Introduction
Lymph node metastasis is a key prognostic indicator
for cancer and a guide for deciding the tumor treatment (1–3). In
the event of lymph node metastasis, tumor lymphatic vessels are
crucial as the tumor cells enter the lymphatic vessels, migrate
inside them and settle on the lymph nodes (4). A number of studies were performed on
blood vessels (5–7) and the molecular mechanisms involved in
the development and maintenance of blood vessels were largely
revealed (8,9). However, lymphangiogenesis and its
roles in pathologic conditions have yet to be elucidated.
The peri-tumoral environment is crucial in lymph
node metastasis. However, it remains unclear whether lymphatic
dissemination is dependent on cancer cell infiltration of newly
formed lymphatic vessels that are induced by the tumor or
pre-existing lymphatic vessels at peripheral sites of the tumor
(10–12).
It was reported that lymphangiogenesis is promoted
by lymphatic growth factors derived from tumor cells in a paracrine
manner (4,13,14).
Therefore, the correlation between the phenotypes of tumor
lymphatic vessels and clinicopathological characteristics in tongue
squamous cell carcinoma (SCC) was investigated.
Materials and methods
Patients and tissue samples
A total of 43 samples were obtained from patients
who were clinicopathologically diagnosed as having tongue SCC at
the Department of Oral Surgery, Hokkaido University Hospital,
Japan. Informed consent was obtained from the patients prior to the
samples being used. The experiment was conducted under the ethics
rules of Hokkaido University Hospital. The clinicopathological
characteristics of these patients are shown in Table I.
 | Table IClinical and pathological
characteristics of the cases examined. |
Table I
Clinical and pathological
characteristics of the cases examined.
| No. of patients |
|---|
| Gender |
| Male/female | 31/12 |
| Tumor size |
| T1/T2 | 37 |
| T3/T4 | 6 |
| Nodal
involvement |
|
N0→N− | 30 |
|
N0→N+ | 6 |
| N+ | 7 |
| Distant
metastasis |
| M0→M1 | 2 |
| Mode of invasion |
| Expansive | 26 |
| Infiltrative | 17 |
None of the patients had received pre-operative
radiation treatment or chemotherapy. There were 31 male and 12
female patients. The mean age of the patients was 64 years (range
28–91). Tumor size was classified according to the TNM
classification of the International Union Against Cancer (UICC).
There were 37 T1 and T2 cases, and 6 T3 and T4 cases. At the first
medical examination, lymph node metastasis (N+; N1 and
N2) was present in 7 cases. During the follow-up period, subsequent
lymph node metastasis (N0 to N+) occurred in 6 of 36
primary N0 patients and distant metastasis in 2 of 43 M0 cases.
Lymph node metastasis (primary and subsequent) and distant
metastasis are indicative of a poor prognosis. Therefore, the cases
that had lymph node metastasis and/or distant organ metastasis were
divided into high metastatic potential cases (high-metastatic) and
cases without any metastasis as low metastatic potential
(low-metastatic). In this study, there were 14 cases of
high-metastatic (7 N+, including 1 N+M0 to
N+M1, 6 N0 to N+ and 1 N0M0 to M1) and 29
cases of low-metastatic.
Immunohistochemical staining
D2-40 was used to detect peri-tumoral lymphatic
vessels. Monoclonal antibody D2-40 was originally raised against
M2A, a 40,000 kDa O-linked sialoglycoprotein that is
expressed in testicular germ cell neoplasias. D2-40 was later shown
to react with lymphatic endothelium in formalin-fixed,
paraffin-embedded tissues and solid tumors (14).
