Introduction
Fibromyalgia (FM) is a chronic, non-inflammatory,
soft-tissue disorder, characterized clinically by widespread pain,
stiffness, fatigue and non-restorative sleep, which affects 2-8% of
the general population (1,2). According to the American College of
Rheumatology 2010 classification criteria, there are 19 areas of
the body which constitute the most painful regions. Furthermore,
for a definitive diagnosis there should be some symptoms for at
least 3 months, which cannot be explained by any other health
disorder (3).
As the etiology and pathogenesis of FM have not yet
been fully elucidated, treatment is usually symptomatic. Both
pharmacological and non-pharmacological treatments are applied for
symptoms, such as pain, fatigue, related depression and sleep
difficulties (4,5). Several treatment approaches have been
described, including drug therapy, exercise, physiotherapy, spa
therapy, acupuncture, diet and cognitive-behavioral therapies
(5). For patients with severe pain,
pharmacological treatment with duloxetine, pregabalin, or tramadol
should be considered, and for those with sleep disturbances,
amitriptyline, cyclobenzaprine, or pregabalin have been suggested.
Rather than individual therapies, multimodal rehabilitation has
been found to improve various short-term outcomes in patients with
severe disability (5). Pregabalin
became the first drug Food and Drug Administration-approved drug
for specifically treating FM and varying responses have been
reported due to the heterogeneity of the FM population (6). Pregabalin, which is a potent inhibitory
ligand for the calcium channel α2-delta subunit in the central
nervous system, has analgesic and anxiolytic properties in addition
to anti-convulsant activity. Patients with FM treated with
pregabalin have exhibited central sensitization. Previous clinical
trials on pregabalin have reported a significant reduction in pain,
improvements in sleep quality and a generally improved
health-related quality of life in the majority of patients with FM
(6,7). Studies on other patient populations
have also demonstrated that exercise programs are beneficial for
patients with FM (8,9). These programs consisting of stretching
exercises, strengthening and aerobic conditioning have been
included as part of standard treatment protocols.
The nociceptive flexion reflex (NFR) is a
polysynaptic withdrawal reflex that occurs following the
stimulation of nociceptive A-delta afferents (10). The level of stimulation needed to
