Introduction
Hepatocellular carcinoma (HCC) is a malignancy of
worldwide significance, and its prevalence is on the increase in
Japan, Western Europe and the US (1). One of the reasons for the poor
prognosis of HCC is its high rate of recurrence. Mounting evidence
indicates that the high rate of recurrence, even after curative
therapy, is due to intrahepatic metastasis or the multi-centric
development of each respective neoplasm clone. Since the high-risk
group of either primary or secondary HCC development appears to be
more discernable than in other types of tumors, it is likely that
chemopreventive agents are beneficial in improving the prognosis of
HCC.
Any solid tumor that has not acquired a new blood
supply cannot grow to more than a few millimeters in size,
including HCC (2). Numerous studies
showed that neovascularization and angiogenic factors, such as the
vascular endothelial cell growth factor (VEGF), are significantly
up-regulated in human HCC samples (3,4). It
has been reported that angiogenesis is also induced at the early
stages of tumor formation and carcinogenesis (5–8). In a
previous study, angiogenesis was found to increase stepwise during
the murine hepatocarcinogenesis process and suppression of the
VEGF-signaling pathway markedly attenuated hepatocarcinogenesis
(9). It has been reported that
alterations in the hepatic microcirculation in the human liver
develop at an early stage of liver carcinogenesis, in association
with liver cell change or within the dysplastic nodules, prior to
the emergence of morphologically identifiable HCC (10).
A number of anti-angiogenic agents have already been
employed in clinical practice, including sorafenib (11). Sorafenib is an oral multi-kinase
inhibitor that acts against several tyrosine kinases (VEGFR-1,
PDGFR and c-kit receptors) and serine/threonine kinases (b-Raf and
p38) (12). A recent study showed
that sorafenib prolonged the median overall survival and delayed
the median time to progression in patients with advanced HCC; thus,
this drug is to become the standard therapy for advanced stages of
HCC (13). However, various serious
concerns exist in employing this agent for chemoprevention against
HCC. Since long-term administration is required and the drug
metabolism is usually hypoactive in patients with cirrhosis, an
agent with proven safety is preferable for chemoprevention against
HCC. Almost all patients show adverse effects upon using sorafenib,
and several of the symptoms are relatively severe (14,15).
Moreover, considering the long-term administration, cost
effectiveness is an issue due to the high cost involved (16). An alternative approach may be to
find a clinically available compound that also exhibits
anti-angiogenic activity, for which the safety of long-term
administration has been proven.
In a previous study, it was reported that the
angiotensin-converting enzyme inhibitor (ACE-I) and vitamin K2 (VK)
exerted strong anti-angiogenic activities. Additionally, the two
agents are widely used in clinical practice as safe drugs for
hypertension (ACE-I) and osteoporosis (VK). The combination
treatment of ACE-I and VK at clinically comparable low doses was
found to show a marked suppressive effect against the development
of HCC in rats via angiogenesis suppression (17). The combined treatment of ACE-I and
VK also ameliorated hepatic dysplastic nodules in a patient with
liver cirrhosis (18). Furthermore,
this combination regimen significantly inhibited the cumulative
recurrence of HCC after curative therapy, at least partly, through
suppression of VEGF-mediated neovascularization (19).
Markers that predict clinical benefit are crucial,
particularly those inhibiting VEGF, since sorafenib occasionally
exerts severe diverse effects, as described above. A study recently
showed that hepatocyte growth factor (HGF) may be a surrogate
marker for the clinical benefit of sorafenib (20). In addition to HGF, various molecules
have been considered as useful biological markers for
anti-angiogenic therapy, such as circulating endothelial progenitor
cells and the soluble form of VEGF receptors (20).
In the present study, a long-term follow-up (54
months) was performed to ascertain whether the combination of ACE-I
and VK inhibits disease recurrence in patients with HCC after
following curative radiofrequency ablation (RFA) therapy.
Furthermore, the identification of non-invasive biological markers
that predict the clinical benefit of this regimen were
investigated.
