Introduction
Radiation recall is described as inflammation
occurring in previously irradiated areas which is triggered by the
administration of a drug (1). It is
most commonly observed when chemotherapeutic drugs, such as
anthracyclines, taxanes, alkylating agents, 5-fluorouracil and
capecitabine, are administered shortly after radiation, although
the reaction may occur years after the completion of radiation
(1,2). Radiation recall most often manifests
as inflammatory reactions of the skin but may also occur in
internal organs and tissues (1,2). In
the event of such a reaction, the offending drug is discontinued.
This case report evaluates a patient with poorly differentiated
adenocarcinoma of the liver. The patient had a recall reaction in
the form of myositis as a result of treatment with gemcitabine.
Radiation recall induced by gemcitabine is a rare and relatively
new phenomenon in the literature. Only two other cases of radiation
recall in a patient being treated for cancer of the liver have been
reported (3,4), and no cases exist involving primary
cancer. It has been reported that the majority of cases of
radiation recall induced by gemcitabine administration involve
inflammation of internal tissues and organs, which differs from the
majority of reactions caused by other chemotherapeutic agents, as
stated above (2). In this case
report, continuing gemcitabine treatment during radiation recall
was analyzed and the related current literature was evaluated.
Case report
A 50-year-old woman initially presented with right
flank discomfort in January 2008. Multiple lesions were evident in
the liver, and a needle biopsy confirmed the presence of poorly
differentiated adenocarcinoma. Due to the fact that multiple
lesions were located in multiple lobes, the patient was not a
candidate for surgery and was therefore considered for radiation
therapy followed by chemotherapy with palliative intent. The
patient received radiation therapy for a total dose of 44.1 Gy in
15 fractions, with a biological equivalent dose of 58.5 Gy. The
treatment was well tolerated, with no side effects greater than the
National Cancer Institute of Canada Grade I to the radiation.
The patient was started on gemcitabine 8 weeks after
completion of radiation. She received a dose of 1000
mg/m2 on days 1 and 8 of a 3-week cycle. On day 8 of her
fourth cycle, the patient complained of a discomfort in the
previously irradiated field. An abdominal examination revealed a
well-demarcated 15-cm rectangular indurated area. The overlying
skin was erythematous and slightly tender to palpation. Consistent
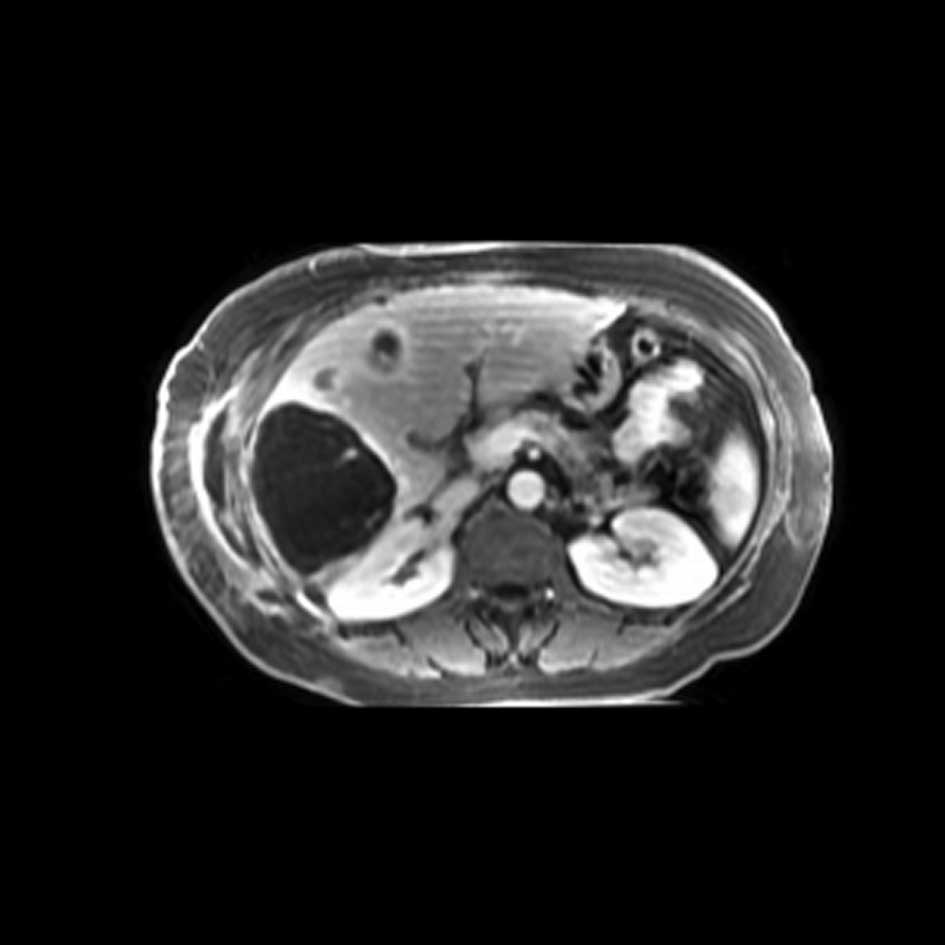
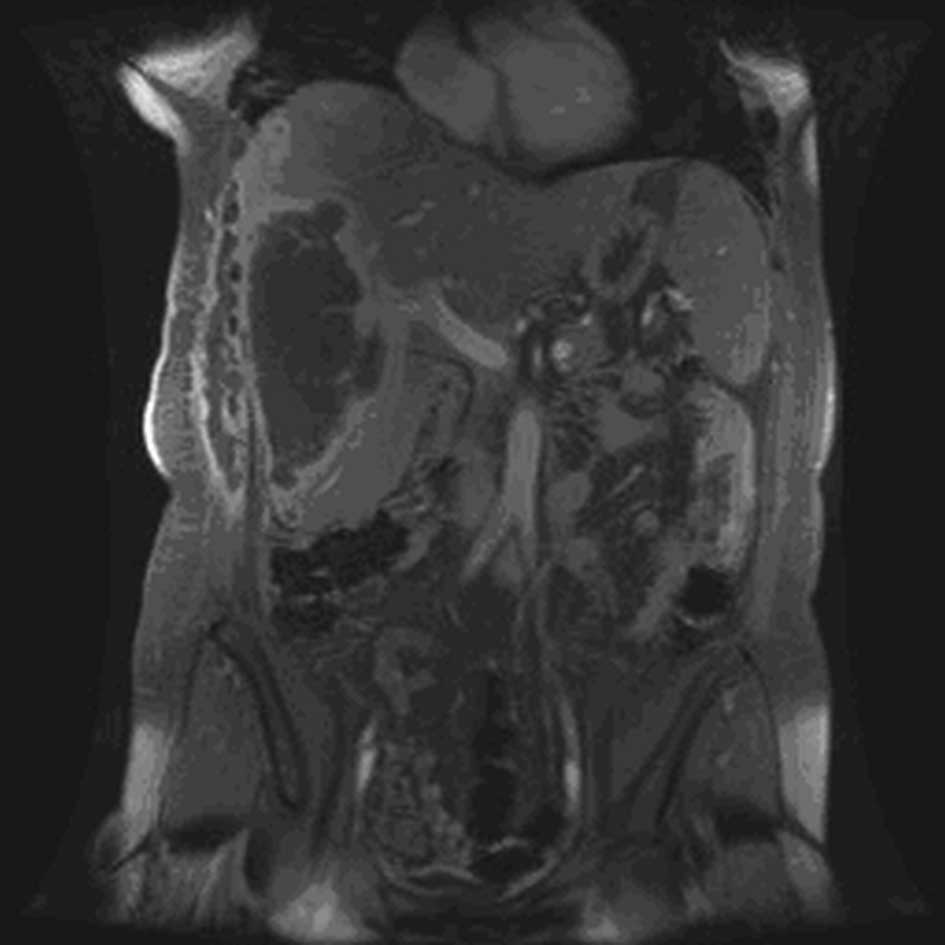
with the literature, clinical and radiological images (Figs. 1 and 2), it was determined that she presented
with a radiation recall reaction induced by gemcitabine treatment,
in the form of myositis.
Following consultation with the patient, the
decision was made to continue with the gemcitabine treatment as the
symptoms appeared to be improving in response to this treatment.
