Introduction
Bladder cancer accounts for approximately 4% of
cancer cases worldwide. Bladder cancer is the fourth most commonly
diagnosed cancer in males (approximately 7%), and the sixth overall
(approximately 5%) in the US (1).
The majority of patients with bladder tumors
(70–80%) present with low-grade, non-muscle-invasive or superficial
tumors confined to the mucosa. The tumors are typically managed
conservatively by transurethral resection, followed by intravesical
adjuvant treatment (2).
The current published clinical guidelines recommend
that patients with an intermediate or high risk of recurrence and
an intermediate risk of progression should be treated with bacillus
Calmette-Guerin (BCG) or mitomycin C (MMC) in an adjuvant setting
following transurethral resection of the bladder tumor (TURBT)
(3). Intravesical immunotherapy
with BCG is currently the most successful adjuvant agent for
treating non-muscle-invasive bladder cancer (NMIBC) (4). However, 60–70% of NMIBCs recur despite
intravesical BCG therapy and 30% of these recurrent tumors present
with a higher grade and more invasive properties, since BCG does
not have long-lasting tumor-specific immunity (5,6). In
addition, BCG has a higher incidence of side effects, often
increasing with the number of instillations, leading to the
discontinuation of intravesical instillation treatment (4,7).
The data show that improvement in the current
standard of intravesical BCG treatment regimens is required. Thus,
novel strategies are crucial for the treatment of NMIBC with a
superior antitumor effect, compared to that of conventional
intravesical BCG therapy.
Numerous clinical trials have shown that combined
treatments are more effective than the single use of any particular
modality. In a study on a bladder cancer cell line, a combination
of BCG, such as the Connaught strain, and MMC inhibited cell growth
more significantly than either agent separately in vitro
(8). In a previous study, bladder
urothelial disruption induced by an intravesical cytostatic agent
enhanced the fibronectin-mediated BCG attachment in murine
experiments (9).
We hypothesized that combined intravesical BCG with
MMC instillation has a positive effect on the adherence of BCG
particles to the bladder wall and consequently stimulates the
induction of the BCG-related immune response. Moreover, the
combined MMC and reduced dose of BCG maintains an antitumor benefit
compared to the standard dose of BCG, possibly with less toxic and
fewer side effects than intravesical BCG instillation.
To improve the efficacy of intravesical BCG therapy,
a combination of MMC and BCG against bladder tumors was examined
using an orthotopic animal model. Additionally, whether the
combined treatment of MMC/BCG induces a beneficial antitumor effect
was examined.
Materials and methods
Tumor cell line and reagents
The murine bladder tumor cell line, MBT-2, was
cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(Life Technologies, Inc., Rockville, MD, USA) at 37°C in a
humidified, 5% CO2 atmosphere.
MMC (Kyowa Hakko Kirin Ltd., Tokyo, Japan) was used
as the chemotherapeutic agent. The reagents were diluted using
phosphate-buffered saline (PBS; Gibco, Invitrogen) to the working
concentrations.
BCG (Connaught substrain, Immucyst,
3.77×108 colony-forming units, dry weight 81 mg; Nihon
Kayaku, Inc., Tokyo, Japan) was used.
Animals
Female C3H/HeN mice (8 weeks old) were purchased
from Japan SLC, Inc. (Hamamatsu, Japan). The animal procedures were
reviewed and approved by the Animal Research Committee of Saitama
Medical School.
Assessment of mitomycin C cytotoxicity to
MBT-2 cells in vitro
To assess the cytotoxic effects of MMC on the growth
of MBT-2 cells, exponentially-growing MBT-2 cells were seeded at a
density of 2×105 per 6-well plate and the cells were
cultured in the absence or presence of MMC (1–100 μg/ml) for the
designated time period (24–72 h; n=3 each). At the indicated time
points, the cells were harvested and counted using a
hemocytometer.
Intravesical implantation of murine
bladder cancer cells
Subconfluent MBT-2 cells were trypsinized and
>95% cell viability was confirmed using the trypan blue
exclusion method. Syngeneic female C3H/HeN mice were anesthetized
using an intraperitoneal injection of 50 mg/kg of sodium
pentobarbital. Prior to each instillation, the bladder was emptied
by gently pressing the bladder between the fingertips. A 24-gauge
Teflon-coated catheter was introduced into the lumen of the bladder
through the urethra. An amount of 1 million MBT-2 cells in a 100 μl
suspension of PBS were then injected into the bladder, as
previously reported (10). The
contact between the MBT-2 cells and the bladder membrane, for the
implantation of the syngeneic orthotopic model, lasted for ~2 h,
until the anesthesia wore off.