Materials and methods
Patients
The study comprised 54 patients with HCC who were
admitted to hospital between July 2004 and July 2009. HCC was
diagnosed via a combination of various imaging modalities, such as
hepatic angiography, enhanced computed tomography (CT) and magnetic
resonance imaging (MRI). In the present study, no unusual lesions
were encountered that required needle biopsy for histological
confirmation; thus, no histological proof of the diagnosis was
obtained. Therapeutic modalities according to the algorithm of HCC
treatment by the Liver Cancer Study Group of Japan (LCSGJ) were
employed. The LCSGJ scoring system is a reliable system comparable
to those of the Cancer of the Liver Italian Program, the Barcelona
Clinic Liver Center and the recently published evidence-based
clinical practice guidelines for HCC in Japan (supported by the
Japanese Ministry of Health, Labor, and Welfare) (21–23).
In this algorithm, the therapeutic strategy is selected based on
the degree of liver damage as determined by LCSGJ and the
characteristics of the tumor itself. The indications of RFA of
LCSGJ are ≤3 tumors measuring ≤3 cm, or a solitary tumor with a
major axis of ≤5 cm. Thus, when RFA is selected as a therapeutic
modality, the patient is admitted to our department.
Study design and treatment
The patients were randomly divided (using sealed
envelopes) into either the control group (Group 1, G1; n=28) or the
combination-treated group (Group 2, G2; n=26). No placebo was used
in G1. The patients in G2 received a constant dose of oral ACE-I
(perindopril; 4 mg/day) and VK (menatetrenone; 45 mg/day) for 54
months. The respective doses of the two compounds are standard in
clinical practice. The clinical profiles, laboratory data and
patient characteristics are shown in Table I. Patients who received medication
that potentially influenced the VK metabolism, such as warfarin,
and those who received other types of anti-hypertensive agents were
excluded from this study. Patients were also required to cease
alcohol intake completely. Until the end of the recurrence
follow-up, no patients received any additional therapy for HCC,
including interferon (IFN). After RFA, follow-ups were conducted
using enhanced CT and ultrasonography (US) once a month for the
first 3 months. Detections of viable HCC during this period
resulted in patient exclusion. All of the patients gave informed
written consent prior to participating in the study. The study
protocol was approved by the Institutional Review Board of the Nara
Medical University and the study was conducted in compliance with
ethical and humane principles. The present study adhered to the
CONSORT statement for randomized studies.
 | Table IClinical characteristics of the
enrolled patients. |
Table I
Clinical characteristics of the
enrolled patients.
| Characteristics | Untreated
control | VK + ACE-I | P-value |
|---|
| No. of patients | 28 | 26 | |
| Age (range;
years) | 61.4±10.3a | 62.5±9.6 | 0.363b |
| Gender (M/F) | 18/10 | 19/7 | 0.491c |
| Etiology
(HCV/HBV/other) | 24/3/1 | 22/3/1 | 0.911c |
| Alcohol (<40
g/>40 g/day) | 16/12 | 13/13 | 0.602c |
| Tumor stage
(I/II/III) | 15/11/2 | 13/12/1 | 0.898c |
| Tumor mean size
(mm) | 21.2±8.7 | 18.9±9.1 | 0.412b |
| No. of tumors | 1.76±1.12 | 1.55±0.83 | 0.068b |
| AFP (ng/ml) | 76.5±213.3 | 83.3±262.5 | 0.150b |
| PIVKA-II
(mAU/ml) | 52.3±60.8 | 60.5±72.6 | 0.188b |
| ALT (IU/l) | 71.4±33.5 | 72.3±36.6 | 0.329b |
| Child-Pugh score
(A/B) | 23/5 | 21/5 | 0.897c |
Assessment of biological markers
The serum tumor markers of α-fetoprotein (AFP),
lectin-reactive AFP (AFP-L3) and des-γ-carboxyprothrombin
(PIVKA-II), were measured every 2 months using routine laboratory
methods. Prior to and at 12 months after drug administration
commenced, the alterations of angiogenic factors were determined
using a TranSignal Angiogenesis Antibody Array (Panomics Inc.,
Redwood city, CA, USA) in the serum, according to the
manufacturer’s manual, after equalization of the protein content.
