Introduction
Transient receptor potential (TRP) channels
constitute a family of unselective cation channels, the majority of
which are permeable to Ca2+ (1). Alterations in the cytosolic
Ca2+ concentration play a pivotal role in fundamental
cellular processes, including the release of transmitters, cell
proliferation, gene transcription and cell death (2). In lymphocytes, the TRP channels play an
essential role in the calcium-mediated inflammatory response of the
immune system (3).
The TRP channels were first described in 1969 in the
fruit fly Drosophila melanogaster and have since been
divided into subfamilies (4,5). The subfamily with the greatest homology
to the channel discovered in the fruit fly is designated as TRP
canonical (TRPC, meaning ‘standard’). The family TRPV vanilloid
(TRPV) is named after the first member of this family, which is
designated TRPV1 (formerly known as vanilloid receptor 1). The
family with homology to melastin-1 is designated as TRPM. The
families TRPP and TRPML are named for their inclusion of polycystin
and mucolipin, respectively. The TRPA family is named for its
abundance of ankyrin repeats (5).
Finally, the TRPN family (NOMP, no mechanopotential) is worthy of
mention. Genomic analysis indicates that this channel is not
expressed in mammals (6).
Seven TRPC subunits have been identified in mammals,
with TRPC2 being pseudogenized in humans and thus, not expressed
(7). The family can be further
subdivided based on homology in the amino acid sequence: TRPC1,
TRPC2 and TRPC3/6/7(8). All TRPC
channels are composed of six transmembrane helices, which then form
a TRPC monomer (9). Four of these
monomers then form a TRPC homotetramer, which represents the
functional cation channel. However, the formation of
heterotetramers is also possible, although these are limited to the
individual subdivisions, such as TRPC3/6/7(10).
All TRPC channels, including TRPC6, require
phospholipase C (PLC) for activation (11,12). The
PLC pathway is most likely initiated by a Gq/11-coupled
receptor, which hydrolyses phosphatidylinositol 4,5-bisphosphate at
the plasma membrane to inositol 1,4,5-trisphosphate
(IP3) and diacylglycerol (DAG). IP3 can then
activate IP3 receptors on the endoplasmic reticulum
(13). DAG has been demonstrated to
directly activate the TRPC6 channel (14). This form of activation and the
resulting influx of Ca2+ into the cell is referred to as
receptor-operated Ca2+ entry (15). However, it appears that DAG is not
the sole means of channel activation (16). Another potential avenue is the
store-operated Ca2+ entry, whereby the TRPC and Orai
channels form a unified entity that becomes active in response to a
decline in intracellular Ca2+ levels within
intracellular stores (17).
A mutation of the TRPC6 gene is a cause of a
genetically inherited form of focal and segmental
glomerulosclerosis. The pathogenic mechanism is considered to be a
gain-of-function mutation and an associated calcium overload
(18,19). It is also known that the calcium
homeostasis influenced by TRPC6 affects immunological processes. A
single nucleotide polymorphism on the TRPC6 gene has been
demonstrated to protect against the development of neuropsychiatric
manifestations associated with systemic lupus erythematosus
(20). Additionally, there is
evidence to suggest that the reduced expression of TRPC6 inhibits
the proliferation of human Burkitt lymphoma cells (21). It was hypothesized that the increase
in intracellular calcium levels, which is mediated by TRPC6,
stimulates cell proliferation (21).
In addition to lymphoma cells, a connection has been established
between TRPC6 and tumor entities, such as breast, cervical,
gastric, esophageal cancer and gliomas. For all these cancer types,
an elevated expression of the channel has been documented (22-26).
In experiments conducted on TRPC6 knockout mice, a
reduction in allergic responses and IgE levels has been observed.
The examined T-helper cells (Th2) exhibited lower levels of
interleukin (IL)-5 and IL-3 secretion in comparison to the
wild-type cells (27). It has been
demonstrated that TRPC6 represents the principal channel regulating
leukocyte migration (28). This
finding builds on the understanding that an increase in
intracellular free Ca2+ plays a pivotal role in this
process, a concept which had already been established (29). Furthermore, the application of
western blotting and calcium imaging techniques enabled the
demonstration that septic peripheral blood T-lymphocytes from rats
exhibited augmented expression of TRPC6(3). This expression is associated with both
the activation of the T-cells and their release of cytokines
(30).
The current literature indicates that the TRPC6
channel may be ubiquitously expressed in human lymphatic tissues,
potentially involved in a range of functions. The TRPC6 channel has
been identified in human lymphatic tissues, particularly the
spleen, using northern blotting (31). Furthermore, the channel was
previously identified using reverse transcriptase-polymerase chain
reaction (RT-PCR) and western blotting in human peripheral blood
T-lymphocytes and Jurkat T-lymphocytes (32). RT-PCR demonstrated TRPC6 expression
in murine tissues, with a differential expression between B- and
T-lymphocytes. The expression of the TRPC6 gene was significantly
higher in B-lymphocytes than in T-lymphocytes (33). Additionally, differences in gene
expression were observed between lymphocytes from different
lymphoid organs. In these experiments, splenocytes exhibited a more
robust expression than lymphocytes from lymph nodes or the thymus.
However, the thymus, spleen, and lymph nodes exhibited a positive
detection of TRPC6 mRNA (33).
A direct examination of the protein and an overview
of the TRPC6 channel in human lymphatic tissues have not yet been
conducted, at least to the best of our knowledge. Consequently, the
present study aimed to investigate human lymphatic tissues using
immunohistochemistry (IHC) to gain a deeper understanding of the
potential immunological function of the channel.
Materials and methods
The objective of the applied methods was to identify
suitable human tissue for subsequent IHC staining to enable the
investigation of the TRPC6 channel. This was achieved by obtaining
tissue samples from lymphatic organs, including lymph nodes,
spleen, vermiform appendix, ileum and thymus from body donors.
Samples of the palatine tonsil were obtained during the course of
planned tonsillectomies. The samples were initially embedded and
cut with a microtome before undergoing hematoxylin and eosin
(H&E) staining. Using the evaluation of the H&E-stained
slides, five samples of each lymphatic organ from body donors with
good morphology were selected and processed for IHC. In total,
three samples of palatine tonsils from patients were processed for
IHC.