The patient was then started on ibuprofen 200 to 400 mg three times
a day for 6 weeks, vitamin E 400 IU two times a day and vitamin C
500 mg three times a day. She continued with two more cycles of
chemotherapy and had a documented stable disease response.
Subsequently, during a follow-up examination, the patient reported
that the discomfort caused by the recall reaction had continued to
subside. On visual examination, the reaction appeared to have
decreased in size as well.
Discussion
Radiation recall occurs when an inciting agent, such
as a chemotherapeutic drug, is administered after radiation. These
agents most commonly produce reactions such as dermatitis or
myositis, but can also produce rarely observed reactions such as
optic neuritis, brainstem necrosis and erysipeloid lesions
(1,5). The first reported case of radiation
recall was in 1959 and was attributed to actinomycin-D (6). Although the term radiation recall and
its implications are well known and various other agents have been
found to cause an occurrence, less than 150 cases have been
published in the literature. The majority of these cases have been
reported since the turn of the century and are likely associated
with the discovery and increased use of new cytotoxic agents. The
exact cause or mechanism remains unknown, which is complicated by
the fact that a variety of drugs have been found to induce
radiation recall with different chemical, biological and metabolic
characteristics. In addition, the timing of the occurrence of
radiation recall has remained variable, and no particular risk
factors from the patient angle have been defined.
Gemcitabine is an anti-metabolite nucleoside analog
that is used against tumors such as pancreatic and lung carcinoma.
Recall reactions attributed to gemcitabine are infrequently
reported in the literature. A literature search of Pub Med,
revealed only 28 cases since the first report in 1999 (1–5,7–21).
Hird et al reported that gemcitabine was involved only 9
times out of 75 cases of radiation recall dermatitis since 1959
(7).
A review of the literature provides some practical
insights into this phenomenon. Table
I summarizes our case along with all other published radiation
recall reactions related to gemcitabine. In 2004, Friedlander et
al described that the majority of cases of radiation recall
related to gemcitabine involved internal tissues and organs
(2). However, our study showed that
50% of such cases involved only skin and another 18% of cases
involved both skin and internal tissues. Due to the paucity of
data, we were not able to correlate radiation dose or dose per
fraction with severity or frequency. In most of the cases,
radiation dose to the skin is likely to have been lower than the
dose to the internal structures. Therefore, it can be suggested
that the radiation dose does not appear to affect the risk of
radiation recall.
 | Table ISummary of our case and all other
published radiation recall reactions related to gemcitabine. |
Table I
Summary of our case and all other
published radiation recall reactions related to gemcitabine.
| Study | Age/Gender | Type of cancer | Radiation dosage | Gemcitabine
dosage | Other chemo
drugs | Time elapsed from
radiation to chemo | Time elapsed from
chemo to reaction | Type of reaction | Continuation of
gemcitabine | Additional
treatment | Outcome |
|---|
| Friedlander et
al (2) | 62/M | Pancreatic | 50.4 Gy in 28
fractions | 1000
mg/m2/week | No | 39 days | 2 months | Myositis | NR | Cortico-
steroids | Full response |
| Fakih (8) | 52/M | Pancreatic | 50.4 Gy in 28
fractions | 1000
mg/m2/week | No | NR | 4 months | Myositis and
dermatitis | No | None | Full response |
| Squire et al
(9) | 54/F | Lung | 30 Gy in 10
fractions | 1000
mg/m2/dose | No | 1 month | 2 months | Myositis | Yes | Prednisone | Symptoms reduced as
prednisone increased |
| Saif et al
(3) | 57/M | Pancreatic | 50.4 Gy in 28
fractions | 150 mg/m2
as 24-h continuous infusion | Irinotecan | 13 weeks | 2 weeks | Antritis and
