Introduction
Intra-axial brain tumors mainly spread within the
brain parenchyma causing a variety of motor, sensory, language and
visual dysfunctions. Apart from recent advancements in
radiochemotherapy, surgery plays an important role in rapidly
reducing intracranial pressure and local adverse effects on brain
function, resulting in the maintenance of the performance status
and prolongation of patient survival.
In the surgery of patients with brain tumors, the
principal aim is to preserve both functional cortical gray and
white matter tracts while maximizing surgical resection of the
lesions (1–3). In cases of lesions originating in the
temporal, parietal, occipital lobe or lateral ventricle, anatomical
and functional preservation of the optic radiation is essential
(4).
Conventional magnetic resonance imaging (MRI)
techniques, such as T2-weighted, gadolinium-enhancing T1-weighted
and fluid-attenuated inversion-recovery (FLAIR) imaging, have been
widely used for the evaluation of the anatomical location of brain
lesions. However, these techniques provide limited information
regarding the integrity and location of white matter tracts
adjacent to the lesions. Similarly, functional MRI (fMRI) provides
limited information on the status of white matter structures,
although it localizes eloquent cortical areas surrounding brain
lesions (2,3,5,6).
Recent advances in magnetic resonance (MR)
technology have enabled major neural tracts to be distinguished in
the white matter. Determining the exact location of the lesion with
respect to clinically eloquent white matter tracts is significant
to neurosurgeons in planning the appropriate surgical approach and
in predicting the extent of safe resection (1–4,6–9).
The present study evaluated the feasibility of diffusion tensor
imaging (DTI)-based tractography in the pre-surgical planning of
brain tumors adjacent to the optic radiation.
Materials and methods
Patient population
This study was approved by the Internal Review Board
of our institution, and written informed consent for the MR studies
was obtained from each patient. Between May 2004 and February 2009,
DTI was performed on 14 consecutive patients with brain tumors
adjacent to the optic radiation. Patients ranged in age from 27 to
75 years (mean 55). Lesions consisted of metastatic tumors in 11
patients and glioma in 3. The location of lesions was the occipital
lobe in 7 patients, the temporal lobe in 5 and the parietal lobe in
2 patients.
MRI techniques
MR images were obtained with a whole-body 1.5-T MR
system (Gyroscan Intera; Philips Medical Systems, Best, The
Netherlands) with a gradient strength of 30 mT/m. A single-shot
echo-planar technique was used to obtain the diffusion-weighted MR
images (repetition time/echo time, 6,000/58 msec) with a
motion-probing gradient in 32 orientations and a field of view of
230 mm. B-values of 0 and 1,000 sec/mm2 were used with
averages of three images. The 128×58 data points were recorded by
using the parallel imaging technique. The parallel-imaging
technique allows image reconstruction with half encoding steps, the
advantage of which lies in its reduction in the geometric image
distortion that is unique to echo-planar imaging. A total of 36
slices with a thickness of 2 mm and without interslice gaps were
obtained (5,10). The DTI studies were performed at the
end of the routine work-up for the pre-surgical evaluation of brain
tumors at our institute with an average acquisition time of 7
min.
Data processing
The DTI data were transferred to an offline
workstation and then PRIDE software was used (Philips Medical
Systems) for image analysis. Diffusion tensor elements and
anisotropy at each voxel were calculated, followed by the
construction of color maps based on the DTI from these data for the
vector in the longest axis (v1). Vector elements were allocated
different colors: red represented the x element (left to right),
green the y element (anteroposterior) and blue the z element
(superoinferior). The intensity of the maps was scaled in
proportion to the fractional anisotropy (FA) (5,10).
