Introduction
Pulmonary hypertension (PH) is a cardiopulmonary
disease characterized by increased pulmonary vascular resistance,
leading to elevated pulmonary arterial pressure, progressive right
heart failure and ultimately, death, caused by various etiologies
(1,2). The ‘2022 ESC/ERS Guidelines for the
diagnosis and treatment of pulmonary hypertension’ define a
hemodynamic criterion for diagnosing PH as a mean pulmonary artery
pressure (mPAP) >20 mmHg, measured during resting right heart
catheterization (3).
Connective tissue disorder (CTD)-associated PH
(CTD-PH) is the second most common cause in group 1 arterial PH,
following idiopathic pulmonary arterial hypertension (4). PH is a severe and life-threatening
complication of CTDs, primarily occurring in systemic sclerosis,
systemic lupus erythematosus, mixed connective tissue disease and
less frequently, in rheumatoid arthritis, inflammatory myopathies
and Sjögren's syndrome (5,6). Among patients with CTDs, those with
concurrent PH have a significantly worse prognosis and lower
survival rates compared to those without PH (7). Therefore, the early detection of PH in
CTDs and proactive intervention are of utmost importance.
As mentioned previously, in the ‘2022 ESC/ERS
Guidelines for the diagnosis and treatment of pulmonary
hypertension’, right heart catheterization is considered to be the
gold standard for the diagnosis of PH (3); however, it is an invasive procedure
associated with potential complications, risks and technical
difficulties, limiting its clinical application. Transthoracic
echocardiography with Doppler is recommended as the primary
non-invasive screening and evaluation method for PH (3). However, there is a certain degree of
mismatch between the pulmonary artery systolic pressure (PASP)
obtained through echocardiography and the mPAP measured by right
heart catheterization, leading to potential underdiagnosis or
misdiagnosis, notably when Doppler envelope quality is fair or poor
(8). Therefore, there is a
requirement to establish a simpler non-invasive tool for
identifying PH. Previous studies have reported a correlation
between the forced vital capacity (FVC)%/diffusing capacity of the
lungs for carbon monoxide (DLCO)% and the presence of PH in
patients with systemic sclerosis (9-11).
Recently, another study indicated that FVC%/DLCO% could identify PH
associated with chronic obstructive pulmonary disease and predict
the 5-year all-cause mortality of patients with chronic obstructive
pulmonary disease (12). Therefore,
the primary focus of the present study was to investigate whether
FVC%/DLCO% can be used to predict PH in a range of CTDs.
Materials and methods
Study subjects
The present study included consecutive patients who
underwent right heart catheterization at the Respiratory and
Critical Care Medicine Center, The First Affiliated Hospital of
Henan University of Science and Technology between July, 2019 and
July, 2022 and were diagnosed with CTD and suspected concurrent PH
by the rheumatology and immunology specialists. The inclusion
criteria required adherence to the following conditions: i) A
diagnosis of relevant CTDs, including systemic lupus erythematosus
(13), rheumatoid arthritis
(14), inflammatory myopathies
(15), systemic sclerosis (16) and Sjögren's syndrome (17), based on the diagnostic criteria of
the American College of Rheumatology/European League Against
Rheumatism (13-17)
and based on the criteria by Sharp et al (18) for mixed connective tissue disease;
ii) transthoracic Doppler ultrasound indicating PASP >30 mmHg.
The exclusion criteria were as follows: i) An age <18 or >70
years; ii) contraindications for pulmonary function testing, such
as pneumothorax, aortic aneurysm, recent myocardial infarction,
active pulmonary tuberculosis, or inability to cooperate with the
test; iii) contraindications for right heart catheterization, such
as bleeding tendency, severe arrhythmia or acute infection; iv)
incomplete clinical data. Finally, a total of 53 patients were
included in the present study and were categorized into two groups
based on a mPAP threshold of 20 mmHg. The groups were the
following: PH group (34 cases) and non-PH group (19 cases). The
present study was approved by the Ethics Committee of the First
Affiliated Hospital of Henan University of Science and Technology
(2019-03-K0027) and all participants provided written informed
consent.