Serial 4-μm sections were cut from formalin-fixed,
paraffin- embedded tissue blocks, and were then dewaxed and
rehydrated through sequential changes of alcohol and distilled
water. The slides were boiled in 10 mM sodium citrate buffer (pH
6.0) for 3 min and incubated for 5 min in 0.3% hydrogen peroxide,
followed by incubation with 1% BSA for 10 min. The slides were then
incubated with primary antibodies D2-40 (1:50 dilution; Signet
Laboratories, Dedham, MA, USA) and MIB-1 (1:100 dilution; Dako,
Tokyo, Japan) for 1 h at room temperature. After being washed with
PBS, the sections were incubated with the Histofine Simple Stain PO
(M) horseradish peroxidase-conjugated anti-mouse secondary antibody
(Nichirei, Tokyo, Japan) for 40 min and washed in PBS. The color
was developed by incubation with the EnVision kit/HRP (DAB; Dako).
The slides were then counterstained with hematoxylin and
mounted.
Assessment of immunohistochemistry
Lymphatic vessels in peri-tumoral
tissue detected by D2-40
The tumor lymphatic vessels were divided into: micro
and large lymphatic vessels, based on diameter. Areas within 500 μm
from the tumor marginal portion were examined under high-power
fields. Lymphatic vessels with diameters <30 μm were defined as
micro lymphatic vessels (MLVs) and those with >30 μm as large
lymphatic vessels (LLVs). Submucosal lymphatic vessels in normal
tissue were also examined as normal counterparts. Three areas were
randomly selected and the number of the two types of vessels was
counted. The lesions were divided into four groups: i) High-MLV
group, in which the mean number of MLVs was ≥5; ii) Low-MLL group,
in which the mean number of MLVs was <5; iii) High-LLV group, in
which the mean number of LLVs was ≥5; and iv) Low-LLV group, in
which the mean number of LLVs was <5.
Proliferative activity of lymphatic
endothelial cells
The proliferative activity of lymphatic endothelial
cells was examined using antibody MIB-1 in 19 tongue SCC samples
from serially-sectioned slides treated with antibody D2-40.
Lymphatic vessels with at least one MIB-1-positive lymphatic
endothelial cell were defined as lymphatic vessels positive for
MIB-1. MIB-1-positive lymphatic vessels were evaluated as
proliferating lymphatic vessels and the lymphatic proliferation
rate was scored as MIB-1-positive lymphatic vessels/total number of
lymphatic vessels (15).
DNA density in tumor lymphatic
endothelial cells
The DNA density of lymphatic endothelial cells was
examined by measuring the chromatin concentration in 28 cases of
tongue SCC. Hematoxylin-stained sections were analyzed by an
imaging device (Image Pro Plus). Serial sections were immunostained
by MIB-1, and MIB-1-positive endothelial cells were eliminated for
scoring in order to select endothelial cells in the resting
stage.
Statistical analysis
Correlations between clinicopathological data and
states of lymphatic vessels were analyzed using the χ2
test. The relationships between lymphatic proliferation and the
tumor metastatic potential, as well as between the nuclear DNA
density of tumor lymphatic endothelial cells and
clinicopathological characteristics were tested using the unpaired
t-test and Mann-Whitney U test.