Specimens
A total of three tonsil samples were provided by the
Department of Otorhinolaryngology (Saarland University, Campus
Homburg/Saar, Germany). All other tissue samples were obtained from
body donors at the Institute of Anatomy (Saarland University,
Campus Homburg/Saar, Germany). Body donors (n=35) were embalmed
with a combination of nitric pickling salt, ethanol and a low
formaldehyde concentration or with a high formaldehyde
concentration (all solutions from Otto Fischar GmbH & Co. KG).
The first method was developed by Janczyk et al (34). The fixation of the corpses occurred
within a period of <72 h post-mortem. A total of 102 samples
were obtained from the body donors. A second collection of samples
was taken from the ileum, vermiform appendix and thymus due to the
difficulty in accessing these tissues. The resulting samples are
presented in Table I. An overview of
the associated body donors of the specimens subjected to IHC is
presented in Table II. The fixation
method, as well as the immediate cause of death, age at death/at
surgery, and sex of the donors are listed in Table II.
 | Table ICollected samples overall. |
Table I
Collected samples overall.
| Sample origin | First
collection | Second
collection | Overall |
|---|
| Lymph nodes | 10 | - | 10 |
| Spleen | 13 | - | 13 |
| Palatine
tonsil | 3 | - | 3 |
| Ileum | 10 | 10 | 20 |
| Vermiform
appendix | 4 | 8 | 12 |
| Thymus | 10 | 34 | 44 |
 | Table IIInformation of corresponding body
donors of the evaluated immunostained samples. |
Table II
Information of corresponding body
donors of the evaluated immunostained samples.
| A, Body donors of
lymph node samples |
|---|
| Sample origin | Fixation | Sex | Age, years | Cause of death | Lymphocyte
aggregations | Lymph node sinus,
capsule and trabeculae | Lymph node
vessels |
|---|
| Lymph nodes | NEP | F | 81 | Cardiac arrest | - | + | N.e. |
| | NEP | F | 87 | Multiorgan
failure | ++ | N.e. | N.e. |
| | Formalin | F | 83 | Cerebral
herniation | + | + | + |
| | Formalin | M | 98 | Cardiac arrest | + | + | N.e. |
| | NEP | M | 85 | Cardio-pulmonary
failure | + | + | N,e. |
| B, Body donors of
spleen samples |
| Sample origin | Fixation | Sex | Age, years | Cause of death | White pulp | Red pulp | Capsule and
trabeculae | Trabecular
arteries |
| Spleen | NEP | F | 87 | Multiorgan
failure | ++ | + | + | + |
| | NEP | F | 79 | Multiorgan
failure | ++ | ++ | + | + |
| | NEP | F | 85 | Cardio-pulmonary
failure | + | + | + | + |
| | NEP | F | 90 | Consumption
coagulopathy | + | + | + | + |
| | NEP | F | 81 | Cardiac arrest | + | + | + | + |
| C, Body donors of
tonsil samples |
| Sample origin | Fixation | Sex | Age, years | Cause of death | Secondary
follicle | T-zone | Follicle-associated
epithelium | High endothelial
venule |
| Palatine
tonsil | Formalin
(D.i.) | M | 47 | - | ++ | + | ++ | ++ |
| | Formalin
(D.i.) | F | 18 | - | ++ | + | ++ | ++ |
| | Formalin
(D.i.) | M | 32 | - | ++ | + | ++ | ++ |
| D, Body donors of
ileum samples |
| Sample origin | Fixation | Sex | Age, years | Cause of death | Lymphocyte
accumulations | Follicle associated
epithelium | Submucosa and
embedded vessels | Muscularis |
| Ileum | NEP | F | 90 | Consumption
coagulopathy | ++ | + | + | + |
| | NEP | F | 87 | Multiorgan
failure | N.e. | N.e. | + | + |
| | Formalin | M | 72 | Cerebral
hypoxia | + | - | + | + |
| | NEP | F | 75 | Advanced anal
carcinoma | + | ++ | + | + |
| | Formalin | F | 84 | Multiorgan
failure | + | + | + | + |
| E, Body donors of
appendix samples |
| Sample origin | Fixation | Sex | Age, years | Cause of death | Lymphocyte
accumulations | Follicle-associated
epithelium | Submucosa and
embedded vessels | Muscularis |
| Vermiform | Formalin | M | 72 | Cerebral
hypoxia | ++ | + | + | + |
| appendix | Formalin | M | 81 | Pneumonia | + | - | + | + |
| | NEP | F | 75 | Advanced anal
carcinoma | ++ | + | + | + |
| | Formalin | M | 92 | Multiorgan
failure | + | - | + | + |
| | Formalin | F | 82 | Age-related
mortality | + | + | + | + |
| F, Body donors of
thymus samples |
| Sample origin | Fixation | Sex | Age, years | Cause of death | Lymphocyte
aggregations | Epithelial
cells |
| Thymus | Formalin | F | 84 | Multiorgan
failure | + | + |
| | Formalin | F | 84 | Multiorgan
failure | + | + |
| | Formalin | M | 80 | Circulatory
failure | - | - |
| | Formalin | F | 89 | Cardiac arrest | + | + |
| | Formalin | M | 92 | Multiorgan
failure | - | + |
All abdominal organs (ileum, vermiform appendix and
spleen) were excised via median laparotomy. Thymus samples were
obtained via median sternotomy from retrosternal adipose tissue.
Lymph nodes were harvested through an incision at the level of the
common femoral artery. A subsequent examination revealed that
retrosternal adipose tissue also contained lymph nodes. Following
excision, the tissue samples were immersed in 4% formaldehyde
(overnight, 4˚C) and subjected to paraffin embedding. The embedded
tissues were then sectioned into 7-µm-thick slices using a
microtome (Leica RM 2026, Leica Microsystems GmbH). H&E
staining was conducted in accordance with standard protocols, and
IHC was performed as previously described (35), with additional details provided
below.Klicken oder tippen Sie hier, um Text einzugeben.
IHC
IHC staining was conducted using a primary
anti-TRPC6 antibody (cat. no. ACC-017; Alomone Labs), which has
been validated for specificity through knockout validation and
peptide incubations conducted in previous studies (36,37).
