Introduction
COVID-19 is a multisystem disease with severe
symptoms following infection with the severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2). With the spread of COVID-19,
some symptoms persist beyond recovery from the acute stage and
become chronic (1,2). Symptoms following illness due to
COVID-19 (sequelae) include both those that persist from the period
soon after COVID-19 was contracted and new symptoms that appear
after recovery. These symptoms continue despite the disappearance
of infectiousness following COVID-19. A Delphi consensus has been
used as a definition of long COVID (LC) as a post-COVID-19
condition. According to this consensus definition, LC is a
post-COVID-19 condition that occurs in individuals with SARS-CoV-2
infection, usually 3 months from onset, with symptoms that last for
at least 2 months with no other explanation (1). These symptoms include, but are not
limited to, disorders of the respiratory, cardiovascular,
musculoskeletal, skin and nervous systems, and LC is considered a
multisystem disease with a wide range of symptoms. In reports to
date, the percentage of patients with post-COVID symptoms has been
considered to range from 10% to ~60% (3-6).
Typical LC presents with fatigue/malaise, joint
pain, myalgia, cough, sputum, dyspnea, chest pain, alopecia, memory
disorders, loss of ability to concentrate, headaches, depressed
mood, olfactory disorder, dysgeusia, palpitations, diarrhea,
abdominal pain, sleeplessness, muscle weakness and skin rash.
Previous studies have noted that LC may severely affect the daily
lives of affected individuals (2-5,7-9).
The precise pathogenic mechanisms of the condition are not yet
fully understood; however, multiple factors, such as direct organ
damage, autoimmune reactions, inflammatory reactions and mental
disorders from SARS-CoV-2 infection, appear to be involved
(10).
Reports of post-COVID-19 symptoms are increasing
globally; however, survey results differ greatly depending on
differences in settings for the patient population, including
patient background, particularly the presence or absence of a
definitive diagnosis, the age of the infected person, the severity
of the disease, and whether they are inpatients or outpatients.
Bias may also occur, for example, in data collection methods.
Differences may also arise from the COVID-19 infection strain. The
omicron strain is generally considered to have fewer sequelae than
earlier strains (11-14).
Moreover, the weakness that is sometimes observed
following the treatment of severe acute diseases not limited to
COVID-19, underlying conditions from before a person became ill
with COVID-19, and the mental and physical effects from changes in
lifestyle due to the pandemic may render the clinical picture of
post-COVID-19 symptoms more complex and difficult to analyze
(15).
In general, a number of these post-COVID-19 symptoms
tend to improve with time (16);
however, the transition of these symptoms over a long-term period,
the extent to which there are differences from the different
infecting strains, and the exact risk factors involved in the
development of post-COVID-19 symptoms are not yet fully
understood.
In the present study, to clarify the actual
incidence of and risk factors for LC in patients with COVID-19 with
both pre-omicron variant and omicron variant in the community, and
to establish strategies for the prevention of LC, a questionnaire
survey of patients with COVID-19 in multiple facilities, including
a regional hospital and associated clinics, was conducted.
Patients and methods
Patients
The present study was a multicenter, cross-sectional
study conducted at one inpatient hospital (Mie General Medical
Center, Yokkaichi, Japan) and four affiliated community clinics
(Sasagawa Clinic, Nakamura Heart Clinic, Tanaka Internal Medicine
Clinic and Kainuma Clinic, Yokkaichi, Japan). The hospital is a
regional hospital that accepts a wide range of patients from mildly
to critically ill. All participants were from the almost same
region, and it was assumed that there were not marked differences
in social backgrounds in this study period. The participants were
individuals diagnosed with COVID-19 up to January, 2023 at the
participating institutions. Their ages ranged from 15 to 89 years,
and they were patients who had suffered from COVID-19 at least 6
months prior to the time they responded to the questionnaire. No
other special exclusion criteria were set. Questionnaires were sent
to 3,399 patients. They included not only Japanese individuals, but
also foreign nationals residing in Japan.
Analysis methods
Questionnaire requests were sent to participants by
post. Responses were received either on mail paper or over the
internet. Subjects were registered by responding to the
questionnaire. The present study was reviewed and approved by the
Ethics Committee of the Mie General Medical Center (O-0142). The
survey included detailed explanations of the purpose of the
study's, and responses implied informed consent. Minors provided
consent to complete the survey with the approval of their parents
or guardians.
According to survey results in Japan, following the
outbreak of COVID-19, the variants of the COVID-19 virus were the
alpha and delta variants for the period to December, 2021; these
variants were then rapidly replaced in nearly all cases by the
omicron variant (17,18). Therefore, in the present study,
patients who developed COVID-19 after that time constituted the
omicron group, whereas those infected prior to that time
constituted the pre-omicron group.
Questionnaire
The questions on the questionnaire could be answered
fairly easily. They included questions on sex, age, smoking and
drinking status, pregnancy, underlying conditions, period of
infection, number of times they had received the novel coronavirus
vaccine prior to infection, symptoms during the time of infection,
treatment during the time of infection, whether there were sequelae
after infection, treatment for the sequelae, symptoms and duration
of the sequelae, and others. Choices for symptoms experienced as
COVID-19 sequelae were made from a list of generally reported
sequelae, but they could also be described freely.
Statistical analysis
Individual questions on whether a sequela had
developed after >1 month were evaluated. For binary questions,
Fisher's exact tests were performed. The Cochran-Armitage test was
used for age, number of infections, number of vaccine doses, and
infection period. Factors with significant associations in the
univariate analysis were included in the multivariable analysis.