Toxicology of intravesical mitomycin C
instillation in mice
The concentration of intravesical MMC instillation
in clinical practice is ~1 mg/ml. The mice were arbitrarily placed
into four groups. The animals (n=6) received 0 μg (PBS: control),
50 μg (0.5 mg/ml), 100 μg (1 mg/ml) or 200 μg (2 mg/ml) of MMC
intravesically five times at 3-day intervals. On day 28, following
the first instillation, body weights were compared to the initial
body weights. Then, new mice (n=6) that received combined MMC and
BCG intravesically were examined in a similar manner.
Intravesical therapy in an orthotopic
bladder cancer model
To assess the antitumoral effect of intravesical
administration treatment, the mice were arbitrarily placed into
groups on day 5 following tumor implantation. The instillations of
each experiment were repeated 5 times at 3-day intervals commencing
on day 5 after tumor implantation under anesthesia. Each
instillation lasted for ~2 h until recovery from anesthesia and the
start of spontaneous voiding. On day 60, the surviving mice were
sacrificed and necropsied. The animal experiments and the
examination of the antitumor effects among the various treatment
groups were performed in the manner described below.
Mitomycin C dose optimization (3
groups)
Intravesical MMC was administered transurethrally at
various doses: 0 (control), 25 and 50 μg in a final volume of 100
μl of PBS (n=8 mice per group), resulting in a concentration
between 0 and 0.5 mg/ml. The intravesical instillation treatment
was performed as described above.
Bacillus Calmette-Guerin dose
optimization (5 groups)
The concentration of intravesical Connaught
substrain BCG instillation in clinical practice is ~2 mg/ml. Our
intravesical BCG was administered transurethrally at various doses:
0 (control), 100, 200, 400 and 800 μg in a final volume of 100 μl
of PBS (n=8 mice per group), resulting in a concentration between 0
and 8 mg/ml. The intravesical instillation treatment was performed
as described above.
Intravesical mitomycin C and/or bacillus
Calmette-Guerin therapy (4 groups)
For the intravesical administration, 100 μl of PBS,
50 μg of MMC in 100 μl of PBS, 100 μg of BCG in 100 μl of PBS, or a
combination of 50 μg of MMC plus 100 μg of BCG in 100 μl of PBS
were instilled transurethrally (n=8 mice per group). The
intravesical instillation treatment was performed as described
above.
Combined intravesical mitomycin
C/bacillus Calmette-Guerin vs. various bacillus Calmette-Guerin
doses (5 groups)
For the intravesical administration, 100 μl of PBS,
a combination of 50 μg of MMC plus 100 μg of BCG in 100 μl of PBS,
100 μg of BCG in 100 μl of PBS, 400 μg of BCG in 100 μl of PBS or
800 μg of BCG in 100 μl of PBS were instilled transurethrally (n=10
mice per group). The intravesical instillation treatment was
performed as previously described.
Bladder tumor following single
intravesical administration (4 groups)
The mice were arbitrarily placed into four groups 17
days after the intravesical implantation of 1×106 MBT-2
cells. A single intravesical administration of 100 μl of PBS, 50 μg
of MMC in 100 μl of PBS, 100 μg of BCG in 100 μl of PBS or a
combination of 50 μg of MMC plus 100 μg of BCG in 100 μl of PBS
were instilled transurethrally (n=5 mice per group). The mice were
sacrificed 1 day after the single intravesical administration and
the whole bladder tissue was obtained. Following removal, the
bladders were filled with 10% formalin and subsequently the samples
were embedded in paraffin. The sections were routinely stained with
hematoxylin and eosin.
Immunostaining (Ki-67, CD68 and CD3)
Immuno-histochemical staining was performed in a
standard manner according to the manufacturer’s instructions
(11). For Ki-67, a primary mouse
monoclonal antibody at a dilution of 1:50 (M7249; Dako, Tokyo,
Japan) was used overnight at 4°C. For CD68 (M0876; Dako) and CD3
(A0452; Dako), primary mouse polyclonal antibodies at a dilution of
1:50 (Dako) were also used overnight at 4°C.
To quantify the Ki-67 expression, the mean of at
least four representative fields was evaluated for each treatment
group. The number of cells was counted under a microscope and the
results were recorded as the percentage of positively-stained
cells.
