Introduction
Non-small cell lung cancer (NSCLC) is a prevalent
malignancy that represents a significant global health concern
(1). There were >1.5 million
new cases of lung cancer from 2010 to 2017, of which ~85.3% were
NSCLC (2). Patients with NSCLC
often face challenges that are associated with the limited
treatment options available and poor prognoses, and these severely
impact survival outcomes (3).
Despite the advancements that have been made in terms of diagnostic
and therapeutic strategies, the overall survival rates for NSCLC
remain low at ~26.4% (2), due to
factors such as drug resistance and adverse treatment effects
(4). Therefore, there is an
urgent need to identify novel therapeutic interventions for NSCLC
and to elucidate their underlying mechanisms of action.
It has been previously shown that dietary
polyphenols exert antitumor effects, including their ability to
inhibit cancer initiation and progression (5). Among these compounds, curcumin is a
natural polyphenolic compound derived from turmeric root that
exhibits anti-inflammatory, antioxidant and antitumor
pharmacological activities (6).
In lung cancer research, curcumin has been shown to possess
potential antitumor effects through the modulation of various
targets and pathways, such as STAT3, EGFR and PI3K/Akt/mTOR
(7). In view of these research
findings, we surmised that curcumin may serve as a promising
candidate for NSCLC therapy.
Pyroptosis is a form of programmed cell death that
is regulated by both intra- and extracellular environmental stimuli
(8). This process is
characterized by increased membrane permeability, cellular
organelle destruction and eventual cell lysis and collapse
(9). Cancer cell pyroptosis has
shown promise as a strategy enabling the inhibition of tumor growth
and metastasis (9). A previous
study reported that natural compounds are able to induce pyroptosis
in cancer cells by targeting inflammasomes (10). For example, cucurbitacin B was
shown to inhibit NSCLC progression via Toll-like receptor
4/NOD-like receptor pyrin domain-containing 3 (NLRP3)/gasdermin D
(GSDMD)-dependent cell pyroptosis (11). Moreover, curcumin was also found
to induce pyroptosis in breast cancer cells through NLRP3
inflammasome activation (12).
Furthermore, the NLRP3 inflammasome serves an essential role in
cancer cell pyroptosis (13). In
response to various stress signals, NLRP3 becomes activated and
cleavage of the precursor forms of the inflammatory cytokines IL-1β
and IL-18 into their biologically active forms occurs (14). On the basis of these findings, it
was hypothesized that inducing NLRP3-dependent cell pyroptosis may
represent an effective strategy for inhibiting cancer progression,
including in cases of NSCLC.
Activation of the NLRP3 inflammasome can be
triggered by various stimuli such as pathogenic microorganisms and
danger signals, which complicates efforts to target NLRP3 directly
(15). Nevertheless, emerging
evidence has suggested that modulating NLRP3 ubiquitination and
deubiquitination may offer a promising therapeutic strategy for
influencing activity of the NLRP3 inflammasome (16,17). A previous study demonstrated that
overexpression of Smad ubiquitination regulatory factor 2 (Smurf2)
promotes the ubiquitination of Forkhead box O4 and suppresses
pyroptosis in oxygen-glucose deprivation/reperfusion-induced
cortical neurons (18). Moreover,
the upregulation of Smurf2 expression has been observed to be
associated with the occurrence, progression and migration of lung
cancer (19). However, at
present, limited evidence is available on whether Smurf2 is able to
regulate NLRP3 ubiquitination and influence pyroptosis in NSCLC
cells, highlighting a gap in our current understanding.
The present study aimed to investigate the potential
therapeutic mechanisms underlying the effects of curcumin in NSCLC.
To meet this aim, experiments utilizing NSCLC cells were performed
and animal models were established, to investigate both the effects
of curcumin on Smurf2 activity and its association with NLRP3
signaling and cell pyroptosis. In addition, through examining the
interplay between NLRP3-dependent pyroptosis and Smurf2 regulation,
the present study sought to identify novel therapeutic targets for
NSCLC.
Materials and methods
Cell culture and treatment
The human lung epithelial cell line BEAS-2B
(iCell-h023; iCell) was cultured in DMEM (cat. no. D5796;
MilliporeSigma) supplemented with 10% Gibco® FBS (cat.
no. 10099141; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (P/S) (cat. no. SV30010; Beyotime Institute
of Biotechnology). The NSCLC cell lines A549 (cat. no. CL-0016) and
NCI-H1299 (cat. no. CL-0165; both purchased from Procell Life
Science & Technology Co., Ltd.) were grown in F-12K medium
(cat. no. iCell-0007; iCell) and RPMI-1640 medium (cat. no. R8758;
MilliporeSigma) respectively, both supplemented with 10% FBS and 1%
P/S. All cells were maintained at 37°C in a humidified atmosphere
containing 5% CO2.
Pyroptosis assay
Pyroptosis was detected using flow cytometric
analysis. To evaluate the role of curcumin in NSCLC cell
pyroptosis, the cells were treated with various concentrations of
curcumin (0, 2.5, 5, 10, 20 and 40 μM) for 24 h at 37°C to
determine the optimal concentration (20). Curcumin was dissolved in 0.5% DMSO
(21) and DMSO concentrations
<1% were investigated to confirm a lack of any significant
cytotoxicity (22). Subsequently,
the NSCLC cells were categorized into the following groups: i) The
control (untreated) group; ii) the curcumin (20 μM curcumin;
cat. no. 78246; MilliporeSigma) group; iii) the curcumin + VX-765
[20 μM curcumin with 20 μM VX-765 (23)] group; iv) the curcumin + INF39 [20
μM curcumin with 10 μM INF39 (24)] group; and v) the curcumin +
disulfram (DSF) [20 μM curcumin with 30 μM DSF
(25)] group. VX-765 (cat. no.
HY-13205), INF39 (cat. no. HY-101868) and DSF (cat. no. HY-B0240;
all purchased from MedChemExpress) were used as inhibitors of
caspase-1, NLRP3 and GSDMD, respectively. Cells were treated with
curcumin alone or in combination with VX-765/INF39/DSF for 24 h at
37°C.
Effect of curcumin on NLRP3
expression
To investigate the effect of curcumin (20 μM)
on NLRP3, NSCLC cells were divided into four groups, namely, the
control, curcumin, MG132 (cat. no. HY-13259; MedChemExpress) and
MG132 + curcumin groups. NLRP3 protein expression levels were
detected by western blotting. Previous studies have reported that
MG132 is a proteasome inhibitor (26, 27). Cells in the MG132 group were
treated with 10 μM MG132 for 6 h at 37°C, whereas those in
the MG132 + curcumin group were treated with 10 μM MG132 for
6 h at 37°C, followed by the application of 20 μM curcumin.
To further explore the association between NLRP3 and curcumin,
NLRP3 expression was knocked down using short interfering RNAs
(siRNAs; si). NSCLC cells were divided into four additional groups,
namely, the control, curcumin, curcumin+si-negative control (NC)
and curcumin+si-NLRP3 groups. The siRNA sequences used were as
follows: the sense si-NC, 5′-TTCTCCGAACGTGTCACGT-3′ and antisense
si-NC, 5′-ACGTGACACGTTCGGAGAA-3′; and the sense si-NLPR3,
5′-CCGTAAGAAGTACAGAAAGTA-3′ and the antisense si-NLPR3,
5′-TACTTTCTGTACTTCTTACGG-3′. These siRNAs were provided by
Honorgene. Cells in the si-NC and si-NLRP3 groups were first
transfected with non-targeting siRNA (si-NC) or NLRP3-specific
siRNA (si-NLRP3) respectively, prior to subsequent treatment with
20 μM curcumin for 24 h at 37°C.
Effect of Smurf2 on NLRP3 and
pyroptosis
NLRP3 protein expression levels and pyroptosis were
detected by western blotting and flow cytometric analysis,
respectively. NSCLC cells were divided into four groups, namely,
the control, overexpression (oe)-NC, oe-Smurf2 and oe-Smurf2 +
MG132 groups. Cells in the oe-NC and oe-Smurf2 groups were
transfected with either an empty vector (oe-NC) or a Smurf2
overexpression plasmid (oe-Smurf2), respectively. The plasmid
backbone of oe-Smurf2 was pUC19 (cat. no. N3041L; New England
BioLabs, Inc.). For the oe-Smurf2 + MG132 group, cells were first
transfected with oe-Smurf2 and subsequently treated with 10
μM MG132 for 6 h at 37°C. Additionally, to further explore
the relationship between Smurf2 and NLRP3, cells were categorized
into five groups, namely, the control, si-NC, si-Smurf2, si-Smurf2
+ si-NC and si-Smurf2 + si-NLRP3 groups. The sense sequence of
si-Smurf2 was 5′-GCTGGATTTCTCGGTTGTGTT-3′ and the antisense
si-Smurf2 sequence was 5′-ACAACACCGAGAAATCCAGC-3′. These siRNAs
were provided by Honorgene. Cells in the si-NC and si-Smurf2 groups
were transfected with si-NC or si-Smurf2, respectively, whereas for
the si-Smurf2 + si-NC and si-Smurf2 + si-NLRP3 groups, cells were
co-transfected with si-Smurf2 together with either si-NC or
si-NLRP3.
Association between Smurf2 and
curcumin
To investigate the association between Smurf2 and
curcumin (20 μM), NSCLC cells were separated into six
groups; namely, the control, curcumin, curcumin + si-NC, curcumin +
si-Smurf2, curcumin + oe-NC and curcumin + oe-Smurf2 groups. The
results of this experiment were determined by western blotting and
flow cytometric analysis. Cells in the si-NC and si-Smurf2 groups
were transfected with si-NC or si-Smurf2, respectively, whereas
cells in the oe-NC and oe-Smurf2 groups were transfected with
either empty vector or the Smurf2 overexpression plasmid. All
groups were treated with 20 μM curcumin following
transfection for 24 h at 37°C.
Cell transfection
Cells were transfected with the plasmids using
Lipofectamine™ 2000 transfection reagent (cat. no. 11668019; Thermo
Fisher Scientific, Inc.). Samples were prepared with 95 μl
of serum-free medium per tube, then 5 μl of siRNA (3
μg) and 5 μl of Lipofectamine™ 2000 were added to the
samples, respectively. After incubating at 22°C for 5 min, the
samples were mixed and incubated at 22°C for 20 min. Finally, the
mixture was homogenized into the transfected wells and incubated at
37°C for 6 h. Subsequent experiments were performed after 48 h of
transfection.