EZR software was used for the analysis (19). The level of significance was 5% and a
P-value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
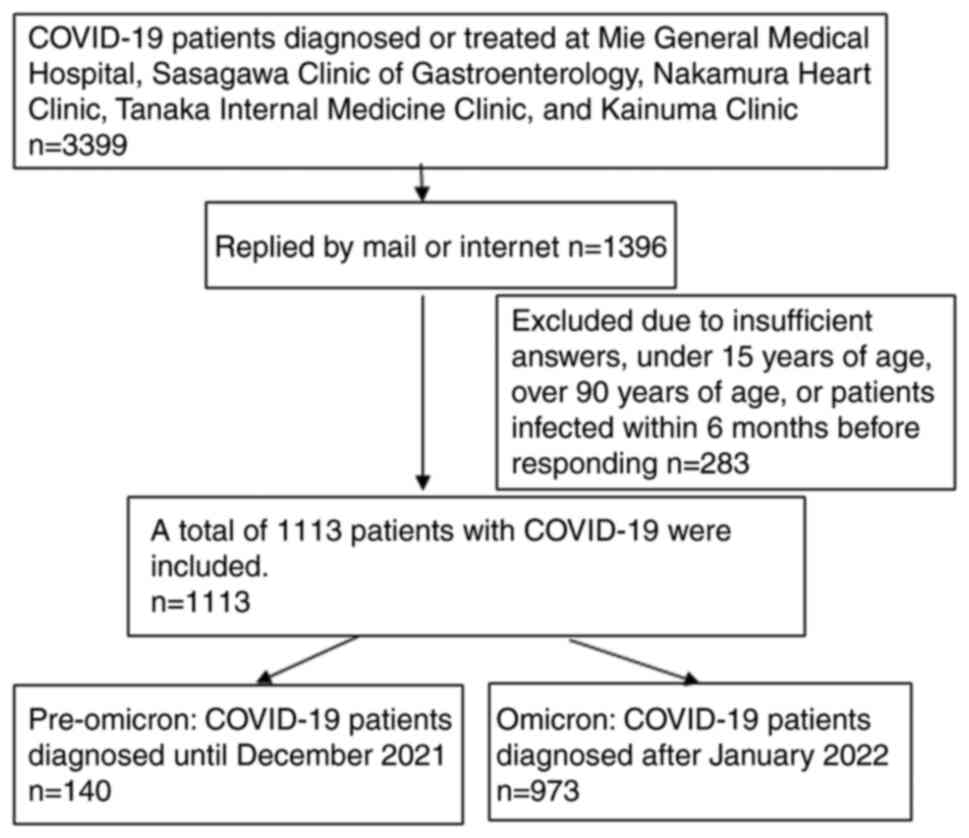
Responses were received from 1,396 individuals, with
valid responses from 1,113 individuals (32.7%) (Fig. 1). Age was distributed over a wide
range in each age group, with 104 individuals in their teens, 137
in their twenties, 138 in their thirties, 175 in their forties, 168
in their fifties, 176 in their sixties, 145 in their seventies, and
70 in their eighties. There were 483 males and 630 females. Of the
female respondents, 36 (5.7%) were pregnant at the time of
infection (Table I).
 | Table IBackground characteristics of the
patients with COVID-19 in the present study. |
Table I
Background characteristics of the
patients with COVID-19 in the present study.
| Characteristic | All (n=1,113)
(%) | Pre-omicron (n=140)
(%) | Omicron (n=973)
(%) | P-value |
|---|
| Age group | | | | 0.256 |
|
Teens | 104 (9.3) | 4 (2.9) | 100 (10.3) | |
|
Twenties | 137 (12.3) | 10 (7.1) | 127 (13.1) | |
| Thirties | 138 (12.4) | 13 (9.3) | 125 (12.8) | |
|
Forties | 175 (15.7) | 25 (17.9) | 150 (15.4) | |
|
Fifties | 168 (15.1) | 28 (20.0) | 140 (14.4) | |
|
Sixties | 176 (15.8) | 31 (22.1) | 145 (14.9) | |
|
Seventies | 145 (13.0) | 20 (14.3) | 125 (12.8) | |
|
Eighties | 70 (6.3) | 9 (6.4) | 61 (6.3) | |
| Sex | | | | 0.145 |
|
Male | 483 (43.4) | 69 (49.3) | 414 (42.5) | |
|
Female | 630 (56.6) | 71 (50.7) | 559 (57.5) | |
| Pregnancy | | | | 0.787 |
|
Yes | 36 (5.7) | 3 (4.2) | 33 (5.9) | |
|
No | 594 (94.3) | 68 (95.8) | 526 (94.1) | |
| Cigarette
smoking | | | |
<0.05a |
|
Never
smoker | 700 (62.9) | 79 (56.4) | 621 (63.8) | |
|
Former
smoker | 325 (29.2) | 53 (37.9) | 272 (28.0) | |
|
Current
smoker | 88 (7.9) | 8 (5.7) | 80 (8.2) | |
| Alcohol
consumption | | | | 0.113 |
|
Never
drinker | 563 (50.6) | 64 (45.7) | 499 (51.3) | |
|
Social
drinker | 333 (29.9) | 50 (35.7) | 283 (29.1) | |
|
Consuming
alcohol at least twice a week | 217 (19.5) | 26 (18.6) | 191 (19.6) | |
| Underlying
disease | | | | |
|
Hypertension | | | | 0.999 |
|
Yes | 241 (21.7) | 30 (21.4) | 211 (21.7) | |
|
No | 872 (78.3) | 110 (78.6) | 762 (78.3) | |
|
Diabetes
mellitus | | | |
<0.001a |
|
Yes | 94 (8.4) | 24 (17.1) | 70 (7.2) | |
|
No | 1019 (91.6) | 116 (82.9) | 903 (92.8) | |
|
Dyslipidemia | | | | 0.353 |
|
Yes | 104 (9.3) | 16 (11.4) | 88 (9.0) | |
|
No | 1009 (90.7) | 124 (88.6) | 885 (91.0) | |
|
Bronchial
asthma | | | | 0.540 |
|
Yes | 58 (5.2) | 9 (6.4) | 49 (5.0) | |
|
No | 1055 (94.8) | 131 (93.6) | 924 (95.0) | |
|
Emphysema | | | | 0.999 |
|
Yes | 12 (1.1) | 1 (0.7) | 11 (1.1) | |
|
No | 1101 (98.9) | 139 (99.3) | 962 (98.9) | |
|
Myocardial
infarction | | | | 0.716 |
|
Yes | 18 (1.6) | 1 (0.7) | 17 (1.7) | |
|
No | 1095 (98.4) | 139 (99.3) | 956 (98.3) | |
|
Malignancy | | | | 0.331 |
|
Yes | 40 (3.6) | 7 (5.0) | 33 (3.4) | |
|
No | 1073 (96.4) | 133 (95.0) | 940 (96.6) | |
|
Rheumatoid,
collagen disease | | | | 0.716 |
|
Yes | 18 (1.6) | 1(0.7) | 17(1.7) | 0.999 |
|
No | 1095 (98.4) | 139(99.3) | 956(98.3) | |
|
Immunological
deficiency | | | | 0.999 |
|
Yes | 15 (1.3) | 2 (1.4) | 13 (1.3) | |
|
No | 1098 (98.7) | 138 (98.6) | 960 (98.7) | |
|
Chronic
kidney disease | | | | 0.999 |
|
Yes | 10 (0.9) | 1 (0.7) | 9 (0.9) | |
|
No | 1103 (99.1) | 139 (99.3) | 964 (99.1) | |
|
Neurological
disease | | | | 0.706 |
|
Yes | 15 (1.3) | 1 (0.7) | 14 (1.4) | |
|
No | 1098 (98.7) | 139 (99.3) | 959 (98.6) | |
| Vaccination prior
to infection | | | | 0.543 |
|
None | 214 (19.2) | 87 (62.1) | 127 (13.1) | |
|
Once | 18 (1.6) | 6 (4.3) | 12 (1.2) | |
|
Twice | 235 (21.1) | 18 (12.9) | 217 (22.3) | |
|
Three
times | 378 (34.0) | 13 (9.3) | 365 (37.5) | |
|
Four
times | 203 (18.2) | 12 (8.6) | 191 (19.6) | |
|
Five
times | 65 (5.8) | 4 (2.9) | 61 (6.3) | |
|
Hospitalization | | | |
<0.001a |
|
Yes | 204 (18.3) | 97 (69.3) | 107 (11.0) | |
|
No | 909 (81.7) | 43 (30.7) | 866 (89.0) | |
| Oxygen
inhalation | | | |
<0.001a |
|
Yes | 50 (4.5) | 35 (25.0) | 15 (1.5) | |
|
No | 1063 (95.5) | 105 (75.0) | 958 (98.5) | |
There were 700 participants (62.9%) in the never
smoker group, 325 (29.2%) in the former smoker group and 88 (7.9%)
in the current smoker group. As regards alcohol consumption, there
were 563 participants (50.6%) in the never drinker group, 333
(29.9%) in the social drinker group, and 217 (19.5%) in the group
who consumed alcohol two or more times per week. For underlying
diseases, a list of options was shown, in addition to which they
could be described freely. An underlying disease of some kind was
reported by 509 participants (45.7%).