obtain this reflex can be utilized as an objective marker of
nociception in patients, and allows for the exploration of the pain
processing pathways at central levels (10).
Therefore, the aim of the present study was to
assess the effects of pregabalin plus exercise vs. pregabalin
treatment alone on the electromyographic NFR threshold in patients
with FM and to determine the associations between the NFR, and the
visual analog scale (VAS), Beck's Depression Inventory (BDI) and
fibromyalgia impact questionnaire (FIQ) scores.
Materials and methods
Study population
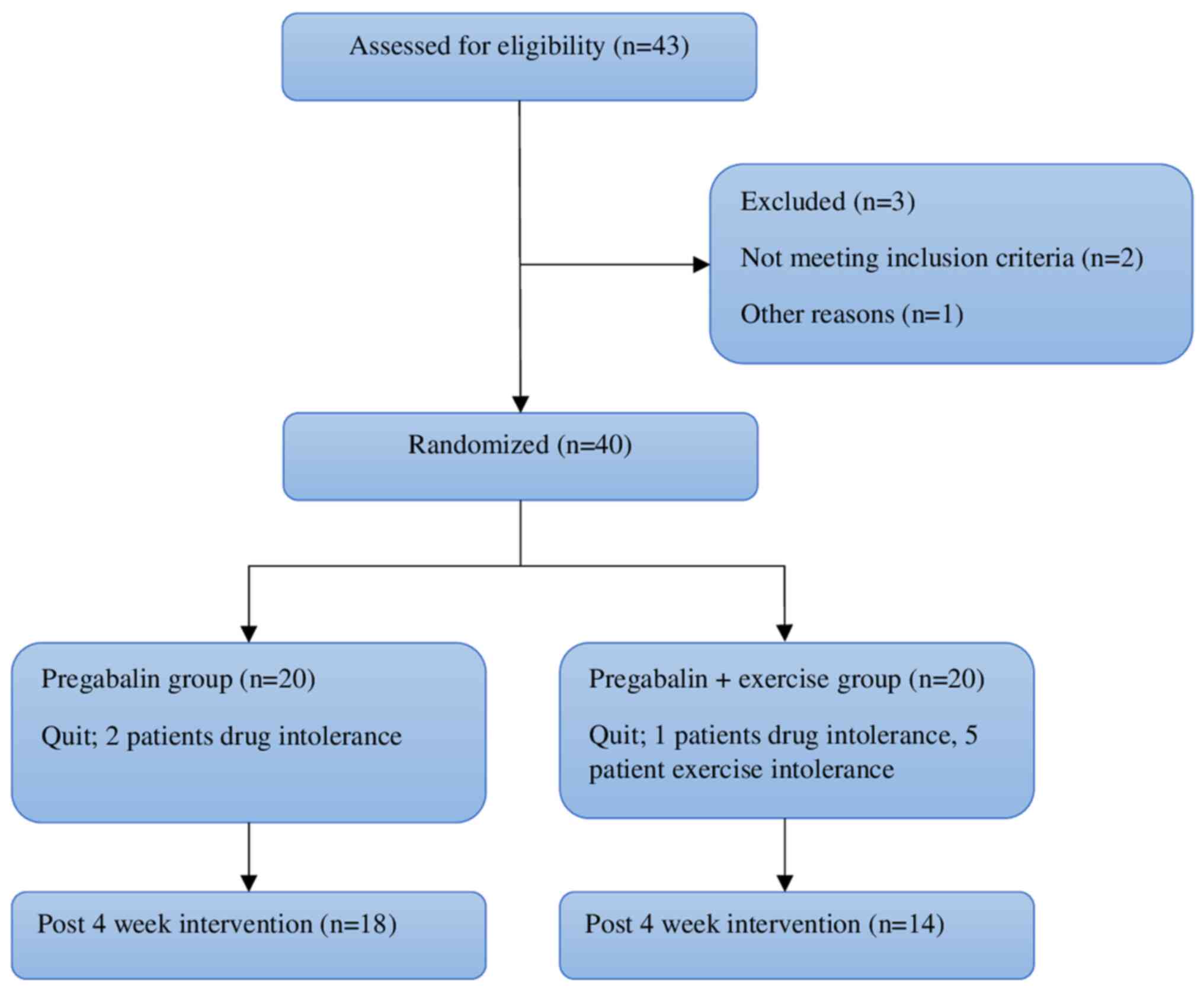
The sample of the present study consisted of 40
patients who met the inclusion criteria of 43 patients between the
ages of 18 and 50 with a confirmed diagnosis of FM according to the
American College of Rheumatology 2010 criteria, who referred to the
Physical Medicine and Rehabilitation Outpatient Clinic of Hatay
Mustafa Kemal University Hospital (Alahan, Turkey) between January,
2016 and July, 2016. The patients were randomly divided into two
groups with 20 patients in each (group 1, pregabalin group; group
2, pregabalin + exercise group). Randomization was performed by
generating random numbers as group 1 and group 2. In groups 1,2
patients withdrew from the study due to drug intolerance and in
group 2, 6 patients withdrew due to drug (1 patient) and exercise
intolerance (5 patients). Eventually, the study was completed with
18 patients in group 1 and 14 patients in group 2. The study
flowchart is illustrated in Fig.
1.
Criteria for inclusion and
exclusion
Patients with a history of cardiac, respiratory,
neurological, psychiatric, malignancy and musculoskeletal problems
including osteoarthritis, joint destruction, spinal degeneration,
rheumatological diseases or trauma within the prior 3 months that
may disrupt the exercise program were excluded from the study.
Patients who were already being treated for FM at the time of the
study initiation or within the prior 3 months, and those with
smoking and alcohol use were also excluded. A clinical assessment
was made on a detailed, standardized form by a physician blinded to
the groups.
Patient data
The demographic and clinical data including age,
body mass index, disease duration and the number of tender points
of the patients were recorded. The Ethics Committee of Hatay
Mustafa Kemal University approved the study and all subjects
provided written informed consent (31.03.2017/06). The study
followed the Declaration of Helsinki.