Prior to the application of the primary antibody, heat-induced
epitope retrieval (HIER) and blocking were performed. For HIER, the
sections were incubated in a 90˚C citrate buffer solution (Abcam,
Cambridge, UK) for 60 min. For blocking, the sections were washed
in phosphate-buffered saline (Carl Roth GmbH & Co. KG) and
incubated with normal goat serum (cat. no. 01-6201; Invitrogen AG;
Thermo Fisher Scientific, Inc.). The sections were incubated with
the primary antibody at a concentration of 1:50 for 12 h, with
negative and positive controls being employed at each staining
cycle. The negative controls were incubated with rabbit serum (cat.
no. PLN5001; Life Technologies; Thermo Fisher Scientific, Inc.) in
place of the primary antibody. The positive controls were of a
section of cardiac muscle of previously conducted studies by Jacobs
et al (36), as this tissue
has already been shown to express the TRPC6 protein by IHC. In
order to achieve the best possible comparability of the individual
staining runs, we used the same sample of cardiac muscle from
Jacobs et al each time. This also allowed for a direct
comparison with the peptide incubations studied by Jacobs et
al (36). A secondary antibody,
horseradish peroxidase (HRP)-conjugated (cat. no. A10547;
Invitrogen AG; Thermo Fisher Scientific Inc.), was used at a
dilution of 1:500. The sections were incubated for 60 min at room
temperature. The chromogen utilized was diaminobenzidine (cat. no.
SK-4103; Vector Laboratories, Inc.), which was incubated with the
sections for 10 min at room temperature. The chromogen exhibited a
brown coloration at the sites of antibody binding, resulting from
the reaction with the HRP of the secondary antibody. The sections
were subsequently counterstained with hematoxylin (Carl Roth GmbH
& Co. KG, Germany) at room temperature for <1 sec to achieve
a minimal counterstain that did not obscure the IHC staining.
Analysis
The sections were examined using a light microscope
with a camera (MikroCam SP 5.1; Bresser, GmbH) to assess the degree
of browning. The sections were classified into three categories
based on the intensity of the brown coloration: Strong, weak and
negative. The negative sections exhibited only blue hematoxylin
counterstaining.
Results
The results of the analysis were divided into the
various lymphatic organs that were evaluated. For each organ,
structures were defined and analyzed individually.
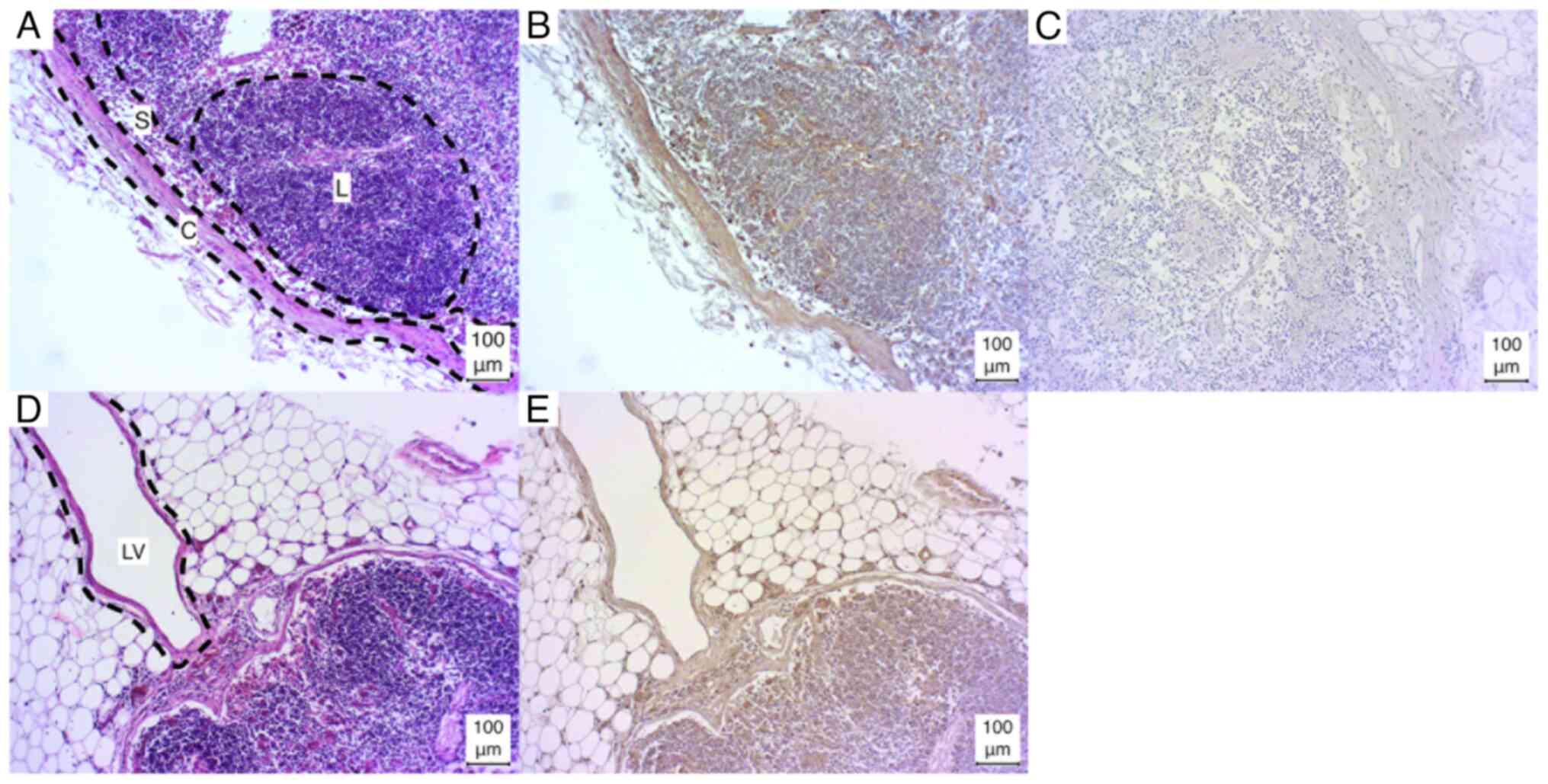
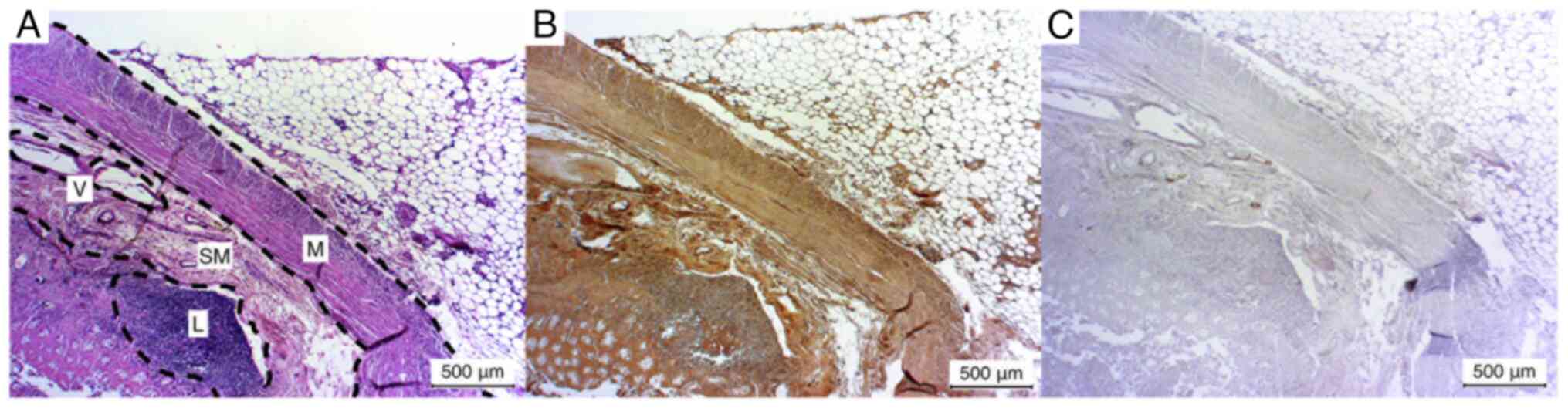
Lymph nodes
The lymph nodes exhibited a weak positive result for
lymphocyte aggregations in three of five cases (3/5), while one
case (1/5) demonstrated a strong positive result, and one case
(1/5) exhibited a negative result. The lymph node capsule,
trabeculae and sinus exhibited weak positive results in all cases.