The omicron group included 973 (87.4%) patients, and
the pre-omicron group included 140 (12.6%) patients. The number of
vaccine doses was 0 for 214 participants (19.2%), 1 for 18
participants (1.6%), 2 for 235 participants (21.1%), 3 for 378
participants (34.0%), 4 for 203 participants (18.2%) and 5 for 65
participants (5.8%). The number of participants who were
hospitalized was 204 (18.3%). Of these, 50 (4.5%) participants were
administered oxygen. The number of smokers was significantly higher
in the omicron group than in the pre-omicron group. By contrast,
the numbers of participants with diabetes mellitus, a history of
hospitalization while ill with COVID-19, and those who received
oxygen were significantly lower in the omicron group (Table I).
Symptoms during initial infection
Symptoms reported by at least 20% of cases during
infection were fever (78.4%), a sore throat (54.4%), cough (50.8%),
fatigue (45.6%), headache (27.8%), phlegm (23.2%) and nasal
discharge (22.1%). Sore throat was significantly more common in the
omicron group, whereas dysgeusia, olfactory disorder, dyspnea and
sleeplessness were more common in the pre-omicron group (Table II).
 | Table IISymptoms during COVID-19 infection in
the pre-omicron and omicron groups. |
Table II
Symptoms during COVID-19 infection in
the pre-omicron and omicron groups.
| Initial symptoms
during infection | Pre-omicron
(%) | Omicron (%) | P-value |
|---|
| Fever | | | 0.821 |
|
Yes | 109 (77.9) | 764 (78.5) | |
|
No | 31 (22.1) | 209 (21.5) | |
| Cough | | | 0.719 |
|
Yes | 69 (49.3) | 496 (51.0) | |
|
No | 71 (50.7) | 477 (49.0) | |
| Fatigue | | | 0.414 |
|
Yes | 59 (42.1) | 449 (46.1) | |
|
No | 81 (57.9) | 524 (53.9) | |
| Sore throat | | |
<0.001a |
|
Yes | 56 (40.0) | 549 (56.4) | |
|
No | 84 (60.0) | 424 (43.6) | |
| Dysgeusia | | |
<0.001a |
|
Yes | 43 (30.7) | 152 (15.6) | |
|
No | 97 (69.3) | 821 (84.4) | |
| Headache | | | 0.999 |
|
Yes | 39 (27.9) | 270 (27.7) | |
|
No | 101 (72.1) | 703 (72.3) | |
| Dyspnea | | |
<0.001a |
|
Yes | 33 (23.6) | 75 (7.7) | |
|
No | 107 (76.4) | 898 (92.3) | |
| Sputum | | | 0.521 |
|
Yes | 29 (20.7) | 229 (23.5) | |
|
No | 111 (79.3) | 744 (76.5) | |
| Joint pain | | | 0.999 |
|
Yes | 24 (17.1) | 172 (17.7) | |
|
No | 116 (82.9) | 801 (82.3) | |
| Olfactory
disorder | | |
<0.01a |
|
Yes | 24 (17.1) | 88 (9.0) | |
|
No | 116 (82.9) | 885 (91.0) | |
| Nasal
discharge | | | 0.102 |
|
Yes | 23 (16.4) | 223 (22.9) | |
|
No | 117 (83.6) | 750 (77.1) | |
| Nasal
obstruction | | | 0.138 |
|
Yes | 16 (11.4) | 162 (16.6) | |
|
No | 124 (88.6) | 811 (83.4) | |
| Sleeplessness | | |
<0.001a |
|
Yes | 16 (11.4) | 37 (3.8) | |
|
No | 124 (88.6) | 936 (96.2) | |
| Myalgia | | | 0.332 |
|
Yes | 13 (9.3) | 120 (12.3) | |
|
No | 127 (90.7) | 853 (87.7) | |
|
Others | 12 (8.6) | 62 (6.4) | |
| Diarrhea | | | 0.438 |
|
Yes | 10 (7.1) | 54 (5.5) | |
|
No | 130 (92.9) | 919 (94.5) | |
| Chest pain | | | 0.114 |
|
Yes | 10 (7.1) | 38 (3.9) | |
|
No | 130 (92.9) | 935 (96.1) | |
| Abdominal pain | | | 0.361 |
|
Yes | 5 (3.6) | 21 (2.2) | |
|
No | 135 (96.4) | 952 (97.8) | |
| Loss of appetite,
nausea | | | 0.052 |
|
Yes | 5 (3.6) | 12 (1.2) | |
|
No | 135 (96.4) | 961 (98.8) | |
Symptoms of LC
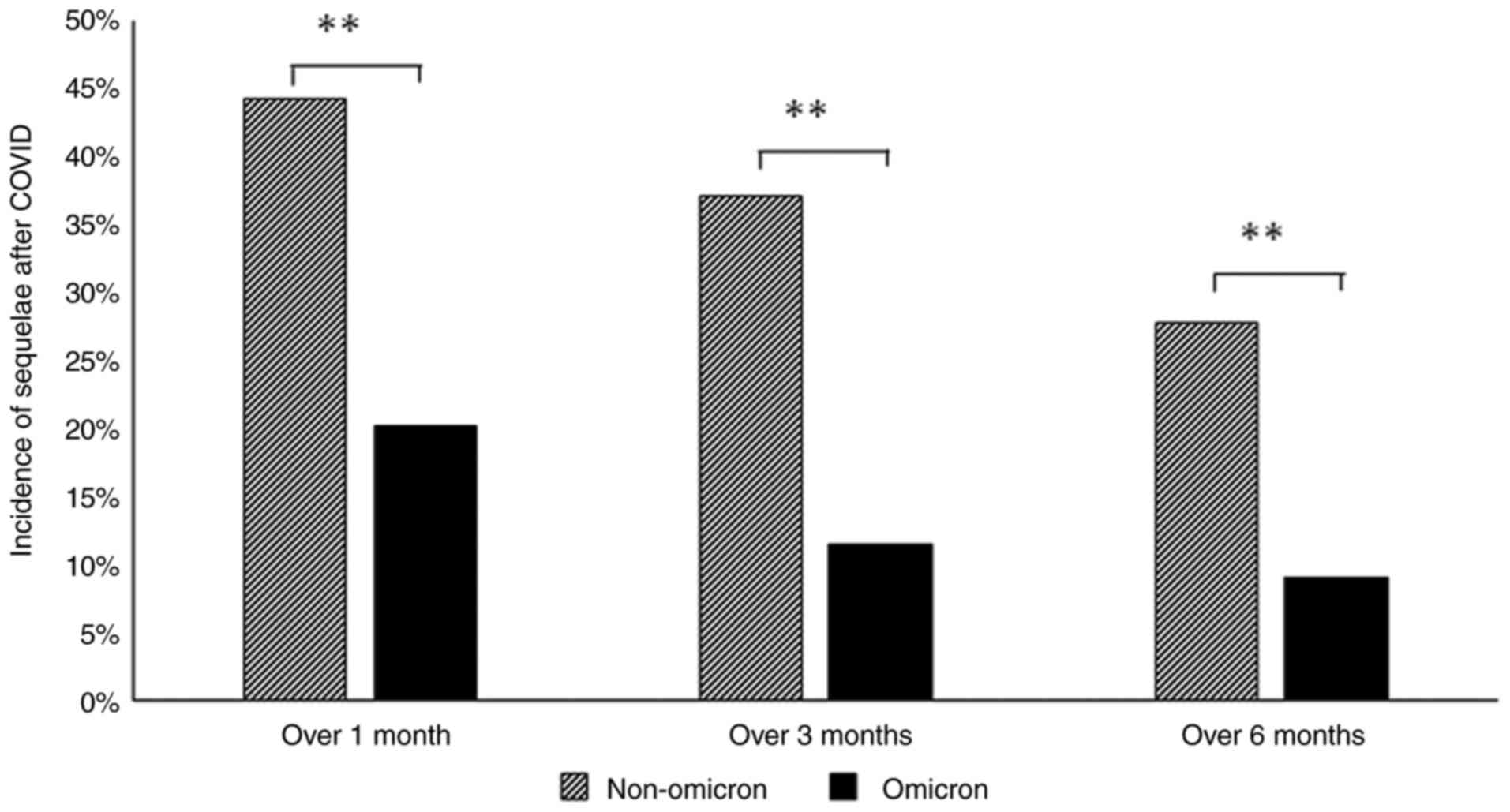
The percentages of patients in whom some type of
sequelae persisted at 1, 3 and 6 months after the infection were
44, 37 and 28%, respectively, in the pre-omicron group, and 20, 12
and 9%, respectively, in the omicron group. The percentages were
significantly lower in the omicron group (Fig. 2). Even at 3 months, which is defined