Treatment and assessment of
parameters
Treatment with pregabalin for all patients in groups
1 and 2 was commenced at 75 mg twice a day in the first week then
at 150 mg twice a day thereafter. Patients in group 2 also
underwent a combined exercise program three times a week for 5
weeks (15 times in total) in the clinic under supervision of a
physiotherapist. The program consisted of a warm-up using a
treadmill for 10 min, aerobic exercises for 15 min, strengthening
exercises for 15 min, and stretching and relaxation exercises for
15 min. The evaluation of the patients was made by a single
researcher blinded to the groups both at baseline, and at the
1-month follow-up examination. The assessment parameters were
calculated as follows: i) By applying ~4 kg/cm2 of thumb
pressure to the predefined 18 tender points, painful points were
identified and the total number was recorded for each patient; ii)
pain intensity was assessed on a VAS. Using a 10-cm long line,
patients recorded the level of pain on the scale where 0
represented ‘no pain’ and 10 ‘the most severe pain’; iii) BDI was
used to assess the intensity of depressive symptoms. This is a
21-item self-report questionnaire often used to assess depression
in patients with chronic pain, with higher total points indicating
more severe depression (11); iv)
the FIQ was used to score functional disability (12). The FIQ has 10 sub-scales of physical
functioning, overall well-being, work missed, job difficulty, pain,
fatigue, morning tiredness, stiffness, anxiety and depression. FIQ
scores range from 0 to 100, with higher scores indicating a greater
negative impact of FM; v) the NFR assessment was applied using a
standardized, validated procedure (13). NFR assessment were performed by the
same physician (ADT), who was blinded to the assignment of the
groups at the beginning and at the end of the study. Repeated
electrical stimuli were applied to the sural nerve endings, using
an up-down staircase method, with stimulation intensity beginning
at 0-mA and increasing in 4-mA increments until the detection of
the first reflex. When the first NFR was detected, the stimulation
intensity was decreased in 2-mA steps until the reflex disappeared.
This process was repeated with stimulation intensity adjusted
upward and downward in 1-mA increments until the NFR appeared and
disappeared two more times. The NFR threshold was calculated as the
average of the peaks and troughs of the stimulation intensities
which produced the second and third occurrence of the NFR.
Therefore, a higher NFR threshold value indicated that higher
stimulus intensities were required to evoke a consistent reflex
response. During the NFR threshold test, the patients rated the
pain sensation of each electrical stimulus using a scale of 0-100,
where 0 indicated no pain and 100 indicated extremely pain.
Statistical analysis
Data analysis was conducted using SPSS version 20.0
(IBM Corp.). Data are presented as the mean ± standard deviation
(SD) and median (min-max) values, and categorical data as number
(n) and percentage (%). Data normality between groups was evaluated
using the Shapiro-Wilk test. If the data was not normally
distributed, the Mann-Whitney U test was used. In the comparisons
the scores before and after treatment, the Wilcoxon signed-rank
test was used according to the distribution of the data.
Correlations between variables were evaluated using Spearman's
correlation analysis.
The power analysis for the estimation of the sample
size was unable to be performed; however, the post hoc power
analysis using the G*Power© 3.1 program
(Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) was
performed. The present study was performed with 32 patients with FM
(group 1, n=18 vs. group 2, n=14 patients). The Wilcoxon
signed-rank test was used for the comparisons if the two groups
revealed an effect size of d=8.0 and the power of the study was
calculated as 99% with 5% type 1 error.
Results
Of the 20 patients in group 1, 2 patients withdrew
from the study due to drug intolerance. In group 2, a total of 6
patients withdrew; 5 due to exercise intolerance and 1 due to drug
intolerance. Therefore, the 5-week intervention period of the study
was completed by 32 patients. The demographic and clinical
characteristics of the study subjects are summarized in Tables I and II. No statistically significant
differences were determined between the groups in terms of age,
body mass index and duration of the disease (Table I). When the groups were compared with
each other, no statistically significant differences were found
between the variables at baseline, and at the 1-month evaluation
(all P> 0.05) (Table II). Both
groups 1 and 2 exhibited significant improvements in activity and
resting VAS scores, FIQ, BDI and NFR scores after 1 month of the
therapy program (all P#x003C;0.05).
 | Table IDemographic characteristics of the
patients with fibromyalgia in the present study. |
Table I
Demographic characteristics of the
patients with fibromyalgia in the present study.