General data collection
The detailed records of demographic data, clinical
information and laboratory test results were collected for all
participants. This included sex, age, body mass index, smoking
status, transthoracic echocardiography PASP, FVC%/DLCO%, 6-min walk
distance, plasma brain natriuretic peptide (BNP) levels, white
blood cell count, red cell distribution width, erythrocyte
sedimentation rate and C-reactive protein levels.
Pulmonary function test
Spirometry was performed using the Jaeger lung
function analyzer (SpringDe Electronic Technology Co., Ltd.) to
assess the patient's pulmonary ventilation and diffusion function.
Forced expiratory volume in one second (FEV1), FVC, FEV1% FVC,
FVC%, DLCO% and other measurements were performed in all subjects
participating in the study in accordance with the recommendations
of the American Thoracic Society and the European Respiratory
Society (19,20). The ‘single breath method’ was used
for DLCO measurements and DLCO measurements were corrected for
serum hemoglobin, as previously described (21).
Echocardiograms
Resting two-dimensional transthoracic
echocardiography was performed using standard techniques. The
transtricuspid pressure gradient was calculated using the modified
Bernoulli equation (4v2), where ‘v’ is the maximum velocity of the
tricuspid valve regurgitant jet. The right atrial pressure (RAP)
was estimated by the respiratory variation in the diameter of the
inferior vena cava. The right ventricular systolic pressure (RVSP)
was calculated by addition of the transtricuspid pressure gradient
to the RAP estimate (22). PASP was
approximately equal to RVSP.
Right heart catheterization
All enrolled patients underwent right heart
catheterization using a Swan-Ganz catheter (Edwards Lifesciences
Corp.). The catheter was inserted via the right femoral vein using
the Seldinger technique. Once a successful puncture was achieved, a
guidewire and sheath were inserted. The distal extension tube of
the catheter was connected to the pressure transducer; the
thermistor connector was connected to the sensor and the inflation
valve of the balloon to the inflation syringe (1.5 ml). The
position of the catheter tip was determined based on the changes in
the pressure waveform. The catheter was advanced until a wedged
waveform was observed, indicating pulmonary artery wedge pressure.
Pressure measurements were recorded at various locations including
the pulmonary artery, right ventricle, right atrium and vena cava
during quiet expiration.
Statistical analysis
Statistical analysis was performed using SPSS 27.0
software. Continuous variables are expressed as the mean ± standard
deviation. The t-test was used to compare continuous variables
between two groups. Categorical variables are presented as numbers
and percentages and the χ2 test was used to compare the
different categorical variables between groups. Logistic regression
analysis was performed to identify independent predictive factors
for significant differences. Pearson's correlation analysis was
conducted to assess the correlation between independent predictive
factors and mPAP. Receiver operating characteristic (ROC) curve
analysis was used to evaluate the diagnostic value of each
predictive factor for the disease and a logistic regression model
was established for joint diagnosis to explore the feasibility of
disease assessment. P<0.05 was considered to indicate a
statistically significant difference.
Results
Disease composition of CTD-PH
A total of 53 eligible patients with six types of
CTDs (systemic lupus erythematosus, rheumatoid arthritis, mixed
CTD, inflammatory myopathies, systemic sclerosis and Sjogren's
syndrome) were included in the present study. Following right heart
catheterization, they were divided into the following two groups
based on a mPAP threshold of 20 mmHg: The PH group (34 cases) and
the non-PH group (19 cases). Among the 34 patients with PH, 14
cases were diagnosed with systemic lupus erythematosus (41.2%), 6
cases with rheumatoid arthritis (17.6%), 6 cases with mixed CTD
(17.6%), 3 cases with inflammatory myopathies (8.8%), 3 cases with
systemic sclerosis (8.8%) and 2 cases with Sjogren's syndrome
(5.9%; Table I).