Results
Micro and large lymphatic vessels and
clinicopathological factors
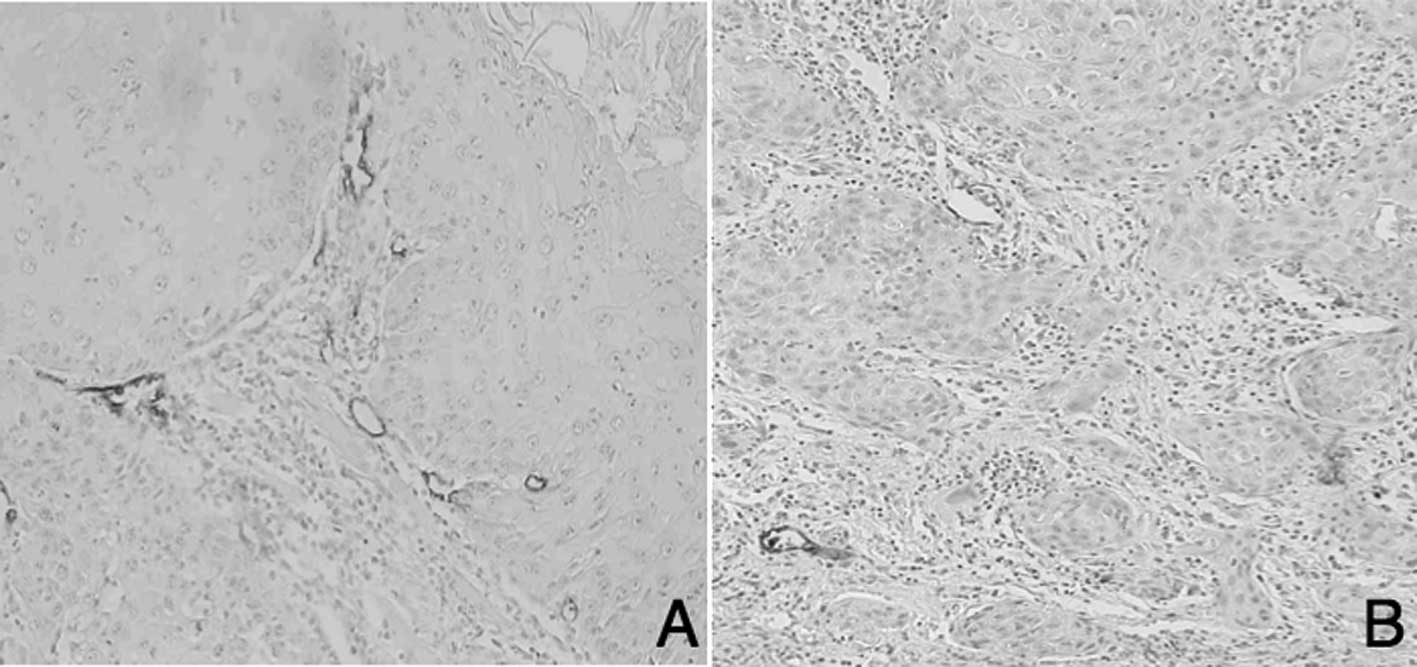
D2-40-positive lymphatic vessels in tongue SCC are
shown in Fig. 1. Numerous MLVs were
located adjacent to the tumor marginal portion in the high-MLV
group (High-MLV, Fig. 1A), whereas
few lymphatic vessels were observed in the low-MLV group (Low-MLV,
Fig. 1B). A total of 20 cases of
High-MLV and 23 of Low-MLV were noted. LLVs were arranged
irregularly and were located at a distance from the tumor marginal
portion than that of MLVs. There were 18 cases of High-LLV
(Fig. 2A) and 25 cases of Low-LLV
(Fig. 2B).