| Parameter | Group 1 (pregabalin)
(n=18) | Group 2 (pregabalin +
exercise) (n=14) | P-value |
|---|
| Age (years) | | | |
|
Mean ±
SD | 42.8±2.4 | 43.3±2.5 | 0.559 |
|
Median
(min-max) | 43 (38-48) | 43 (38-48) | |
| Height (cm) | | | |
|
Mean ±
SD | 158.0±3.2 | 158.3±3.0 | 0.791 |
|
Median
(min-max) | 158 (152-164) | 158 (154-164) | |
| Weight (kg) | | | |
|
Mean ±
SD | 72.1±5.4 | 72.8±5.6 | 0.708 |
|
Median
(min-max) | 72 (62-82) | 72.5 (62-82) | |
| BMI
(kg/m2) | | | |
|
Mean ±
SD | 28.9±2.7 | 29.0±2.3 | 0.877 |
|
Median
(min-max) | 28.6 (24.8-34.5) | 28.8 (25.8-34.5) | |
| Disease duration
(months) | | | |
|
Mean ±
SD | 71.3±29.3 | 70.2±28.6 | 0.920 |
|
Median
(min-max) | 72 (6-132) | 72 (12-132) | |
 | Table IIGroup comparisons at baseline and at
the 1st month after treatment. |
Table II
Group comparisons at baseline and at
the 1st month after treatment.
| | Group 1 | Group 2 | |
|---|
| Parameter | Mean ± SD | Median (min-max) | Mean ± SD | Median (min-max) | P-valueb |
|---|
| VAS resting | | | | | |
|
Baseline | 6.0±1.3 | 6 (4-8) | 6.0±1.4 | 6 (4-8) | 0.889 |
|
At 1st
month | 4.3±1.0 | 4.5 (3-6) | 3.5±1.0 | 3.5 (2-5) | 0.048 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
| VAS activity | | | | | |
|
Baseline | 7.5±1.1 | 8 (6-9) | 7.4±1.2 | 7 (6-9) | 0.867 |
|
At 1st
month | 5.8±0.9 | 6 (5-7) | 5.0±1.1 | 5 (4-7) | 0.022 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
| FIQ | | | | | |
|
Baseline | 62.5±4.9 | 62.5
(54.4-70.7) | 62.3±4.8 | 62.6
(53.6-71.2) | 0.937 |
|
At 1st
month | 53.5±5.0 | 52.7
(46.6-63.6) | 51.2±4.5 | 51.0
(42.3-60.2) | 0.166 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
| BDI | | | | | |
|
Baseline | 24.0±2.6 | 23 (21-29) | 23.8±2.7 | 24 (20-28) | 0.838 |
|
At 1st
month | 13.7±2.6 | 13 (10-19) | 13.5±2.4 | 13.5 (10-17) | 0.833 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
| Tender points | | | | | |
|
Baseline | 14.5±1.6 | 14 (12-17) | 14.8±1.7 | 15 (12-17) | 0.559 |
|
At 1st
month | 12.0±1.5 | 12 (10-14) | 11.8±1.6 | 12 (10-15) | 0.726 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
| NFR threshold | | | | | |
|
Baseline | 11.5±4.4 | 11.5
(2.4-18.4) | 10.8±3.4 | 12 (4.2-16) | 0.610 |
|
At 1st
month | 13.4±3.4 | 13.5 (8-20) | 12.8±2.3 | 13.5 (10-16.2) | 0.555 |
|
P-valuec |
#x003C;0.001a |
#x003C;0.001a | |
The correlations between clinical measurements, pain
scores, depression scores and NFR scores obtained at baseline and
at 1 month after treatment are presented in Table III. At baseline, a strong negative
significant correlation was found between NFR and pain (resting and
activity) scores and physical function (Rho=-0.62, Rho=-0.69 and
Rho=-0.60, respectively). At 1 month following treatment, in group
1, a strong negative significant correlation was identified between
the NFR scores, pain (resting and activity) scores and FIQ scores
(Rho=-0.82, Rho=-0.61 and Rho=-0.68, respectively). In group 2, a
strong negative significant correlation was detected between the
NFR scores and pain and FIQ scores (Rho=-0.66 and Rho=-0.66,
respectively).
 | Table IIICorrelations of NFR scores with
clinical parameters obtained before and at 1 month after
treatment. |
Table III
Correlations of NFR scores with
clinical parameters obtained before and at 1 month after
treatment.
| Parameter | VAS resting | VAS activity | FIQ | BDI |
|---|
| All the
patients | | | | |
|
Baseline |
P#x003C;0.001b |
P#x003C;0.001b |
P#x003C;0.001b | P=0.05a |
|
NFR
scores | Rho=-0.62 | Rho=-0.69 | Rho=-0.60 | Rho=-0.35 |
| Group 1 | | | | |
|
At 1
month |
P#x003C;0.001b |
P=0.007b |
P=0.002b | P=0.13 |
|
NFR
scores | Rho=-0.82 | Rho=-0.61 | Rho=-0.68 | Rho=-0.37 |
| Group 2 | | | | |
|
At 1
month | P=0.07 | P=0.01b |
P=0.009b | P=0.66 |
|
NFR
scores | Rho=-0.49 | Rho=-0.66 | Rho=-0.66 | Rho=-0.51 |
Discussion
The present study investigated the effects of the
combination of pregabalin and exercise on the NFR threshold in
patients with FM. The results demonstrated a significant
improvement in the NFR threshold at the 1st month after treatment.