 | Table IDisease manifestation of CTD-PH. |
Table I
Disease manifestation of CTD-PH.
| Classification of
CTD-PH | Count, n (%) |
|---|
| Systemic lupus
erythematosus | 14 (41.2) |
| Rheumatoid
arthritis | 6 (17.6) |
| Mixed connective
tissue disease | 6 (17.6) |
| Inflammatory
myopathies | 3 (8.8) |
| Systemic
sclerosis | 3 (8.8) |
| Sjogren's
syndrome | 2 (5.9) |
Comparison of population
characteristics and laboratory test results
Following comparison of the population
characteristics and the laboratory test results between the PH and
the non-PH groups, no statistically significant differences were
found as regards the parameters age, sex, body mass index, smoking
index, white blood cell count, red cell distribution width,
erythrocyte sedimentation rate and C-reactive protein. However, the
PH group exhibited significantly higher values of FVC%/DLCO%, mPAP
(which was used for grouping), echocardiographic PASP and plasma
BNP compared with those noted in the non-PH group. The 6-min
walking distance was significantly lower in the PH group (Table II).
 | Table IIComparison of the demographic
characteristics and general information of the patients. |
Table II
Comparison of the demographic
characteristics and general information of the patients.
| Characteristic | PH (n=34) | non-PH (n=19) | P-value |
|---|
| Age (years) | 45.85±12.87 | 41.15±14.78 | 0.233 |
| Sex
(male/female) | 2/32 | 1/18 | 1.000 |
| BMI
(kg/m2) | 20.72±1.85 | 20.34±2.5 | 0.541 |
| Smoking
indexa | 26.47±18.55 | 48.42±33.76 | 0.537 |
| FVC%/DLCO% | 1.54±0.43 | 1.11±0.33 | <0.001 |
| mPAP (mmHg) | 38.06±11.25 | 15.53±3.72 | <0.001 |
| Echocardiographic
PASP (mmHg) | 54.97±18.55 | 40.26±9.12 | <0.001 |
| Plasma BNP
(pg/ml) |
1,010.72±1,848.83 | 117.16±138.36 | 0.008 |
| WBC
(x109/l) | 5.26±2.26 | 4.78±2.45 | 0.505 |
| RDW (%) | 28.19±15.85 | 26.33±12.84 | 0.643 |
| ESR (mm/h) | 32.41±26.87 | 44.42±26.27 | 0.122 |
| CRP (mg/l) | 15.02±23.15 | 14.25±18.11 | 0.902 |
| 6MWD (m) | 348.71±90.66 | 431.95±59.26 | <0.001 |
Multivariable logistic regression
analysis of factors associated with pulmonary hypertension
A multivariable logistic regression analysis was
conducted on the significantly different indicators (FVC%/DLCO%,
PASP, plasma BNP and 6-min walking distance) to determine their
independent predictive factors for PH. The results indicated that
the 6-min walking distance was not an independent predictive factor
for CTD-PH (P=0.470), while FVC%/DLCO%, PASP, and plasma BNP could
serve as independent predictive factors for CTD-PH (Table III).
 | Table IIIMultivariate logistic regression
analysis related to PH. |
Table III
Multivariate logistic regression
analysis related to PH.
| Characteristic | OR | 95% CI | P-value |
|---|
| FVC%/DLCO% | 1.45 | 1.120-1.890 | 0.027 |
| Echocardiographic
PASP | 1.893 | 1.512-2.482 | 0.019 |
| Plasma BNP | 1.994 | 1.290-2.399 | 0.019 |
| 6MWD | 1.005 | 0.991-1.019 | 0.470 |
Correlation analysis of FVC%/DLCO%,
PASP, plasma BNP and mPAP
Pearson's correlation analysis was performed to
examine the correlation between the selected independent predictive
factors, including FVC%/DLCO%, PASP, plasma BNP and mPAP. The
results indicated no statistically significant correlation between
plasma BNP and mPAP (P=0.054). However, a significant correlation
was noted between FVC%/DLCO% and mPAP (R=0.499, P<0.001;
Fig. 1), as well as between
echocardiographic PASP and mPAP (R=0.571, P<0.001; Fig. 2).