Fiber tracking
The procedure for mapping neural connections
commenced by designating two arbitrary regions of interest (ROIs)
in the three-dimensional (3D) space. To visualize the optic
radiation, a pair of ROIs was placed on the lateral geniculate
nucleus of the thalamus and near the primary visual cortex,
including the calcarine fissure in the two hemispheres on the
coronal vector-color map. The extent of the axonal projections was
traced from the seed pixels within the ROIs in the anterograde
(forward) and retrograde (backward) directions. Tracking was
terminated (stop criterion) when a pixel with low FA, a
pre-determined trajectory curvature between two contiguous vectors,
or both were reached. In cases when no continuous tracts between
two previously defined ROIs were confirmed, one ROI at the lateral
geniculate nucleus and an additional large ROI covering a wide area
of the ipsilateral occipital lobe were employed. Consequently,
fiber tracts passing through the two ROIs were identified as the
final tract of interest. The FA and/or a pre-determined trajectory
curvature between two contiguous vectors (inner product) were
variously defined until final values that provided the most
complete reconstruction of the tracts of interest were determined
(5,10).
Assessment and treatment
On the basis of the pre-surgical fiber tracking
results overlaid in the T2-weighted MR image, the structural
integrity and location of the eloquent optic radiation relevant to
the brain tumors were determined. Conventional MR images, such as
T1-, and T2-weighted MR imaging, FLAIR MR imaging, and
gadolinium-enhancing T1-weighted MR imaging in three orthogonal
directions were evaluated. Moreover, surgically significant
extracranial and intracranial vascular structures were thoroughly
studied by means of MR angiography, 3D computerized tomography
angiography or conventional cerebral angiography when necessary.
Finally, optimal surgical approaches were employed in each patient
to avoid injury to the eloquent cortex and white matter as well as
to preserve normal vascular structures to the utmost extent.
Pre-operative and postsurgical visual field defects
were assessed by ophthalmologists using the Goldmann perimeter.
Results
The optic radiations on the lesional side were
evident in all 14 patients, and the exact location of each brain
tumor relevant to the eloquent white matter tracts was discernible.
In all 14 cases, the optic radiation was displaced superiorly,
inferiorly or medially, depending on the location of the lesion,
and no evidence of white matter infiltration by each brain tumor
was noted.
A total of 11 metastatic tumors and 3 gliomas (2
anaplastic astrocytomas and 1 glioblastoma) were extensively
resected using the transcortical approach. Thus, violation to the
eloquent cortex was avoided to the utmost extent, while the
shortest surgical tract to each brain lesion was selected.
Debulking the tumor and obtaining sufficient working space was
initially carried out. Maximal delicate surgical manipulation,
including the limitation of both mechanical and heat injury, was
then achieved particularly for the lesion adjacent to the supposed
optic radiation.
One of the 2 patients presenting with
quadrantanopsia and 1 of the 2 patients with hemianopsia during the
pre-operative period showed improvement in their visual field
postoperatively. The remaining 2 patients with pre-operative visual
defects and 10 patients with normal visual function experienced no
visual deterioration after surgery. Table I shows the characteristics and
surgical results of the patients.
 | Table ICharacteristics and outcome of the 14
patients examined with diffusion tensor imaging. |
Table I
Characteristics and outcome of the 14
patients examined with diffusion tensor imaging.
| No. | Gender | Age | Diagnosis | Location | Size (cm) | Visual
disturbance | Removal | Neurological
outcome |
|---|
| 1 | F | 63 | Metastasis | Occipital lobe | 2 | None | Total | NC |
| 2 | F | 60 | Metastasis | Occipital lobe | 3 | Quad | Total | NC |
| 3 | F | 46 | Metastasis | Occipital lobe | 3 | None | Total | NC |
| 4 | M | 75 | Metastasis | Occipital lobe | 3 | None | Partial | NC |
| 5 | F | 55 | Metastasis | Occipital lobe | 4 | None | Total | NC |
| 6 | M | 63 | Metastasis | Occipital lobe | 4 | None | Partial | NC |
| 7 | M | 42 | Metastasis | Occipital lobe | 5 | Hemi | Total | Improved |
| 8 | F | 60 | Metastasis | Parietal lobe | 3 | None | Total | NC |
| 9 | M | 74 | Metastasis | Parietal lobe | 5 | None | Total | NC |
| 10 | F | 55 | Metastasis | Temporal lobe | 5 | None | Total | NC |
| 11 | M | 41 | Metastasis | Temporal lobe | 7 | Hemi | Total | Improved |
| 12 | F | 27 | Glioma | Temporal lobe | 2 | None | Subtotal | NC |
| 13 | M | 42 | Glioma | Temporal lobe | 3 | Quad | Subtotal | NC |
| 14 | M | 65 | Glioma | Temporal lobe | 4 | None | Subtotal | NC |
Illustrative case
A 63-year-old woman with a medical history of lung
cancer presented to our institute after experiencing a sudden onset
of headaches. An examination showed no evidence of neurological
deficits, and a Goldmann perimeter showed a full visual field.