Animal experiments
Male nude mice (weight, 14-15 g; aged, 4 weeks) were
obtained from Hunan SJA Laboratory Animal Co., Ltd. The mice were
kept in an environment of 25±2°C, 50±5% humidity and a 12-hour
light-dark cycle with free access to food and water. After a 7 day
acclimatization period, NSCLC in vivo models were
established as described previously (28). Briefly, 2×106 A549
cells (100 μl) were resuspended in PBS (cat. no. AWC0409;
Changsha Abiwei Biotechnology Co., Ltd.) and injected
subcutaneously into the dorsal side of each mouse. The total number
of mice used was 30. The tumor volume was recorded every 3 days
using the formula: Volume=0.5 × length × width2. The
maximum tumor volume we observed was 999.35 mm3. At 7
days following injection, the formation of tumors was confirmed and
intervention procedures were subsequently initiated.
To investigate the association between curcumin and
NLRP3 in vivo, the mice were divided into four groups
(n=3/group), namely, the model, model + curcumin,
modelsi-NC + curcumin and modelsi-NLRP3 +
curcumin groups. The model group comprised untreated NSCLC nude
mice, whereas the model + curcumin group received curcumin
treatment via intraperitoneal injection (30 mg/kg) once daily for
14 consecutive days (29). The
modelsi-NC + curcumin and modelsi-NLRP3 +
curcumin groups were established by injecting 2×106 A549
cells (100 μl) transfected with si-NC or si-NLRP3 into the
mice, followed by the same curcumin treatment regimen.
Furthermore, to evaluate the correlation between
curcumin and Smurf2 in vivo, the mice were separated into
six groups (n=3/group), namely, the model, model+curcumin,
modelsi-NC + curcumin, modelsi-Smurf2 +
curcumin, modeloe-NC + curcumin and
modeloe-Smurf2 + curcumin groups. In these groups, the
nude mice were injected with A549 cells (2×106 cells in
100 μl) transfected with si-NC or si-Smurf2 or oe-NC or
oe-Smurf2 to initiate tumorigenesis, followed by subsequent
curcumin intervention.
At the end of the experiments, the mice were
euthanized by intravenous injection with 150 mg/kg sodium
pentobarbital (30) (cat. no.
P3761; Merck KGaA). Mice were determined to be dead when their
breathing and heartbeat stopped and their pupils dilated. The
humane endpoint we used was a tumor volume that reached 10% of the
mouse's body weight (approximately 2,000 mm3). There
were no animals that reached the humane endpoint before the end of
the experiment. Tumors and peripheral blood samples from the tail
vein (~0.5 ml) were collected for subsequent analyses. All
experimental procedures were approved by the Medical Ethics
Committee of the Second Affiliated Hospital, University of South
China (approval no. NHFE2022010601).
Cell counting kit-8 (CCK-8) assay
For the CCK-8 assay, cells were digested by
trypsin-based digestive solution (cat. no. AWC0232; Changsha Abiwei
Biotechnology Co., Ltd.) and seeded at a density of
5×103 cells/well, with three replicate wells set up for
each group. Each well was supplemented with 100 μl medium
containing 10% CCK-8 solution (cat. no. NU679; Dojindo
Laboratories, Inc.). The cells were incubated at 37°C in an
atmosphere containing 5% CO2 atmosphere for 4 h.
Following incubation, the optical density at 450 nm was measured
using a microplate reader (cat. no. MB-530; Shenzhen Hele
Technology Co., Ltd.).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
Cell proliferation was assessed using an EdU assay
kit (cat. no. C10310; Guangzhou RiboBio Co., Ltd.). EdU solution
was diluted 1:1,000 in cell culture medium and the cells
(5×103 cells/well) were subsequently incubated with 100
μl medium containing 50 μM EdU overnight at 22°C.
Cells were then fixed at 22°C for 30 min using the fixative
provided in the kit and stained sequentially with 1X Apollo
staining reaction solution and 1X Hoechst 33342 reaction solution
for 1.5 h at room temperature, protected from the light.
Subsequently, images were captured using a fluorescence microscope
(cat. no. BA210T; Motic Microscopes) and the proliferation rate was
calculated as the ratio of EdU-positive cells to Hoechst-stained
cells (proliferation rate=EdU:Hoechst) using the ImageJ software
(version 1.8.0.112; National Institutes of Health).
Transwell assay
For the migration assay, 500 μl Complete™
medium (cat. no. AW-MC028; Changsha Abiwei Biotechnology Co., Ltd.)
containing 10% FBS was added to the lower chamber of a Transwell
plate (cat. no. 3428; Corning, Inc.). NSCLC cells undergoing drug
treatment were resuspended at a concentration of 2×106
cells/ml and 100 μl cell suspension was placed in the upper
chamber, without serum. Following incubation at 37°C for 48 h,
cells in the upper chamber were removed and the cells on the
underside of the membrane that had migrated were transferred to
clean wells. The cells were stained with 0.1% crystal violet (cat.
no. AWC0333; Shanghai Biotechwell Co., Ltd.) at 22°C for 5 min,
washed with water, visualized using a fluorescence microscope (cat.
no. BA210T; Motic Microscopes) and analyzed using ImageJ software
(version 1.8.0.112; National Institutes of Health).
For the invasion assay, the EP tubes, Matrigel™
(cat. no. 354262; Becton, Dickinson and Company) and Transwell
inserts were preconditioned at 4°C overnight. On the day of the
experiment, Matrigel was diluted to a final concentration of 200
μg/well and added to the upper chamber. Following incubation
at 37°C for 30 min, the supernatant was aspirated and the remaining
steps were performed as described for the migration assay above.
The invasive cells were stained with crystal violet at 22°C for 5
min, visualized using a fluorescence microscope and analyzed using
ImageJ software (version 1.8.0.112; National Institutes of
Health).
Flow cytometric analysis
Cell cycle assay
NSCLC cells (2×106 cells/ml) were
resuspended in pre-cooled PBS and fixed by adding pre-cooled 100%
ethanol dropwise to a final concentration of 75%. The samples were
stored at 4°C overnight. The following day, ethanol was removed by
centrifugation at 400 × g for 5 min at 4°C and the cells were
resuspended in pre-cooled PBS. Cells were subsequently stained with
PI (cat. no. MB2920; Dalian Meilune Biology Technology Co., Ltd.)
working solution at 4°C for 30 min in the dark. The cell cycle
distribution was analyzed using a flow cytometer (cat. no.
A00-1-1102; Beckman Coulter, Inc.) and the percentage of cells in
each phase was determined from the PI fluorescence histogram.
GraphPad Prism 8 (version 8.0.2.263; Dotmatics) was used to produce
the PI fluorescence histogram.
Detection of cell pyroptosis
NSCLC cells (2×106 cells/ml) were
collected using trypsin-based digestive solution (cat. no. AWC0232;
Changsha Abiwei Biotechnology Co., Ltd.) for 5 min at 22°C and
washed thoroughly. The cells were subsequently treated with
caspase-1 working solution (cat. no. ab219935; Abcam) for 5 min at
22°C, followed by staining with PI for 30 min at 22°C. The mixture
was subsequently incubated at 22°C for 1 h, protected from the
light. Finally, pyroptosis was analyzed using a flow cytometer
(cat. no. A00-1-1102; Beckman Coulter, Inc.) and the GraphPad Prism
8 software (version 8.0.2.263; Dotmatics).
Scanning electron microscopy (SEM)
analysis
NSCLC cells (2×106 cells/ml) were fixed
with 2.5% glutaraldehyde (cat. no. AWI0097; Changsha Abiwei
Biotechnology Co., Ltd.) for 12 h at 22°C and washed using PBS.
Subsequently, cells were treated with 1% osmium acid (cat. no.
AWI0136; Changsha Abiwei Biotechnology Co., Ltd.) for 2 h at 22°C.
Dehydration was performed sequentially using increasing
concentrations of ethanol (30, 50, 70, 80, 90, 95 and 100%; 15
min/concentration), after which the samples were treated with a 1:1
mixture of 100% ethanol and isoamyl acetate (cat. no. 10003118;
Sinopharm Chemical Reagent Co., Ltd.) for 30 min at 22°C. After
overnight drying at 22°C, the samples were observed using an SEM
microscope (Helios 5 UC; Thermo Fisher Scientific, Inc.) and
analyzed using ImageJ software (version 1.8.0.112; National
Institutes of Health).
Western blot analysis
Total protein was extracted from cells
(2×106 cells/ml) and the tumor tissues of nude mice
using 300 μl RIPA lysis buffer (cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with a
protease inhibitor cocktail (cat. no. AWH0645) and a protein
phosphatase inhibitor (cat. no. AWH0650; both purchased from
Changsha Abiwei Biotechnology Co., Ltd.). The BCA protein
quantification kit (cat. no. AWB0156, Changsha Abiwei Biotechnology
Co., Ltd) was utilized to determine the protein concentration. The
densitometry was performed using GraphPad Prism 8 software (version
8.0.2.263; Dotmatics). Protein samples (20 μg) were used for
electrophoresis. The constant voltage of electrophoresis was 75 V
for 130 min. Subsequently, the proteins were separated using
SDS-PAGE (12% gel) and transferred to PVDF membranes pre-treated
with methanol (99.8%) for 15 sec at 22°C. The membranes were
blocked with 5% skimmed milk for 90 min at 22°C and incubated with
primary antibodies (Table I) at
4°C overnight. β-actin was used as the internal reference control.