ROC curve of FVC%/DLCO% and
echocardiographic PASP for the diagnosis of CTD-PH
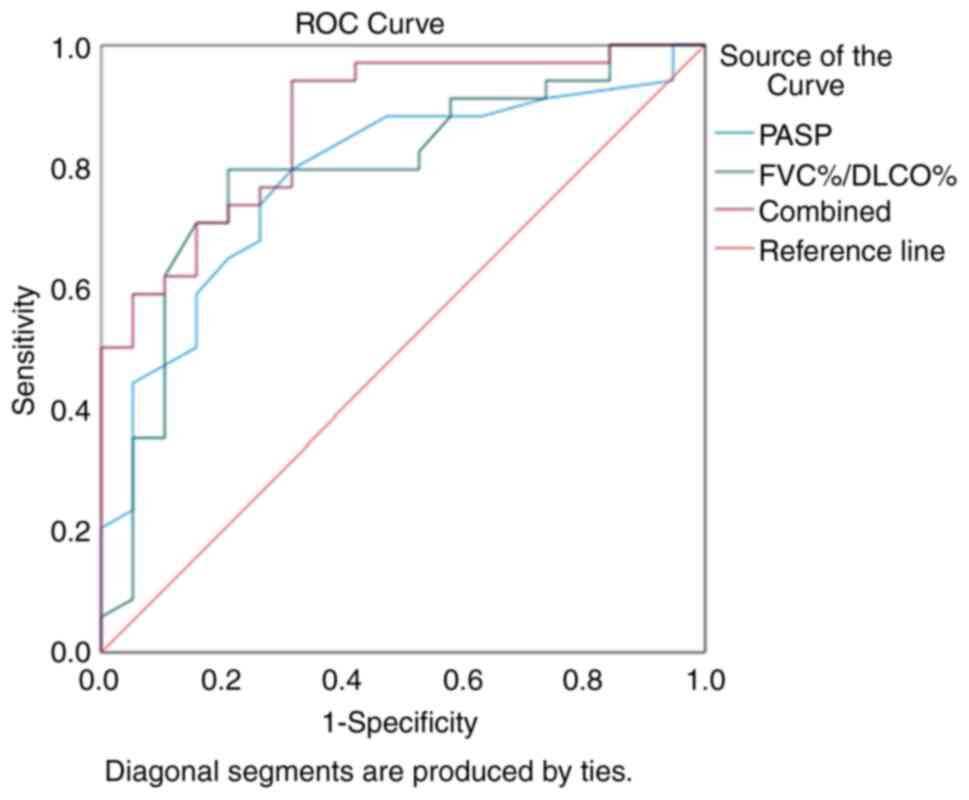
The values of FVC%/DLCO% and PASP in diagnosing
CTD-PH were determined using ROC curve analysis. The AUC for using
FVC%/DLCO% alone to diagnose CTD-PH was 0.791, with an optimal
diagnostic threshold of 1.35. The sensitivity was 0.794 and the
specificity 0.789. The AUC for using PASP alone to diagnose CTD-PH
was 0.783, with an optimal diagnostic threshold of 39.5 mmHg. The
sensitivity was 0.794 and the specificity 0.684. When combining
both FVC%/DLCO% and PASP for the diagnosis of CTD-PH, the AUC was
0.872, with a sensitivity of 0.941 and a specificity of 0.684.
These results indicated that the combined use of FVC%/DLCO% and
PASP markedly improved the diagnostic rate of CTD-PH (Fig. 3).