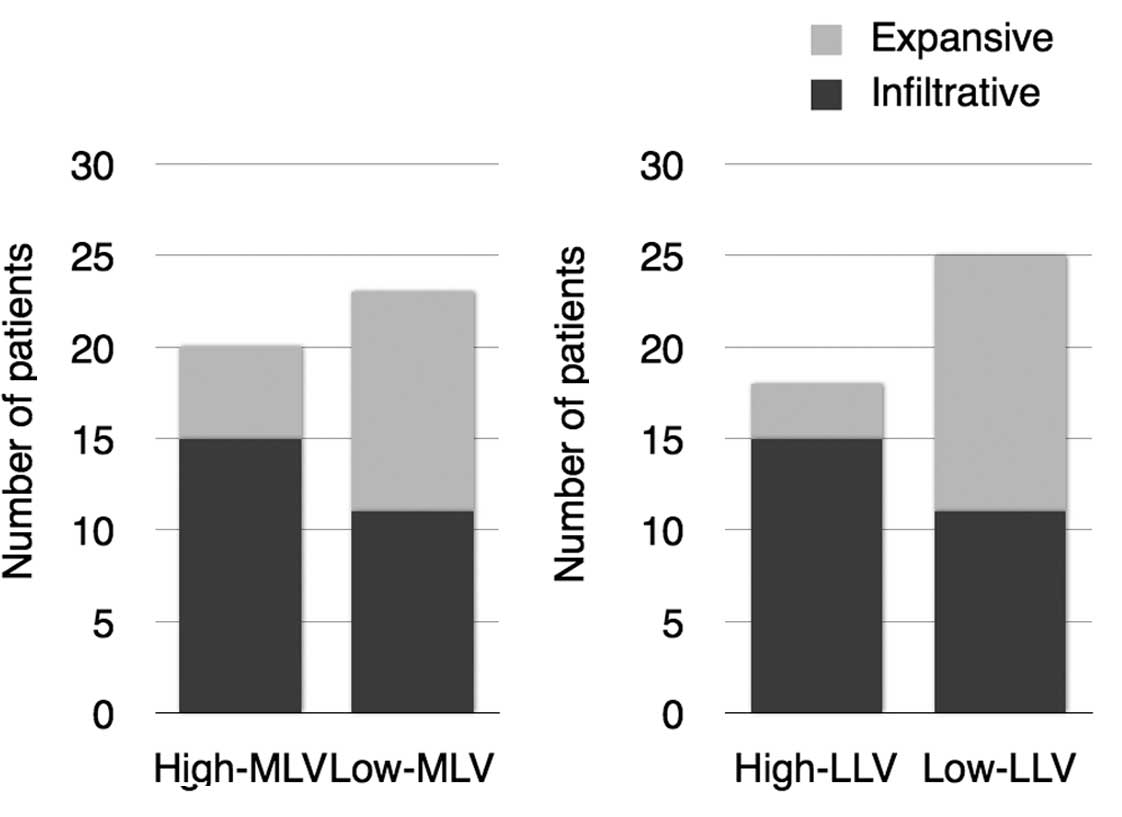
No correlation was found between the characteristics
of the lymphatic vessels and age, gender or T stage. On the other
hand, a highly significant correlation was found between cases with
high-metastatic and High-MLV (P=0.0034, χ2 test) and
High-LLV groups (P=0.00069, χ2 test) (Table II, Fig.
3). In addition, an increasing tendency of the infiltrating
mode of invasion and High-MLV (P=0.069) and a significant
correlation between cases with an infiltrating invasion pattern of
cancer and High-LLV (P=0.00925) were observed (Fig. 4).
 | Table IIRelationship between lymphatic vessel
characteristics and clinicopathological findings. |
Table II
Relationship between lymphatic vessel
characteristics and clinicopathological findings.
| High-MLV | Low-MLV | High-LLV | Low-LLV |
|---|
| Gender |
| Male | 15 | 16 | 14 | 17 |
| Female | 5 | 7 | 4 | 8 |
| Tumor size |
| T1, T2 | 16 | 21 | 15 | 22 |
| T3, T4 | 4 | 2 | 3 | 3 |
| Tumor metastatic
potential |
| High-metastatic | 11 | 3 | 11 | 3 |
| Low-metastatic | 9 | 20 | 7 | 22 |
| Mode of invasion |
| Expansive | 5 | 12 | 3 | 14 |
| Infiltrative | 15 | 11 | 15 | 11 |
Proliferative activity of lymphatic
endothelial cells and tumor metastasis
MIB-1 immunostaining was performed for 19 cases of
tongue SCC, using serial sections stained with D2-40. Lymphatic
vessels with at least one MIB-1-positive lymphatic endothelial cell
were defined as MIB-1-positive lymphatic vessels and tumor
lymphatic endothelial cell proliferation was investigated.