Subsequently, the membranes were incubated with secondary
antibodies (Table I) at 37°C for
1.5 h. Protein bands were visualized using ECL Plus super-sensitive
luminescent liquid (cat. no. K-12049-D50; Advansta, Inc.) and
imaged using a chemiluminescence imaging system (ChemiScope6100;
Clinx Science Instruments Co., Ltd.).
 | Table IAntibodies used in the present
study. |
Table I
Antibodies used in the present
study.
| Antibody
target | Cat. no. | Dilution | Supplier |
|---|
| NOD-like receptor
pyrin domain-containing 3 | ab263899 | 1:1,000 | Abcam |
| Caspase-1 | ab207802 | 1:1,000 | Abcam |
| Gasdermin D
(GSDMD)/cleaved GSDMD | ab225867 | 1:1,000 | Abcam |
| Smad ubiquitination
regulatory factor 2 | AWA55626 | 1:1,000 | Changsha Abiwei
Biotechnology Co., Ltd. |
| β-actin | 66009-1-Ig | 1:5,000 | Proteintech Group,
Inc. |
| HRP goat anti-mouse
IgG | SA00001-1 | 1:5,000 | Proteintech Group,
Inc. |
| HRP goat
anti-rabbit IgG | SA00001-2 | 1:6,000 | Proteintech Group,
Inc. |
To assess the protein stability of NLRP3, cells were
treated with the protein synthesis inhibitor cycloheximide (CHX) at
a concentration of 100 μg/ml (cat. no. 583794; Gentihold)
for 0, 4, 8 or 12 h at 4°C (31).
Following CHX treatment, proteins were analyzed using the standard
western blotting procedure described above.
Co-immunoprecipitation (Co-IP)
assay
The cells were washed and lysed using 400 μl
of IP cell lysate (cat. no. AWB0144; Changsha Abiwei Biotechnology
Co., Ltd.). The lysate was subsequently centrifuged at 4,000 × g
for 15 min at 4°C to isolate the proteins, which were then
incubated at 4°C overnight with NLRP3 antibodies (cat. no.
ab263899; Abcam) which was diluted by PBST containing 0.05% Tween
20 (cat. no. AWI0130, Changsha Abiwei Biotechnology Co., Ltd).
Subsequently, Protein A/G agarose beads (20 μl) were added
to capture the antigen-antibody complexes and the mixture was
incubated at 4°C for 2 h with gentle shaking. The agarose beads
were washed four times with 400 μl of IP lysate and the
final precipitate was collected. Following Co-IP assay, both the
ubiquitination level of NLRP3 and the expression levels of NLRP3
and Smurf2 were analyzed using western blotting analysis, as
described above.
ELISA
The concentrations of IL-1β in the cell supernatants
and animal serum samples were measured using either a human IL-1β
ELISA kit (cat. no. CSB-E08053h) or a mouse IL-1β ELISA kit (cat.
no. CSB-E08054m; both kits purchased from Cusabio Technology, LLC),
following the manufacturer's instructions. Similarly, the levels of
IL-18 were determined using a human IL-18 ELISA kit (cat. no.
CSB-E07450h) or mouse IL-18 ELISA kit (cat. no. CSB-E04609m; both
kits purchased from Cusabio Technology, LLC).
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from NSCLC cell lines
(2×106 cells/ml) using TRIzol® reagent (cat.
no. 15596026; Invitrogen; Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. RNA was subsequently
reverse transcribed into cDNA using an mRNA reverse transcription
kit (cat. no. CW2569; Cwbio). The relative expression levels of
NLRP3 and Smurf2 were determined using the UltraSYBR Mixture (cat.
no. CW2601; Cwbio) on an ABI 7900 system (cat. no. QuantStudio1;
Thermo Fisher Scientific, Inc.). The thermocycling conditions used
were as follows: Denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 30 sec. The
2−ΔΔCq method was employed to calculate the relative
mRNA levels of target genes (32). β-actin used as the internal
reference control and the specific primer sequences are shown in
Table II.
 | Table IIPrimer sequences used in the present
study. |
Table II
Primer sequences used in the present
study.
| Gene | Sequence
(5′-3′) | Product length,
bp |
|---|
| H-NOD-like receptor
pyrin domain-containing 3 | F:
GCCACGCTAATGATCGACT | 170 |
| R:
TCTTCCTGGCATATCACAGT | |
| H- Smad
ubiquitination regulatory factor 2 | F:
CATGTCTAACCCCGGAGGC | 138 |
| R:
TGCCCAGATCCATCAACCAC | |
| H-β-actin | F:
ACCCTGAAGTACCCCATCGAG | 224 |
| R:
AGCACAGCCTGGATAGCAAC | |
Hematoxylin and eosin (H&E)
staining
Tumor tissues from nude mice were treated with
xylene (cat. no. 10023418; Sinopharm Chemical Reagent Co., Ltd.)
for 20 min at 22°C, fol lowed by sequential immersion in a series
of decreasing ethanol solutions (100, 100, 95, 85 and 75%; 5 min
each). After rinsing the tissues in distilled water for 5 min,
tissue sections were stained with hematoxylin (cat. no. AWI0009a;
Changsha Abiwei Biotechnology Co., Ltd.) for 10 min at 22°C, and
subsequently with eosin (cat. no. G1100; Beijing Solarbio Science
& Technology Co., Ltd.) for 5 min at 22°C. The sections were
dehydrated using gradient ethanol (95-100%), treated with xylene
for 10 min at 22°C, mounted with neutral gum (cat. no. ZLI-9555;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) and imaged using
an optical microscope (cat. no. BA310E; Motic Microscopes).
Molecular docking analysis
Molecular docking analysis of curcumin with the
Smurf2 protein was performed using VINA software (version 1.1.2;
Scripps Research). PyMOL software (version 2.3; Schrödinger) was
used to visualize binding of the ligand to the receptor-binding
pocket and to predict receptor-ligand interactions and binding
energies.
Immunohistochemical analysis
Tumor tissues from nude mice were fixed in 4%
paraformaldehyde for 30 min at 22°C. Paraffin sections were 4-5 μm
thick. The tissues were treated sequentially with xylene and
ethanol of decreasing concentrations (100, 95, 85 and 75%). The
tissues were subsequently immersed in 0.01 mol/l citrate buffer
(cat. no. ZLI-9065; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) and microwaved until the solution began to boil. After
cooling to room temperature, the sections were treated with 1%
periodic acid (cat. no. BSBA-4245; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) to inactivate endogenous enzymes for 15
min at room temperature. The sections were blocked with 3% BSA
(cat. no. AWT0368a, Changsha Abiwei Biotechnology Co., Ltd) at 22°C
for 30 min. The primary antibodies against Ki67 (cat. no.
28074-1-AP; 1:400; Proteintech Group, Inc.) were applied and
incubated at 4°C overnight. Subsequently, the sections were
incubated with HRP-conjugated goat anti-rabbit IgG secondary
antibodies (1:1,000; Table I) at
37°C for 30 min, after which the sections were treated with a DAB
working solution (cat. no. ZLI-9017; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) and counterstained with hematoxylin at
22°C for 5 min. The tissues were subsequently dehydrated with
ethanol in ascending concentrations (60-100%), treated with xylene
at 22°C for 10 min, mounted for fluorescence microscopic (cat. no.
BA210T; Motic Microscopes) examination and analyzed using the
ImageJ software (version 1.8.0.112; National Institutes of
Health).
Bioinformatics analysis
To predict which ubiquitin ligases are associated
with NLRP3, bioinformatics analysis was performed using the
UbiBrowser 1.0 database (http://ubibrowser.bio-it.cn/ubibrowser/). The data
were downloaded from the public repository on the UbiBrowser 1.0
database (http://ubibrowser.bio-it.cn/ubibrowser/strict/networkview/networkview/name/Q96P20/jobId/ubibrowse-I2025-02-06-15867-1738804849).
The significance cut-off level used was P<0.05.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 software (version 8.0.2.263; Dotmatics). All experiments
were performed in triplicate and data are expressed as the mean±SD.
For data following a normal distribution, comparisons between two
groups were performed using the unpaired t-test, whereas
comparisons among multiple groups were performed using a one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Curcumin inhibits the growth of NSCLC
cells by inducing pyroptosis
To evaluate the role of curcumin in the pyroptosis
of NSCLC cells, the IC50 of curcumin, reflecting its
effect on cell growth, was determined (33). The results showed that the
IC50 for curcumin in the experiments performed using
BEAS-2B cells was 233.6 μM, whereas the IC50
values for A549 and NCI-H1299 cells were 14.85 and 13.14 μM,
respectively. To effectively inhibit the target activity in the
specific cell lines under investigation, the concentration of 20
μM for curcumin was selected for subsequent experiments
(Fig. 1A).
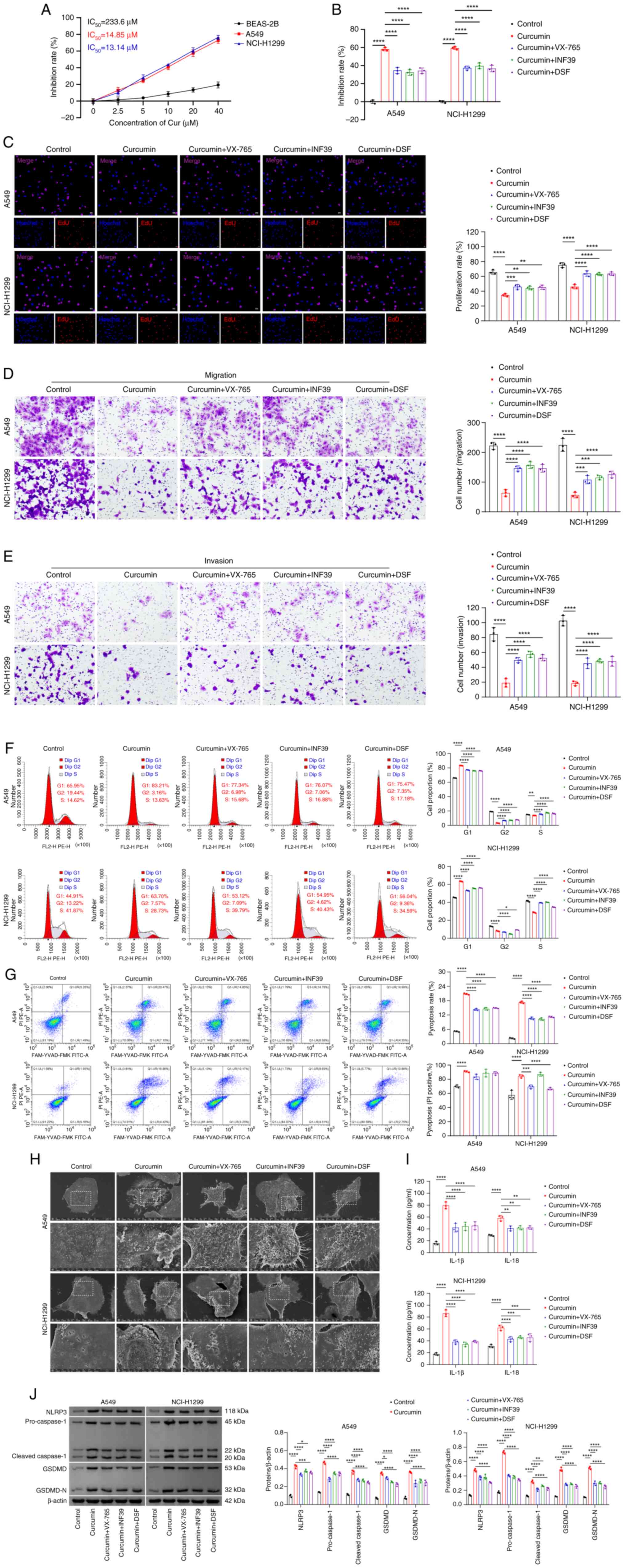 | Figure 1Curcumin inhibits the growth of NSCLC
cells by inducing pyroptosis. (A) The IC50 value of
curcumin was determined by CCK-8 assay. (B) Cell viability of NSCLC
cells was assessed using the CCK-8 assay. (C) NSCLC cell
proliferation was analyzed via EdU assay. Scale bar, 50 μm.