In one sample, the sinus was fibrotic and could not be evaluated
(Fig. 1A-C).
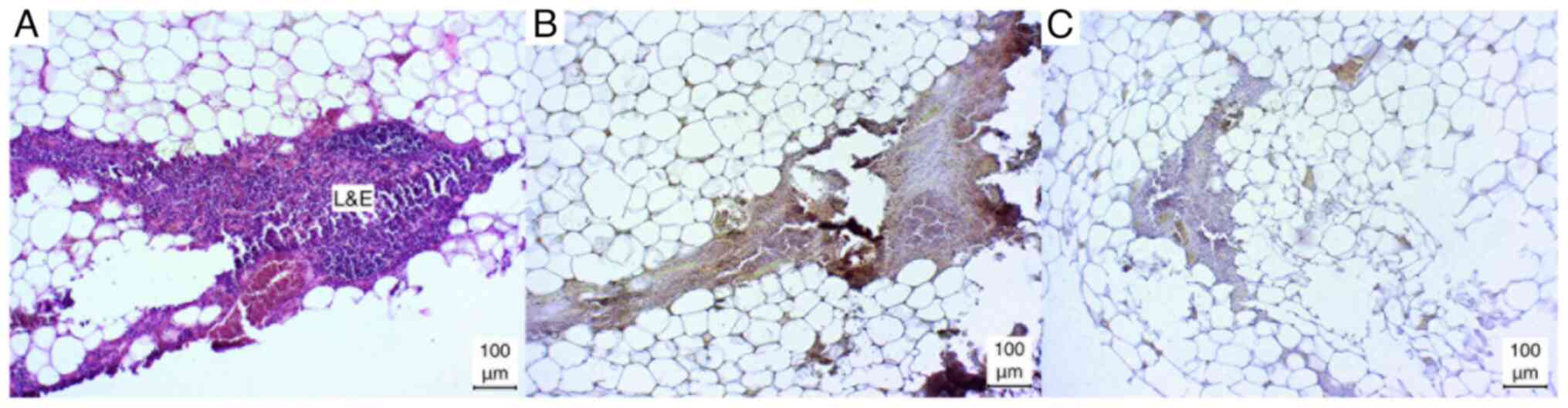
Additionally, in certain sections (1/5), the lymph
vessels were incised, resulting in a positive staining outcome.
However, due to the limited sample size, a definitive analysis
could not be conducted (Fig. 1D and
E).
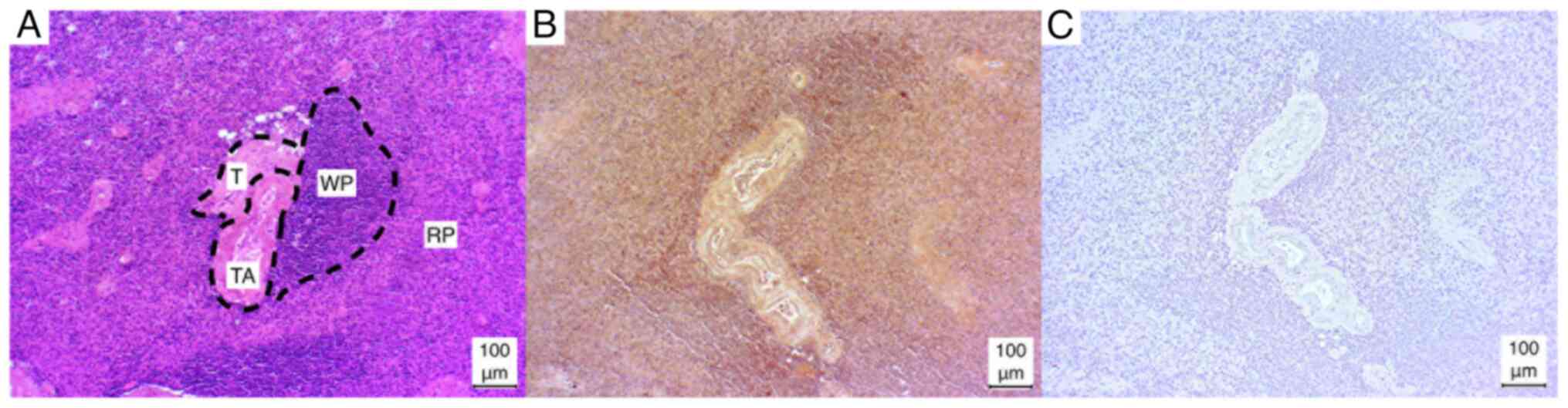
Spleen
In the case of the lymphocyte aggregations in the
white pulp, either a strong positive (2/5) or a weak positive (3/5)
result was observed. By contrast, the red pulp exhibited a weak
positive result in the majority of cases (4/5), with only one
instance of a strong positive result (1/5). In addition, the
capsule and trabeculae exhibited a consistently weak positive
staining result in all cases (5/5). Similarly, the trabecular
arteries exhibited a consistent, weak positive result, with the
media of the vessels particularly susceptible to staining (Fig. 2).