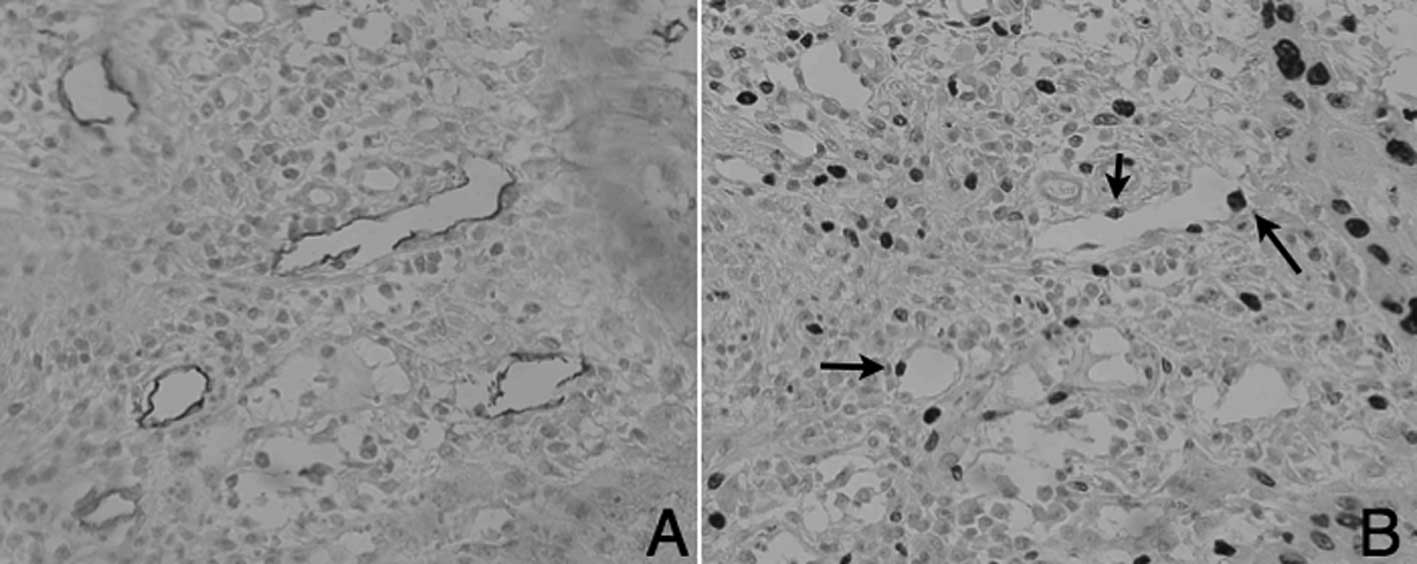
The identification of MIB-1-positive lymphatic
vessels in the normal tissue samples proved difficult. However,
MIB-1-positive lymphatic vessels were increased around the tumor
area of tongue SCC. D2-40-positive lymphatic endothelial cells
(Fig. 5A) and MIB-1 expression of
lymphatic endothelial cells (Fig.
5B) is shown in the serial sections.