Cell migration (D) and invasion (E) were evaluated by Transwell
assays. Scale bar, 100 μm. (F) Cell cycle distribution was
analyzed by flow cytometric analysis. (G) Pyroptosis detection was
performed via flow cytometry, where UR/(UL+UR) represents the
percentage of cell death that corresponds to death by pyroptosis.
(H) Pyroptosis-associated morphological characteristics were
observed by scanning electron microscopy. Top scale bar, 10
μm and bottom scale bar, 5 μm. (I) Levels of IL-1β
and IL-18 in cell supernatants were measured using ELISA kits. (J)
The protein expression levels of NLRP3, caspase-1, GSDMD and
GSDMD-N were detected by western blotting analysis.
*P<0.05; **P<0.01;
***P<0.001; and ****P<0.0001. NSCLC,
non-small cell lung cancer; NLRP3, NOD-like receptor pyrin
domain-containing 3; GSDMD, gasdermin D; DSF, disulfram; EdU,
5-ethynyl-2′-deoxyuridine; GSDMD-N, cleaved GSDMD; CCK-8, Cell
Counting Kit-8; UR, upper right; UL, upper left. |
To further investigate the association between
curcumin and pyroptosis, NSCLC cells were treated either with
curcumin alone or in combination with inhibitors of caspase-1
(VX-765), NLRP3 (INF39) or GSDMD (DSF). These results demonstrated
that treatment with curcumin led to a significant reduction in the
viability (Fig. 1B),
proliferation (Fig. 1C),
migration (Fig. 1D) and invasion
(Fig. 1E) of NSCLC cells compared
with untreated controls. However, inhibition of caspase-1, NLRP3 or
GSDMD reversed the effects of curcumin on the viability (Fig. 1B), proliferation (Fig. 1C), migration (Fig. 1D) and invasion (Fig. 1E) of cells. Additionally, curcumin
intervention resulted in cell cycle arrest in NSCLC cells, which
was also reversed by treating the cells with the inhibitors of
caspase-1, NLRP3 and GSDMD (Fig.
1F).
Furthermore, treatment with curcumin increased the
levels of cell pyroptosis and cell death via pyroptosis when
compared with the control group (Fig.
1G). The morphological changes characteristic of pyroptosis,
including increased cell swelling, reduced cytoplasmic density and
pore formation in the cell membrane, were observed in the
curcumin-treated NSCLC cells (Fig.
1H). These changes induced by curcumin, however, were markedly
attenuated when caspase-1, NLRP3 or GSDMD inhibitors were added
(Fig. 1G and H). Furthermore, the
levels of the pyroptosis-associated cytokines, IL-1β and IL-18, in
the cell supernatant were increased upon treatment with curcumin
(Fig. 1I). Similarly, curcumin
treatment increased the expression levels of pyroptosis-associated
proteins, including NLRP3, caspase-1, GSDMD and GSDMD-N, but
reduced these expression levels upon inhibition of caspase-1, NLRP3
or GSDMD (Fig. 1J). Taken
together, these findings suggested that curcumin suppresses the
growth of NSCLC cells, potentially through the induction of
pyroptosis.
Curcumin induces pyroptosis in NSCLC cell
lines and cancerous tissues from NSCLC nude mice via upregulation
of NLRP3
Given the close association between NLRP3 and cell
pyroptosis (34), the present
study aimed to further investigate the association between curcumin
and NLRP3. The stability and ubiquitination levels of NLRP3 were
assessed in NSCLC cells. The results obtained demonstrated that
curcumin increased the protein stability of NLRP3, while reducing
its ubiquitination levels (Fig. 2A
and B). Moreover, curcumin increased NLRP3 protein expression
levels and inhibited its degradation, similar to the effects
exhibited by MG132, a proteasome inhibitor (Fig. 2C). The combination of curcumin and
MG132 resulted in a higher level of NLRP3 expression compared with
curcumin alone, demonstrating that curcumin promotes NLRP3
expression through reducing its ubiquitination, and that this
effect is dependent on the proteasomal degradation pathway.
 | Figure 2Curcumin induces pyroptosis in NSCLC
cell lines and NSCLC nude mice cancer tissues via the upregulation
of NLRP3. (A) NLRP3 protein stability was assessed by western
blotting analysis. (B) The ubiquitination of NLRP3 was evaluated by
Co-IP assay. (C) NLRP3 protein expression levels were measured by
western blotting analysis. (D) NLRP3 knockdown efficiency was
assessed by reverse transcription-quantitative PCR and western
blotting analyses. (E) Pyroptosis was detected by flow cytometry,
with UR/(UL+UR) representing the percentage of cell death that
corresponds to death by pyroptosis. (F) The protein expression
levels of NLRP3, caspase-1, GSDMD and GSDMD-N in cells were
detected by western blotting analysis. (G) IL-1β and IL-18 levels
in cell supernatants was measured by ELISA. (H) Representative
images of the nude mice excised tumors were presented. (I) The
tumor volumes were measured. (J) Pathological changes in tumor
tissues were analyzed by hematoxylin and eosin staining. Top scale
bar, 100 μm and bottom scale bar, 25 μm. (K) The
tumor weights were measured. (L) Protein expression levels of
NLRP3, caspase-1, GSDMD and GSDMD-N in tumors were detected by
western blotting analysis. (M) Serum concentrations of IL-1β and
IL-18 were determined using ELISA kits. *P<0.05;
**P<0.01; ***P<0.001; and
****P<0.0001. NSCLC, non-small cell lung cancer;
Co-IP, co-immunoprecipitation; NLRP3, NOD-like receptor pyrin
domain-containing 3; GSDMD, gasdermin D; CHX, cycloheximide; si,
short interfering RNA; NC, negative control; GSDMD-N, cleaved
GSDMD; UR, upper right; UL, upper left. |
To further elucidate the role of NLRP3 in curcumin's
action on NSCLC cells, NLRP3 was knocked down in NSCLC cells. Among
the siRNA groups, si-NLRP3#2 exhibited the highest knockdown
efficiency and this was therefore used in subsequent experiments
(Fig. 2D). Compared with the
curcumin + si-NC group, the curcumin + si-NLRP3 group demonstrated
a significant decrease in pyroptosis (Fig. 2E) and was associated with
significant reductions in the protein expression levels of NLRP3,
caspase-1, GSDMD and GSDMD-N in cells (Fig. 2F), as well as marked reductions in
the concentrations of IL-1β and IL-18 in the culture supernatant
(Fig. 2G). Comparing the results
obtained in A549 and NCI-H1299 cells, there was an almost one-fold
difference in the level of change of GSDMD expression, which was
potentially due to the fact that gene or protein expression levels
vary by differing degrees in different types of cells. This finding
may also have been due to the cells' unique properties, or the
regulatory expression mechanisms, such as cell specificity or cell
cycle regulation. Moreover, this approximately one-fold difference
in the GSDMD expression level comparing between the curcumin + siNC
and curcumin + siNLRP3 groups may have resulted from the effect
that knocking down NLRP3 has on reducing the expression levels of
GSDMD and GSDMD-N (Fig. 2F).
Collectively, these findings suggested that NLRP3 knockdown
diminishes the ability of curcumin to induce pyroptosis in NSCLC
cells (Fig. 2E-G).
To corroborate these findings in vivo,
experiments were performed using NSCLC nude mouse models (Fig. 2H). These experiments showed that
treatment with curcumin led to a significant reduction in tumor
volume (Fig. 2I) and weight
(Fig. 2K) compared with the model
group. Moreover, compared with the modelsi-NC+curcumin
group, the knockdown of NLRP3 caused a significant increase in
tumor volume (Fig. 2I) and weight
(Fig. 2K), demonstrating that
NLRP3 exerted a crucial role in mediating the effects of curcumin.
Subsequently, H&E staining further demonstrated that NLRP3
knockdown inhibited curcumin-induced NSCLC cell death in tumor
tissues (Fig. 2J). Consistent
with the in vitro findings, curcumin intervention in tumors
led to a significantly increase in the protein expression levels of
NLRP3, caspase-1, GSDMD and GSDMD-N, as well significant increases
in the concentrations of IL-1β and IL-18 in the serum when compared
with the model group. Knockdown of NLRP3 reversed these effects,
suppressing curcumin-induced pyroptosis in the NSCLC tumors
(Fig. 2L and M). Taken together,
the results from both the in vitro and the in vivo
experiments suggested that curcumin induced pyroptosis in NSCLC
cell lines and tumor tissues via upregulating NLRP3.