The alterations of VEGF (24) and
the soluble form of VEGF receptor-1 (sVEGFR-1) and -2 (sVEGFR-2) in
the serum were also examined using an enzyme-linked immunosorbent
assay (ELISA) kit (R&D Systems) according to the manufacturer’s
instructions as previously described (9).
Statistical analysis
Patient characteristic variables were analyzed with
the Mann-Whitney U test and the Fisher’s exact probability test.
The cumulative recurrence of HCC was plotted using the Kaplan-Meier
method, and the differences in recurrence curves were tested using
the log-rank test.
Results
Patient characteristics and HCC
recurrence
The clinical characteristics of the enrolled
patients are shown in Table I. No
significant differences were noted among the patients of the two
groups regarding age, gender, etiology of the disease or background
liver function (Child-Pugh score). In addition, the tumor baseline,
such as stage, number of tumors, AFP, AFP-L3 and PIVKA-II levels,
did not differ between the groups. Since no abnormal laboratory
data were found that correlated to treatment with either ACE-I or
VK, we were able to follow up all of the enrolled patients in each
group until detection of recurrence.
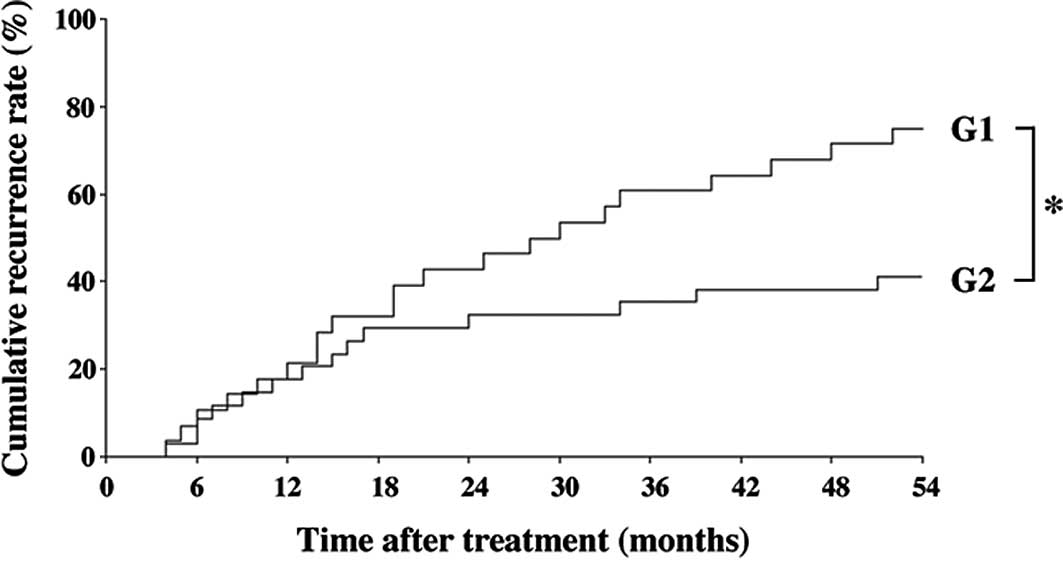
The inhibitory effect of the combined treatment of
ACE-I and VK is shown in Fig. 1.
The combination treatment of ACE-I and VK (G2) significantly
inhibited the cumulative recurrence of HCC as compared to the
control group (G1) for 54 months after the treatment (P<0.01).
Although the primary end-point of the study was the cumulative
recurrence rates, we also examined whether or not the survival
curves of the patients were altered during the follow-up period. No
statistical differences were noted between the groups (data not
shown).
Alteration of angiogenic factors
To elucidate which angiogenic factors were altered
by treatment with ACE-I and VK, we employed the angiogenic antibody
array that detects 19 different angiogenic factors in the serum of
the enrolled patients following the 12-month treatment, as
previously described (19). In the
control group, the hot spot of VEGF was significantly increased,
whereas it was markedly attenuated by the combined treatment of
ACE-I and VK. One recent report showed that the HGF level may be a
biomarker of sorafenib clinical efficiency. However, in our ACE-I
and VK combination treatment, no significant alteration of HGF was
detected (data not shown).