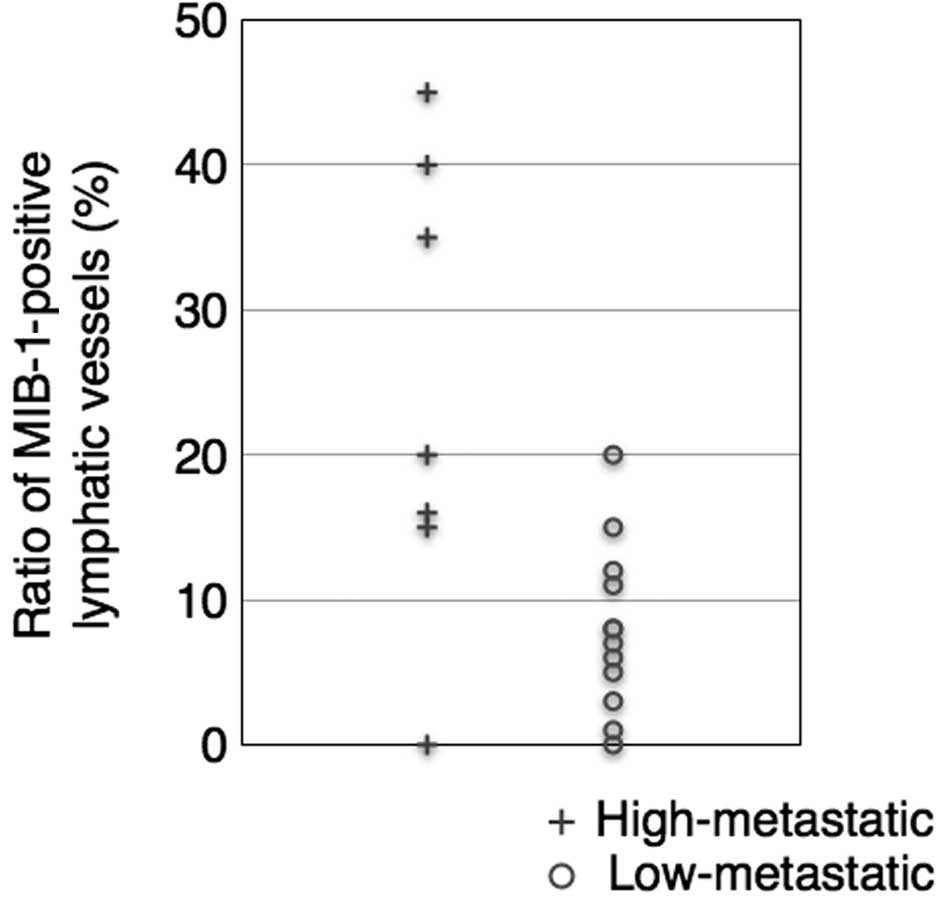
MLV and LLV exhibited MIB-1-positive lymphatic
endothelial cells. High-metastatic cases had a higher ratio of
MIB-1-positive MLVs (23.6%). By contrast, the mean percentage of
proliferating lymphatic vessels was 8% in the low-metastatic cases
(unpaired one-tailed t-test, P<0.05, Fig. 6). Thus, lymphatic endothelial cells
were noted to be actively proliferating in the high-metastatic
cases.
DNA contents of tumor lymphatic
endothelial cells
The nuclear DNA density of tumor lymphatic vessels
was estimated in 28 cases. The tumor endothelial cells had a
significant higher nuclear DNA density compared to the lymphatic
endothelial cells in normal tissue. A higher correlation was
observed between the nuclear DNA density of tumor lymphatic
endothelial cells and the metastatic potential. The mean DNA
density of endothelial cells in the low-metastatic cases was
1.17-fold higher than that in normal tissue, and 1.38-fold higher
in the high-metastatic cases, which was statistically significant
(two-tailed Mann-Whitney U test, P<0.01, Fig. 7).
Discussion
Tumor lymphatic vessels were shown to be induced by
vascular endothelial cell growth factors (VEGFs) that are secreted
by tumor cells, and this induction is thought to promote lymph node
metastasis (1,4,16,17).
Lymphatic vessels are classified into initial and collecting
lymphatic vessels (18).
Morphological differences between initial and collecting lymphatic
vessels include the size of the vessels and the existence of smooth
muscle cells and intraluminal valves. Initial lymphatic vessels are
smaller and have primary valves that are composed of partial
overlaps between lymphatic endothelial cells that are involved in
the activity of lymphatic vessels (18,19).