NLRP3 stability is regulated via Smurf2
activity
To identify additional potential targets associated
with the stability and ubiquitination of NLRP3, the UbiBrowser 1.0
database was utilized, which predicted that the E3 ligase Smurf2
was involved in NLRP3 ubiquitination (Fig. 3A and B). A previous study also
reported an association between Smurf2 and NSCLC (19). Consistent with the findings of the
aforementioned study, the experimental results from the present
study demonstrated that Smurf2 was highly expressed in NSCLC cells
(Fig. 3C) and that Smurf2 could
interact with NLRP3 (Fig. 3D). To
further investigate the role of Smurf2 in NSCLC, Smurf2 was
overexpressed or knocked down in NSCLC cells. The transfection
efficiencies were confirmed, showing successful overexpression and
knockdown of Smurf2, respectively (Fig. 3E). Overexpression of Smurf2 caused
a significant reduction in NLRP3 protein expression levels,
although this effect was significantly reversed following treatment
with the proteasome inhibitor, MG132 (Fig. 3F). Additionally, Smurf2
overexpression led to an increase in the ubiquitination of NLRP3
(Fig. 3G). Compared with the
si-NC group, knocking down Smurf2 led to a significantly increased
expression level of NLRP3 (Fig.
3H). Moreover, the knockdown of Smurf2 markedly increased both
the activation and cleavage of caspase-1 and GSDMD (Fig. 3H), significantly increasing the
secretion of IL-1β and IL-18 (Fig.
3I) and cell pyroptosis (Fig.
3J) when compared with the si-NC group. However, knocking down
NLRP3 markedly decreased the activation and cleavage of caspase-1
and GSDMD (Fig. 3H), the
secretion of IL-1β and IL-18 (Fig.
3I), and pyroptosis (Fig. 3J)
when compared with the si-Smurf2 + si-NC group. Collectively, these
findings suggested that the inhibition of Smurf2 activity reduced
NLRP3 ubiquitination, thereby promoting its stability and enhancing
cell pyroptosis.
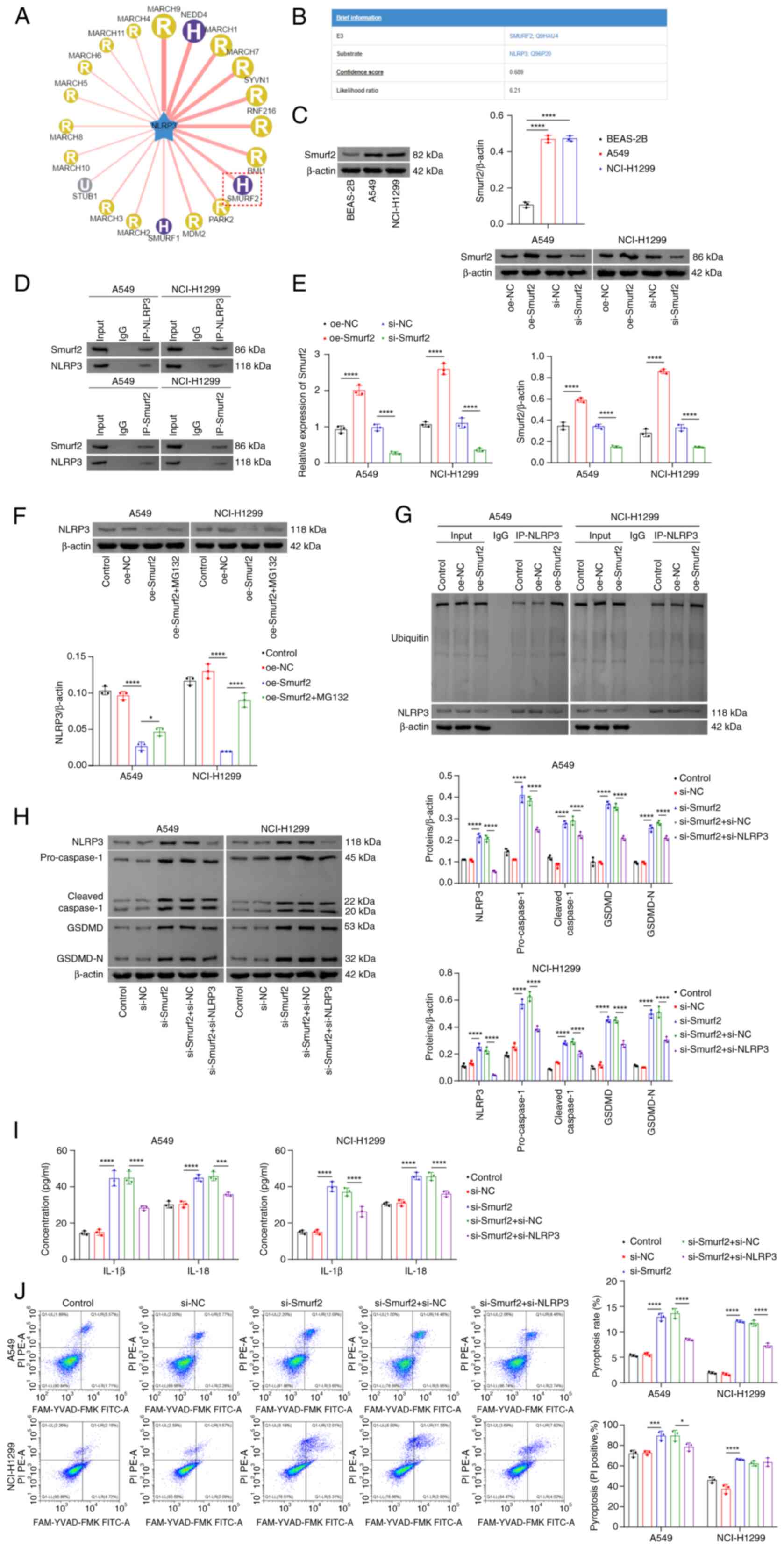 | Figure 3NLRP3 stability is regulated by
Smurf2 activity. (A and B) Prediction of Smurf2 as an E3 ligase for
NLRP3 was detected using the UbiBrowser 1.0 database. (C) Smurf2
protein expression levels were analyzed by western blotting. (D)
Co-IP assay results confirmed the interaction between Smurf2 and
NLRP3. (E) Smurf2 transfection efficiency was determined via both
reverse transcription-quantitative PCR and western blotting
analyses. (F) The detection of NLRP3 protein expression levels was
performed using western blotting analysis. (G) The ubiquitination
of NLRP3 was detected by Co-IP assay. (H) The protein expression
levels of NLRP3, caspase-1, GSDMD and GSDMD-N in cells were
determined using western blotting analysis. (I) Secreted IL-1β and
IL-18 levels were measured using ELISA kits. (J) Flow cytometric
analysis was performed for the detection of cell pyroptosis. The
UR/(UL+UR) represents the percentage of cell death that corresponds
to death by pyroptosis. *P<0.05;
***P<0.001; and ****P<0.0001. NLRP3,
NOD-like receptor pyrin domain-containing 3; Smurf2, Smad
ubiquitination regulatory factor 2; Co-IP, co-immunoprecipitation;
GSDMD, gasdermin D; oe, overexpression; NC, negative control; si,
short interfering RNA; GSDMD-N, cleaved GSDMD; UR, upper right; UL,
upper left. |
Curcumin targets Smurf2 to inhibit NLRP3
ubiquitination and promote pyroptosis
Building on the observed association between NLRP3
and Smurf2 in NSCLC cells, the potential interaction between
curcumin and Smurf2 was subsequently further explored. The chemical
structure of curcumin is shown in Fig. 4A. Molecular docking analysis
confirmed that curcumin could bind to Smurf2, with a binding energy
of −8.5 kcal/mol (Fig. 4B).
Although the interaction between Smurf2 and NLRP3 had already been
demonstrated in the present study, curcumin treatment was found to
reduce the strength of this interaction (Fig. 4C). Additionally, overexpression of
Smurf2 also attenuated NLRP3 expression as a consequence of their
mutual interaction (Fig. 4D).
Moreover, the knockdown of Smurf2 was shown to significantly
enhance the ability of curcumin to stabilize NLRP3 compared with
the curcumin + si-NC group, whereas overexpression of Smurf2
circumvented this effect compared with the curcumin + oe-NC group
(Fig. 4E). Similarly, Smurf2
knockdown markedly augmented the curcumin-induced increase in the
protein expression levels of NLRP3, caspase-1, GSDMD and GSDMD-N,
as well as the secretion levels of IL-1β and IL-18 compared with
the curcumin + si-NC group. On the other hand, overexpression of
Smurf2 reversed these curcumin-induced effects compared with the
curcumin + oe-NC group (Fig. 4F and
G). Furthermore, the knockdown of Smurf2 led to a significant
enhancement in the extent of pyroptosis in NSCLC cells compared
with the curcumin + si-NC group, whereas Smurf2 overexpression
inhibited pyroptosis compared with the curcumin + oe-NC group
(Fig. 4H). Taken together, these
findings suggested that curcumin both inhibited NLRP3
ubiquitination and promoted pyroptosis, in NSCLC cells through
targeting Smurf2.