duodenitis | No | Proton pump,
inhibitors blood transfusion | Full response |
| Jeter et al
(1) | 41/F | Breast | 30 Gy in 10
fractions | 1000
mg/m2/week | Herceptin | 5.5 months | 2 weeks | Rash | No | None | Slow improvement |
| Jeter et al
(1) | 59/M | NSCLC | 40 Gy in 16
fractions | 1000
mg/m2/week every 2 weeks | No | 3 months | 3 months | Optic neuritis with
subacute loss of vision | No | Dexamethasone | Improvement by MRI,
no recovery of vision |
| Jeter et al
(1) | 79/M | NSCLC | 30 Gy in 10
fractions | 1 dose of 1000
mg/m2 | No | 11 days | 10 days | Dermatitis,
typhlitis, colitis | Yes | Gel pads and bowel
rest | Full response |
| Jeter et al
(1) | 52/F | Pancreatic | 50.4 Gy in 28
fractions | 1000 mg/m2
for 1 dose, then 750 mg/m2/week | No | 3 weeks | 9 weeks | Dermatitis,
myositis | Yes | Ibuprofen | Full response |
| Jeter et al
(1) | 54/M | Unknown primary | 35 Gy in 14
fractions | 600
mg/m2/week | Docetaxel | 1 week | 7¾ months | Brainstem
radionecrosis | No | Dexamethasone | Symptoms recurred
after steroids lessened |
| Jeter et al
(1) | 63/F | Unknown primary | 35 Gy in 12
fractions | 1000 mg/m2
for 1 dose | No | 3.4 months | 3 days | Lymphangitis with
erythema and edema | No | Dexamethasone | Minimal response |
| Tan et al
(10) | 53/F | Ovarian | 25 Gy in 10
fractions | 1000
mg/m2/week | No | 6 months | 1 day | Dermatitis | Yes | Empirical oral
antibiotics | Symptoms gradually
resolved until next cycle of treatment |
| Tan et al
(10) | 67/M | Mesothelioma | 54 Gy in 30
fractions | 1 dose of 1000
mg/m2 | No | 9 days | 2 days | Erysipeloid
lesions | No | None | Full response |
| Schwartz et al
(5) | 67/F | Ovarian | 45 Gy in 25
fractions | 800 mg/m2,
then 600 mg/m2 | No | 3.5 months | 1 day | Dermatitis | No | Topical
steroids | Quick recovery |
| Schwarte et
al (11) | 64/F | Esophageal | 50.4 Gy in 28
fractions | 1000
mg/m2/week | Docetaxel | 8 months | 2 days | Pneumonitis | No | Prednisone | Recovery over 2
months |
| Marisavljević et
al (12) | 32/F | Stage IIB Hodgkin
lymphoma | Total of 60 Gy | 1250
mg/m2/week | No | 2.5 years | 2 days | Dermatitis | Yes | None | Symptoms recurred
after each administration |
| Castellano et
al (13) | NR | NSCLC | NR | Total dose of 3000
mg/m2 | Vinorelbine | NR | 2 weeks | Dermatitis and
pneumonitis | No | None | Full response |
| Hird et al
(7) | 55/F | Metastatic breast
adeno-carcinoma | 20 Gy in 5
fractions to thoracic spine and 20 Gy in 5 fractions, whole brain
radiation | 1000
mg/m2 | Paclitaxel | 10 days | 2 days | Dermatitis | No | Silver
sulphadiazine cream | Discoloration still
apparent after 8 weeks |
| Bar-Sela et
al (14) | 65/M | NSCLC | 45 Gy in 25
fractions | 1000
mg/m2/week | No | 2 months | 6 weeks | Dermatitis | | i.v. antibiotics
and steroids | |
| Welsh et al
(15) | 60/M | Transitional cell
carcinoma of the bladder | 45 Gy in 18
fractions | NR | Cisplatin | 4 weeks | 4 months | Myositis | Yes | Non-steroidal anti-
inflammatory drugs and prednisone | Symptoms resolved
over 6 weeks |
| Ganem et al
(16) | 58/F | Squamous cell
carcinoma of the lung | 33 Gy in 11
fractions | 1000
mg/m2/week | Cisplatin | 1 month | 3 months | Myositis | NR | Oral opiates,
antibiotics and steroids | Progressively
resolved over 3 months |
| Burstein et
al (17) | 41/F | Stage II breast
cancer | NR | NR | No | 6 months | 2 weeks | Dermatitis | No | None | Slow
improvement |
| Castellano et
al (4) | 61/M | Stage IV NSCLC | 24 Gy in 8
fractions | 1250
mg/m2/week | No | 4 weeks | 1 week | Dermatitis | No | Dexamethasone and
diphenhydramine | Completely resolved
within 10 days |
| Fogarty et al
(18) | 65/F | Squamous cell
carcinoma of the lung | 36 Gy in 12
fractions | 1000
mg/m2/week | Carboplatin | 4 months | 6 weeks | Dermatitis and
myositis | No | Oral steroids | Symptoms partially
resolved |
| Horan et al
(19) | 58/M | Squamous cell
carcinoma of the lung | 24 Gy in 8
fractions | 1000
mg/m2/week | No | 2 months | 4 weeks | Necrosis | No | None | Symptoms gradually
resolved |
| Monne et al