Plasma levels of VEGF, sVEGFR-1 and
sVEGFR-2
Since VEGF expression was predominantly altered by
ACE-I and VK combination treatment, we measured the expression
levels of VEGF-related molecules using ELISA in all of the
patients. The serum VEGF level in the control group increased after
12 months, whereas the combination treatment of ACE-I and VK
markedly attenuated the VEGF level as compared to the pre-treatment
level (Fig. 2A). Additionally, the
expression levels of the soluble forms of sVEGFR-1 and sVEGFR-2
were elucidated. As shown in Fig.
2B, no significant differences were noted in sVEGFR-1 between
the the pre-treatment and post-treatment levels in the two groups.
On the other hand, sVEGFR-2 was markedly decreased by the
combination treatment of ACE-I and VK, whereas the serum level of
sVEGFR-2 in the control group increased (Fig. 2C). The chronological alterations of
VEGF and sVEGFR-2 in the treated group were then examined until 12
months after treatment in the patients without HCC recurrence. The
VEGF expression level gradually decreased, but at 6 months of
treatment it was statistically significant compared to the
pre-treatment basal level. The decrease in sVEGFR-2 expression
occurred earlier than that of VEGF. sVEGFR-2 expression was
observed to have already significantly decreased at 3 months
following the ACE-I and VK combination treatment (Fig. 3).
Discussion
The identification of surrogate markers that predict
the clinical outcome of anti-angiogenic therapies is crucial, since
a number of currently available anti-angiogenic agents exert severe
diverse effects. The present study showed that a combination
treatment with ACE-I and VK significantly inhibited the cumulative
HCC recurrence for 54 months after curative therapy along with the
suppression of VEGF and sVEGFR-2. Since the two agents are widely
used in clinical practice without serious side effects, this
combined treatment is a potential new strategy for secondary
chemoprevention against HCC.
One of the first biomarkers for evaluating
anti-angiogenic therapy was the pre-treatment level of VEGF in
tissue and circulation, but it failed to predict the outcome of
anti-VEGF therapy in several types of cancer. Only the E4559 trial
on non-small cell lung cancer revealed that the pre-treatment
plasma VEGF level was of prognostic significance (25). In addition to VEGF, sVEGFR-1 and
sVEGFR-2 have also been studied as potential biomarkers. These two
full-length receptors have an extracellular domain containing seven
immunoglobulin-like loops, a transmembrane domain and an
intracellular split catalytic tyrosine kinase domain. sVEGFR-1 is
the product of alternative mRNA splicing and comprises only six of
seven immunoglobulin-like domains. sVEGFR-1 is reportedly a
potential surrogate marker for disease progression in several types
of cancer, such as breast cancer and renal cell carcinoma. In HCC,
sVEGFR-1 was found to be correlated with poor prognosis, whereas it
was not an independent prognostic factor for HCC (26). In the present study, no significant
correlation was found between the alteration of sVEGFR-1 and HCC
recurrence.
The natural form, sVEGFR-2, was detected more
recently than sVEGFR-1. Similar to sVEGFR-1, sVEGFR-2 is unable to
carry out tyrosine transphosphorylation and activate the downstream
signal transduction of angiogenesis, due to the lack of the
tyrosine kinase domain (20). The
activation of VEGFR-2 reportedly plays a significant role in tumor
angiogenesis. We previously reported that VEGF and VEGFR-2
interaction plays a pivotal role in HCC growth and
hepatocarcinogenesis (9,27). The neutralizing VEGFR-2 monoclonal
antibody significantly attenuated hepatocarcinogenesis and HCC
growth along with VEGF-mediated neovascularization. Clinical
studies have evaluated the plasma levels of sVEGFR-2 as a potential
surrogate marker of disease progression in various malignancies
based on the hypothesis that the circulating sVEGFR-2 level may
provide insight into VEGFR-2 activation (28). In the present study, it was noted
that both VEGF and sVEGR-2 were significantly decreased by the
combination treatment of ACE-I and VK. These results indicate that
the suppressive effect of this combination regimen on HCC
recurrence is, at least partly, mediated by the suppression of VEGF
and VEGFR-2-mediated neovascularization. However, no specific
histological evidence of alteration of neovascularization in the
liver was found. Although the serum VEGF level reportedly reflects
the intrahepatic VEGF expression level in patients with chronic
liver diseases (29), chronological
immunohistochemical studies are required to confirm whether
alterations of VEGF and sVEGFR-2 reflect intrahepatic
neovascularization.