To quantify the CD68 and CD3 expression, the number
of positive cells were counted manually and the number of control
bladder-positive cells were subtracted from the number of
infiltrating cells in the tumor. The numbers were then normalized
to those for 1 mm2, expressed as a percentage to the
total cell number per mm2.
Statistical methods
The values are presented as the mean ± standard
deviation (SD). Variations among the groups were assessed using a
one-way ANOVA. Statistical significance was determined as
p<0.05. The survival curves were generated using the
Kaplan-Meier method and were compared using the log-rank test.
Results
Cytotoxic effect of mitomycin C against
MBT-2 cells
MMC exerted moderate cytotoxic effects against MBT-2
cells in vitro. After 1 day of exposure to MMC at
concentrations of 0 (control), 1, 10 or 100 μg/ml, the number of
MBT-2 cells (x105) were 2.60±0.10, 1.63±0.40, 1.47±0.25
and 0.23±0.06, respectively. After 2 days of exposure, the number
of cells were 4.83±0.61, 0.73±0.15, 0.53±0.25 and 0.10±0.0,
respectively. After 3 days of exposure, the number of cells were
15.50±2.34, 0.63±0.29, 0.27±0.06 and 0.0±0.0, respectively.
Compared to the untreated cells, MMC caused both a dose- and a
time- dependent inhibition of growth during the 3-day culture
period.
Intravesical instillation in an
orthotopic bladder cancer model
Toxicology of intravesical mitomycin C
instillation in mice
Of the 6 mice in each group treated with a dose of 0
(control), 50, 100 or 200 μg of MMC, 6, 6, 1 and 0 mice survived,
respectively, (Table I). Of the 6
mice that received combined MMC (50 μg) and BCG (100 μg), all 6
survived (Table I).
 | Table IToxicology of intravesical mitomycin C
instillation in mice (n=6). |
Table I
Toxicology of intravesical mitomycin C
instillation in mice (n=6).
| Dose (μg) | Death after
instillation | Body weight at
initiation (g) | Body weight at end
point (g) | Body weight
difference (g) | Survival (days) |
|---|
| Mitomycin C |
| 50 | 0 | 19.4±0.4 | 21.2±1.4 | 1.8±1.1 | 28 |
| 100 | 5 (83%) | 19.0±0.8 | 14.3±1.6 | −4.7±1.6 | 14.0±9.0 |
| 200 | 6 (100%) | 20.9±0.4 | 15.6±0.7 | −5.3±0.5 | 7.3±5.0 |
| 50 + BCG 100 | 0 | 20.7±0.9 | 21.4±2.3 | 0.7±1.4 | 28 |
| Control (PBS) | 0 | 19.8±1.3 | 22.7±1.8 | 2.9±1.1 | 28 |
The pathological findings for the murine bladders of
the mice that succumbed due to adverse events associated with
intravesical MMC instillation showed acute inflammation, submucosal
edema and detachment of the urothelium membrane on the bladder. The
bladders in the 200 μg group exhibited these findings more
significantly compared to the 100 μg group. In addition, the
survival period in the 200 μg group (7.3±5.0 days) was
significantly shorter than that of the 100 μg group (14.0±9.0
days).
Based on the preceeding toxicology experiment, 25 or
50 μg of MMC were used for subsequent MMC dose optimization studies
on the orthotopic bladder cancer model.
Dose optimization of mitomycin C (5
groups)
Of the 8 mice in each group treated with a dose of 0
(control), 25 or 50 μg of MMC, 0, 0 and 2 mice, respectively,
failed to develop tumors and survived (Table II). The mean survival period in the
50 μg treatment group was 40.0±13.2 days, while the mean survival
periods in the 25 and 0 μg (control) groups were 34.8±7.0 and
26.4±3.2 days, respectively. The survival advantage for
intravesical MMC in the 50 μg administration group was
statistically significant, compared to the control group
(p=0.002).
 | Table IIDose optimization of mitomycin C in an
orthotopic bladder cancer model (n=8). |
Table II
Dose optimization of mitomycin C in an
orthotopic bladder cancer model (n=8).
| Dose (μg) | Death due to bladder
tumor | Failure to produce
tumor | Tumor-taking rate (%)
(no. of tumor appearance/no. of observation subjects) | Survival (days) |
|---|
| 25 | 8 | 0 | 100% (8/8) | 34.8±7.0 |
| 50 | 6 | 2 | 75% (6/8) | 40.0±13.2 |
| Control (PBS) | 8 | 0 | 100% (8/8) | 26.4±3.2 |
Dose optimization of bacillus
Calmette-Guerin (5 groups)
Of the 8 mice in each group treated with a dose of 0
(control), 100, 200, 400 or 800 μg of BCG, 1, 2, 2, 3 and 4 mice,
respectively, failed to develop tumors and survived (Table III). Compared to the PBS treatment
(control), intravesical BCG administration resulted in a longer
survival period, presumably demonstrating a beneficial antitumor
effect in a dose-dependent manner.