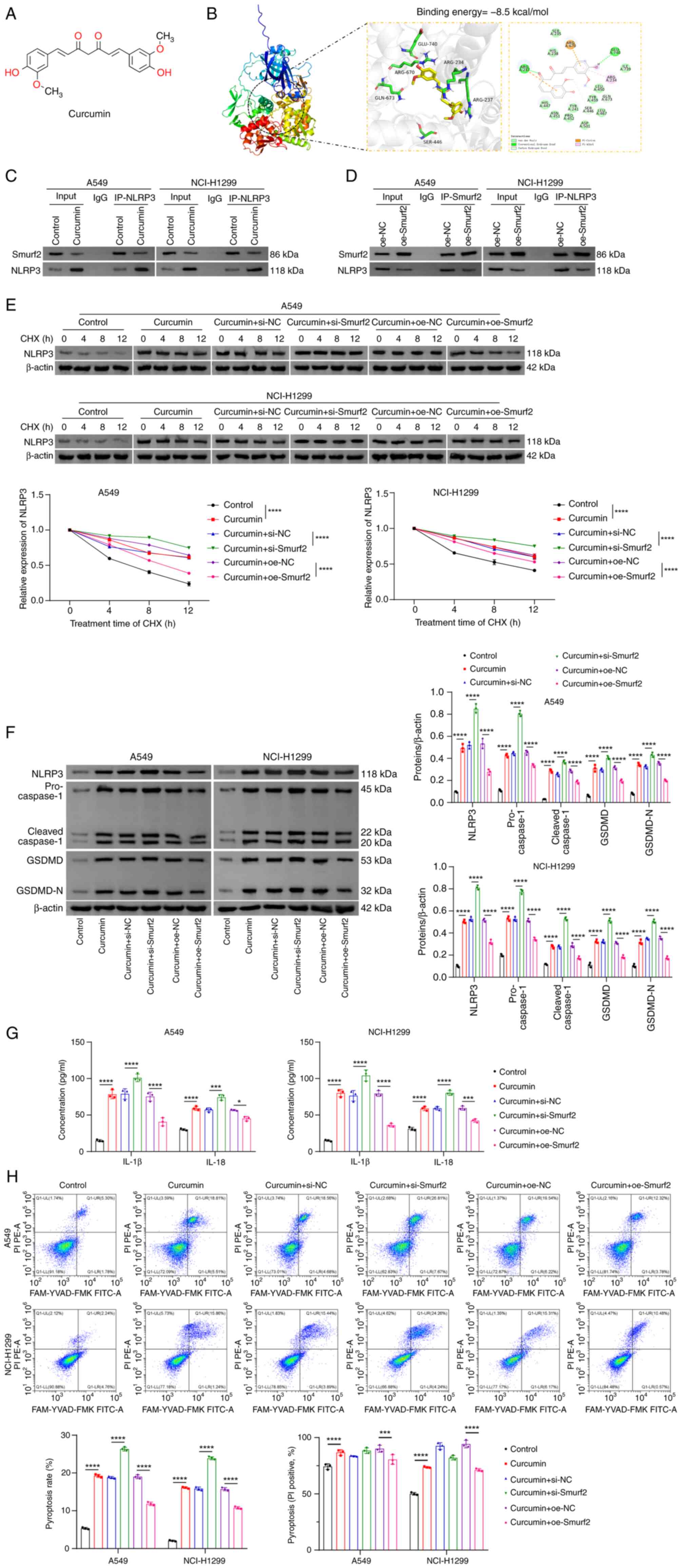 | Figure 4Curcumin targets Smurf2 to inhibit
NLRP3 ubiquitination and promote pyroptosis. (A) The chemical
structure of curcumin. (B) Molecular docking analysis was
performed, showing the binding of curcumin to Smurf2. (C and D)
Co-IP analysis was performed for the interaction strength between
Smurf2 and NLRP3. (C) The immunoprecipitation protein was NLRP3.
(D) The immunoprecipitation protein was Smurf2. (E) The protein
expression levels of NLRP3 were assessed using western blot
analysis. CHX was used to detect the protein stabilities of NLRP3.
(F) The protein expression levels of NLRP3, caspase-1, GSDMD and
GSDMD-N were determined using western blot analysis. (G) The
ability of cells to secrete IL-1β and IL-18 was measured using
ELISA kits. (H) Cell pyroptosis was determined using flow
cytometric analysis. UR/(UL+UR) represents the percentage of cell
death that corresponds to death by pyroptosis.
*P<0.05; ***P<0.001; and
****P<0.0001. Smurf2, Smad ubiquitination regulatory
factor 2; NLRP3, NOD-like receptor pyrin domain-containing 3;
Co-IP, co-immunoprecipitation; IP, immunoprecipitated; GSDMD,
gasdermin D; si, short interfering RNA; NC, negative control; oe,
overexpression; GSDMD-N, cleaved GSDMD; CHX, cycloheximide; UR,
upper right; UL, upper left. |
Curcumin inhibits NSCLC progression
through regulating the Smurf2/NLRP3 axis in vivo
To validate the effects of curcumin on NSCLC
progression in vivo and to assess the involvement of the
Smurf2/NLRP3 axis, experiments were performed using NSCLC
tumor-bearing modelled nude mice (Fig. 5A). These results showed that,
following curcumin intervention, the tumor volumes and weights were
significantly reduced. These effects were further enhanced by
Smurf2 knockdown, although they were significantly reversed upon
overexpressing Smurf2 (Fig. 5B).
Subsequent histological analysis using H&E staining
demonstrated that curcumin treatment promoted NSCLC cell death.
This effect was enhanced by Smurf2 knockdown but inhibited by
Smurf2 overexpression (Fig. 5C).
Consistently with these findings, the expression levels of Ki67, a
marker of cell proliferation (35), was markedly reduced in tumor
tissues following curcumin treatment compared with the model group.
Compared with the modelsi-NC + curcumin group, Smurf2
knockdown led to a further suppression of Ki67 expression, whereas
Smurf2 overexpression caused a significant increase in Ki67 levels
compared with the modeloe-NC + curcumin group.
Collectively, these results suggested that curcumin influenced cell
proliferation in NSCLC tumors via Smurf2 regulation (Fig. 5D and E). Furthermore, Smurf2
knockdown led to a significant augmentation of the curcumin-induced
increases in the protein expression levels of Smurf2, NLRP3,
caspase-1, GSDMD and GSDMD-N, as well as marked increases in the
secretion of IL-1β and IL-18 in tumor tissues compared with the
modelsi-NC + curcumin group. On the other hand, Smurf2
overexpression reversed these curcumin-induced effects compared
with the modeloe-NC + curcumin group (Fig. 5F and G). Taken together, these
findings suggested that curcumin inhibited NSCLC progression in
vivo via regulating the Smurf2/NLRP3 axis, suggesting its
potential use as a therapeutic agent for NSCLC.
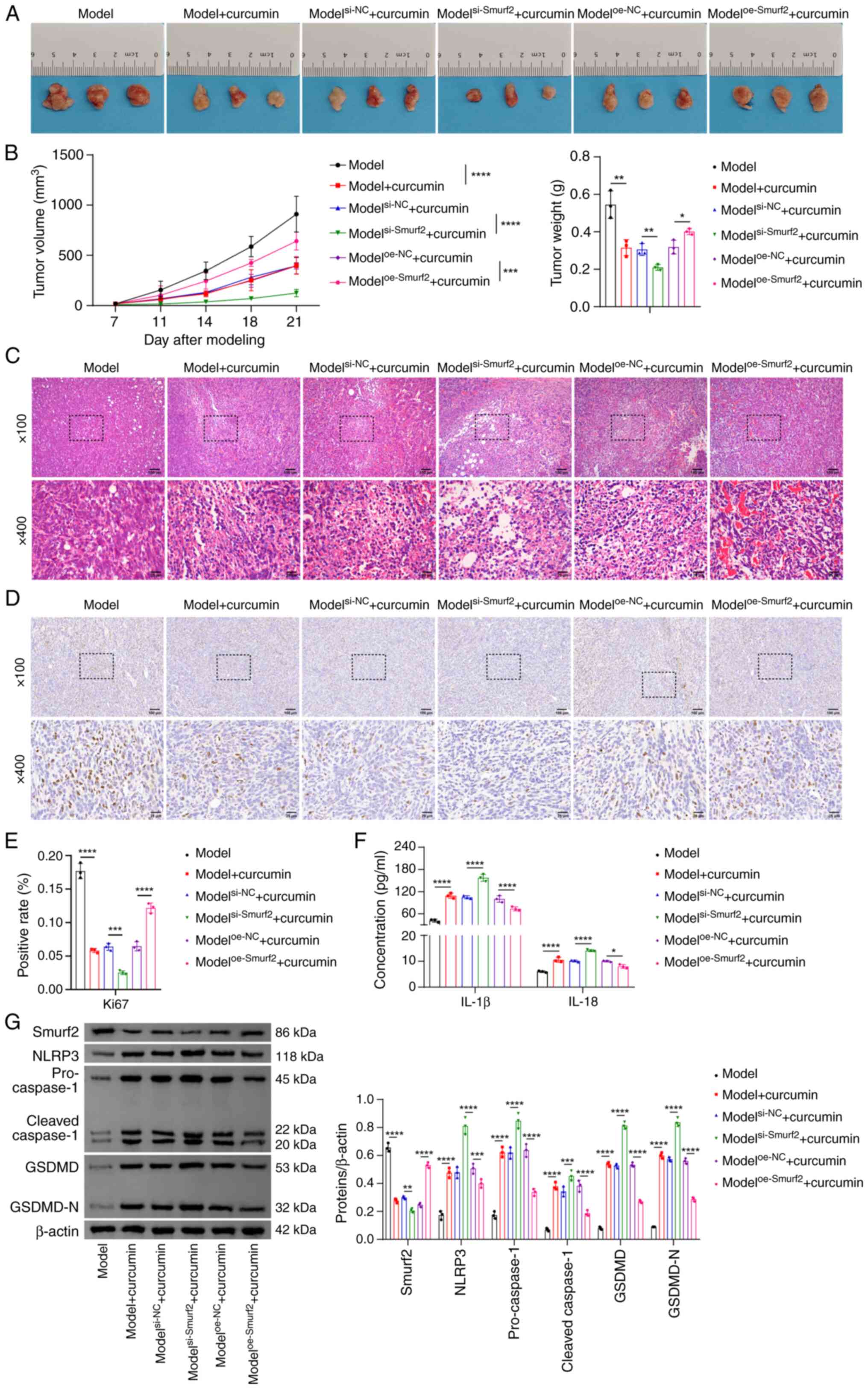 | Figure 5Curcumin inhibits non-small cell lung
cancer progression by regulating the Smurf2/NLRP3 axis in
vivo. (A) Representative images of tumors are shown. (B) Tumor
volume and weight measurements are shown. (C) Pathological
examination of tumor tissues was performed by hematoxylin and eosin
staining. Top scale bar, 100 μm and bottom scale bar, 25
μm. (D and E) Ki67 expression in tumors was assessed using
immunohistochemical analysis. Top scale bar, 100 μmand
bottom scale bar, 25 μm. (F) Serum concentrations of IL-1β
and IL-18 were measured using ELISA kits. (G) The protein
expression levels of Smurf2, NLRP3, caspase-1, GSDMD and GSDMD in
tumors were detected by western blotting analysis.