The results of the present study indicated that the treatment in
both groups had positive effects on the NFR threshold of patients
with FM. A significant improvement was also observed in the tender
points, VAS, BDI and FIQ scores at the 1st month after treatment.
There were no significant differences between pregabalin and
pregabalin-exercise combination as regards efficacy.
The patients with FM have a combined symptom
experience with widespread pain, sleep disturbance, fatigue and an
impaired work capacity. The management consists of
multidisciplinary treatment using pharmacological and
non-pharmalogical strategies. Exercise therapy, including aerobic
exercise, group exercise, resistance and strength training, tai chi
and yoga may help prevent chronic pain and fatigue. Exercise may
also help with other symptoms of FM, including depression,
difficulty concentrating and sleep issues (8,9).
Aerobic exercise, such as running or brisk walking,
can help with a number of symptoms of FM. Resistance training also
strengthens the muscles and can improve FM symptoms. In the present
study, the combination of aerobic and strengthening exercises was
preferred, the effectiveness of which has been shown in previous
studies (14,15). Standard strengthening or aerobic
exercise is an essential non-pharmacological management method.
Moreover, pain control is another critical achievement of exercise,
which appears to be a secondary consequence of the improvement in
muscle strength and endurance. It is known that exercise increases
endorphin levels, and has antioxidant and anti-inflammatory
effects. In the present study, the lack of additional benefits in
symptoms with exercise may be explained by the short follow-up
period.
The NFR is mediated by an anatomic substrate
comprised of a complex network of interneurons that are located at
various levels of the spinal cord, and these spinal interneurons
are modulated by supraspinal pathways (13). Studies that have used the NFR to show
the excitability of dorsal horn neurons of the spinal cord, which
are formed with peripheral C fibers (a nociceptive afferent), have
reported that this excitability in patients with FM causes central
sensitization and chronic pain (16). Furthermore, central sensitization has
been determined in patients with FM using NFR as an objective
assessment of spinal nociception (16). For this reason, NFR is used in the
management of central sensitization and descending pain control
systems. In addition, the NFR procedure has been shown to be a
valuable tool used to evaluate pharmacologically active therapeutic
agents at the spinal level.
To the best of our knowledge, there are only a few
studies available to date evaluating the effect of FM treatment on
the NFR threshold. In a previous study comparing cognitive
behavioral therapy with other medical treatments, the NFR
thresholds increased significantly in the cognitive behavioral
treatment group following therapy, whereas a decrease in the NFR
threshold was found in the medical treatment group. Allowing both
groups to use pain relief medication may have affected the NFR
responses (17). In the present
study, pain medication was not permitted, apart from pregabalin, as
it could affect the NFR threshold. In another study, amitriptyline
was shown to lead to an increase in NFR threshold (18). Matthey et al (19) demonstrated that milnacipran exerted a
predominantly supraspinal analgesic effect, as evidenced by the
significant clinical benefits and the absence of changes in the NFR
threshold in patients with FM. According to the findings of the
study by Matthey et al (19),
although milnacipran led to a reduction in pain intensity and VAS
scores, it did not modulate thermal allodynia and potentially
restored normal diffuse noxious inhibitory control activity. The
findings of the present study have demonstrated that the treatment
in both groups had positive effects on the NFR threshold. The
improvement in the NFR threshold following treatment with
pregabalin may be interpreted as reduced central sensitization due
to pregabalin. The changes in the NFR threshold values following
treatment were similar in both groups; thus, it can be stated that
exercise does not appear to add to the efficacy of the treatment.
This may have been due to the short-term exercise therapy. Further
studies with longer durations of exercise regimens are required to
investigate this aspect.
Desmeules et al (16) found lower baseline NFR threshold
values (average, 22.7 mA) in patients with FM vs. healthy controls.