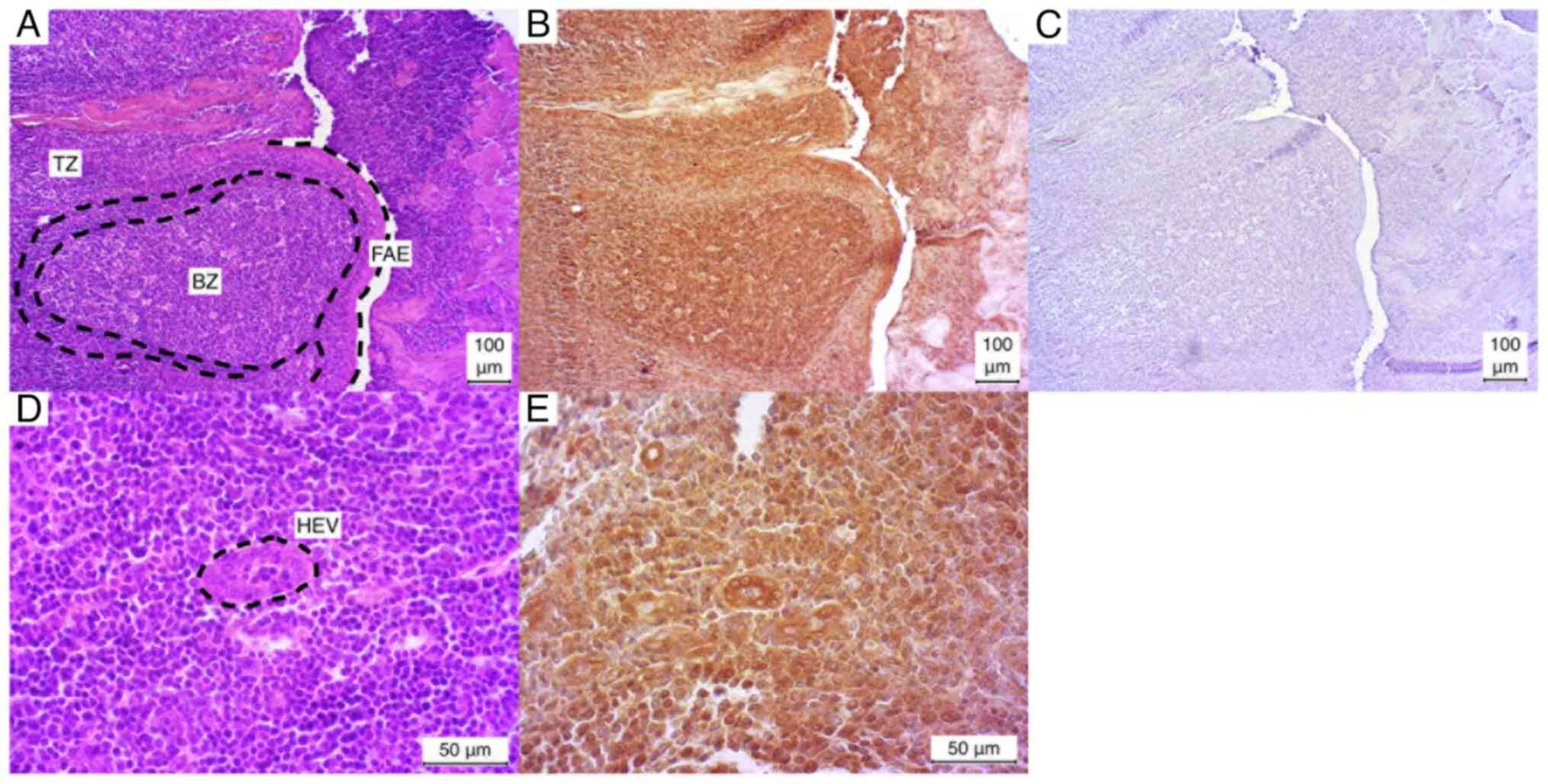
Palatine tonsil
The palatine tonsil exhibited a markedly positive
IHC staining pattern for the observed secondary follicles in all
three cases (3/3). The germinal center was particularly
well-stained in comparison to the surrounding edge. In contrast,
the T-zone demonstrated a relatively weak positive staining pattern
in all three cases (3/3). The follicle associated epithelium (FAE)
and the incised high endothelial venules (HEVs) exhibited a
similarly robust positive result in all three cases (Fig. 3).
Ileum
The ileum exhibited a weak positive staining result
for lymphocyte accumulations in the majority of cases (3/5), while
in one additional case (1/5), the lymphocytes demonstrated a
negative staining result. In one instance (1/5), Peyer's patches
were not observed, thus precluding the assessment of the
lymphocytes and FAE. In one of five cases (1/5), the FAE exhibited
a markedly positive staining result. In the remaining cases, the
staining was weak positive (3/5). The submucosa and the embedded
vessels and muscularis exhibited a consistently weak positive
result in all five cases (Fig.
4).