 | Table IIIDose optimization of bacillus
Calmette-Guerin in an orthotopic bladder cancer model (n=8). |
Table III
Dose optimization of bacillus
Calmette-Guerin in an orthotopic bladder cancer model (n=8).
| Dose (μg) | Death due to
bladder tumor | Failure to produce
tumor | Tumor-taking rate
(%) (no. of tumor appearance/no. of observation subjects) | Survival
(days) |
|---|
| 100 | 6 | 2 | 75.0% (6/8) | 37.9±17.4 |
| 200 | 6 | 2 | 75.0% (6/8) | 41.8±14.3 |
| 400 | 5 | 3 | 62.5% (5/8) | 42.3±16.7 |
| 800 | 4 | 4 | 50.0% (4/8) | 44.3±16.9 |
| Control (PBS) | 7 | 1 | 87.5% (7/8) | 31.3±13.4 |
Intravesical mitomycin C and/or
bacillus Calmette-Guerin therapy (4 groups)
Of the 8 mice in each group treated with PBS
(control), MMC-alone, BCG-alone or combined MMC/BCG, 1, 2, 2 and 3
mice, respectively, failed to develop tumors and survived (Table IV). The tumor-taking rates were
87.5, 75, 75 and 62.5% in the control group, the BCG-alone, the
MMC-alone and the combined MMC/BCG group, respectively. A
significant survival advantage was observed for the combined
MMC/BCG group (51.5±8.1 days), compared to the BCG-alone group
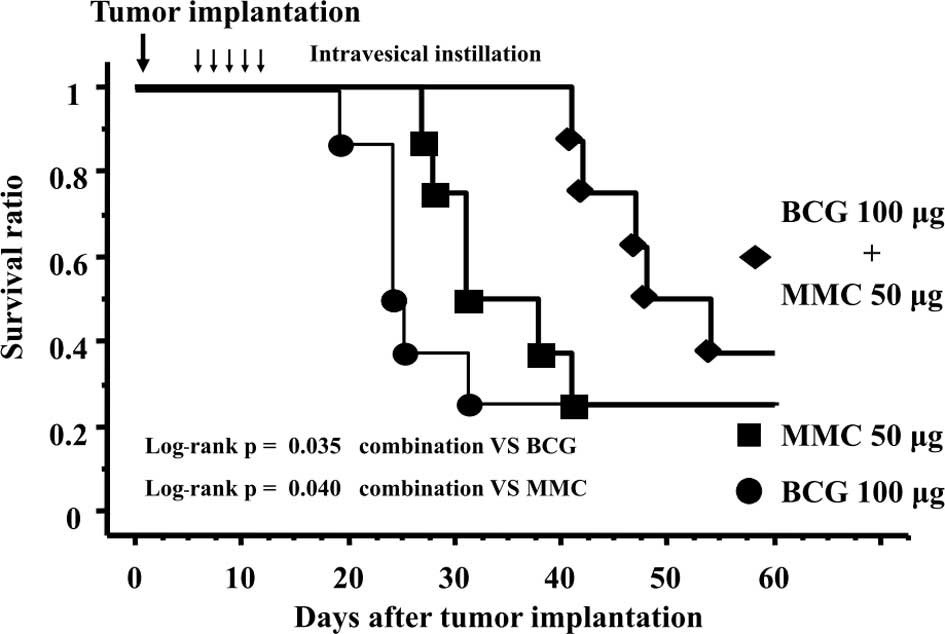
(33.4±16.8 days, p=0.035, Fig. 1)
and the MMC-alone group (39.5±13.5 days, p=0.040, Fig. 1).
 | Table IVBacillus Calmette-Guerin and/or
mitomycin C therapy in an orthotopic bladder cancer model
(n=8). |
Table IV
Bacillus Calmette-Guerin and/or
mitomycin C therapy in an orthotopic bladder cancer model
(n=8).