as LC, >10% of the patients in the omicron group were
symptomatic. Symptoms as sequelae after 3 months in the pre-omicron
group were, in order of frequency, fatigue, alopecia, loss of
concentration, dyspnea, olfactory disorder, dysgeusia, muscle
weakness, memory disorder, headache, cough and others. In the
omicron group, the frequency of all LC symptoms was lower than that
in the pre-omicron group; in particular, the frequencies of
alopecia and olfactory disorder were low (Table III).
 | Table IIILong COVID following COVID infection
in the pre-omicron and omicron groups. |
Table III
Long COVID following COVID infection
in the pre-omicron and omicron groups.
| Symptom | Pre-omicron
(%) | Omicron (%) | P-value |
|---|
| Fatigue | | |
<0.001a |
|
Yes | 17 (12.1) | 29 (3.0) | |
|
No | 123 (87.9) | 944 (97.0) | |
| Alopecia | | |
<0.001a |
|
Yes | 11 (7.9) | 10 (1.0) | |
|
No | 129 (92.1) | 963 (99.0) | |
| Loss of
concentration | | |
<0.01a |
|
Yes | 10 (7.1) | 23 (2.4) | |
|
No | 130 (92.9) | 950 (97.6) | |
| Dyspnea | | |
<0.01a |
|
Yes | 10 (7.1) | 20 (2.1) | |
|
No | 130 (92.9) | 953 (97.9) | |
| Olfactory
disorder | | |
<0.001a |
|
Yes | 10 (7.1) | 3 (0.3) | |
|
No | 130 (92.9) | 970 (99.7) | |
| Dysgeusia | | |
<0.001a |
|
Yes | 9 (6.4) | 9 (0.9) | |
|
No | 131 (93.6) | 964 (99.1) | |
| Muscle
weakness | | |
<0.05a |
|
Yes | 8 (5.7) | 19 (2.0) | |
|
No | 132 (94.3) | 954 (98.0) | |
| Memory
disorder | | |
<0.05a |
|
Yes | 6 (4.3) | 14 (1.4) | |
|
No | 134 (95.7) | 959 (98.6) | |
| Headache | | |
<0.05a |
|
Yes | 6 (4.3) | 11 (1.1) | |
|
No | 134 (95.7) | 962 (98.9) | |
| Cough | | | 0.116 |
|
Yes | 6 (4.3) | 19 (2.0) | |
|
No | 134 (95.7) | 954 (98.0) | |
| Sputum | | | 0.095 |
|
Yes | 5 (3.6) | 15 (1.5) | |
|
No | 135 (96.4) | 958 (98.5) | |
| Sleeplessness | | | 0.079 |
|
Yes | 5 (3.6) | 14 (1.4) | |
|
No | 135 (96.4) | 959 (98.6) | |
| Chest pain | | |
<0.05a |
|
Yes | 4 (2.9) | 4 (0.4) | |
|
No | 136 (97.1) | 969 (99.6) | |
| Palpitations | | | 0.691 |
|
Yes | 4 (2.9) | 9 (0.9) | |
|
No | 136 (97.1) | 964 (99.1) | |
| Depressed mood | | | 0.093 |
|
Yes | 3 (2.1) | 6 (0.6) | |
|
No | 137 (97.9) | 967 (99.4) | |
| Muscle pain | | | 0.364 |
|
Yes | 2 (1.4) | 8 (0.8) | |
|
No | 138 (98.6) | 965 (99.2) | |
| Joint pain | | | 0.999 |
|
Yes | 1 (0.7) | 8 (0.8) | |
|
No | 139 (99.3) | 965 (99.2) | |
| Diarrhea | | | 0.999 |
|
Yes | 0 (0.0) | 4 (0.4) | |
|
No | 140 (100.0) | 969 (99.6) | |
| Abdominal pain | | | 0.999 |
|
Yes | 0 (0.0) | 2 (0.2) | |
|
No | 140 (100.0) | 971 (99.8) | |
Risk factors for LC
The factors related to LC symptoms at 3 months after
infection were analyzed. In all subjects, significant risk factors
in the univariate analysis were underlying disease (P<0.001),
two or more vaccine doses (P<0.001), oxygen inhalation
(P<0.001), hospitalization (P<0.001) and the pre-omicron
variant (P<0.001). Significant differences were also observed in
age, with the highest number of LC cases (23.81%) observed in
patients in their fifties, with a tendency to be lower in younger
groups. No significant differences were observed in sex, smoking
status, drinking habits, antiviral drug history and pregnancy
(Table IV). In the multivariate
logistic regression analysis, each factor which exhibited a
significant difference in the univariate analysis, hospital
admission [odds ratio (OR), 1.950; P<0.01] and age (OR, 1.160;
P<0.01) were found to be significant risk factors. Conversely,
the incidence of LC was significantly lower with vaccination (OR,
0.831; P<0.01) and in the omicron group (OR, 0.506; P<0.01).