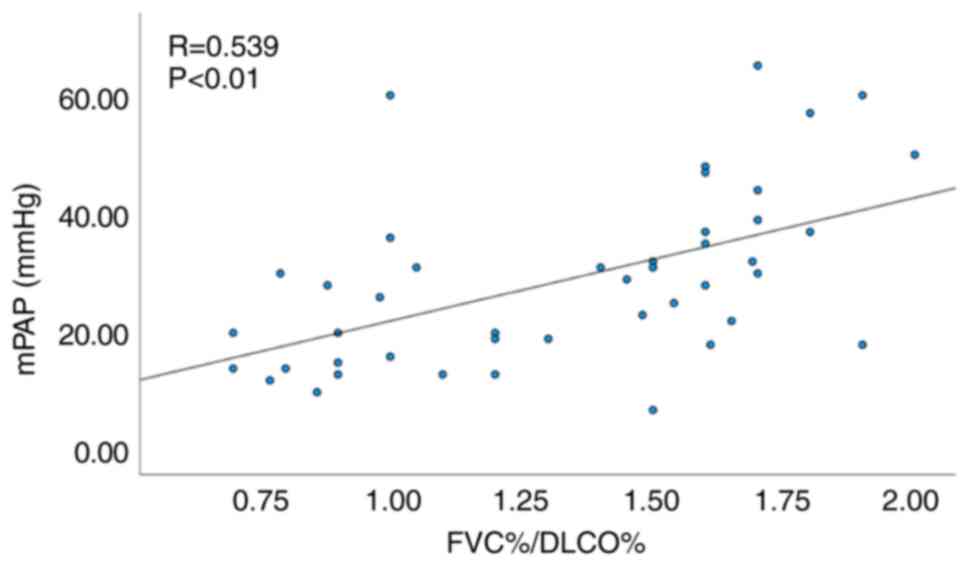
Subgroup analysis excluding patients
with concomitant interstitial lung disease (ILD)
Since 9 patients presented with concomitant
interstitial lung disease in the present study, the assessment of
the effect of ILD was examined using a subgroup analysis.
Similarly, among the 44 patients without ILD a significant
correlation was noted between FVC%/DLCO% and mPAP (R=0.539,
P<0.01; Fig. 4), as well as
between echocardiographic PASP and mPAP (R=0.620, P<0.01;
Fig. 5).
Discussion
PH is associated with a significant impact on the
quality of life of patients and high mortality rates, similar to
other cardiovascular diseases (23).
In a registry study on PH in the USA, CTD-PH was found to be
particularly prominent in group 1 pulmonary arterial hypertension
classification (24). Therefore, the
timely identification and treatment of PH in patients with CTD is
of utmost importance. The 2022 ESC/ERS guidelines and the
Australian Scleroderma Interest Group recommend the annual
screening of patients with PH with systemic sclerosis, including
asymptomatic individuals, using transthoracic echocardiography,
pulmonary function tests and serum biomarkers (3,25,26).
However, these guidelines (3,25,26)
do not specify screening for PH in other types of CTD, which may be
due to the higher prevalence of systemic sclerosis-associated PH in
Western countries, such as 74% in the UK and 68% in France
(27,28). In Asian countries, the highest
proportion of PH incidence is noted for systemic lupus
erythematosus, with 58.4% in China and 35.3% in Korea (29,30). In
the present study, the highest incidence of CTD-associated PH was
found in systemic lupus erythematosus (41.2%), followed by
rheumatoid arthritis (17.6%) and mixed connective tissue disease
(17.6%). The relatively low incidence of systemic sclerosis (8.8%)
in the present study aligns with data derived from large-scale
studies in Asia. Therefore, active screening for PH in Asian
countries, including systemic sclerosis and other types of CTDs, is
necessary.
The definitive diagnosis of PH is typically based on
right heart catheterization; however, this procedure is limited by
its technical requirements and the inherent risks of invasive
operations. The conduct of this examination for all suspected
patients with PH is not practical (31). Therefore, the non-invasive screening
methods explored in the present study, which include systemic lupus
erythematosus and systemic sclerosis, are of utmost importance.