| Treatment (μg) | Death due to
bladder tumor | Failure to produce
tumor | Tumor-taking rate
(%) (no. of tumor appearance/no. of observation subjects) | Survival
(days) |
|---|
| BCG (100) | 6 | 2 | 75.0% (6/8) | 33.4±16.8 |
| MMC (50) | 6 | 2 | 75.0% (6/8) | 39.5±13.5 |
| BCG (100) + MMC
(50) | 5 | 3 | 62.5% (5/8) | 51.5±8.1 |
| Control (PBS) | 7 | 1 | 87.5% (7/8) | 29.6±13.0 |
Intravesical combined mitomycin
C/bacillus Calmette-Guerin vs. various BCG doses (total of 5
groups)
Of the 10 mice in each group treated with PBS
(control), 100, 400 or 800 μg of BCG or combined MMC (50 μg) plus
BCG (100 μg), 1, 3, 4, 6 and 4 mice, respectively, failed to
develop tumors and survived (Table
V). A significant survival advantage for the combined MMC/BCG
group (47.7±11.9 days) was observed, compared to the 100 μg BCG
group (34.5±17.9, p=0.04). The survival period of the combined
MMC/BCG group was similar to that of the group treated with a dose
eight times higher than that of BCG alone (46.7±12.3 days).
 | Table VCombined bacillus
Calmette-Guerin/mitomycin C vs. various bacillus Calmette-Guerin
doses (n=10). |
Table V
Combined bacillus
Calmette-Guerin/mitomycin C vs. various bacillus Calmette-Guerin
doses (n=10).
| Treatment (μg) | Death due to
bladder tumor | Failure to produce
tumor | Tumor-taking rate
(%) (no. of tumor appearance/no. of observation subjects) | Survival
(days) |
|---|
| BCG (100) | 7 | 3 | 70% (7/10) | 34.5±17.9 |
| BCG (400) | 6 | 4 | 60% (6/10) | 42.4±16.8 |
| BCG (800) | 4 | 6 | 40% (4/10) | 46.7±12.3 |
| BCG (100) + MMC
(50) | 6 | 4 | 60% (6/10) | 47.7±11.9 |
| Control (PBS) | 9 | 1 | 90% (9/10) | 27.3±11.9 |
Immunostaining of cancers, macrophages
and T-lymphocytes
Three antibodies reactive to Ki-67, CD68 and CD3
antigens were used to evaluate the response to the combined
intravesical MMC/BCG treatment against inoculated bladder cancer
cells. The Ki-67 antibody labeled the nuclei of proliferating
cells. Antibodies to CD68 and CD3 labeled the cell membrane and
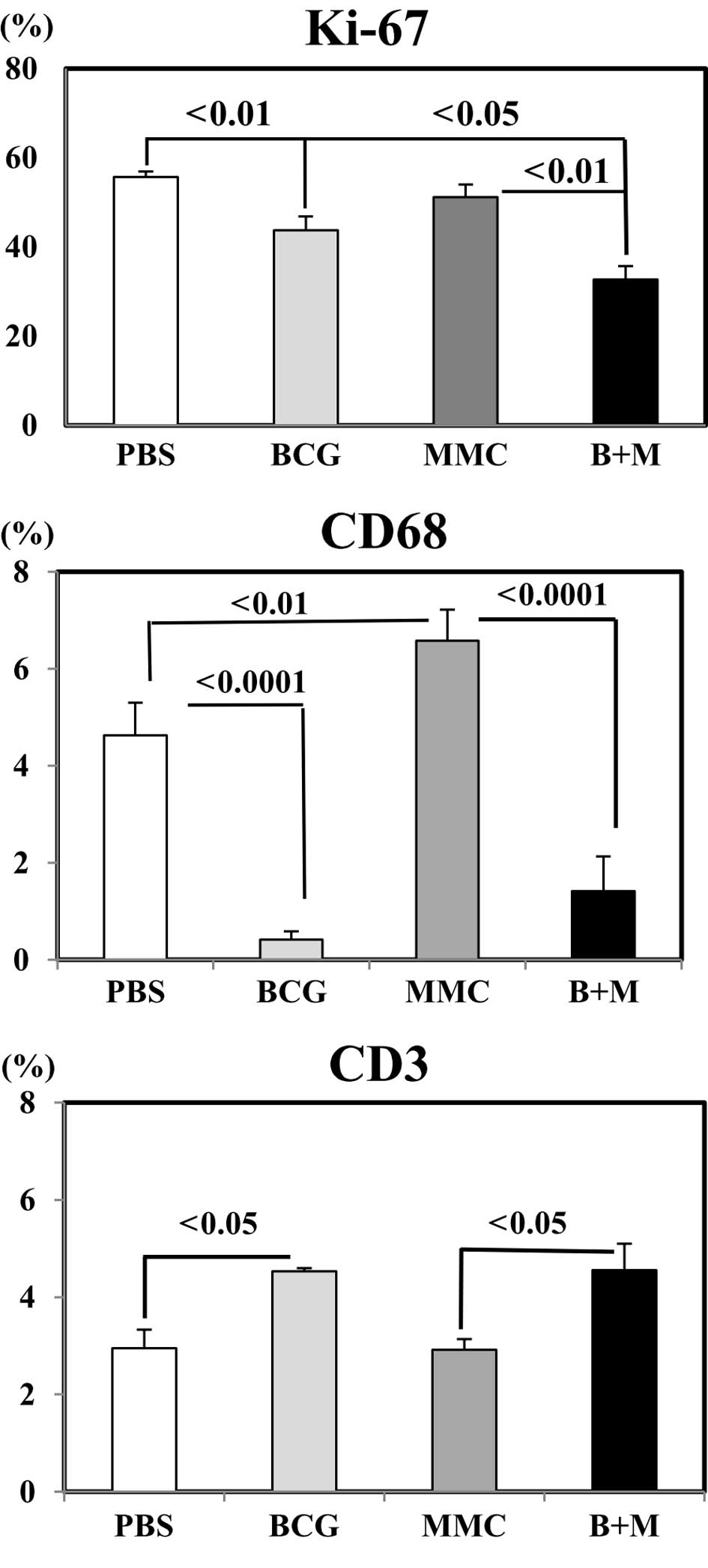
cytoplasm of infiltrated macrophages and T-lymphocytes. Fig. 2 shows the statistical analysis
regarding the difference among the treatment groups.
Combined treatment of mice by MMC/BCG resulted in a
significant decrease of the Ki-67 labeling index of cancer cells,
compared to the control (PBS: 55.7±1.2% vs. MMC/BCG: 32.7±3.0%,
p<0.01), BCG-alone (43.8±3.1%, p<0.05) and MMC-alone groups
(51.2±2.8%, p<0.01; Fig. 2,
Ki-67). BCG-alone significantly reduced, while MMC-alone increased
CD68+ cells as compared to the control (p<0.0001 and
p<0.01, respectively). This trend was the same when two drugs