Conventional MRI revealed a 2-cm heterogeneous signal mass lesion
on the T1- and T2-weighted images in the left occipital lobe. The
lesion was accompanied with repeated hemorrhage during the
follow-up period (Fig. 1a).
Pre-operative diffusion-tensor tractography showed that the optic
radiation was deviated superiorly and medially relative to the mass
lesion (Fig. 1b). The patient
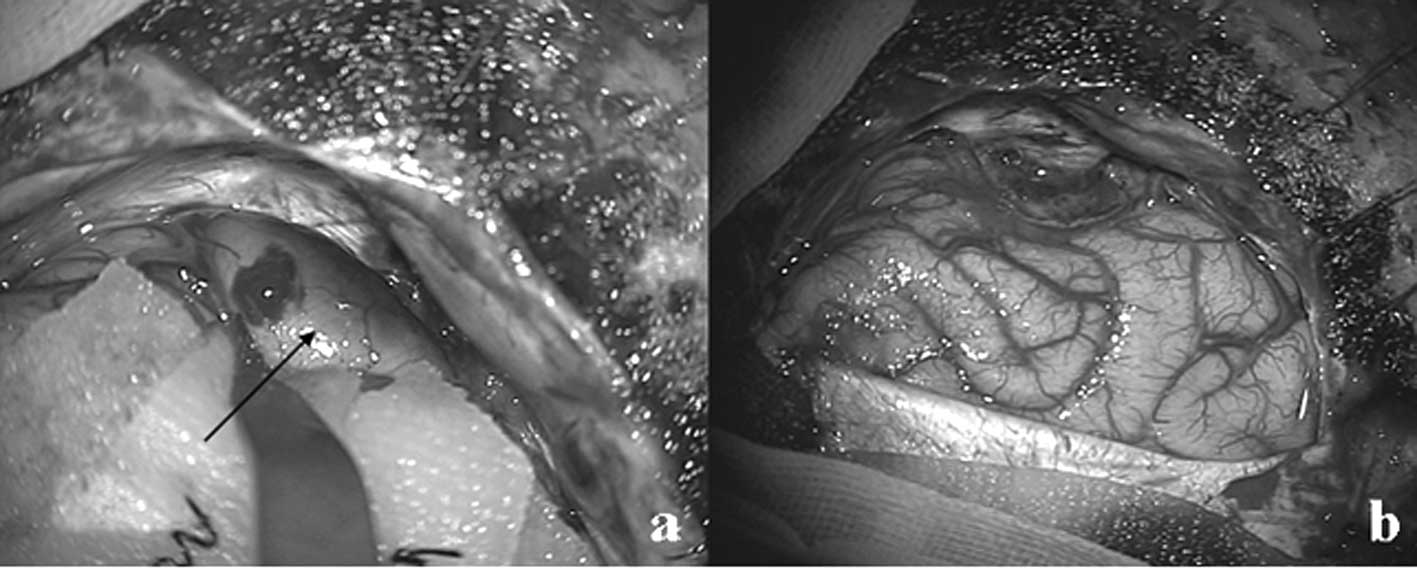
underwent a left occipital craniotomy, and gross total resection of
the mass lesion was performed using the transcortical lingual gyrus
approach (Figs. 1c and 2a and b). The lesion was intentionally
approached through an inferior occipital surface for preserving the
eloquent optic radiation running at the opposite end. Pathological
findings were consistent with a metastatic adenocarcinoma. The
patient was discharged from our institute without any neurological
deficit.