(20) | NR | Bone metastatic
breast cancer | 55.2 Gy in 24
fractions | 1250
mg/m2 | No | 20 days | 1 day | Acute
dermatitis | NR | Corticosteroids and
dismutase superoxide | Cutaneous and
subcutaneous fibrosis was experienced |
| Monne et al
(20) | NR | Lung cancer | 20 Gy in 5
fractions | 1250
mg/m2/week | Cisplatin | 7 days | 2 months | Acute
dermatitis | NR | Corticosteroids and
dismutase superoxide | Cutaneous and
subcutaneous fibrosis was experienced |
| Monne et al
(20) | NR | Pancreatic
cancer | 35 Gy in 14
fractions | 1250
mg/m2/week | 5-FU | 12 days | 3 months | Acute
dermatitis | NR | Corticosteroids and
dismutase superoxide | Cutaneous and
subcutaneous fibrosis was experienced |
| Duvic et al
(21) | NR | Cutaneous T cell
lymphoma | NR | 1000
mg/m2/week | | NR | NR | Skin
ulceration | | | |
| Present study | 50/F | Hepatocellular
carcinoma | 44.1 Gy in 15
fractions | 1000
mg/m2/week | Capecitabine on day
1 of each regimen | 8 weeks | 8 weeks | Myositis | Yes | Ibuprofen, vitamins
E and C | |
In that same study, Friedlander et al also
documented that a shorter time interval between radiation therapy
and chemotherapy was correlated with recall reactions involving
internal tissues (2). The averages
of this interval confirm this in that the average time period for
cutaneous reactions was 4 months while the average time period for
reactions involving internal tissues was 2.5 months, although the
medians were found to be the same at 1.5 months. The relationship
between the interval from commencement of chemotherapy and the type
of reaction suggests a variable sensitivity.
Another significant observation, noted by Camidge
and Price in 2001, is that the risk of succumbing to a recall
reaction is not affected by whether the patient receives
monotherapy or if chemotherapeutic agents are administered in
combination (22). We also noted
that there is no significant difference between the number of
gemcitabine-induced radiation recall reactions presented while the
patient is receiving monotherapy or a combination treatment, nor
does this appear to affect the type of reaction presented. In our
case, gemcitabine administration was continued while the recall
reaction was treated with conservative supportive care. Only six
other studies in the literature of radiation recall induced by
gemcitabine report the continuation of gemcitabine treatment,
whether to maintain the current regimen or lower the dose (1,9,10,12,15).
Four of these cases reported that the patients had complete
improvement of their symptoms while still on gemcitabine. The other
two cases reported that the symptoms improved, but then recurred
following each administration. It should be noted that in one of
these cases the patient received no treatment for the reaction
(12). Including our case, none of
the cases in which gemcitabine was continued noted an increase in
symptoms or pain at any time during chemotherapy. In the case in
which the symptoms recurred after each administration, the symptoms
returned to their original form and did not present at a higher
grade (10,12). Moreover, two of the cases in the
literature documenting a discontinuation of gemcitabine treatment
reported that the cancer had metastasized, leading to patient death
(8,19). Clearly this is a primary
consideration for the patient and health care provider when a
reaction occurs. In our case, the symptoms experienced by the
patient gradually improved while on gemcitabine treatment and did
not worsen after administration.
Our case report, along with other similar cases in
the literature, lends support to treating clinicians who decide to
continue chemotherapy with gemcitabine in cases with a radiation
recall reaction. Our data do not suggest that a gemcitabine recall
reaction heralds a more resistant disease or greater metastatic
potential. Radiation techniques or regimens do not need to be
adjusted. Gemcitabine recall remains an enigmatic and rare event
that should not affect primary cancer management decisions.