Padera et al (20) reported
that newly formed small intratumoral lymphatic vessels did not have
any correlation with lymphatic metastasis since intratumoral
lymphatic vessels were not functional and enlarged lymphatic
vessels were correlated with tumor metastasis. On the other hand,
Skobe et al (21) reported
that breast cancer cells secreted VEGF-C, which increased
intratumoral lymphangiogenesis and resulted in significantly
enhanced metastasis to the regional lymph nodes and lungs. Thus,
the significance of the lymphatic vessels around tumors remains to
be determined.
We examined the correlation between the phenotypes
of tumor lymphatic vessels and the clinicopathological
characteristics in tongue SCC. Highly significant metastatic
potential was observed in cases in the High-MLV and -LLV groups.
Skobe et al transplanted VEGF-C-overexpressing breast cancer
cell lines into nude mice and found numerous small lymphatic
vessels in the stromal tissue of transplanted VEGF-C-overexpressing
MDA-MB-435 cells. These authors also reported that
VEGF-C-overexpressing tumors were infiltrated throughout the
central tumor areas by lymphatic vessels, whereas control tumors
were not invaded by lymphatic vessels (21). Our results were in agreement with
the report (15) that tumor MLVs
may be regarded as newly formed lymphatic vessels, indicating the
ability for tumor lymphangiogenesis. Our results suggest that lymph
node metastasis of tongue SCC is predicted by estimating lymphatic
vessels around the tumor, even in the early stages of tumor
progression.
In the High-MLV and -LLV groups, a correlation to
the infiltrative growth pattern of tumors was noted. Ueda et
al (16) reported that
VEGF-C-expressing ovarian carcinoma cell lines showed an invasive
phenotype with correlated expression levels of VEGF-C and MMP-2.
The precise mechanisms of VEGF-C and MMP-2 expression have yet to
be elucidated, but it may be postulated that cases in the high
tumor lymphatic vessel group express a high level of VEGF-C as well
as MMP-2 or other extracellular matrix-degrading enzymes that may
be involved in invasive potential.
We examined the proliferative activity of tumor
lymphatic vessels by immunostaining serial sections with MIB-1 and
D2-40 antibodies. In 19 cases of tongue SCC, both MLV and LLV had a
greater number of MIB-1-positive lymphatic endothelial cells
compared to lymphatic vessels in normal tissue. Moreover, the
high-metastatic group showed an increasing ratio of MIB-1-positive
endothelial cells. These results suggested that newly formed
lymphatic vessels were closely associated with regional lymph node
metastases.
It is known that the nuclear DNA contents of tumor
cells increase due to chromosome abnormalities (22). Malignant tumor cells are known to
have a broad range of nuclear DNA content compared to normal or
benign neoplastic cells. However, stromal cells in cancer are
considered to be intact, including endothelial cells in epithelial
tumors. Hida et al reported that tumor vascular endothelial
cells exhibit genetic abnormalities (23–25).
We measured the DNA content of tumor lymphatic
vessels using an imaging device. The nuclear DNA content was found
to increase in tumor lymphatic compared to normal lymphatic
endothelial cells. Cases in the high-metastatic group showed an
increased DNA content in tumor lymphatic endothelial cells compared
to those in the low-metastatic cells. Our results indicate that
tumor lymphatic endothelial cells may have genetic abnormalities
simialr to those observed in tumor vascular endothelial cells.
Further studies are required to determine the characteristics of
tumor lymphatic endothelial cells and the inhibitory mechanism of
lymph node metastasis, which may contribute to the welfare of
patients diagnosed with tongue SCC.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
He Y, Karpanen T and Alitalo K: Role of
lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta.
1654:3–12. 2004.PubMed/NCBI
|
|
2
|
Achen MG, McColl BK and Stacker SA: Focus
on lymphangiogenesis in tumor metastasis. Cancer Cell. 7:121–127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bird-Lieberman EL and Fitzgerald RC: Early
diagnosis of oesophageal cancer. Br J Cancer. 101:1–6. 2009.
View Article : Google Scholar
|
|
4
|
Karpanen T, Egeblad M, Karkkainen MJ, et
al: Vascular endothelial growth factor C promotes tumor
lymphangiogenesis and intralymphatic tumor growth. Cancer Res.