were infused into the test subjects when compared to MMC-alone
treatment (p<0.0001; Fig. 2,
CD68). Notably, more CD3+ cells infiltrated into the
tumors when BCG was injected, irrespective of the presence
(p<0.05) or absence of (p<0.05) MMC (Fig. 2, CD3).
Discussion
BCG is currently regarded as the most successful
immunotherapy for solid tumors. The intravesical instillation of
BCG is the most effective means of prophylaxis for the recurrence
and progression of non-muscle-invasive bladder cancer (4). Although the mechanism of the antitumor
effects of BCG remains to be elucidated, it is generally accepted
that intravesical BCG instillation causes the non-specific
stimulation of the local immune system, inducing the local
infiltration of the bladder wall with activated T cells (6). Repeat intravesical BCG instillations
induce a cascade of immunological reactions, including local
cytokine and chemokine production which are probably released by
T-lymphocytes and other cells infiltrating the bladder wall
following intravesical BCG treatment (12).
In patients that do not respond to intravesical BCG
therapy, cystectomy remains the treatment of choice, when patients
are compliant. Patients in whom BCG fails are a challenge to
urologists and careful individualization of therapy in experienced
hands appears warranted. Therefore, new conservative possibilities
should be explored.
The effector mechanisms of intravesical MMC (a
cystostatic drug) and BCG (immunotherapy) differ. Local
administration of low doses of various anticancer drugs
(cystostatic drugs) at the site of antigenic stimulation may
potentiate the generation of T-effector cells, as detected by the
delayed-type hypersensitivity measurements in murine models
(13). Evidence from murine
experiments indicated that certain chemotherapy anticancer agents
(cystostatic drugs), such as doxorubicin or gemcitabine, promote
the activation of immune effectors (14,15).
The rationale for combining anticancer drugs is
based on the need to increase efficacy and reduce the emergence of
resistant malignant cells. MMC plays a dual role in that it
promotes antitumor action and has a tissue-scarifying effect that
enables BCG to attach more efficiently to the urothelium (9,16).
Therefore, we hypothesize that an enhanced antitumor effect would
be demonstrated by the combination of MMC and BCG. Although
sequential intravesical administration of MMC and BCG has been used
in patients with bladder cancer, our proposed combined intravesical
administration of MMC/BCG regimen, infusing the two drugs
simultaneously, has yet to be examined (17–20).