The present study indicated a strong correlation
between the FVC%/DLCO% ratio in pulmonary function tests and mPAP
measured by right heart catheterization. The use of FVC%/DLCO%
alone for the diagnosis of CTD-PH yielded a high sensitivity
(0.794) and specificity (0.789), with an optimal diagnostic
threshold of 1.35. The sensitivity for diagnosing PH improved to
0.94 when the aforementioned method was combined with
echocardiography; the value obtained was significantly higher than
that of echocardiography alone. This combination approach is more
favorable for the early detection of CTD-PH. This finding can be
explained by the pathological mechanisms of CTD-PH. In pulmonary
function tests, FVC primarily reflects the ventilatory function of
the patient, while DLCO reflects the diffusion function.
Inflammatory stimulation in CTD leads to the excessive
proliferation and differentiation of endothelial cells, smooth
muscle cells and fibroblasts in pulmonary arteries, resulting in
the thickening of the pulmonary arterial intima and decreased
peripheral vascular beds, thereby causing a decline in DLCO. By
contrast, FVC does not decrease proportionally (9,32,33).
Therefore, the FVC%/DLCO% ratio may serve as a diagnostic criterion
for CTD-PH. It is recommended that clinicians regularly conduct
pulmonary function tests in the routine management of patients with
CTD to identify individuals with PH at an early stage and to
intervene early, in order to minimize adverse outcomes. The 2022
ESC/ERS guidelines (3) incorporate
various indicators, including the 6-min walking distance and plasma
BNP, into the risk stratification of PH. However, in the present
study, the 6-min walking distance did not exhibit an independent
predictive value for PH. It was speculated that this may be due to
the potential influence of joint involvement and restricted
activity in certain patients with CTD. Plasma BNP was identified as
an independent predictive factor for PH; however, no significant
correlation was noted between plasma BNP and mPAP (P=0.054). This
may be attributed to the relatively small sample size and certain
biases in the present study.
Considering that a relatively large proportion of
patients with CTD exhibited concomitant ILD with 20.58% (7/34) in
the PH group and 10.53% (2/19) in the non-PH group, subgroup
analysis was conducted to exclude the influence of decreased DLCO
in ILD on the final results. The analysis revealed an optimal
correlation between FVC%/DLCO% and mPAP even following the
exclusion of patients with ILD.
To the best of our knowledge, the present study is
the first to propose the application of the FVC%/DLCO% ratio as a
predictive tool for the identification of PH associated with a
range of CTDs. However, this was is a single-center study with a
relatively small sample size and further clinical validation is
required. Further larger multicenter cohort studies are warranted
in order to further verify the established threshold of FVC%/DLCO%
for the diagnosis of CTD-PH and explore its association with
overall disease mortality.
Acknowledgements
The authors would like to thank Professor Tongsheng
Wang (Respiratory and Critical Care Medicine Center of the First
Affiliated Hospital of Henan University of Science and Technology,
Luoyang, China) for his assistance during the revision of the
manuscript. He provided assistance with correcting the diction and
grammar of the article.
Funding
Funding: The present study was sponsored by the Henan Provincial
Medical Science and Technology Research Project (grant no.
LHGJ20200594).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and XH designed the theme of the study. HL and JS
assisted in the collection of data. HS wrote and reviewed the
article. PG and XH had made significant contributions to the
analysis and interpretation of the data. All authors have read and
approved the final manuscript. HS and XH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Henan University of
Science and Technology (2019-03-K0027), and all participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Uden D, Boomars K and Kool M:
Dendritic cell subsets and effector function in idiopathic and
connective tissue disease-associated pulmonary arterial
hypertension. Front Immunol. 10(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang HMF: Progress of diagnosis and
treament of connective tissue disease related pulmonary arterial
hypertension. J Med Recapitulate. 18:1686–1689. 2012.
|
|
3
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Heart J.
