin K, Travelin B, Bergh J, Grankvist K and Henriksson
R: Prognostic correlation of basic fibroblast growth factor and
vascular endothelial growth factor in 1307 primary breast cancers.
Clin Breast Cancer. 4:340–347. 2003. View Article : Google Scholar
|
|
107
|
Surowiak P, Murawa D, Materna V,
Maciejczyk A, Pudelko M, Ciesla S, Breborowicz J, Murawa P, Zabel
M, Dietel M and Lage H: Occurence of stromal myofibroblasts in the
invasive ductal breast cancer tissue is an unfavourable prognostic
factor. Anticancer Res. 27:2917–2924. 2007.PubMed/NCBI
|
|
108
|
Yiangou C, Gomm JJ, Coope RC, Law M,
Luqmani YA, Shousha S, Coombes RC and Johnston CL: Fibroblast
growth factor 2 in breast cancer: Occurrence and prognostic
significance. Br J Cancer. 75:28–33. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Granato AM, Nanni O, Falcini F, Folli S,
Mosconi G, De Paola F, Medri L, Amadori D and Volpi A: Basic
fibroblast growth factor and vascular endothelial growth factor
serum levels in breast cancer patients and healthy women: Useful as
diagnostic tools? Breast Cancer Res. 6:R38–R45. 2004. View Article : Google Scholar :
|
|
110
|
Faridi A, Rudlowski C, Biesterfeld S,
Schuh S, Rath W and Schröder W: Long-term follow-up and prognostic
significance of angiogenic basic fibroblast growth factor (bFGF)
expression in patients with breast cancer. Pathol Res Pract.
198:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Smith K, Fox SB, Whitehouse R, Taylor M,
Greenall M, Clarke J and Harris AL: Upregulation of basic
fibroblast growth factor in breast carcinoma and its relationship
to vascular density, oestrogen receptor, epidermal growth factor
receptor and survival. Ann Oncol. 10:707–713. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Meijer D, Sieuwerts AM, Look MP, van
Agthoven T, Foekens JA and Dorssers LCJ: Fibroblast growth factor
receptor 4 predicts failure on tamoxifen therapy in patients with
recurrent breast cancer. Endocr Relat Cancer. 15:101–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ivanović V, Demajo M, Krtolica K,
Krajnović M, Konstantinović M, Baltić V, Prtenjak G, Stojiljković
B, Breberina M, Nesković-Konstantinović Z, et al: Elevated plasma
TGF-beta1 levels correlate with decreased survival of metastatic
breast cancer patients. Clin Chim Acta. 371:191–193. 2006.
View Article : Google Scholar
|
|
114
|
El-Abd E, El-Tahan R, Fahmy L, Zaki S,
Faid W, Sobhi A, Kandil K and El-Kwisky F: Serum metastasin mRNA is
an important survival predictor in breast cancer. Br J Biomed Sci.
65:90–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tripsianis G, Papadopoulou E, Romanidis K,
Katotomichelakis M, Anagnostopoulos K, Kontomanolis E, Botaitis S,
Tentes I and Kortsaris A: Overall survival and clinicopathological
characteristics of patients with breast cancer in relation to the
expression pattern of HER-2, IL-6, TNF-α and TGF-b1. Asian Pac J
Cancer Prev. 14:6813–6820. 2013. View Article : Google Scholar
|
|
116
|
Zhu X, Xu M, Zhao X, Shen F, Ruan C and
Zhao Y: The detection of plasma soluble podoplanin of patients with
breast cancer and its clinical signification. Cancer Manag Res.
12:13207–13214. 2020. View Article : Google Scholar :
|
|
117
|
Tripsianis G, Papadopoulou E,
Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K,
Kontomanolis E, Tentes I and Kortsaris A: Coexpression of IL-6 and
TNF-α: Prognostic significance on breast cancer outcome. Neoplasma.
61:205–212. 2014. View Article : Google Scholar
|
|
118
|
Cai S, Zheng J, Song H, Wu H and Cai W:
Relationship between serum TGF-β 1, MMP-9 and IL-1β and
pathological features and prognosis in breast cancer. Front Genet.
13:10953382023. View Article : Google Scholar
|
|
119
|
Al-Ashkar N and Zetoune AB: S100A14 serum
level and its correlation with prognostic factors in breast cancer.
J Egypt Natl Canc Inst. 32:372020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yahia S, Tahari Z, Medjdoub A, Tahari FZ,
Bessaih N, Messatfa M, Deblaoui F, Raiah M, Ouldcadi H, Seddiki S
and Sahraoui T: Expression profile of interleukin-6,
4-hydroxy-2-nonenal, and hypoxia-inducible factor 1-α in women with
breast cancer and their association with clinicopathological
parameters. Contemp Oncol (Pozn). 27:14–21. 2023.
|
|
121
|
Panis C, Herrera AC, Victorino VJ, Aranome
AM and Cecchini R: Screening of circulating TGF-β levels and its
clinicopathological significance in human breast cancer. Anticancer
Res. 33:737–742. 2013.PubMed/NCBI
|
|
122
|
Milovanović J, Todorović-Raković N and
Radulovic M: Interleukin-6 and interleukin-8 serum levels in
prognosis of hormone-dependent breast cancer. Cytokine. 118:93–98.
2019. View Article : Google Scholar
|
|
123
|
Wang RX, Ji P, Gong Y, Shao ZM and Chen S:
Value of CXCL8-CXCR1/2 axis in neoadjuvant chemotherapy for
triple-negative breast cancer patients: A retrospective pilot
study. Breast Cancer Res Treat. 181:561–570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Paccagnella M, Abbona A, Michelotti A,
Geuna E, Ruatta F, Landucci E, Denaro N, Vanella P, Lo Nigro C,
Galizia D, et al: Circulating cytokines in metastatic breast cancer
patients select different prognostic groups and patients who might
benefit from treatment beyond progression. Vaccines (Basel).
10:782022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Denys H, Derycke L, Hendrix A, Westbroek
W, Gheldof A, Narine K, Pauwels P, Gespach C, Bracke M and De Wever
O: Differential impact of TGF-beta and EGF on fibroblast
differentiation and invasion reciprocally promotes colon cancer
cell invasion. Cancer Lett. 266:263–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kjær IM, Olsen DA, Brandslund I, Bechmann
T, Jakobsen EH, Bogh SB and Madsen JS: Prognostic impact of serum
levels of EGFR and EGFR ligands in early-stage breast cancer. Sci
Rep. 10:165582020. View Article : Google Scholar : PubMed/NCBI
|