We hypothesized that the combination of MMC/BCG is
beneficial as an alternate intravesical treatment following TURBT.
Thus, we examined whether this combination strategy would augment
intravesical BCG therapy in an orthotopic animal model. The
orthotopic bladder cancer model closely mimics the clinical
situation and this model has led to a substantially greater
understanding of the mechanisms involved, a pre-requisite for a
meaningful conclusion (21). The
immunologic aspects of intravesical BCG-related experiments should
be evaluated with a syngeneic orthotopic bladder cancer model, such
as our MBT-2 cells/C3H mouse orthotopic model. However, there are
significant shortcomings since the outcomes of a murine orthotopic
bladder cancer model may present differently compared to human
clinical practice.
The combined intravesical instillation of BCG and
antifibrinolytic agents was reported to be a safe and effective
method of improving BCG immunotherapy as noted in a pilot study of
257 patients with TURBT (22). From
a clinical point of view, the intravesical instillation of MMC/BCG
is an attractive strategy since it is simple to perform,
cost-effective and easy to prepare without requiring additional
time for catheterization.
In the present study, a significant survival
advantage was observed in the combined instillation of the MMC/BCG
group, compared to that in the BCG-alone and MMC-alone groups.
Clinical research in the field currently focuses on
the means of reducing BCG-induced side effects. A number of authors
have proposed lowering the dose of BCG by one third to reduce
BCG-related adverse events while maintaining an antitumor effect
against bladder tumors (23).
The number and severity of the side effects caused
by 6 weeks of BCG were not significantly different between the
sequential combination group, to which 4 weeks of MMC was
administered prior to BCG administration, and the BCG monotherapy
group, indicating that BCG-related toxicity does not increase when
BCG instillations are started immediately after a period of
intravesical MMC instillation (17).
In the present study, a repeated intravesical BCG
instillation protocol exhibited a dose-dependent antitumor effect
in an orthotopic bladder cancer model. A significant survival
advantage was observed in the combined MMC/BCG group, compared to
that in the BCG-alone and MMC-alone groups. A similar survival
period was also noted between the combined MMC/BCG group and the
BCG-alone group with a dose eight times higher than that of
BCG.
Thus, it can be speculated that a combined,
locally-applied MMC/BCG regimen enables the BCG dose to be reduced,
resulting in fewer adverse events as a result of BCG instillation,
while maintaining an antitumor effect against bladder cancer.
However, we must consider that a substantial safety concern exists
for this combined instillation regimen, since the combination of
BCG-induced bladder inflammation and MMC-induced bladder irritation
may cause a breach in the normal bladder barrier function, enabling
systemic exposure to high levels of MMC or systemic infection with
BCG.
The antitumor mechanism of intravesical BCG
immunotherapy is complex in that the cell death pathway induced by
intravesical BCG administration in bladder tumors is associated
with apoptosis, necrosis and a decrease in cell proliferation
(24). On the other hand, the
antitumor effect of BCG in the human bladder tumor cell line T24
was associated with a decrease in proliferation, and apoptosis was
not considered to be a significant mechanism of antitumor effects
(25). The Ki-67 immunostaining
method is widely used as a cell proliferation marker in various
types of tumors (26). Asakura
et al reported that the Ki-67 labeling index was an
independent predictor of the recurrence of non-muscle-invasive
bladder tumor and of progression (27). Santos et al reported that a
high Ki-67 labeling index and multifocality were significantly
related to recurrence and progression-free survival, and were
independent prognostic factors for non-muscle-invasive bladder
tumor (28). In this study, the
Ki-67 labeling index of the bladder tumors following intravesical
instillation in the combined MMC/BCG group was found to be
significantly lower than that in either the BCG-alone or MMC-alone
groups. We consider Ki-67 immunostaining using TURBT specimens to
aid analysis and to be a prognostic marker for patients following
TURBT. However, the use of Ki-67 immunostaining as a marker for
evaluating the response to intravesical therapy may be limited.
Notably, no parameter for predicting the response to intravesical
BCG treatment has been introduced clinically (4).
In an animal model using guinea pigs, the sequential
combination of intravesical MMC and BCG was studied for
immunological effects (16). A
slight increase in the MHC class II expression on the bladder
urothelium was detected when MMC and BCG treatment was combined.
However, these authors concluded that MMC did not enhance the
immune response of BCG, but that the increased antitumor activity
was due more to the separate activity of the two drugs, i.e., a
cytostatic effect of MMC on tumor cells and a local immune response
in the bladder evoked by BCG.