We observed that the expression of VEGF and sVEGFR-2
was chronologically decreased by the combined treatment of ACE-I
and VK in patients without HCC recurrence. The decrease in sVEGFR-2
expression occurred earlier than that of VEGF. sVEGFR-2 expression
was noted to have already significantly decreased at 3 months
following ACE-I and VK combination treatment. In renal cell
carcinoma, sVEGFR-2 levels were found to be significantly decreased
by treatment with sunitinib, which is also a multi-kinase
inhibitor, including VEGFR during treatment cycle 1 (30). Moreover, the levels tended to return
to near baseline 2 weeks after treatment, suggesting that sVEGFR-2
is potentially a sensitive marker of anti-angiogenic therapy. These
results show that sVEGFR-2 may be utilized as a useful clinical
predictive marker of the combination treatment of ACE-I and VK.
Although the primary end-point of the study was the
cumulative recurrence rates, we also examined whether the survival
curves of the patients were altered or not during the follow-up
period. No statistical differences were found between the groups
(data not shown). Due to a short follow-up period, no statistical
differences were detected between the two groups in this study.
Further long-term and large-scale studies are required to verify
whether or not the suppressive effects of ACE-I and VK on
cumulative recurrence improve prognosis.
In conclusion, we demonstrated that the combination
treatment of ACE-I and VK significantly attenuated the cumulative
recurrence of HCC for 54 months following curative therapy along
with the suppression of VEGF and sVEGFR-2 serum levels. In patients
without recurrence, a significant suppression of VEGF and sVEGFR-2
was achieved within 6 and 3 months following treatment,
respectively. These results suggest that the combination treatment
of ACE-I and VK is a potential new anti-angiogenic strategy for
secondary chemoprevention against HCC, since the two agents are
widely used in clinical practice without serious side effects.
Furthermore, sVEGFR-2 may be a useful clinical predictive marker of
this combination treatment.
Abbreviations:
|
AT-II
|
angiotensin-II
|
|
ACE
|
angiotensin-converting enzyme
|
|
AT1-R
|
angiotensin type-1 receptor
|
|
ACE-I
|
ACE inhibitor
|
|
ARB
|
angiotensin type-1 receptor
blocker
|
|
EC
|
endothelial cell
|
|
HCC
|
hepatocellular carcinoma
|
|
IFN
|
interferon
|
|
RFA
|
percutaneous radiofrequency
ablation
|
|
sVEGFR-2
|
soluble form of VEGF receptor-2
|
|
VK
|
vitamin K2
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Schafer DF and Sorrell MF: Hepatocellular
carcinoma. Lancet. 353:1253–1257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerbel RS: Tumor angiogenesis: past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo RP, Zhong C, Shi M, et al: Clinical
value of apoptosis and angiogenesis factors in estimating the
prognosis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
132:547–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iavarone M, Lampertico P, Iannuzzi F, et
al: Increased expression of vascular endothelial growth factor in
small hepatocellular carcinoma. J Viral Hepat. 14:133–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CY, Shan S, Huang Q, et al: Initial
stages of tumor cell-induced angiogenesis: evaluation via skin
window chambers in rodent models. J Natl Cancer Inst. 92:143–147.
2000. View Article : Google Scholar
|
|
6
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
7
|
Bergers G, Javaherian K, Lo KM, Folkman J
and Hanahan D: Effects of angiogenesis inhibitors on multistage
carcinogenesis in mice. Science. 284:808–812. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brandvold KA, Neiman P and Ruddell A:
Angiogenesis is an early event in the generation of myc-induced
lymphomas. Oncogene. 19:2780–2785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshiji H, Kuriyama S, Yoshii J, et al:
Halting the interaction between vascular endothelial growth factor
and its receptors attenuates liver carcinogenesis in mice.