In another study which compared patients with FM with healthy
controls, the NFR threshold value in patients with FM was found to
be 12 mA on average (20). In the
present study, the mean NFR threshold was determined as 11.2 mA in
the patients with FM before treatment. Differences have been
observed in various studies as regards the mean NFR threshold
values in patients with FM. These differences may be explained by
methodological differences in measurement or that different
cultures and/or ethnicities have different pain sensitivities
(21).
In a multicenter double-blind randomized trial,
Crofford et al (6)
demonstrated a significant difference in VAS values in the
pregabalin group which received pregabalin at 300 mg compared with
the placebo group in patients with FM. Hooten et al
(8), in a study on 72 patients with
FM, investigated the effects of aerobic and strengthening exercises
on pain scores after 3 weeks, and a decrease in pain scores
following treatment was reported. In the present study, the
baseline VAS resting and activity scores were similar in both
groups and a significant decrease was observed in both groups
following treatment.
The study by Straube et al (22) demonstrated that pregabalin at 300,
450 and 600 mg/day was effective on the FIQ scores. Similarly, the
results of the present study revealed a significant improvement in
FIQ scores following treatment in both groups.
As regards signs of depression, both groups of
patients exhibited a significant reduction in BDI; however, there
was no significant difference between the BDI scores of the
pregabalin plus exercise combination and the pregabalin only group
at the 1st month following treatment. From these results, it can be
inferred that exercise provides no additional benefit in the
short-term, although long-term exercise therapy may become more
effective as regards the BDI scores. In the study by Sañudo et
al (23), long-term aerobic
exercise was compared with combined exercise and the BDI scores
were lower in the aerobic exercise group. Strengthening and
stretching rather than aerobic exercises may have the effect of
decreasing symptoms of depression.
It was expected, based on previous literature that
describes the NFR as a physiological correlate of pain, that a
reduction in pain with increasing exercise intensity would be
associated with higher NFR thresholds and lower NFR responding
(24). To the best of our knowledge,
the present study was the first to assess the effects of exercise
(treadmill, aerobic exercises, strengthening, stretching and
relaxation exercises) involving the NFR of muscles. Contrary to the
initial expectations, no significant differences between the groups
were found in the NFR threshold. The findings demonstrated that a
short-term exercise program did not affect the NFR.
There are some important limitations to the present
study that should be mentioned. The main limitation of the present
study was the small sample size. Therefore, acknowledging this as a
preliminary study, there is a need for further studies using a
long-term exercise program to determine the efficacy of
exercise.
In conclusion, the present study demonstrated that
both treatment groups had a significant symptomatic improvement in
the VAS, BDI, FIQ and NFR threshold values at the 1st month
following treatment. However, short-term aerobic and strengthening
exercises did not appear to provide additional benefits, which
could be considered a chronic training effect. Further studies
investigating long-term efficacy are thus required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (OV, MTY, HO, HG and ADT) conceptualized
the study. OV, MTY and HO made substantial contributions to data
analysis and interpretation, and wrote and prepared the draft of
the manuscript. HG and ADT analyzed the data and provided critical
revisions. HG and ADT confirm the authenticity of all the data. All
authors contributed to manuscript revision, and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the local Ethics
Committee of Hatay Mustafa Kemal University (Alahan, Turkey).
Written informed consent was obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clauw DJ: Fibromyalgia: A clinical review.
JAMA. 311:1547–1555. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guler H, Yildizgoren MT, Ustun N, Paksoy H
and Turhanoglu AD: Isokinetic assessment of the wrist muscles in
females with fibromyalgia. Arch Rheumatol. 31:215–220.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolfe F, Clauw DJ, Fitzcharles MA,
Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield
JB and Yunus MB: The American college of rheumatology preliminary
diagnostic criteria for fibromyalgia and measurement of symptom
severity. Arthritis Care Res (Hoboken). 62:600–610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kurt EE, Kocak FA, Erdem HR, Tuncay F and
Kelez F: Which non-pharmacological treatment is more effective on
clinical parameters in patients with fibromyalgia: Balneotherapy or
aerobic exercise? Arch Rheumatol. 31:162–169. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Macfarlane GJ, Kronisch C, Dean LE, Atzeni
F, Häuser W, Fluß E, Choy E, Kosek E, Amris K, Branco J, et al:
EULAR revised recommendations for the management of fibromyalgia.