Diabetes and oxygen inhalation did not exhibit any significant
difference in this multivariate analysis (Table V).
 | Table IVFactors related to long COVID
following infection with COVID-19. |
Table IV
Factors related to long COVID
following infection with COVID-19.
| Factor | No LC, n=949
(%) | LC, n=164 (%) | P-value |
|---|
| Age group | | |
<0.001a |
|
Teens | 101 (10.6) | 3 (1.8) | |
|
Twenties | 124 (13.1) | 13 (7.9) | |
|
Thirties | 121 (12.8) | 17 (10.4) | |
|
Forties | 150 (15.8) | 25 (15.2) | |
|
Fifties | 128 (13.5) | 40 (24.4) | |
|
Sixties | 147 (15.5) | 29 (17.7) | |
|
Seventies | 119 (12.5) | 26 (15.9) | |
|
Eighties | 59 (6.2) | 11 (6.7) | |
| Sex | | | 0.932 |
|
Male | 411 (43.3) | 72 (43.9) | |
|
Female | 538 (56.7) | 92 (56.1) | |
| Pregnancy | | | 0.339 |
|
Non-pregnancy | 505 (53.2) | 89 (54.3) | |
|
Pregnancy | 33 (3.5) | 3 (1.8) | |
| Smoking status | | | 0.106 |
|
Never
smoker | 606 (63.9) | 94 (57.3) | |
|
Former
smoker | 271 (28.6) | 54 (32.9) | |
|
Current
smoker | 72 (7.6) | 16 (9.8) | |
| Alcohol
consumption | | | 0.743 |
|
Never
drinker | 482 (50.8) | 81 (49.4) | |
|
Social
drinker | 277 (29.2) | 56 (34.1) | |
|
Consuming
alcohol at least twice a week | 190 (20.0) | 2 7 (16.5) | |
| Vaccination
status | | |
<0.001a |
|
0-1
times | 171 (18.0) | 61 (37.2) | |
|
2-6
times | 778 (82.0) | 103 (62.8) | |
| Underlying
disease | | |
<0.001a |
|
None | 536 (56.5) | 68 (41.5) | |
|
Any | 413 (43.5) | 96 (58.5) | |
| Antiviral drug
use | | | 0.131 |
|
Yes | 95 (10.0) | 23 (14.0) | |
|
No | 854 (90.0) | 141 (86.0) | |
| Hospital
admission | | |
<0.001a |
|
Admission | 138 (14.5) | 66 (40.2) | |
|
No
admission | 811 (85.5) | 98 (59.8) | |
| Oxygen
inhalation | | |
<0.001a |
|
Oxygen
required | 28 (3.0) | 22 (13.4) | |
|
No oxygen
demands | 921 (97.0) | 142 (86.6) | |
| Omicron
variant | | |
<0.001a |
|
Non-omicron | 88 (9.3) | 52 (31.7) | |
|
Omicron | 861 (90.7) | 112 (68.3) | |
 | Table VFactors related to long COVID
following infection with COVID-19 in the multivariate logistic
regression analysis. |
Table V
Factors related to long COVID
following infection with COVID-19 in the multivariate logistic
regression analysis.
| | CI | |
|---|
| Factor | OR | 2.5% | 97.5% | P-value |
|---|
| Admission | 1.950 | 1.220 | 3.120 |
<0.01a |
| Diabetes
mellitus | 1.110 | 0.626 | 1.950 | 0.729 |
| Oxygen
inhalation | 1.290 | 0.626 | 2.580 | 0.470 |
| Age | 1.160 | 1.050 | 1.280 |
<0.01a |
| Vaccination | 0.831 | 0.729 | 0.948 |
<0.01a |
| Omicron | 0.506 | 0.301 | 0.851 |
<0.01a |
Subsequently, further investigations of LC in
patients in the omicron group were conducted. The incidence of LC
was significantly higher in patients with an underlying disease
(P<0.001) and those who were hospitalized (P<0.01) (Table VI). In the multivariate logistic
regression analysis for the type of underlying disease, emphysema
(OR, 6.66; P<0.001), bronchial asthma (OR, 5.13; P<0.001),
rheumatoid/collagen disease (OR, 3.31; P<0.05) and hypertension
(OR, 1.77l P<0.05) were significant risk factors for LC
(Table VII).
 | Table VIFactors related to long COVID
following infection with COVID-19 in the omicron group. |
Table VI
Factors related to long COVID
following infection with COVID-19 in the omicron group.