Patients can be informed that the reaction usually resolves and
does not change their prognosis.
References
|
1
|
Jeter M, Jänne P, Brooks S, et al:
Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys.
53:394–400. 2002. View Article : Google Scholar
|
|
2
|
Friedlander P, Bansal R, Schwartz L,
Wagman R, Posner J and Kemeny N: Gemcitabine-induced radiation
recall preferentially involves internal tissues and organs. Cancer.
100:1793–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif M, Sellers S and Russo S:
Gemcitabine-related radiation recall in a patient with pancreatic
cancer. Anticancer Drugs. 17:107–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castellano D, Hitt R, Cortés-Funes H,
Romero A and Rodrigues-Peralto J: Diagnosis in oncology. Case 2
Radiation recall reaction induced by gemcitabine. J Clin Oncol.
3:695–696. 2000.
|
|
5
|
Schwartz B, Khuntia D, Kennedy A and
Markham M: Gemcitabine-induced radiation recall dermatitis
following whole pelvic radiation therapy. Gynecol Oncol.
91:421–422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Angio G, Farber S and Maddock C:
Potentiation of X-ray effects of actinomycin D. Radiology.
73:174–177. 1959.
|
|
7
|
Hird A, Wilson J, Symons S, Sinclair E,
Davis M and Chow E: Radiation recall dermatitis: case report and
review of the literature. Curr Oncol. 15:53–62. 2008. View Article : Google Scholar
|
|
8
|
Fakih M: Gemcitabine-induced rectus
abdominus radiation recall. JOP. 7:306–310. 2006.PubMed/NCBI
|
|
9
|
Squire S, Chan M, Feller E, Mega A and
Gold R: An unusual case of gemcitabine-induced radiation recall. Am
J Clin Oncol. 29:6362006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan D, Bunce P, Liles W and Gold W:
Gemcitabine-related ‘pseudocellulitis’: report of 2 cases and
review of the literature. Clin Infect Dis. 45:e72–e76. 2007.
|
|
11
|
Schwarte S, Wagner K, Karstens J and
Bremer M: Radiation recall pneumonitis induced by gemcitabine.
Strahlenther Onkol. 183:215–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marisavljević D, Ristić B and Hajder J:
Gemcitabine-induced radiation recall dermatitis in a patient with
resistant Hodgkin lymphoma. Am J Hematol. 80:912005.PubMed/NCBI
|
|
13
|
Castellano D, Hitt R, Ciruelos E,
Cortés-Funes H and Colomer R: Biweekly vinorelbine and gemcitabine:
a phase I dose-finding study in patients with advanced solid
tumors. Ann Oncol. 14:783–787. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bar-Sela G, Beny A, Bergman R and Kuten A:
Gemcitabine-induced radiation recall dermatitis: case report.
Tumori. 87:428–430. 2001.
|
|
15
|
Welsh J, Torre T, DeWeese T and O'Reilly
S: Radiation myositis. Ann Oncol. 10:1105–1108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ganem G, Solal-Celigny P, Joffroy A, Tassy
D, Delpon A and Dupuis O: Radiation myositis: The possible role of
gemcitabine. Ann Oncol. 11:1615–1616. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burstein H: Diagnosis in oncology. Case 1
Radiation recall dermatitis from gemcitabine. Am J Clin Oncol.
18:693–698. 2000.PubMed/NCBI
|
|
18
|
Fogarty G, Ball D and Rischin D: Radiation
recall reaction following gemcitabine. Lung Cancer. 33:299–302.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horan G, Smith S and Podd T:
Gemcitabine-induced radiation necrosis of the pectoralis major
muscle. Clin Oncol. 18:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sole Monne JM, Macia Garau M, Cambra
Serest MJ and Sureda Gonzalez M: Radiation recall dermatitis from
gemcitabine. a new toxic effect in 3 patients. Radiother Oncol.
57:S32–S34. 2000.
|
|
21
|
Duvic M, Talpur R, Wen S, Kurzrock R,
David CL and Apisarnthanarax N: Phase II evaluation of gemcitabine
monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma.
7:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Camidge R and Price A: Characterizing the
phenomenon of radiation recall dermatitis. Radiother Oncol.
59:237–245. 2001. View Article : Google Scholar : PubMed/NCBI
|