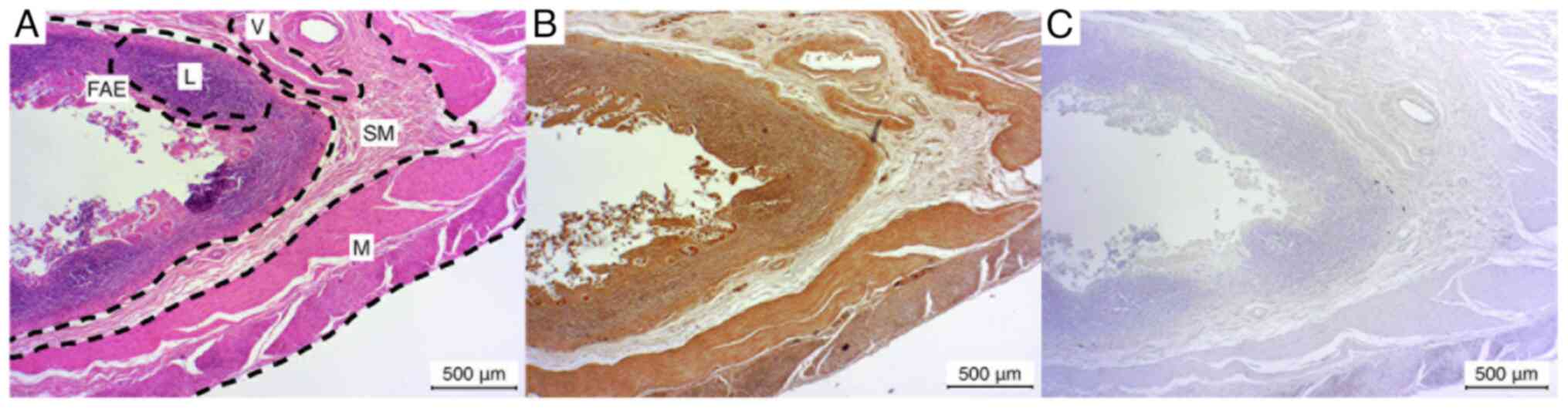
Vermiform appendix
The results of the vermiform appendix exhibited a
comparable trend to those of the ileum. In the majority of cases
(3/5), a weak positive staining of the lymphocyte aggregation in
the Peyer's patches was observed. In the remaining cases (2/5), the
lymphocyte staining was negative. The FAE exhibited a markedly
positive staining result in two of the five cases (2/5), while the
remaining three cases demonstrated a weak positive result (3/5).
The submucosa and the embedded vessels and muscular layer exhibited
a consistently weak positive staining result in all five cases
(Fig. 5).
Thymus
In the majority of cases (3/5), the thymus tissue
exhibited a weak positive result for lymphocyte immunostaining.
Conversely, in the remaining cases (2/5), the lymphocyte
immunophenotyping yielded a negative result. The intermediate
epithelial cells demonstrated a weak positive immunostaining in
four of the five cases (4/5). In one instance, a negative result
was observed (1/5). The overall result of the thymus tissue
staining was the weakest (Fig.
6).
Discussion
The present study demonstrated the presence of TRPC6
protein in all investigated lymphatic tissues. To the best of our
knowledge, this represents the first direct detection of the
protein in lymphatic tissues.
The specificity of the antibody has been
corroborated in previous studies that utilized the same antibody
from Alomone Labs through peptide incubation procedures and
knockout validation experiments (36,37). Our
study established positive control samples for the same tissue
type, cardiac muscle, which allowed for comparison with the
previously mentioned study by Jacobs et al (30).
The TRPC6 protein was identified in lymphocytes in
all examined tissues, with notable variations observed across
different organs. In the spleen, the white pulp exhibited stronger
immunostaining than the lymphocyte aggregates in lymph nodes,
ileum, appendix and thymus. This pattern aligns with the previously
described expression patterns of the TRPC6 gene in murine lymphatic
tissues (38).
However, the strongest staining signal was observed
in the lymphocytic population of the palatine tonsil. This
observation may be attributed to the fact that the tissue was
obtained from young patients undergoing tonsillectomy. A
differential staining behavior between T- and B-lymphocytes was
also observed, with the B-zone exhibiting a darker staining pattern
than the T-zone in the tonsil. The results are consistent with the
previously described TRPC6 expression patterns in murine T- and
B-lymphocytes. In this case, the B-lymphocytes exhibited a
significantly stronger expression than the T-lymphocytes (33).
The TRPC6 protein was identified in
follicle-associated lymphatic tissue in both the tonsil and
gut-associated lymphatic tissue. TRPC6 may be involved in molecular
processes of the immune response.
The lymph vessels in lymph nodes, the vessels in the
submucosa of the vermiform appendix and ileum, as well as the
trabecular arteries of the spleen expressed TRPC6. This was
similarly described for vessels in non-lymphatic organs in the
study by Abdinghoff et al (39). The expression of the TRPC6 protein in
murine lymph vessels has already been described (40). The intima and adventitia exhibited
particularly robust staining in the IHC analysis. It is noteworthy
that the lymphatic vessels were only incised in one of the observed
sections, limiting the ability to draw conclusions about TRPC6
expression lymphatic vessels.
The observation of HEVs in the palatine tonsil
demonstrated a notable presence of TRPC6 protein. As TRPC6
functions as a mediator in leukocyte migration (28), it was hypothesized that this process
in HEVs may be associated with TRPC6. This assertion is limited to
granulocytes at present, however. A connection between TRPC6 and
the diapedesis of lymphocytes through the HEV has not been
described and cannot be proven by the methodology of the present
study. Unfortunately, HEV could not be observed in the other
examined tissues, which can be attributed to processes of
immunosenescence (41).
The most notable limitation of the present study was
the advanced age of the donors, with an average age of 83 years at
death. Consequently, the evidence presented herein is limited to
the lymphatic tissue of the elderly, with the exception of the
tonsils. As only tonsillar tissue from young subjects was examined,
it cannot be ruled out that the comparability of the results to the
other examined tissues may be affected by the age difference. It
was possible to identify age-related morphological changes in the
lymph nodes and thymus, as well as in the Peyer's patches in the
ileum and vermiform appendix. The thymus tissue exhibited the
presence of lymphocytic aggregates in the form of thymal residual
tissue networks within the retrosternal adipose tissue. Hassall's
corpuscles could not be observed. These findings were corroborated
upon repeated tissue sampling. The observed morphology of the
thymus tissue aligns with the previously described age-related
changes in morphology in the study by Ströbel et al
(42). In the lymph nodes, there was
a shift in the boundary between the cortex and the medulla, a
fibrotic transformation, a reduction in the number of HEVs and
secondary follicles (41,43-45).
Consequently, it was not possible to differentiate between the T-
and B-zones in the tissues, apart from the tonsilla, as these zones
merge during the immunosenescence (44,46).
The consistent result of the immunostaining of the
connecting tissues in lymphatic organs can be linked to the
discovery that TRPC6 plays a central role in the differentiation of
fibroblasts (47). This suggests a
potential link between TRPC6 and the development of fibrosis.
The utilization of IHC immunostaining to detect
TRPC6 in human lymphatic tissues represents a limitation of the
present study. Further assays, such as western blotting or RT-PCR,
need to be performed to substantiate the evidence that TRPC6 is
ubiquitously present in human lymphatic tissues.