61:1786–1790. 2001.PubMed/NCBI
|
|
5
|
Nor JE and Polverini PJ: Role of
endothelial cell survival and death signals in angiogenesis.
Angiogenesis. 3:101–116. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moldovan NI and Asahara T: Role of blood
mononuclear cells in recanalization and vascularization of thrombi:
past, present, and future. Trends Cardiovasc Med. 13:265–269. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blagosklonny MV: Antiangiogenic therapy
and tumor progression. Cancer Cell. 5:13–17. 2004. View Article : Google Scholar
|
|
8
|
Coultas L, Chawengsaksophak K and Rossant
J: Endothelial cells and VEGF in vascular development. Nature.
438:937–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stoeltzing O, Meric-Bernstam F and Ellis
LM: Intracellular signaling in tumor and endothelial cells: the
expected and, yet again, the unexpected. Cancer Cell. 10:89–91.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, et al: Angiosarcomas express mixed endothelial phenotypes of
blood and lymphatic capillaries: podoplanin as a specific marker
for lymphatic endothelium. Am J Pathol. 154:385–394. 1999.
View Article : Google Scholar
|
|
11
|
Oliver G: Lymphatic vasculature
development. Nat Rev Immunol. 4:35–45. 2004. View Article : Google Scholar
|
|
12
|
Akishima Y, Ito K, Zhang L, et al:
Immunohistochemical detection of human small lymphatic vessels
under normal and pathological conditions using the LYVE-1 antibody.
Virchows Arch. 444:153–157. 2004. View Article : Google Scholar
|
|
13
|
Pepper MS and Skobe M: Lymphatic
endothelium: morphological, molecular and functional properties. J
Cell Biol. 163:209–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukunaga M: Expression of D2-40 in
lymphatic endothelium of normal tissues and in vascular tumours.
Histopathology. 46:396–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, et al: LYVE-1 immunohistochemical assessment of
lymphangiogenesis in endometrial and lung cancer. J Clin Pathol.
58:202–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueda M, Hung YC, Terai Y, et al: Vascular
endothelial growth factor-C expression and invasive phenotype in
ovarian carcinomas. Clin Cancer Res. 11:3225–3232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aishima S, Nishihara Y, Iguchi T, et al:
Lymphatic spread is related to VEGF-C expression and D2-40-positive
myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol.
21:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagendoorn J, Padera TP, Kashiwagi S, et
al: Endothelial nitric oxide synthase regulates microlymphatic flow
via collecting lymphatics. Circ Res. 95:204–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagendoorn J, Padera TP, Fukumura D and
Jain RK: Molecular regulation of microlymphatic formation and
function: role of nitric oxide. Trends Cardiovasc Med. 15:169–173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padera TP, Kadambi A, di Tomaso E, et al:
Lymphatic metastasis in the absence of functional intratumor
lymphatics. Science. 296:1883–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skobe M, Hawighorst T, Jackson DG, et al:
Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rubio Bueno P, Naval Gias L, Garcia
Delgado R, et al: Tumor DNA content as a prognostic indicator in
squamous cell carcinoma of the oral cavity and tongue base. Head
Neck. 20:232–239. 1998.
|
|
23
|
Hida K, Hida Y, Amin DN, et al:
Tumor-associated endothelial cells with cytogenetic abnormalities.
Cancer Res. 64:8249–8255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hida K, Hida Y and Shindoh M:
Understanding tumor endothelial cell abnormalities to develop ideal
anti-angiogenic therapies. Cancer Sci. 99:459–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akino T, Hida K, Hida Y, et al:
Cytogenetic abnormalities of tumor-associated endothelial cells in
human malignant tumors. Am J Pathol. 175:2657–2667. 2009.
View Article : Google Scholar : PubMed/NCBI
|