Discussion
In planning the surgery of brain lesions adjacent to
the optic radiation, it is important to pre-operatively confirm the
3D relationship between the lesion and affected tract as well as
the eloquent gray matter, including the visual cortex, to avoid
postoperative permanent visual impairments (1,4,7). The
usefulness of functional mapping using fMRI, positron-emission
tomography or visual evoked potential in surgical planning was
previously confirmed in patients with occipital lobe tumors
(11–13).
DTI provides information on the direction water
molecules follow at the cellular level, indicating the orientation
of fiber tracts. DTI-based tractography is currently the only
imaging method used to show white matter tracts in vivo
(5,14). This technique is widely used to
visualize motor, sensory, speech and visual pathways for
neurosurgical planning and postoperative assessment (1,3). Our
imaging technique facilitated the visualization of targeted white
matter in a short time period and with less invasiveness. This
technique is also feasible for patients with a poor performance
status.
The optic radiation starts in the lateral geniculate
body and forms a band that winds around the inferior and posterior
horns of the lateral ventricles, terminating in the visual cortex
or striate area, with the three-bundled arrangement comprising the
anterior, central and posterior bundles (10,15).
The anterior bundle runs anteriorly prior to making a sharp turn
around the tip of the inferior horn (known as Meyer’s loop) and
passes posteriorly along the lateral wall of the ventricle
ultimately terminating on the lower lip of the calcarine fissure
(16). The central bundle initially
runs in the lateral direction, then posteriorly along the lateral
wall of the ventricle and finally radiates into the calcarine
cortex. In addition, the posterior bundle runs directly in the
posterior direction along the roof of the ventricle and converges
on the upper lip of the calcarine fissure. Specifically, the
anterior bundle subserves the superior quadrant; the central
bundle, the macula; and the posterior bundle, the inferior visual
quadrant (10,15). Particularly in the pre-surgical
planning of anterior temporal lobe resection in patients with
refractory temporal lobe epilepsy due to hippocampal sclerosis and
resection of tumors originating in medial temporal structures or
within the temporal horn, knowledge of the boundary of the anterior
fibers of the Meyer’s loop and their relationship to the temporal
pole is required, but remains problematic (15,16). A
recent study demonstrated that tractography of the optic radiation
is clinically feasible in patients undergoing temporal lobe
resections in spite of the technical difficulty of visualizing the
anterior extent of Meyer’s loop (17).
Prior to the surgical exposure of tumors seated in
the temporal, occipital or parietal lobe, our neuroimaging
technique assessed the anatomical relationships between the optic
radiations and these lesions. The aim of surgery for brain tumors
adjacent to the functional cortical gray and white matter tracts is
to preserve vital cerebral tissue in order to avoid postoperative
neurological deficits as well as to maximize lesion resectability
(1–3). In patients with mass lesions located
in the posterior part of the temporal lobe or in the
temporo-parieto-occipital junction, we selected an appropriate
surgical tract that was distant to the eloquent fiber running
beside the lesion. Subsequently, less surgical manipulation of the
visual tracts occurred and violation of the functionally eloquent
cortex, areas involved in vision or language, during cortical
incision was avoided. On the other hand, we used the subtemporal
approach through the fusiform or parahippocampal gyri or the
inferior temporal gyrus approach particularly to avoid Meyer’s
loop, resulting in the safe resection of the medial temporal tumors
(15).