| Factor | No LC, n=861
(%) | LC, n=112 (%) | P-value |
|---|
| Sex | | | 0.839 |
|
Male | 365 (42.4) | 49 (43.8) | |
|
Female | 496 (57.6) | 63 (56.3) | |
| Pregnancy | | | 0.567 |
|
Non-pregnancy | 465 (54.0) | 61 (54.5) | |
|
Pregnancy | 31 (3.6) | 2 (1.8) | |
| Smoking status | | | 0.669 |
|
Never
smoker | 554 (64.3) | 67 (59.8) | |
|
Former
smoker | 240 (27.9) | 32 (28.6) | |
|
Current
smoker | 67 (7.8) | 13 (11.6) | |
| Alcohol
consumption | | | 0.513 |
|
Never
drinker | 440 (51.1) | 59 (52.7) | |
|
Social
drinker | 245 (28.5) | 38 (33.9) | |
|
Consuming
alcohol at least twice a week | 176 (20.4) | 15 (13.4) | |
| Vaccination
status | | | 0.999 |
|
0-1
times | 123 (14.3) | 16 (14.3) | |
|
2-6
times | 738 (85.7) | 96 (85.7) | |
| Underlying
disease | | |
<0.001b |
|
None | 496 (57.6) | 42 (37.5) | |
|
Any | 365 (42.4) | 70 (62.5) | |
| Hospital
admission | | |
<0.01a |
|
Admission | 84 (9.8) | 23 (20.5) | |
|
No
admission | 777 (90.2) | 89 (79.5) | |
| Oxygen
inhalation | | | 0.0833 |
|
Oxygen
required | 11 (1.3) | 4(3.6) | |
|
No oxygen
demands | 850 (98.7) | 108 (96.4) | |
 | Table VIIAssociations between underlying
diseases and long COVID following infection with COVID-19 in the
omicron group in the multivariate logistic regression analysis. |
Table VII
Associations between underlying
diseases and long COVID following infection with COVID-19 in the
omicron group in the multivariate logistic regression analysis.
| Underlying
disease | OR | 2.5% CI | 97.5% CI | P-value |
|---|
| Emphysema | 6.66 | 2.088 | 7.999 |
<0.001a |
| Bronchial
asthma | 5.13 | 2.386 | 5.465 |
<0.001a |
| Rheumatoid,
collagen disease | 3.31 | 1.232 | 5.606 |
<0.05a |
| Chronic kidney
disease | 2.22 | 0.566 | 6.695 | 0.312 |
| Hypertension | 1.77 | 1.135 | 2.375 |
<0.01a |
| Malignancy | 1.75 | 0.765 | 3.399 | 0.222 |
| Dyslipidemia | 1.67 | 0.945 | 2.558 | 0.088 |
| Myocardial
infarction | 1.67 | 0.546 | 4.388 | 0.424 |
| Diabetes
mellitus | 1.66 | 0.896 | 2.676 | 0.125 |
| Immunological
deficiency | 1.40 | 0.371 | 4.863 | 0.660 |
| Neurological
disease | 0.59 | 0.093 | 4.113 | 0.606 |
Discussion
In the present study, a questionnaire survey of
community-dwelling patients with COVID-19, including patients
admitted to the hospital and those observed as outpatients in
multiple institutions, was conducted. The prevalences of symptoms
and sequelae were investigated, comparing cases that occurred
during the omicron epidemic period (omicron group) and those that
occurred earlier (pre-omicron group).
Of the symptoms at onset, there were significantly
more cases of sore throat in the omicron group and significantly
more cases of dysgeusia and olfactory disorder in the pre-omicron
group. In a previous study, loss of smell and infiltration of the
lower respiratory tract were less common in participants infected
during the omicron pandemic than in those infected during the delta
pandemic (20). In addition, sore
throat was known to have been more common in patients during the
omicron pandemic than during the delta pandemic. Furthermore, the
hospitalization rate was reported to have been lower during the
omicron period than during the delta period (20). The omicron variant is also markedly
more transmissible than previous variants; however, in groups that
have been vaccinated, the severity has been shown to be lower
(21). A similar finding was
observed in the present study. These findings suggest that
different SARS-CoV-2 subtypes have different associations with
symptoms and severity, and they also affect the frequency of LC
(15).
In line with the definition of LC, in the present
study, sequelae of some kind were confirmed in 37% of the
pre-omicron group and 12% of the omicron group at 3 months. As for
the prevalence of LC, there is a possibility of a lower risk of
developing LC symptoms when infected with the omicron strain than
when infected with the delta variant or other earlier variants
(7,11,15).
However, it should be noted that these studies were historical
case-control studies.
The results of the present study are in agreement
with these reports. That is, in the area in which the study was
conducted, initial symptoms and the frequency of LC were low during
the omicron pandemic. Of the LC symptoms, numerous patients
experienced fatigue in particular; this was observed in >10% of
patients in the pre-omicron group, and in only ~3% of the omicron
group. A number of other symptoms, for example, loss of
concentration, dyspnea, muscle weakness and memory loss, had lower
frequencies in the omicron group; however, the frequencies of
alopecia and olfactory disorders in particular were markedly lower
in the omicron group. In previous studies as well, tiredness and
fatigue were the LC symptoms with the highest frequencies (15,22-25).
Fatigue is observed at even 100 days following the initial symptoms
of acute COVID-19. Moreover, in syndromes, such as acute
respiratory distress syndrome, it has been observed that more than
two-thirds of patients report clinically significant fatigue
symptoms after 1 year (3,26). The symptoms observed in patients who
have suffered from COVID-19 are considered to resemble those of
chronic fatigue syndrome, and exacerbations of severe
incapacitating fatigue, pain, neurocognitive disability,
compromised sleep and symptoms suggestive of autonomic dysfunction
are also observed (3,27). The etiology of neuropsychiatric
symptoms in patients with COVID-19 is complex and multifactorial.
These symptoms may be related to the direct effect of the
infection, as well as to cerebrovascular disease (including
hypercoagulation) (3). In fact, the
loss of concentration, memory disorder, sleeplessness, depressed
mood and various neuropsychiatric symptoms were also reported in
the present study, and measures for their diagnosis and treatment
are desired.
The question remains regarding which types of
patients are at a high risk of developing LC. In the present study,
underlying disease, the number of vaccine doses (≤1), oxygen
administration and hospitalization were related to the risk of
developing LC. By age, the highest numbers of patients were in
their fifties. In previous studies, an older age, the female sex, a
high body mass index, complications, hospital admission or a large
number of symptoms in the acute stage and disease severity were
identified (4,9,10,24,28-29).
In a previous study on adults in the UK, the female sex, an older
age, obesity, smoking, vaping, hospitalization with COVID-19,
poverty and being a healthcare worker were associated with a higher
probability of persistent symptoms following COVID-19 infection
(30). An Asian ethnicity was
associated with a lower probability (30).
In the present study as well, which is considered to
reflect the infection status in a region in Japan, the risk of LC
was similarly increased with more severe symptoms in the early
stage. This suggests that attention should be paid to LC in
individuals with factors for more severe disease and patients with
severe symptoms in the early stage. The risk of developing LC was
also higher in patients with the underlying diseases, such as
hypertension, diabetes mellitus, hyperlipidemia, bronchial asthma,
emphysema, chronic kidney disease, hypertension and rheumatoid or
collagen disease. These are also factors related to a greater
severity of COVID-19(31), and they
are considered to be related to LC.