*P<0.05; **P<0.01;
***P<0.001; and ****P<0.0001. Smurf2,
Smad ubiquitination regulatory factor 2; NLRP3, NOD-like receptor
pyrin domain-containing 3; GSDMD, gasdermin D; GSDMD-N, cleaved
GSDMD; oe, overexpression; NC, negative control; si, short
interfering RNA. |
Discussion
NSCLC remains one of the most prevalent cancers
globally. Although mortality rates are decreasing as a result of
therapeutic advances, they currently remain high (36). The present study demonstrated a
potential therapeutic role of curcumin through performing a series
of in vitro and in vivo experiments. These findings
showed that curcumin promoted NSCLC cell pyroptosis via modulating
the Smurf2/NLRP3 axis, thereby inhibiting tumor progression;
moreover, these results may provide valuable insights into
developing novel strategies for NSCLC therapy.
As a polyphenolic compound, curcumin has shown
significant potential for cancer treatment (37). It has been reported to inhibit
colorectal cancer metastasis through activating the reactive oxygen
species/Kelch-like ECH-associated protein 1/nuclear factor
erythroid 2-related factor 2/microRNA (miR)-34a/b/c pathway
(38). In esophageal squamous
cell carcinoma, curcumin has been shown to alleviate cancer
progression by downregulating the circular RNA nuclear receptor
interacting protein 1/miR-532-3p/AKT signaling pathway (39). In head and neck squamous cell
carcinoma, curcumin exerts anticancer effects through modulating
the tumor microenvironment (39).
Similarly, the present study showed that curcumin inhibited NSCLC
cell growth via promoting pyroptosis and the addition of inhibitors
targeting pyroptosis-associated factors led to a reversal of
curcumin's effects, thereby highlighting its mechanistic role in
inducing apoptosis and pyroptosis in NSCLC cells.
NLRP3 is an important component of the inflammasome
protein complex, which promotes the maturation and secretion of
pro-IL-1β and pro-IL-18, the precursor forms of IL-1β and IL-18
(40). As an essential regulator
of pyroptosis (41), NLRP3 has
been shown to mediate pyroptosis through natural active substances
in a variety of cancer types. For example, anthocyanin was shown to
activate pyroptosis in oral squamous cell carcinoma cells via
NLRP3, caspase-1 and GSDMD signaling (42). Similarly, polyphyllin VI was found
to induce caspase-1-mediated pyroptosis in NSCLC through inducing
the NLRP3/GSDMD axis (43) and
citric acid triggers pyroptosis in ovarian cancer (OC) cells via
caspase-4, NLRP3 and GSDMD signaling (44). In the present study, curcumin
increased the stability of NLRP3 and reduced its level of
ubiquitination, underscoring its regulatory role in pyroptosis.
Both in vitro and in vivo experiments demonstrated
that knockdown of NLRP3 reversed the effects of curcumin-induced
pyroptosis, including the activation and cleavage of caspase-1 and
GSDMD, increased secretion of IL-1β and IL-18 and suppression of
NSCLC tumor growth in nude mice. Collectively, these results
underlined the pivotal role of NLRP3 in curcumin-mediated
pyroptosis, suggesting that targeting NLRP3 may be a promising
strategy for NSCLC treatment.
Upon western blot analysis, cleaved-caspase-1
appears with two bands. This is due to the fact that pro-caspase-1
undergoes cleavage upon activation, generating several fragments of
different sizes, including fragments of 20 and 22 kDa in size
(45). These fragments represent
the cleavage products of caspase-1 and they result in the formation
of the active caspase-1 enzyme (45). Furthermore, changes in the
expression level of pro-caspase-1 reflect the activation status of
caspase-1. The expression level of cleaved-caspase-1 has been shown
to reflect the activity level of caspase-1 more directly during
apoptosis and the inflammatory response (46). The independent detection of the
expression levels of pro-caspase-1 and cleaved-caspase-1 in the
present study has contributed to a more detailed understanding of
the underlying regulatory mechanisms of the caspase-1 signaling
pathway by showing the effects of certain factors, such as curcumin
and Smurf2, on the cleavage and activation of caspase-1. Therefore,
the results for pro-caspase-1 and cleaved-caspase-1, the 20 and 22
kDa cleaved products, respectively, were presented
individually.
The investigation of NLRP3 ubiquitination and
deubiquitination has emerged as an important area of research in
the context of inflammatory diseases (15). The UbiBrowser 1.0 database was
utilized to identify potential E3 ligases that interacted with
NLRP3, which predicted that Smurf2 was among the candidate E3
ligases. Smurf2 is a ubiquitin E3 ligase involved in the
degradation of various proteins via the 26S proteasome (47). Moreover, other studies have
demonstrated diverse roles for Smurf2 in cancer. Xie et al
(45) reported that Smurf2
mediates glutathione S-transferase P1 ubiquitination, leading to
ferroptosis in cancer cells through a glutathione peroxidase
4-independent mechanism. Han et al (48) showed that Smurf2 interacts with
DNA-binding inhibitor 2 (ID2) in lung cancer cells, thereby
promoting the polyubiquitination and degradation of ID2 via the
ubiquitin-proteasome pathway, which ultimately induces cell cycle
arrest. Furthermore, Pi et al (49) found that the deletion of Smurf2
expression in human OC reduces the ubiquitination of receptor for
activated C kinase 1, thereby promoting OC progression. In the
present study, it was demonstrated that Smurf2 interacted with
NLRP3 and that the upregulation of Smurf2 enhanced NLRP3
ubiquitination. Furthermore, Smurf2 knockdown promoted NLRP3
expression and cell pyroptosis, demonstrating that Smurf2 can both
regulate NLRP3 ubiquitination and exert a significant impact on the
process of pyroptosis in NSCLC cells.
A previous study showed that curcumin can inhibit
TGF-β/Smad signaling through activating autophagy (50). Additionally, Smad2 and Smad3
degradation may occur through Smurf2-mediated ubiquitination and
proteasomal degradation (50).
The associated involvement of curcumin and Smurf2 was also
demonstrated in a rat model of paraquat-induced pulmonary fibrosis,
where curcumin was found to modulate Smurf2 activity (51). In the present study, molecular
docking analysis demonstrated that curcumin binds to Smurf2.
Additionally, treatment with curcumin inhibited the strength of the
interaction between Smurf2 and NLRP3. Knockdown of Smurf2 led to an
augmentation of curcumin's effects on the stability of NLRP3,
pyroptosis and the expression of pyroptosis-associated factors in
NSCLC cells. On the other hand, the overexpression of Smurf2 led to
a reversal of these effects, underscoring the crucial role of
Smurf2 in curcumin-mediated regulation of NLRP3-dependent
pyroptosis. Based on the in vivo mouse model experiments,
curcumin treatment reduced both the volume and the weight of NSCLC
tumors, decreased the level of Ki67 expression and increased the
expression levels of NLRP3 and pyroptosis-associated factors. These
findings were consistent with the results obtained from the in
vitro experiments, further supporting the potential of curcumin
as a therapeutic agent for NSCLC. Moreover, the in vivo
experiments confirmed that curcumin inhibited NSCLC progression by
modulating the Smurf2/NLRP3 axis, thereby offering a novel
perspective for NSCLC treatment.
However, the present study had certain limitations.
First, the precise mechanism underlying Smurf2′s interactions with
other proteins requires further, more detailed studies, such as the
GSDMD protein. Secondly, clinical studies are required to confirm
the efficacy of curcumin in treating NSCLC. These challenges
highlight areas for future research and should be the focus for
ongoing efforts aiming to address these gaps in current
knowledge.
In conclusion, the present study showed that
curcumin promoted NLRP3-dependent pyroptosis in NSCLC cells through
inhibiting Smurf2 activity, highlighting a potential mechanism
underlying the antitumor effects exerted by curcumin and suggesting
novel therapeutic targets and strategies for the treatment of
NSCLC.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX, SZ, XT and XD performed the experiments and
collected data. YX analyzed the data and wrote the manuscript. XD
revised the manuscript. All authors read and approved the final
version of the manuscript. YX and XD confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Experiments in the present study were approved by
the Medical Ethics Committee of the Second Affiliated Hospital,
University of South China (approval no. NHFE2022010601; Hengyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by the Key Research Project of Nanhua
University Funding (grant no. nk2020108).
References
|
1
|
Srivastava S, Mohanty A, Nam A, Singhal S
and Salgia R: Chemokines and NSCLC: Emerging role in prognosis,
heterogeneity, and therapeutics. Semin Cancer Biol. 86:233–246.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ganti AK, Klein AB, Cotarla I, Seal B and
Chou E: Update of incidence, prevalence, survival, and initial
treatment in patients with non-small cell lung cancer in the US.
JAMA Oncol. 7:1824–1832. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark SB and Alsubait S: Non-small cell
lung cancer. StatPearls. StatPearls Publishing LLC; Treasure
Island, FL: 2024
|
|
4
|
Jonna S and Subramaniam DS: Molecular
diagnostics and targeted therapies in non-small cell lung cancer
(NSCLC): An update. Discov Med. 27:167–170. 2019.
|
|
5
|
Huang X, Wang Y, Yang W, Dong J and Li L:
Regulation of dietary polyphenols on cancer cell pyroptosis and the
tumor immune microenvironment. Front Nutr. 9:9748962022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kotha RR and Luthria DL: Curcumin:
Biological, pharmaceutical, nutraceutical, and analytical aspects.
Molecules. 24:29302019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan Mohd Tajuddin WNB, Lajis NH, Abas F,
Othman I and Naidu R: Mechanistic understanding of curcumin's
therapeutic effects in lung cancer. Nutrients. 11:29892019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang
Y, Yu T, Wu X, Shi Y, Ma P and Shu Y: Pyroptosis: A new frontier in
cancer. Biomed Pharmacother. 121:1095952020. View Article : Google Scholar
|
|
9
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Yan W, Duan H, Wang D, Zhou Y, Feng
D, Zheng Y, Zhou S, Liu G and Qin X: Therapeutic effects of natural
products on liver cancer and their potential mechanisms. Nutrients.