In the present study, the efficacy of tractography
was tested to determine the appropriate surgical corridor and the
extent of resection in patients with brain tumors adjacent to the
optic radiations. Tractography successfully designated the site of
cortical incision in combination with the findings of conventional
MRI, MR angiography, MR venography and conventional angiography. It
was previously reported that the combination of fMRI and DTI-based
tractography may permit the achievement of more favorable results
in pre-surgical planning in brain tumor resection (2,8,14). In
all 14 patients, tractography facilitated the visualization of the
geniculo-calcarine tract passing close to the lesions. The size and
site of lesions, as well as perifocal changes such as edema and
hemorrhage, may interfere with the visualization of the optic
tracts (5,6). In the present study, the optic tracts
were examined for large lesions (up to 7 cm) with or without
perifocal edema in the pre-surgical planning stage. Regarding the
site, the relationship of the posterior temporal, occipital or
parietal lesions to the optic radiation posterior to the geniculate
ganglion was achieved, and the choice of surgical approach was
facilitated. In contrast, in a few cases of medial temporal
lesions, technical difficulty in obtaining the trajectory curvature
of Meyer’s loop on DTI occurred. Consequently, the interpretation
of tractographic images should be undertaken with caution due to
considerable heterogeneity in the anterior extent (17).
To predict the anatomical relationship between the
eloquent fiber and the mass lesion, an understanding of the
pathological behavior of the lesions is crucial, i.e., whether they
are expansive or infiltrative. Observation with an operative
microscope clearly shows the demarcated border in the majority of
expansive growth tumors, such as metastatic ones. Therefore,
peritumoral structure, including the white matter bundle, may tend
to be displaced by a mass effect. During the resection of expansive
growth tumors, it is essential to avoid violation of the displaced
optic radiation through mechanical or heat injury. After achieving
a sufficient surgical working space by maximal internal
decompression, tumor dissection near the presumed eloquent fiber
should be meticulous, and the vascular component feeding the
eloquent neural tissue should be similarly preserved. Consequenlty,
gross total removal can be achieved without permanent neurological
deficit in the majority of cases.
In contrast, infiltrative growth tumors such as
glioma may easily invade or involve the contiguous eloquent fiber.
Unlike well-demarcated lesions, surgery of infiltrative lesions is
more likely to cause deficits. Therefore, intra-operatively, while
a maximal resection of a poorly demarcated mass is attempted, the
dissection of white matter bundles around the optic radiations
noted in the pre-operative DTI sequences is limited to avoid
postoperative neurological deterioration. Reports have described
the need for pre-operative and intra-operative integrated
neurosurgical planning combined with electrophysiological and
functional image guidance to define the safe resection limit
(1,8,9,11,13).
Multimodal navigation, in addition to pre-operative tractography,
in the microsurgical management of eloquent lesions is expected to
allow surgeons to maximize surgical resectability, as well as to
minimize postoperative morbidity and prolong patient survival.
At the time of surgical planning, the reliability of
tractographic images in defining trajectories that are anatomically
compatible with eloquent fiber should be considered (14). An important limitation to the
clinical use of DTI-based tractography is poor quantification. The
threshold of degrees, whether there is clinical significance or not
in tract visualization, remains to be determined. It is reported
that intra-operative subcortical neurostimulation may underestimate
the fiber tracts (18). However,
other authors report the accordance of findings of DTI tractography
and subcortical stimulation (4,11).
Using this innovative technique, we showed the
anatomical relationship between brain tumors and the optic
radiations. However, tractography is a subjective process,
dependent on user-defined parameters, and the visualized tract
themselves are ‘virtual’ representations of the underlying white
matter (17). Therefore, further
studies are required to validate this method and establish clinical
significance through pre-operative imaging, intra-operative
electrophysiological data and postoperative clinico-radiological
results (3,13).
In conclusion, our study showed that DTI
tractography is clinically feasible and provides useful information
regarding the surgical strategy for lesions located in eloquent
visual areas. Despite the existence of limitations, improvements in
pre-operative visual dysfunction and the absence of postoperative
deficits indicate the potential of this technique as a
neurosurgical planning tool.
