Introduction
Scrotal massive lymphedema, also known as scrotal
elephantiasis, represents a rare condition that accounts for ~0.6%
of all cases of lymphedema (1-4).
Although relatively common in areas with endemic filariasis, it is
rarely observed in industrialized areas, where such a condition is
almost exclusively iatrogenic or congenital (1-4).
Scrotal massive lymphedema, is a disease usually caused by
obstruction, aplasia, or hypoplasia of the lymphatic vessels
draining the scrotum. It can be either congenital or acquired. The
most common acquired etiology is infection due to lymphogranuloma
venereum or filarial infestation with Wuchereria bancrofti.
The scrotal skin usually becomes thickened and in severe cases, it
can be affected by ulcerations (5).
Scrotal elephantiasis can cause severe functional, social and
emotional limitations due to recurrent skin infections, sexual
dysfunction, pain and cosmetic deformity, as well as limitations of
mobility and ambulation (1-4).
The response to medical therapy is poor and the complete excision
of the affected tissues, together with reconstructive methods can
provide a definitive solution, irrespective of the etiology
(1-4).
The purpose of surgery is to excise the mass, to reconstruct the
scrotum and repair the skin of the penis (6). Although a variety of surgical
procedures have been reported, the ideal solution has not yet been
identified and no definitive recommendations exist to date, at
least to the best of our knowledge (1-4).
The present study describes the case of a Caucasian
patient with massive idiopathic penoscrotal lymphedema. A novel
surgical technique for scrotal elephantiasis correction was used
for the treatment of this patient.
Case report
In May, 2020, a 43-year-old Caucasian male patient
was admitted to the Department of Urology and Andrology of
University Federico II of Naples (Naples, Italy) with massive
scrotal elephantiasis. The past medical history of the patient was
relevant for arterial hypertension. His previous surgical history
included a circumcision at the age of 16. There was no history of
sexually transmitted diseases, irradiation, or travel to endemic
regions. The onset was spontaneous, and the swelling had
substantially increased over the past 4 years. He reported
psychological discomfort mainly due to the esthetic appearance of
the genitalia and a burning sensation during micturition. No other
notable findings were evident at the physical examination. The
weight and height of the patient at the time of surgery were 140 kg
and 190 cm, respectively (body mass index, 38.7 kg/m2;
obesity class II). He presented a massive swelling of the scrotum
with cutaneous indurations covering the vast majority of the area
(Fig. 1). His penis was completely
buried, and the glans was not visible. The penis, the testes and
the spermatic cords were not palpable. Laboratory testing
(including human immunodeficiency virus, markers for testicular
cancer, antibodies to schistosomes, Chlamydia trachomatis
and filariae) were negative. Magnetic resonance imaging of the
abdomen revealed the integrity of the corpora cavernosa, the
spermatic cord, as well as the testis. On the other hand, the
status of the glans was uncertain. The patient was thus scheduled
for a complete scrotectomy. The various stages of surgery included
the following:
Preparation prior to surgery
Intravenous broad-spectrum antibiotics (cefazolin, 2
g, intravenous) were administered. Skin preparation and draping of
the patient was performed with the patient placed in a simple
lithotomy position, with the calves placed on Allen stirrups. A
Mayo table was used to sustain the scrotum. The skin to be excised
was marked out and a ‘hanger-shaped incision’, allowing the
preservation of the penis, spermatic cords and both testes was
performed (Fig. 2).
Demolitive phase
Following the superior incision, the dissection of
the tissues was commenced with the aid of LigaSure
ImpactTM (Medtronic) aiming to identify the penis first
and subsequently, the spermatic cords. Once the penis was
identified, a midline incision of the scrotum was performed to
reveal the glans (Fig. 3). In this
case, the glans appeared completely dysmorphic (Fig. 4). The external meatus was stenotic;
however, the patient was we successfully catheterized with the use
of a hydrophilic guidewire and urethral dilators. A frozen section
of the prepuce and glandular tissue was made to exclude the
possibility of a cancerous degeneration. The report was concordant
to a non-specific chronic inflammation with areas of epidermal and
dermal fibrosis. The spermatic cords and testis were recognized and
secured away. Once the noble structures were identified (Fig. 5) the mass was excised from the bottom
to the top. The excised scrotal tissue weighed 4,250 g.