Furthermore, the morphology and antigen preservation
are also affected by autolytic processes that arise during the time
periods immediately preceding the fixation process (48). It is not possible to exclude the
possibility of autolytic processes occurring as a result of the
methodology employed in this study. Moreover, it cannot be ruled
out that the applied fixation method influenced the antigen
preservation, despite the absence of differences in staining
behavior between fixed specimens of the same organ, where
dissimilar fixation techniques were employed.
In conclusion, research on TRPC6 in lymphatic tissue
is still in its infancy. Nevertheless, the present study indicates
that the widespread presence of TRPC6 in this tissue suggests a
diverse potential range of functions of the TRPC6 channel in
lymphatic tissue. The identification of drugs that interact with
TRPC6 (21,49) could open new avenues for therapeutic
intervention in autoimmune diseases, septic syndromes and malignant
lymphatic diseases. Potential treatments could target TRPC6 to
control the release of cytokines in sepsis and inhibit abnormal
calcium signaling in cancers such as B-cell lymphomas. The results
of the present study emphasize the promising future applications of
pharmacological interventions in these diseases. However, the
ubiquity of TRPC6 in lymphatic tissue demonstrated in our study is
likely to pose a major challenge to the selectivity of any
potential therapy. Further IHC studies of tissue from young
subjects, as well as pathological tissue, are necessary. Of
particular interest would be an immunohistochemical study of TRPC6
in lymphoma cells or the staining of lymph nodes of septic
patients, with a direct comparison to physiological tissue. This
could provide deeper insight into the role of TRPC6 in
pathophysiological functions in lymphatic tissue and could aid the
development of pharmaceutical therapies.
Acknowledgements
Th authors would like to express their gratitude to
Ms. Irina Scheck, Ms. Katja Schäfer, Mr. Alexander Grissmer, and
Anja Beckmann (Institute of Anatomy and Cell Biology, Saarland
University, Homburg, Germany) for their invaluable technical
assistance. Furthermore, the authors extend their gratitude to Dr.
Silke Wemmert of the Department of Otorhinolaryngology for
facilitating the procurement of the tonsillar samples.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT and FD planned and conducted the study. FD
performed the experiments. FD evaluated the data. FD and TT wrote
the manuscript. FF, AB and BS designed the study and wrote the
manuscript. FD and TT confirm the authenticity of all raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Commission of the Saarland Medical Association (Ärztekammer des
Saarlandes) under the approval no. 163/20. All donors provided
written consent for the use of their tissue samples for scientific
research during their lifetime. Additionally, patients from the
Department of Otorhinolaryngology at Saarland University Hospital
provided written consent for the use of their tissue samples for
scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pedersen SF, Owsianik G and Nilius B: TRP
channels: An overview. Cell Calcium. 38:233–252. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Wu QY, Sun MR, Wu CL, Li Y, Du JJ, Zeng
JY, Bi HL and Sun YH: Activation of calcium-sensing receptor
increases TRPC3/6 expression in T lymphocyte in sepsis. Mol
Immunol. 64:18–25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cosens DJ and Manning A: Abnormal
Electroretinogram from a Drosophila Mutant. Nature. 224:285–287.
1969.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol.
12(218)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nilius B, Owsianik G, Voets T and Peters
JA: Transient Receptor Potential Cation Channels in Disease.
Physiol Rev. 87:165–217. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wes PD, Chevesich J, Jeromin A, Rosenberg
C, Stetten G and Montell C: TRPC1, a human homolog of a Drosophila
store-operated channel. Proc Natl Acad Sci USA. 92:9652–9656.
1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Venkatachalam K and Montell C: TRP
Channels. Annu Rev Biochem. 76:387–417. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ramsey IS, Delling M and Clapham DE: An
introduction To TRP channels. Annu Rev Physiol. 68:619–647.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hofmann T, Schaefer M, Schultz G and
Gudermann T: Subunit composition of mammalian transient receptor
potential channels in living cells. Proc Natl Acad Sci USA.
99:7461–7466. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Montell C: The TRP superfamily of cation
channels. Sci STKE. 2005(re3)2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Trebak M, Vazquez G, Bird GS and Putney JW
Jr: The TRPC3/6/7 subfamily of cation channels. Cell Calcium.
33:451–461. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang H, Cheng X, Tian J, Xiao Y, Tian T,
Xu F, Hong X and Zhu MX: TRPC channels: Structure, function,
regulation and recent advances in small molecular probes. Pharmacol
Ther. 209(107497)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hofmann T, Obukhov AG, Schaefer M,
Harteneck C, Gudermann T and Schultz G: Direct activation of human
TRPC6 and TRPC3 channels by diacylglycerol. Nature. 397:259–263.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Abramowitz J and Birnbaumer L: Physiology
and pathophysiology of canonical transient receptor potential
channels. FASEB J. 23:297–328. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y
and Inoue R: Multiple regulation by calcium of murine homologues of
transient receptor potential proteins TRPC6 and TRPC7 expressed in
HEK293 cells. J Physiol. 561 (Pt 2):415–432. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao Y, Erxleben C, Abramowitz J,
Flockerzi V, Zhu MX, Armstrong DL and Birnbaumer L: Functional
interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated
heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad
Sci USA. 105:2895–2900. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Winn MP, Conlon PJ, Lynn KL, Farrington
MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S,
Burchette JL, et al: A Mutation in the TRPC6 cation channel causes
familial focal segmental glomerulosclerosis. Science.
308:1801–1804. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ramirez GA, Lanzani C, Bozzolo EP,
Citterio L, Zagato L, Casamassima N, Canti V, Sabbadini MG,
Rovere-Querini P, Manunta P and Manfredi AA: TRPC6 gene variants
and neuropsychiatric lupus. J Neuroimmunol. 288:21–24.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song X, Liu BC, Lu XY, Yang LL, Zhai YJ,
Eaton AF, Thai TL, Eaton DC, Ma HP and Shen BZ: Lovastatin inhibits
human B lymphoma cell proliferation by reducing intracellular ROS
and TRPC6 expression. Biochim Biophys Acta. 1843:894–901.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guilbert A, Dhennin-Duthille I, Hiani YE,
Haren N, Khorsi H, Sevestre H, Ahidouch A and Ouadid-Ahidouch H:
Expression of TRPC6 channels in human epithelial breast cancer
cells. BMC Cancer. 8(125)2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan Q, Zheng A, Liu X, Chen Y and Han L:
Expression of transient receptor potential channel 6 in cervical
cancer. Onco Targets Ther. 5:171–176. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G,
Jin Y and Wang Y: Blockade of TRPC6 channels induced G2/M phase
arrest and suppressed growth in human gastric cancer cells. Int J
Cancer. 125:2281–2287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ding X, He Z, Zhou K, Cheng J, Yao H, Lu
D, Cai R, Jin Y, Dong B, Xu Y and Wang Y: Essential Role of TRPC6
Channels in G2/M phase transition and development of human glioma.