16:16422024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan R, Zhao W, Wang QQ, He J, Han S, Gao
H, Feng Y and Yang S: Cucurbitacin B inhibits non-small cell lung
cancer in vivo and in vitro by triggering
TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res.
170:1057482021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan H, Jiang L, Sun X, Liu X, Yang G, Sun
X, Cheng T, Ji Y, Zhang F, Du Y, et al: Curcumin induces MCF-7
cells pyroptosis via autophagy/CTSB/NLRP3/caspase-1/GSDMD signaling
pathway in vitro and vivo. J Vet Heal Sci. 3:250–261. 2022.
|
|
13
|
Feng SH, Zhao B, Zhan X, Li RH, Yang Q,
Wang SM and Li A: Quercetin-induced pyroptosis in colon cancer
through NEK7-mediated NLRP3 inflammasome-GSDMD signaling pathway
activation. Am J Cancer Res. 14:934–958. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krishnan SM, Ling YH, Huuskes BM, Ferens
DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK,
et al: Pharmacological inhibition of the NLRP3 inflammasome reduces
blood pressure, renal damage, and dysfunction in salt-sensitive
hypertension. Cardiovasc Res. 115:776–787. 2019. View Article : Google Scholar :
|
|
15
|
Akther M, Haque ME, Park J, Kang TB and
Lee KH: NLRP3 ubiquitination-A new approach to target NLRP3
inflammasome activation. Int J Mol Sci. 22:87802021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Fang Y, Lv X, Hu C, Chen G, Zhang
L, Jin B, Huang L, Luo W, Liang G and Wang Y: Deubiquitinase OTUD6A
in macrophages promotes intestinal inflammation and colitis via
deubiquitination of NLRP3. Cell Death Differ. 30:1457–1471. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu T, Yu W, Fang H, Wang Z, Chi Z, Guo X,
Jiang D, Zhang K, Chen S, Li M, et al: Ubiquitination of NLRP3 by
gp78/Insig-1 restrains NLRP3 inflammasome activation. Cell Death
Differ. 29:1582–1595. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan B, Jin Y, Mao S, Zhang Y, Yang D, Du M
and Yin Y: Smurf2-mediated ubiquitination of FOXO4 regulates
oxygen-glucose deprivation/reperfusion-induced pyroptosis of
cortical neurons. Curr Neurovasc Res. 20:443–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaudhary KR, Kinslow CJ, Cheng H, Silva
JM, Yu J, Wang TJ, Hei TK, Halmos B and Cheng SK: Smurf2 inhibition
enhances chemotherapy and radiation sensitivity in non-small-cell
lung cancer. Sci Rep. 12:101402022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang FR, Li SY, Hu XW, Li XR and Li HJ:
Identifying the antitumor effects of curcumin on lung
adenocarcinoma using comprehensive bioinformatics analysis. Drug
Des Devel Ther. 16:2365–2382. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schamberger B and Plaetzer K:
Photofungizides based on curcumin and derivates thereof against
candida albicans and aspergillus niger. Antibiotics (Basel).
10:13152021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galvao J, Davis B, Tilley M, Normando E,
Duchen MR and Cordeiro MF: Unexpected low-dose toxicity of the
universal solvent DMSO. FASEB J. 28:1317–1330. 2014. View Article : Google Scholar
|
|
23
|
Li M, Wu R, Wang L, Zhu D, Liu S, Wang R,
Deng C, Zhang S, Chen M, Lu R, et al: Usenamine A triggers
NLRP3/caspase-1/GSDMD-mediated pyroptosis in lung adenocarcinoma by
targeting the DDX3X/SQSTM1 axis. Aging (Albany NY). 16:1663–1684.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leng B, Zhang Y, Liu X, Zhang Z, Liu Y,
Wang H and Lu M: Astragaloside IV suppresses high glucose-induced
NLRP3 inflammasome activation by inhibiting TLR4/NF-κB and CaSR.
Mediators Inflamm. 2019:10824972019. View Article : Google Scholar
|
|
25
|
Zhao C, Liang F, Ye M, Wu S, Qin Y, Zhao
L, Zhang L, He J, Cen L and Lin F: GSDMD promotes neutrophil
extracellular traps via mtDNA-cGAS-STING pathway during lung
ischemia/reperfusion. Cell Death Discov. 9:3682023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge X, Cai F, Shang Y, Chi F, Xiao H, Xu J,
Fu Y and Bai C: PARK2 attenuates house dust mite-induced
inflammatory reaction, pyroptosis and barrier dysfunction in
BEAS-2B cells by ubiquitinating NLRP3. Am J Transl Res. 13:326–335.
2021.PubMed/NCBI
|
|
27
|
Zhang X, Zhang K and Zhang Y: Pigment
epithelium-derived factor facilitates NLRP3 inflammasome activation
through downregulating cytidine monophosphate kinase 2: A potential
treatment strategy for missed abortion. Int J Mol Med.
45:1436–1446. 2020.PubMed/NCBI
|
|
28
|
Chen Z, Li P, Shen L and Jiang X: Heat
shock protein B7 (HSPB7) inhibits lung adenocarcinoma progression
by inhibiting glycolysis. Oncol Rep. 50:1962023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shih KC, Chan HW, Wu CY and Chuang HY:
Curcumin enhances the abscopal effect in mice with colorectal
cancer by acting as an immunomodulator. Pharmaceutics. 15:15192023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kollias NS, Hess WJ, Johnson CL, Murphy M
and Golab G: A literature review on current practices, knowledge,
and viewpoints on pentobarbital euthanasia performed by
veterinarians and animal remains disposal in the United States. J
Am Vet Med Assoc. 261:733–738. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai B, Zhao J, Zhang Y, Liu Y, Ma C, Yi F,
Zheng Y, Zhang L, Chen T, Liu H, et al: USP5 attenuates NLRP3
inflammasome activation by promoting autophagic degradation of
NLRP3. Autophagy. 18:990–1004. 2022. View Article : Google Scholar :
|
|
32
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calibasi-Kocal G, Pakdemirli A, Bayrak S,
Ozupek NM, Sever T, Basbinar Y, Ellidokuz H and Yigitbasi T:
Curcumin effects on cell proliferation, angiogenesis and metastasis
in colorectal cancer. J BUON. 24:1482–1487. 2019.PubMed/NCBI
|
|
34
|
Jing X, Yun Y, Ji X, Yang E and Li P:
Pyroptosis and inflammasome-related genes-NLRP3, NLRC4 and NLRP7
polymorphisms were associated with risk of lung cancer.
Pharmgenomics Pers Med. 16:795–804. 2023.PubMed/NCBI
|
|
35
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Howlader N, Forjaz G, Mooradian MJ, Meza
R, Kong CY, Cronin KA, Mariotto AB, Lowy DR and Feuer EJ: The
effect of advances in lung-cancer treatment on population
mortality. N Engl J Med. 383:640–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giordano A and Tommonaro G: Curcumin and
cancer. Nutrients. 11:23762019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu C, Rokavec M, Huang Z and Hermeking H:
Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress
colorectal cancer metastasis. Cell Death Differ. 30:1771–1785.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo T, Guan H, Liu J, Wang J and Zhang Y:
Curcumin inhibits esophageal squamous cell carcinoma progression
through down-regulating the circNRIP1/miR-532-3p/AKT pathway.
Environ Toxicol. 38:2705–2716. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu J and Wu H: Structural Mechanisms of
NLRP3 inflammasome assembly and activation. Annu Rev Immunol.
41:301–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng X, Wan J and Tan G: The mechanisms
of NLRP3 inflammasome/pyroptosis activation and their role in
diabetic retinopathy. Front Immunol. 14:11511852023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yue E, Tuguzbaeva G, Chen X, Qin Y, Li A,
Sun X, Dong C, Liu Y, Yu Y, Zahra SM, et al: Anthocyanin is
involved in the activation of pyroptosis in oral squamous cell
carcinoma. Phytomedicine. 56:286–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al: Polyphyllin VI
induces caspase-1-mediated pyroptosis via the induction of
ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer.
Cancers (Basel). 12:1932020. View Article : Google Scholar
|
|
44
|
Wang X, Yin Y, Qian W, Peng C, Shen S,
Wang T and Zhao S: Citric acid of ovarian cancer metabolite induces
pyroptosis via the caspase-4/TXNIP-NLRP3-GSDMD pathway in ovarian
cancer. FASEB J. 36:e223622022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie W, Peng M, Liu Y, Zhang B, Yi L and
Long Y: Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD
pathway in colon cancer. Cell Commun Signal. 21:3292023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu X, Li M, Chen Z, Yu Y, Shi H, Yu Y,
Wang Y, Chen R and Ge J: Mitochondrial calpain-1 activates NLRP3
inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in
CVB3-induced myocarditis. Basic Res Cardiol. 117:402022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chae DK, Park J, Cho M, Ban E, Jang M, Yoo
YS, Kim EE, Baik JH and Song EJ: MiR-195 and miR-497 suppress
tumorigenesis in lung cancer by inhibiting SMURF2-induced TGF-β
receptor I ubiquitination. Mol Oncol. 13:2663–2678. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Han M, Guo Y, Li Y, Zeng Q, Zhu W and
Jiang J: SMURF2 facilitates ubiquitin-mediated degradation of ID2
to attenuate lung cancer cell proliferation. Int J Biol Sci.
19:3324–3340. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pi Y, Feng Q, Sun F, Wang Z, Zhao Y, Chen
D, Liu Y and Lou G: Loss of SMURF2 expression enhances RACK1
stability and promotes ovarian cancer progression. Cell Death
Differ. 30:2382–2392. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kong D, Zhang Z, Chen L, Huang W, Zhang F,
Wang L, Wang Y, Cao P and Zheng S: Curcumin blunts
epithelial-mesenchymal transition of hepatocytes to alleviate
hepatic fibrosis through regulating oxidative stress and autophagy.
Redox Biol. 36:1016002020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen H, Yang R, Tang Y and Fu X: Effects
of curcumin on artery blood gas index of rats with pulmonary
fibrosis caused by paraquat poisoning and the expression of Smad 4,
Smurf 2, interleukin-4 and interferon-γ. Exp Ther Med.
17:3664–3670. 2019.PubMed/NCBI
|



