Reconstructive phase
The ‘hanger-shaped incision’ allowed for the
reconstruction of a trapezoidal shaped cavity. The perineal
subcutaneous tissues were deeply dissected to create a pouch as a
‘neoscrotum’ with a ‘neoseptum’. The latter was created using 2-0
Vicryl stitches between the midline fat and the dermis bilaterally.
The reconstruction of the neoscrotum was obtained by closing the
trapezoidal area of the perineum into a T-shaped line. More
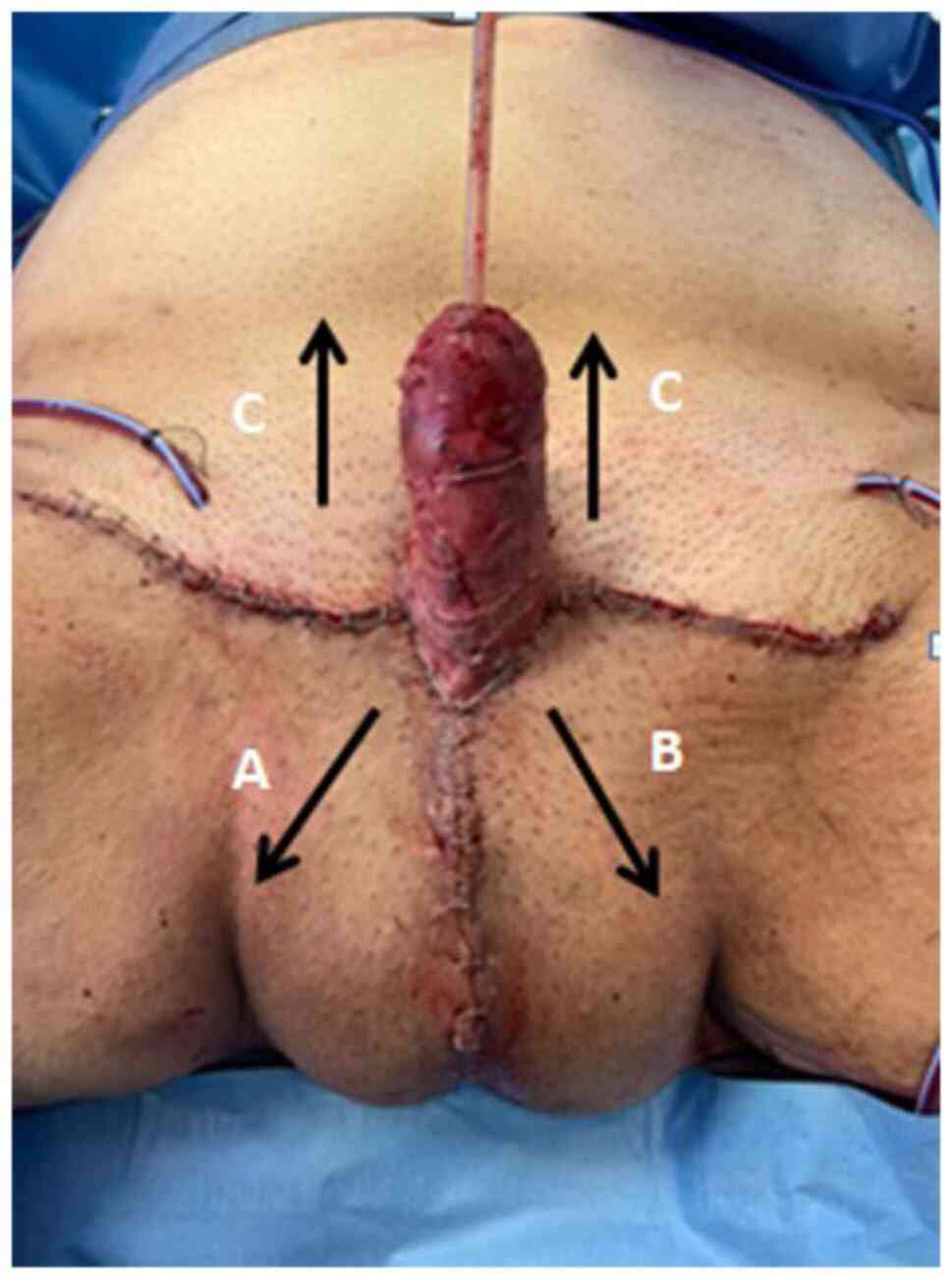
precisely, the inferior corners of the trapezium (Fig. 6A and B) were lifted up and sutured to the base of
the penis at the 3' and 9' o'clock positions (Fig. 6A1 and B1). This allowed for the creation of a
‘neo-raphe’ (Fig. 7). The superior
corners were approximated using a V-Y advancement flap to solve the
‘doggy ears’ (Fig. 8). A
split-thickness skin graft (STSG) of 0.016 inches was harvested
from the inner surface of the right leg, in order to cover the
penis. The STSG was quilted onto the dartos layer of the penis in a
spiral way using 4-0 Vicryl Rapid suture (Fig. 9). The whole glans was completely
affected by the inflammatory degeneration (Fig. 4); therefore, a resurfacing of the
glans was performed; two 10 ch Redivac (closed drain system) drains
were positioned bilaterally.
Post-operative management
The total time for the procedure was 5 h and 45 min,
with an estimated blood loss of 250 ml. Post-operative therapy
included paracetamol at 1,000 mg three times a day, cefazoline (2
g) once a day and enoxaparin (6,000 IU) twice a day. The drains
were removed after 4 days.
The duration of the hospital stay was 10 days. The
post-operative hospitalization was uneventful. The post-operative
appearance at 2 weeks is illustrated in Fig. 10 and no complications were reported
following 1 year of follow up. The excised tissue was sent for
histopathological analysis. Histologically, the tissue was
characterized by marked edema with dermal fibrosis, dilatation of
lymphatic vessels and chronic inflammatory infiltrates, mainly
lymphoplasmacellular into the dermis separated by fibrous bands
(data not shown).
Discussion
The management of penoscrotal elephantiasis should
be focused on treating the underlying causes. A neoplastic process