J Natl Cancer Inst. 102:1052–1068. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shi Y, Ding X, He ZH, Zhou KC, Wang Q and
Wang YZ: Critical role of TRPC6 channels in G2 phase transition and
the development of human oesophageal cancer. Gut. 58:1443–1450.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sel S, Rost BR, Yildirim AÖ, Sel B, Kalwa
H, Fehrenbach H, Renz H, Gudermann T and Dietrich A: Loss of
classical transient receptor potential 6 channel reduces allergic
airway response. Clin Exp Allergy. 38:1548–1558. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weber EW, Han F, Tauseef M, Birnbaumer L,
Mehta D and Muller WA: TRPC6 is the endothelial calcium channel
that regulates leukocyte transendothelial migration during the
inflammatory response. J Exp Med. 212:1883–1899. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang AJ, Manning JE, Bandak TM, Ratau MC,
Hanser KR and Silverstein SC: Endothelial cell cytosolic free
calcium regulates neutrophil migration across monolayers of
endothelial cells. J Cell Biol. 120:1371–1380. 1993.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ramirez GA, Coletto LA, Sciorati C,
Bozzolo EP, Manunta P, Rovere-Querini P and Manfredi AA: Ion
channels and transporters in inflammation: Special Focus on TRP
Channels and TRPC6. Cells. 7(70)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hofmann T, Schaefer M, Schultz G and
Gudermann T: Transient receptor potential channels as molecular
substrates of receptor-mediated cation entry. J Mol Med (Berl).
78:14–25. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gamberucci A, Giurisato E, Pizzo P, Tassi
M, Giunti R, Mcintosh DP and Benedetti A: Diacylglycerol activates
the influx of extracellular cations in T-lymphocytes independently
of intracellular calcium-store depletion and possibly involving
endogenous TRP6 gene products. Biochem J. 364 (Pt 1):245–254.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Inada H, Iida T and Tominaga M: Different
expression patterns of TRP genes in murine B and T lymphocytes.
Biochem Biophys Res Commun. 350:762–767. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Janczyk P, Weigner J, Luebke-Becker A,
Kaessmeyer S and Plendl J: Nitrite pickling salt as an alternative
to formaldehyde for embalming in veterinary anatomy-A study based
on histo- and microbiological analyses. Ann Anat. 193:71–75.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Diebolt CM, Schaudien D, Junker K,
Krasteva-Christ G, Tschernig T and Englisch CN: New insights in the
renal distribution profile of TRPC3-Of mice and men. Ann Anat.
252(152192)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jacobs T, Abdinghoff J and Tschernig T:
Protein detection and localization of the non-selective cation
channel TRPC6 in the human heart. Eur J Pharmacol.
924(174972)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kistler AD, Singh G, Altintas MM, Yu H,
Fernandez IC, Gu C, Wilson C, Srivastava SK, Dietrich A, Walz K, et
al: Transient receptor potential channel 6 (TRPC6) protects
podocytes during complement-mediated glomerular disease. J Biol
Chem. 288:36598–36609. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jang Y, Lee Y, Kim SM, Yang YD, Jung J and
Oh U: Quantitative analysis of TRP channel genes in mouse organs.
Arch Pharm Res. 35:1823–1830. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Abdinghoff J, Servello D, Jacobs T,
Beckmann A and Tschernig T: Evaluation of the presence of TRPC6
channels in human vessels: A pilot study using
immunohistochemistry. Biomed Rep. 16(42)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Davis MJ, Castorena-Gonzalez JA and
Zawieja SD: Electric field stimulation unmasks a subtle role for
T-type calcium channels in regulating lymphatic contraction. Sci
Rep. 13(15862)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hadamitzky C, Spohr H, Debertin AS, Guddat
S, Tsokos M and Pabst R: Age-dependent histoarchitectural changes
in human lymph nodes: An underestimated process with clinical
relevance? J Anat. 216:556–562. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ströbel P, Moritz R, Leite MI, Willcox N,
Chuang WY, Gold R, Nix W, Schalke B, Kiefer R, Müller-Hermelink HK,
et al: The ageing and myasthenic thymus: A morphometric study
validating a standard procedure in the histological workup of
thymic specimens. J Neuroimmunol. 201-202:64–73. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Denz FA: Age changes in lymph nodes. J
Pathol Bacteriol. 59:575–591. 1947.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Luscieti P, Hubschmid T, Cottier H, Hess
MW and Sobin LH: Human lymph node morphology as a function of age
and site. J Clin Pathol. 33:454–461. 1980.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cakala-Jakimowicz M, Kolodziej-Wojnar P
and Puzianowska-Kuznicka M: Aging-Related cellular, structural and
functional changes in the lymph nodes: A significant component of
immunosenescence? An overview. Cells. 10(3148)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kwok T, Medovich SC, Silva-Junior IA,
Brown EM, Haug JC, Barrios MR, Morris KA and Lancaster JN:
Age-Associated changes to lymph node fibroblastic reticular cells.
Front Aging. 3(838943)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hofmann K, Fiedler S, Vierkotten S, Weber
J, Klee S, Jia J, Zwickenpflug W, Flockerzi V, Storch U, Yildirim
AÖ, et al: Classical transient receptor potential 6 (TRPC6)
channels support myofibroblast differentiation and development of
experimental pulmonary fibrosis. Biochim Biophys Acta Mol Basis
Dis. 1863:560–568. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pelstring RJ, Allred DC, Esther RJ,
Lampkin SR and Banks PM: Differential antigen preservation during
tissue autolysis. Hum Pathol. 22:237–241. 1991.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ding M, Wang H, Qu C, Xu F, Zhu Y, Lv G,
Lu Y, Zhou Q, Zhou H, Zeng X, et al: Pyrazolo[1,5-a]pyrimidine
TRPC6 antagonists for the treatment of gastric cancer. Cancer Lett.
432:47–55. 2018.PubMed/NCBI View Article : Google Scholar
|