43:3618–3731. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cansu DU and Korkmaz C: Pulmonary
hypertension in connective tissue diseases: Epidemiology,
pathogenesis, and treatment. Clin Rheumatol. 42:2601–2610.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rhee RL, Gabler NB, Sangani S, Praestgaard
A, Merkel PA and Kawut SM: Comparison of treatment response in
idiopathic and connective tissue disease-associated pulmonary
arterial hypertension. Am J Respir Crit Care Med. 192:1111–1117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mukerjee D, St George D, Coleiro B, Knight
C, Denton CP, Davar J, Black CM and Coghlan JG: Prevalence and
outcome in systemic sclerosis associated pulmonary arterial
hypertension: Application of a registry approach. Ann Rheum Dis.
62:1088–1093. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Clements PJ, Tan M, McLaughlin VV, Oudiz
RJ, Tapson VF, Channick RN, Rubin LJ and Langer A: Pulmonary
Arterial Hypertension Quality Enhancement Research Initiative
(PAH-QuERI) Investigators. The pulmonary arterial hypertension
quality enhancement research initiative: comparison of patients
with idiopathic PAH to patients with systemic sclerosis-associated
PAH. Ann Rheum Dis. 71:249–252. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang RF, Zhou L, Ma GF, Shao FC, Wu XH
and Ying KJ: Diagnostic value of transthoracic Doppler
echocardiography in pulmonary hypertension: a meta-analysis. Am J
Hypertens. 23:1261–1264. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Donato L, Giovanna Elisiana C, Giuseppe G,
Pietro S, Michele C, Brunetti ND, Valentina V, Matteo DB and Maria
Pia FB: Utility of FVC/DLCO ratio to stratify the risk of mortality
in unselected subjects with pulmonary hypertension. Intern Emerg
Med. 12:319–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sivova N, Launay D, Wémeau-Stervinou L, De
Groote P, Remy-Jardin M, Denis G, Lambert M, Lamblin N,
Morell-Dubois S, Fertin M, et al: Relevance of partitioning DLCO to
detect pulmonary hypertension in systemic sclerosis. PLoS One.
8(e78001)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hsu VM, Chung L, Hummers LK, Wigley F,
Simms R, Bolster M, Silver R, Fischer A, Hinchcliff ME, Varga J, et
al: Development of pulmonary hypertension in a high-risk population
with systemic sclerosis in the pulmonary hypertension assessment
and recognition of outcomes in scleroderma (PHAROS) cohort study.
Semin Arthritis Rheum. 44:55–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Zhang R, Shan H, Shi W, Feng X, Chen
H, Yang X, Li Y, Zhang J and Zhang M: FVC/D(LCO) identifies
pulmonary hypertension and predicts 5-year all-cause mortality in
patients with COPD. Eur J Med Res. 28(174)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aringer M, Costenbader K, Daikh D, Brinks
R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen
DL, et al: 2019 european league against rheumatism/American college
of rheumatology classification criteria for systemic lupus
erythematosus. Ann Rheum Dis. 78:1151–1159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American college of rheumatology/european league against
rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lundberg IE, Tjärnlund A, Bottai M, Werth
VP, Pilkington C, Visser M, Alfredsson L, Amato AA, Barohn RJ,
Liang MH, et al: 2017 european league against rheumatism/American
college of rheumatology classification criteria for adult and
juvenile idiopathic inflammatory myopathies and their major
subgroups. Ann Rheum Dis. 76:1955–1964. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
van den Hoogen F, Khanna D, Fransen J,
Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP,
Medsger TA Jr, Carreira PE, et al: 2013 classification criteria for
systemic sclerosis: An American college of rheumatology/European
league against rheumatism collaborative initiative. Ann Rheum Dis.
72:1747–1755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shiboski CH, Shiboski SC, Seror R,
Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H,
Vitali C, Bowman SJ, et al: 2016 American college of
rheumatology/european league against rheumatism classification
criteria for primary Sjögren's syndrome: A consensus and
data-driven methodology involving three international patient
cohorts. Arthritis Rheumatol. 69:35–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharp GC, Irvin WS, Tan EM, Gould RG and
Holman HR: Mixed connective tissue disease-an apparently distinct
rheumatic disease syndrome associated with a specific antibody to
an extractable nuclear antigen (ENA). Am J Med. 52:148–159.