has to be excluded and medical treatment should be administered
when necessary. To complete the treatment a surgical approach is
essential. There are several ways to approach such conditions
(7-9). In
such cases, an excisional approach is the only possible treatment.
Several surgical techniques have been presented over the years. In
2008, Garaffa et al (10)
described the ‘Scrotal inverted ‘W’ incision’. In 2014, Machol
et al (11) demonstrated that
lateral-based scrotal flaps (with or without mid-raphe Z-plasty)
allowed for a correct anatomical reconstruction. In addition, in
2018, Irdam and Fadhly (12)
described a circular incision encircling the scrotum.
Furthermroe, in 2019, Alnajjar et al
(13) suggested an innovative
approach with a ‘batman incision’ that avoids the need for pedicled
flaps and their associated risks of flap ischemia/necrosis, longer
surgical times and additional donor site wounds. On the other hand,
such technique requires penile transposition and lower abdomen
extensive mobilization (13).
The present study ‘redrew’ the surgical approach to
this uncommon disease using a ‘hanger-shaped incision’ in order to
obtain a trapezoidal cavity. Such an incision permits the formation
of neo-raphe, neo-septum and eventually, the neo-scrotum as a
method with which to restore as much as possible the natural
appearance of the genitalia. The neo-septum was designed in order
to avoid the risk of spermatic cords twisting. In fact, the
continuous stretching of the tissues, due to the weight of the
mass, could irremediably cause a lengthening of the cords. When
approaching such large masses, a top-down approach is suggested, as
the identification of the noble structures becomes more feasible
and safe. Moreover, the ‘hanger-shaped incision’ permits a
T-closure of the wound, preventing the extensive mobilization of
the pre-pubic area which will be mandatory for a penile
transposition. In addition, a penile transposition requires a
perfect ring excision to avoid either difficulty with the grafting
or constriction during erection. On the other hand, the T closure
ensures a redistribution of the tensile strengths on three
different unconnected flaps (Figs.