Abbreviations:
|
DTI
|
diffusion tensor imaging
|
|
MRI
|
magnetic resonance imaging
|
|
FLAIR
|
fluid-attenuated
inversion-recovery
|
|
fMRI
|
functional MRI
|
|
ROIs
|
regions of interest
|
|
3D
|
three-dimensional
|
|
FA
|
fractional anisotropy
|
References
|
1
|
Romano A, D’Andrea G, Minniti G,
Mastronardi L, Ferrante L, Fantozzi LM and Bozzao A: Pre-surgical
planning and MR-tractography utility in brain tumour resection. Eur
Radiol. 19:2798–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witwer BP, Moftakhar R, Hasan KM, et al:
Diffusion-tensor imaging of white matter tracts in patients with
cerebral neoplasm. J Neurosurg. 97:568–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu CS, Li KC, Xuan Y, Ji XM and Qin W:
Diffusion tensor tractography in patients with cerebral tumors: a
helpful technique for neurosurgical planning and postoperative
assessment. Eur J Radiol. 56:197–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shinoura N, Suzuki Y, Yamada R, Tabei Y,
Saito K and Yagi K: Relationships between brain tumor and optic
tract or calcarine fissure are involved in visual deficits after
surgery for brain tumor. Acta Neurochir. 152:637–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada K, Kizu O, Mori S, et al: Brain
fiber tracking with clinically feasible diffusion-tensor MR
imaging: initial experience. Radiology. 227:295–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark CA, Barrick TR, Murphy MM and Bell
BA: White matter fiber tracking in patients with space-occupying
lesions of the brain: a new technique for neurosurgical planning?
Neuroimage. 20:1601–1608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikuta K, Takagi Y, Nozaki K, et al: Early
experience with 3-T magnetic resonance tractography in the surgery
of cerebral arteriovenous malformations in and around the visual
pathway. Neurosurgery. 58:331–337. 2006. View Article : Google Scholar
|
|
8
|
González-Darder JM, González-López P,
Talamantes F, Quilis V, Cortes V, Garcia-March G and Rordán P:
Multimodal navigation in the functional microsurgical resection of
intrinsic brain tumors located in eloquent motor areas: role of
tractography. Neurosurg Focus. 28:E52010.PubMed/NCBI
|
|
9
|
Nimsky C, Ganslandt O, Hastreiter P, Wang
R, Benner T, Sorensen AG and Fahlbusch R: Preoperative and
intraoperative diffusion tensor imaging-based fiber tracking in
glioma surgery. Neurosurgery. 56:130–138. 2005.
|
|
10
|
Yamamoto T, Yamada K, Nishimura T and
Kinoshita S: Tractography to depict three layers of visual field
trajectories to the calcarine gyri. Am J Ophthalmol. 140:781–785.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamada K, Todo T, Morita A, et al:
Functional monitoring for visual pathway using real-time visual
evoked potentials and optic-radiation tractography. Neurosurgery.
57:121–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roux FE, Ibarrola D, Lotterie JA, Chollet
F and Berry I: Perimetric visual field and functional MRI
correlation: implications for image-guided surgery in occipital
brain tumours. J Neurol Neurosurg Psychiatry. 71:505–514. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duffau H, Velut S, Mitchell MC, Gatignol P
and Capelle L: Intra-operative mapping of the subcortical visual
pathways using direct electrical stimulations. Acta Neurochir.
146:265–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada K, Sakai K, Akazawa K, Yuen S and
Nishimura T: MR tractography: a review of its clinical
applications. Magn Reson Med Sci. 8:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sincoff EH, Tan Y and Abdulrauf SI: White
matter fiber dissection of the optic radiations of the temporal
lobe and implications for surgical approaches to the temporal horn.
J Neurosurg. 101:739–746. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powell HWR, Parker GJM, Alexander DC, et
al: MR tractography predicts visual field defects following
temporal lobe resection. Neurology. 65:596–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yogarajah M, Focke NK, Bonelli S, et al:
Defining Meyer’s loop-temporal lobe resections, visual field
deficits and diffusion tensor tractography. Brain. 132:1656–1668.
2009.
|
|
18
|
Kinoshita M, Yamada K, Hashimoto N, et al:
Fiber-tracking does not accurately estimate size of fiber bundle in
pathological condition: initial neurosurgical experience using
neuronavigation and subcortical white matter stimulation.
Neuroimage. 25:424–429. 2005. View Article : Google Scholar
|