1972.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wanger J, Clausen JL, Coates A, Pedersen
OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der
Grinten CP, et al: Standardisation of the measurement of lung
volumes. Eur Respir J. 26:511–522. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Graham BL, Brusasco V, Burgos F, Cooper
BG, Jensen R, Kendrick A, MacIntyre NR, Thompson BR and Wanger J:
2017 ERS/ATS standards for single-breath carbon monoxide uptake in
the lung. Eur Respir J. 49(1600016)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Johnson DC: Importance of adjusting carbon
monoxide diffusing capacity (DLCO) and carbon monoxide transfer
coefficient (KCO) for alveolar volume. Respir Med. 94:28–37.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fisher MR, Criner GJ, Fishman AP, Hassoun
PM, Minai OA, Scharf SM and Fessler HE: NETT Research Group.
Estimating pulmonary artery pressures by echocardiography in
patients with emphysema. Eur Respir J. 30:914–921. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sarzi-Puttini P, Atzeni F, Gerli R,
Bartoloni E, Doria A, Barskova T, Matucci-Cerinic M, Sitia S,
Tomasoni L and Turiel M: Cardiac involvement in systemic rheumatic
diseases: An update. Autoimmun Rev. 9:849–852. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McGoon MD and Miller DP: REVEAL: A
contemporary US pulmonary arterial hypertension registry. Eur
Respir Rev. 21:8–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thakkar V, Stevens WM, Prior D, Moore OA,
Byron J, Liew D, Patterson K, Hissaria P, Roddy J, Zochling J, et
al: N-terminal pro-brain natriuretic peptide in a novel screening
algorithm for pulmonary arterial hypertension in systemic
sclerosis: A case-control study. Arthritis Res Ther.
14(R143)2012.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Coghlan JG, Denton CP, Grünig E, Bonderman
D, Distler O, Khanna D, Müller-Ladner U, Pope JE, Vonk MC, Doelberg
M, et al: Evidence-based detection of pulmonary arterial
hypertension in systemic sclerosis: The DETECT study. Ann Rheum
Dis. 73:1340–1349. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Condliffe R, Kiely DG, Peacock AJ, Corris
PA, Gibbs JS, Vrapi F, Das C, Elliot CA, Johnson M, DeSoyza J, et
al: Connective tissue disease-associated pulmonary arterial
hypertension in the modern treatment era. Am J Respir Crit Care
Med. 179:151–157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Humbert M, Sitbon O, Chaouat A, Bertocchi
M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot
F, et al: Pulmonary arterial hypertension in France: Results from a
national registry. Am J Respir Crit Care Med. 173:1023–1030.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao J, Wang Q, Liu Y, Tian Z, Guo X, Wang
H, Lai J, Huang C, Yang X, Li M and Zeng X: Clinical
characteristics and survival of pulmonary arterial hypertension
associated with three major connective tissue diseases: A cohort
study in China. Int J Cardiol. 236:432–437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jeon CH, Chai JY, Seo YI, Jun JB, Koh EM
and Lee SK: pulmonary hypertension study group of Korean College of
Rheumatology. Pulmonary hypertension associated with rheumatic
diseases: Baseline characteristics from the Korean registry. Int J
Rheum Dis. 15:e80–e89. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen HHC: The utilization of right heart
catheterization in the context of pulmonary arterial hypertension.
J Clin Int Med. 39:156–158. 2022.
|
|
32
|
Meyrick B and Reid L: Pulmonary
hypertension. Anatomic and physiologic correlates. Clin Chest Med.
4:199–217. 1983.PubMed/NCBI
|
|
33
|
Sun XG, Hansen JE, Oudiz RJ and Wasserman
K: Pulmonary function in primary pulmonary hypertension. J Am Coll
Cardiol. 41:1028–1035. 2003.PubMed/NCBI View Article : Google Scholar
|