11 and 12).
In conclusion, in the case described in the present
study, the ‘hanger-shaped incision’ minimized the use of rotation
flaps and should reduced the risk of recurrence by eliminating all
the cellulitis tissue, restoring the natural compartmentalization
of the scrotum and improving the esthetic appearance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MCa was involved in the conceptualization of the
study and in the study supervision. ADG was involved in data
curation and in the conceptualization of the study. MCr was
involved in in the conceptualization of the study and in formal
analysis. AG, CDA and LoC were involved in the conceptualization of
the study and in the writing of the original draft. AP was involved
in the validation of the patient data and in the development or
design of the study. LN was involved in patient data analysis and
methodology. CMi and GO were involved the study methodology and
performed the novel type of surgery. GCe was involved in the study
methodology and advised on patient treatment. GCa, LuC, CMa and GMF
were involved in the conceptualization of the study, and in the
writing, reviewing and editing of the manuscript. VM was involved
in the development or design of the study and performed the novel
type of surgery. RLR was involved in project administration and
methodology, and has performed the novel type of surgery. MCa and
ADG confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Written consent has been obtained from the patient
for the inclusion of his data in the present case report.
Patient consent for publication
Written consent was obtained from the patient. This
consent also included consent for the publication of the patient
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Modolin M, Mitre AI, da Silva JC, Cintra
W, Quagliano AP, Arap S and Ferreira MC: Surgical treatment of
lymphedema of the penis and scrotum. Clinics (Sao Paulo).
61:289–294. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Muehlberger T, Homann HH, Kuhnen C, Vogt
PM and Steinau HU: Etiology, clinical aspects and therapy of
penoscrotal lymphedema. Chirurg. 72:414–418. 2001.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
3
|
García-Tutor E, Botellé del Hierro J, San
Martín Maya A, Castro García J, España A, Fernández Montero J and
Robles García JE: Surgical treatment of penile lymphedema
associated with hidradenitis suppurativa. Actas Urol Esp.
29:519–522. 2005.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
4
|
Lobato RC, Zatz RF, Cintra Junior W,
Modolin MLA, Chi A, Van Dunem Filipe de Almeida YK and Gemperli R:
Surgical treatment of a penoscrotal massive localized lymphedema:
Case report. Int J Surg Case Rep. 59:84–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pastor C and Granick MS: Scrotal
lymphedema. Eplasty. 11(ic15)2011.PubMed/NCBI
|
|
6
|
Lin T, Lin YZ, Wu YP, Lin TT, Chen DN, Wei
Y, Xue XY and Xu N: Penoscrotal edema: A case report and literature
review. BMC Urol. 19(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aulia I and Yessica EC: Surgical
management of male genital lymphedema: A systematic review. Arch
Plast Surg. 47:3–8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pacheco YD, García-Duque O and
Fernández-Palacios J: Penile and scrotal lymphedema associated with
hidradenitis suppurativa: Case report and review of surgical
options. Cir Cir. 86:84–88. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zugor V, Horch RE, Labanaris AP, Schreiber
M and Schott GE: Penoscrotal elephantiasis: Diagnostics and
treatment options. Urologe A. 47:472–476. 2008.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
10
|
Garaffa G, Christopher N and Ralph DJ: The
management of genital lymphoedema. BJU Int. 102:480–484.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Machol JA IV, Langenstroer P and Sanger
JR: Surgical reduction of scrotal massive localized lymphedema
(MLL) in obesity. J Plast Reconstr Aesthet Surg. 67:1719–1725.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Irdam GA and Fadhly S: Scrotal
reconstruction on scrotal lymphedema. Urol Case Rep. 20:9–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alnajjar HM, Castiglione F, Ahmed K,
Haider A, Nigam R and Muneer A: A novel ‘Batman’ scrotectomy
technique for the management of scrotal lymphoedema following
treatment for penile cancer. Transl Androl Urol. 8:448–456.
2019.PubMed/NCBI View Article : Google Scholar
|