Suggested mechanisms for LC include direct tissue
damage from SARS-CoV-2 infection, constitutive inflammatory
responses including autoimmunity and microvascular coagulation
disorders with endothelial dysfunction (2,32). In
addition, SARS-CoV-2 is neurotropic, and viral RNA and protein can
be detected persistently in multiple organs including the brain,
suggesting deep involvement in the pathology (33,34). The
consideration of LC as a systemic disease, in addition to viral
eradication in the early stage, and the handling of immune and
coagulation disorders may also be required.
Individuals with a history of ≥2 vaccine doses in
the present study had a lower risk of developing LC. In fact, this
is supported by evidence which indicates that vaccination prior to
infection lowers the risk of developing LC (35). Individuals who have had two vaccine
doses have also been reported to have a significantly lower
likelihood of developing long-duration symptoms (≥28 days) than
unvaccinated individuals (10). In
individuals who are vaccinated prior to acute infection or after
infection, the risk of LC symptoms, such as sleeping disturbances,
myalgia, renal injury and cognitive deficits has been reported to
be lower with vaccination, and there is increasing evidence to
suggest that lasting improvements are observed following a second
vaccine dose (10,36). These findings suggest that
vaccination contributes to mitigating the development of LC and
symptoms; however, further clinical research on the effects of
vaccination is required.
In addition to vaccines, the control of underlying
disease symptoms is also considered to be critical in preventing
LC. Early antiviral therapy has been reported to be associated with
a decreased risk of developing LC, associated hospitalization and
mortality. Therefore, early antiviral therapy is recommended for
individuals who are at risk (37,38).
The present study has several limitations and
biases, which should be mentioned. These include the fact that
there are two methods of data collection, the fact that the
infecting strain was determined according to the time of infection,
the fact that the effect of treatment was not taken into account,
the relatively low response rate, the non-response bias, and the
fact that the accuracy of the answers was not guaranteed.
Furthermore, the sample size may not have been sufficient to reach
conclusions regarding the risk of developing LC. It was also not
possible to objectively evaluate each symptom.
In conclusion, in the present study, a questionnaire
survey of Japanese community-dwelling individuals who had been ill
with COVID-19 was conducted. LC was found in >10% of the omicron
group, although this was lower in patients in the pre-omicron
group. These results demonstrate that this could have a notable
impact on healthcare in the community. Risk factors for LC were
found to include disease severity in the acute stage and underlying
diseases, especially pulmonary diseases. Research on this topic is
associated various issues in terms of methodology, and there is
marked variability between studies in risk factors, definitions,
follow-up duration and other matters. However, a notable number of
patients require long-term care following infection even with the
omicron variant. Clinically, it will be crucial to establish a
medical strategy by predicting the progression to LC based on the
background characteristics of patients with COVID-19.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data are not publicly
available due to privacy or ethical policy.
Authors' contributions
IM, KY, TN, JT, KK, HW and KS designed the study and
performed data curation. IM, MO and TI were responsible for data
collection and statistical analyses. HW, KS and IM were major
contributors to the writing of the original draft of the
manuscript. HW and KS confirm the authenticity of all the raw data
and supervised the study. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of the Mie General Medical Center (O-0142). The
survey included detailed explanations of the purpose of the
study's, and responses implied informed consent. Minors provided
consent to complete the survey with the approval of their parents
or guardians. All procedures were performed in accordance with the
principles of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soriano JB, Murthy S, Marshall JC, Relan P
and Diaz JV: WHO Clinical Case Definition Working Group On
Post-COVID-19 Condition. A clinical case definition of
post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis.
22:e102–e107. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Davis HE, McCorkell L, Vogel JM and Topol
EJ: Long COVID: Major findings, mechanisms and recommendations. Nat
Rev Microbiol. 21:133–146. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lopez-Leon S, Wegman-Ostrosky T, Perelman
C, Sepulveda R, Rebolledo PA, Cuapio A and Villapol S: More than 50
long-term effects of COVID-19: A systematic review and
meta-analysis. Sci Rep. 11(16144)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sugiyama A, Miwata K, Kitahara Y, Okimoto
M, Abe K, Bunthen E, Ouoba S, Akita T, Tanimine N, Ohdan H, et al:
Long COVID occurrence in COVID-19 survivors. Sci Rep.
12(6039)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miyazato Y, Tsuzuki S, Morioka S, Terada
M, Kutsuna S, Saito S, Shimanishi Y, Takahashi K, Sanada M, Akashi
M, et al: Factors associated with development and persistence of
post-COVID conditions: A cross-sectional study. J Infect Chemother.
28:1242–1248. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Woodrow M, Carey C, Ziauddeen N, Thomas R,
Akrami A, Lutje V, Greenwood DC and Alwan NA: Systematic review of
the prevalence of long COVID. Open Forum Infect Dis.
10(ofad233)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han Q, Zheng B, Daines L and Sheikh A:
Long-term sequelae of COVID-19: A systematic review and
meta-analysis of one-year follow-up studies on post-COVID symptoms.
Pathogens. 11(269)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morioka S, Tsuzuki S, Maruki T, Terada M,
Miyazato Y, Kutsuna S, Saito S, Shimanishi Y, Takahashi K, Sanada
M, et al: Epidemiology of post-COVID conditions beyond 1 year: A
cross-sectional study. Public Health. 216:39–44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Imoto W, Yamada K, Kawai R, Imai T,
Kawamoto K, Uji M, Kanda H, Takada M, Ohno Y, Ohtani H, et al: A
cross-sectional, multicenter survey of the prevalence and risk
factors for Long COVID. Sci Rep. 12(22413)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Perumal R, Shunmugam L, Naidoo K, Karim
SS, Wilkins D, Garzino-Demo A, Brechot C, Parthasarathy S, Vahlne A
and Nikolich JŽ: Long COVID: A review and proposed visualization of
the complexity of long COVID. Front Immunol.
14(1117464)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morioka S, Tsuzuki S, Suzuki M, Terada M,
Akashi M, Osanai Y, Kuge C, Sanada M, Tanaka K, Maruki T, et al:
Post COVID-19 condition of the Omicron variant of SARS-CoV-2. J
Infect Chemother. 28:1546–1551. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Srikanth S, Boulos JR, Dover T, Boccuto L
and Dean D: Identification and diagnosis of long COVID-19: A
scoping review. Prog Biophys Mol Biol. 182:1–7. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kutsuna S, Onozuka D, Asano K, Matsunami K
and Matsuoka T: Cross-sectional surveillance study of long COVID in
Toyonaka city, Osaka prefecture, Japan. J Infect Chemother.