Ann Rheum. 76:318–328. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crofford LJ, Rowbotham MC, Mease PJ,
Russell IJ, Dworkin RH, Corbin AE, Young JP Jr, LaMoreaux LK,
Martin SA and Sharma U: Pregabalin 1008-105 study group. Pregabalin
for the treatment of fibromyalgia syndrome: Results of a
randomized, double-blind, placebo-controlled trial. Arthritis
Rheum. 52:1264–1273. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Smith MT and Moore BJ: Pregabalin for the
treatment of fibromyalgia. Expert Opin Pharmacother. 13:1527–1533.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hooten WM, Qu W, Townsend CO and Judd JW:
Effects of strength vs aerobic exercise on pain severity in adults
with fibromyalgia: A randomized equivalence trial. Pain.
153:915–923. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meiworm L, Jakop E, Walker UA, Peter HH
and Keul J: Patients with fibromyalgia benefit from aerobic
endurance exercise. Clin Rheumatol. 19:253–257. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sandrini G, Arrigo A, Bono G and Nappi G:
The nociceptive flexion reflex as a tool for exploring pain control
systems in headache and other pain syndromes. Cephalalgia.
13:21–27. 1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hisli N: A study on validity and
reliability test of the beck depression scale. J Psychol.
6:118–122. 1988.
|
|
12
|
Sarmer S, Ergin S and Yavuzer G: The
validity and reliability of the Turkish version of the fibromyalgia
impact questionnaire. Rheumatol Int. 20:9–12. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rhudy JL and France CR: Defining the
nociceptive flexion reflex (NFR) threshold in human participants: A
comparison of different scoring criteria. Pain. 128:244–253.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bidonde J, Busch AJ, Schachter CL, Overend
TJ, Kim SY, Góes SM, Boden C and Foulds HJ: Aerobic exercise
training for adults with fibromyalgia. Cochrane Database Syst Rev.
6(CD012700)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Andrade A, Steffens RA, Sieczkowska SM,
Tartaruga LA and Vilarino GT: A systematic review of the effects of
strength training in patients with fibromyalgia: Clinical outcomes
and design considerations. Adv Rheumatol. 58(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Desmeules JA, Cedraschi C, Rapiti E,
Baumgartner E, Finckh A, Cohen P, Dayer P and Vischer TL:
Neurophysiologic evidence for a central sensitization in patients
with fibromyalgia. Arthritis Rheum. 48:1420–1429. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ang DC, Chakr R, Mazzuca S, France CR,
Steiner J and Stump T: Cognitive-behavioral therapy attenuates
nociceptive responding in patients with fibromyalgia: A pilot
study. Arthritis Care Res (Hoboken). 62:618–623. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Amelin AV, Zaĭtsev AA, Ivanov VE, Ignatov
IuD, Korenko LA and Skoromets AA: Study of mechanisms of action of
amitriptyline and acupuncture using nociceptive flexor reflex in
patients with chronic forms of headache. Anesteziol Reanimatol.
5:19–21. 1998.PubMed/NCBI(In Russian).
|
|
19
|
Matthey A, Cedraschi C, Piguet V, Besson
M, Chabert J, Daali Y, Courvoisier D, Montagne A, Dayer P and
Desmeules JA: Dual reuptake inhibitor milnacipran and spinal pain
pathways in fibromyalgia patients: A randomized, double-blind,
placebo-controlled trial. Pain Physician. 16:E553–E562.
2013.PubMed/NCBI
|
|
20
|
Ang DC, Chakr R, France CR, Mazzuca SA,
Stump TE, Hilligoss J and Lengerich A: Association of nociceptive
responsivity with clinical pain and the moderating effect of
depression. J Pain. 12:384–389. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Al-Harthy M, Ohrbach R, Michelotti A and
List T: The effect of culture on pain sensitivity. J Oral Rehabil.
43:81–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Straube S, Derry S, Moore RA and McQuay
HJ: Pregabalin in fibromyalgia: Meta-analysis of efficacy and
safety from company clinical trial reports. Rheumatology (Oxford).
49:706–715. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sañudo B, Galiano D, Carrasco L,
Blagojevic M, de Hoyo M and Saxton J: Aerobic exercise versus
combined exercise therapy in women with fibromyalgia syndrome: A
randomized controlled trial. Arch Phys Med Rehabil. 91:1838–1843.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sandrini G, Serrao M, Rossi P, Romaniello
A, Cruccu G and Willer JC: The lower limb flexion reflex in humans.
Prog Neurobiol. 77:353–395. 2005.PubMed/NCBI View Article : Google Scholar
|