Hepatology. 39:1517–1524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frachon S, Gouysse G, Dumorti J, et al:
Endothelial cell marker expression in dysplastic lesions of the
liver: an immunohistochemical study. J Hepatol. 34:850–857. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors: a review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar
|
|
15
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berenson A: A cancer drug shows promise,
at a price that many can’t pay. The New York Times.
A1:C22006.PubMed/NCBI
|
|
17
|
Yoshiji H, Kuriyama S, Noguchi R, et al:
Combination of vitamin K(2) and the angiotensin-converting enzyme
inhibitor, perindopril, attenuates the liver enzyme-altered
preneoplastic lesions in rats via angiogenesis suppression. J
Hepatol. 42:687–693. 2005. View Article : Google Scholar
|
|
18
|
Yoshiji H, Noguchi R, Yamazaki M, et al:
Combined treatment of vitamin K2 and angiotensin-converting enzyme
inhibitor ameliorates hepatic dysplastic nodule in a patient with
liver cirrhosis. World J Gastroenterol. 13:3259–3261. 2007.
|
|
19
|
Yoshiji H, Noguchi R, Toyohara M, et al:
Combination of vitamin K2 and angiotensin-converting enzyme
inhibitor ameliorates cumulative recurrence of hepatocellular
carcinoma. J Hepatol. 51:315–321. 2009. View Article : Google Scholar
|
|
20
|
Murukesh N, Dive C and Jayson GC:
Biomarkers of angiogenesis and their role in the development of
VEGF inhibitors. Br J Cancer. 102:8–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kokudo N and Makuuchi M: Evidence-based
clinical practice guidelines for hepatocellular carcinoma in Japan:
the J-HCC guidelines. J Gastroenterol. 44:119–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makuuchi M, Kokudo N, Arii S, et al:
Development of evidence-based clinical guidelines for the diagnosis
and treatment of hepatocellular carcinoma in Japan. Hepatol Res.
38:37–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okita K: Management of hepatocellular
carcinoma in Japan. J Gastroenterol. 41:100–106. 2006. View Article : Google Scholar
|
|
24
|
Ferrara N: VEGF as a therapeutic target in
cancer. Oncology. 69(Suppl 3): 11–16. 2005. View Article : Google Scholar
|
|
25
|
Dowlati A, Gray R, Sandler AB, Schiller JH
and Johnson DH: Cell adhesion molecules, vascular endothelial
growth factor, and basic fibroblast growth factor in patients with
non-small cell lung cancer treated with chemotherapy with or
without bevacizumab – an Eastern Cooperative Oncology Group Study.
Clin Cancer Res. 14:1407–1412. 2008.
|
|
26
|
Nagaoka S, Yoshida T, Akiyoshi J, et al:
The ratio of serum placenta growth factor to soluble vascular
endothelial growth factor receptor-1 predicts the prognosis of
hepatocellular carcinoma. Oncol Rep. 23:1647–1654. 2010.
|
|
27
|
Yoshiji H, Kuriyama S, Yoshii J, et al:
Synergistic effect of basic fibroblast growth factor and vascular
endothelial growth factor in murine hepatocellular carcinoma.
Hepatology. 35:834–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wierzbowska A, Robak T, Wrzesien-Kus A,
Krawczynska A, Lech-Maranda E and Urbanska-Rys H: Circulating VEGF
and its soluble receptors sVEGFR-1 and sVEGFR-2 in patients with
acute leukemia. Eur Cytokine Netw. 14:149–153. 2003.PubMed/NCBI
|
|
29
|
Corradini SG, Morini S, Liguori F, et al:
Differential vascular endothelial growth factor A protein
expression between small hepatocellular carcinoma and cirrhosis
correlates with serum vascular endothelial growth factor A and
alpha-fetoprotein. Liver Int. 29:103–112. 2009. View Article : Google Scholar
|
|
30
|
Deprimo SE, Bello CL, Smeraglia J, et al:
Circulating protein biomarkers of pharmacodynamic activity of
sunitinib in patients with metastatic renal cell carcinoma:
modulation of VEGF and VEGF-related proteins. J Transl Med.
5:322007. View Article : Google Scholar : PubMed/NCBI
|