30:511–515. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khanh HN, Cornelissen L,
Castanares-Zapatero D, De Pauw R, Van Cauteren D, Demarest S,
Drieskens S, Devleesschauwer B, De Ridder K, Charafeddine R and
Smith P: Association between SARS-CoV-2 variants and post COVID-19
condition: Findings from a longitudinal cohort study in the Belgian
adult population. BMC Infect Dis. 23(774)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fernández-de-Las-Peñas C, Notarte KI,
Peligro PJ, Velasco JV, Ocampo MJ, Henry BM, Arendt-Nielsen L,
Torres-Macho J and Plaza-Manzano G: Long-COVID symptoms in
individuals infected with different SARS-CoV-2 variants of concern:
A systematic review of the literature. Viruses.
14(2629)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cai J, Lin K, Zhang H, Xue Q, Zhu K, Yuan
G, Sun Y, Zhu F, Ai J, Wang S and Zhang W: A one-year follow-up
study of systematic impact of long COVID symptoms among patients
post SARS-CoV-2 omicron variants infection in Shanghai, China.
Emerg Microbes Infect. 12(2220578)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kodera S, Niimi Y, Rashed EA, Yoshinaga N,
Toyoda M and Hirata A: Estimation of mRNA COVID-19 vaccination
effectiveness in Tokyo for Omicron variants BA.2 and BA.5: Effect
of social behavior. Vaccines (Basel). 10(1820)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakano Y, Otsuka Y, Honda H, Sunada N,
Tokumasu K, Sakurada Y, Matsuda Y, Hasegawa T, Ochi K, Hagiya H, et
al: Transitional changes in fatigue-related symptoms due to long
COVID: A single-center retrospective observational study in Japan.
Medicina (Kaunas). 58(1393)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Menni C, Valdes AM, Polidori L, Antonelli
M, Penamakuri S, Nogal A, Louca P, May A, Figueiredo JC, Hu C, et
al: Symptom prevalence, duration, and risk of hospital admission in
individuals infected with SARS-CoV-2 during periods of omicron and
delta variant dominance: A prospective observational study from the
ZOE COVID Study. Lancet. 399:1618–1624. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Torjesen I: Covid-19: Omicron may be more
transmissible than other variants and partly resistant to existing
vaccines, scientists fear. BMJ. 375(n2943)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Michelen M, Manoharan L, Elkheir N, Cheng
V, Dagens A, Hastie C, O'Hara M, Suett J, Dahmash D, Bugaeva P, et
al: Characterising long COVID: A living systematic review. BMJ Glob
Health. 6(e005427)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Surapaneni KM, Singhal M, Saggu SR, Bhatt
A, Shunmathy P and Joshi A: A scoping review on long COVID-19:
Physiological and psychological symptoms post-acute, long-post and
persistent post COVID-19. Healthcare (Basel).
10(2418)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo J, Zhang J, Tang HT, Wong HK, Lyu A,
Cheung CH and Bian Z: Prevalence and risk factors of long COVID
6-12 months after infection with the Omicron variant among
nonhospitalized patients in Hong Kong. J Med Virol.
95(e28862)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shang H, Chang T, Yang W, Shi L, Hu S,
Tian L, Ren J, Wang T, Wang J, Guo J and Cui Y: Analysis of
influencing factors on long COVID in COVID-19 patients infected
with omicron variant three months after discharge: A
cross-sectional study. BMC Infect Dis. 24(36)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Neufeld KJ, Leoutsakos JS, Yan H, Lin S,
Zabinski JS, Dinglas VD, Hosey MM, Parker AM, Hopkins RO and
Needham DM: Fatigue symptoms during the first year following ARDS.
Chest. 158:999–1007. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pallanti S, Grassi E, Makris N, Gasic GP
and Hollander E: Neurocovid-19: A clinical neuroscience-based
approach to reduce SARS-CoV-2 related mental health sequelae. J
Psychiatr Res. 130:215–217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Maglietta G, Diodati F, Puntoni M,
Lazzarelli S, Marcomini B, Patrizi L and Caminiti C: Prognostic
factors for Post-COVID-19 syndrome: A systematic review and
meta-analysis. J Clin Med. 11(1541)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsampasian V, Elghazaly H, Chattopadhyay
R, Debski M, Naing TKP, Garg P, Clark A, Ntatsaki E and Vassiliou
VS: Risk factors associated with post-COVID-19 condition: A
systematic review and meta-analysis. JAMA Intern Med. 183:566–580.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Whitaker M, Elliott J, Chadeau-Hyam M,
Riley S, Darzi A, Cooke G, Ward H and Elliott P: Persistent
COVID-19 symptoms in a community study of 606,434 people in
England. Nat Commun. 13(1957)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamamoto A, Wada H, Ichikawa Y, Mizuno H,
Tomida M, Masuda J, Makino K, Kodama S, Yoshida M, Fukui S, et al:
Evaluation of biomarkers of severity in patients with COVID-19
infection. J Clin Med. 10(3775)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wada H, Shiraki K, Shimpo H, Shimaoka M,
Iba T and Suzuki-Inoue K: Thrombotic mechanism involving platelet
activation, hypercoagulability and hypofibrinolysis in coronavirus
disease 2019. Int J Mol Sci. 24(7975)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Matsunaga A, Tsuzuki S, Morioka S,
Ohmagari N and Ishizaka Y: Long COVID: current status in Japan and
knowledge about its molecular background. Glob Health Med. 4:83–93.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ding Q and Zhao H: Long-term effects of
SARS-CoV-2 infection on human brain and memory. Cell Death Discov.
9(196)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Notarte KI, Catahay JA, Velasco JV,
Pastrana A, Ver AT, Pangilinan FC, Peligro PJ, Casimiro M, Guerrero
JJ, Gellaco MML, et al: Impact of COVID-19 vaccination on the risk
of developing long-COVID and on existing long-COVID symptoms: A
systematic review. EClinicalMedicine. 53(101624)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao P, Liu J and Liu M: Effect of COVID-19
vaccines on reducing the risk of long COVID in the real world: A
systematic review and meta-analysis. Int J Environ Res Public
Health. 19(12422)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie Y, Choi T and Al-Aly Z: Association of
treatment with Nirmatrelvir and the risk of post-COVID-19
condition. JAMA Intern Med. 183:554–564. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Choi YJ, Seo YB, Seo JW, Lee J, Nham E,
Seong H, Yoon JG, Noh JY, Cheong HJ, Kim WJ, et al: Effectiveness
of antiviral therapy on long COVID: A systematic review and
meta-analysis. J Clin Med. 12(7375)2023.PubMed/NCBI View Article : Google Scholar
|