Numerous aspects in our study, including a combined
local administration, the use of an orthotopic murine bladder
cancer model and varying concentrations of the two drugs, account
for discrepancies between our results and previously reported
animal studies examining the combination of MMC and BCG (16).
As anticipated, the cytotoxic effect of BCG on the
murine bladder tumors was enhanced when injected together with MMC
in an orthotopic bladder cancer model. We invetigated an underlying
mechanism of this enhanced effect by evaluating macrophages (CD68
immunostaining) and T cells (CD3 immunostaining), since these
agents play a significant role in cellular immunity. The number of
macrophages decreased, while the T cells increased within tumors
when BCG was given to animals. This is an unexpected result, as we
assumed that BCG generates granuloma which involves macrophages.
MMC, on the other hand, increased macrophages within tumors, in
order to eliminate apoptotic cells.
Only macrophages within tumors, and not in the
surrounding tissue were examined, due to the experimental
methodological limitations of this study. Immunohistochemical
analysis using samples following a single intravesical
administration were regarded as unsuitable for the investigation of
the mechanism among distinct treatment groups. It is presumed that
an optimal immnostaining method for examining the mechanisms
following multiple intravesical instillation using bladder
specimens in orthotopic murine bladder cancer model has yet to be
determined. Thus, it appears that our findings utilizing bladder
tumor specimens may provide less valuable information regarding the
response of multiple intravesical instillations protocol.
Although no difference was found between the
combined group and the BCG-alone group with regard to CD3, T-cell
infiltration and CD68 macrophage activity, our results suggest that
T cells are responsible for attacking cancer cells, while
macrophages remove dead cells. Therefore, it can be assumed that
other cell populations, such as B-cells, natural killer cells or
the cytokine or chemokine levels, may differ among different
treatment groups, possibly contributing to the enhanced antitumor
effect of the combined administration regimen.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Hsieh JT, Dinney CP and Chung LW: The
potential role of gene therapy in the treatment of bladder cancer.
Urol Clin North Am. 27:103–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malmstrom PU, Sylvester RJ, Crawford DE,
et al: An individual patient data meta-analysis of the long-term
outcome of randomised studies comparing intravesical mitomycin C
versus bacillus Calmette-Guerin for non-muscle-invasive bladder
cancer. Eur Urol. 56:247–256. 2009. View Article : Google Scholar
|
|
4
|
Bohle A and Brandau S: Immune mechanisms
in bacillus Calmette-Guerin immunotherapy for superficial bladder
cancer. J Urol. 170:964–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ratliff TL, Ritchey JK, Yuan JJ, Andriole
GL and Catalona WJ: T-cell subsets required for intravesical BCG
immunotherapy for bladder cancer. J Urol. 150:1018–1023.
1993.PubMed/NCBI
|
|
6
|
Alexandroff AB, Jackson AM, O’Donnell MA
and James K: BCG immunotherapy of bladder cancer: 20 years on.
Lancet. 353:1689–1694. 1999.PubMed/NCBI
|
|
7
|
Witjes JA: Management of BCG failures in
superficial bladder cancer: a review. Eur Urol. 49:790–797. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajala P, Kaasinen E, Rintala E, et al:
Cytostatic effect of different strains of Bacillus Calmette-Guerin
on human bladder cancer cells in vitro alone and in combination
with mitomycin C and interferon-alpha. Urol Res. 20:215–217. 1992.
View Article : Google Scholar
|
|
9
|
Kavoussi LR, Brown EJ, Ritchey JK and
Ratliff TL: Fibronectin-mediated Calmette-Guerin bacillus
attachment to murine bladder mucosa. Requirement for the expression
of an antitumor response. J Clin Invest. 85:62–67. 1990. View Article : Google Scholar
|
|
10
|
Horiguchi Y, Larchian WA, Kaplinsky R,
Fair WR and Heston WD: Intravesical liposome-mediated interleukin-2
gene therapy in orthotopic murine bladder cancer model. Gene Ther.
7:844–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacob DA, Bahra M, Langrehr JM, et al:
Combination therapy of poly (ADP-ribose) polymerase inhibitor
3-aminobenzamide and gemcitabine shows strong antitumor activity in
pancreatic cancer cells. J Gastroenterol Hepatol. 22:738–748.
2007.
|
|
12
|
De Boer EC, Rooijakkers SJ, Schamhart DH
and Kurth KH: Cytokine gene expression in a mouse model: the first
instillations with viable bacillus Calmette-Guerin determine the
succeeding Th1 response. J Urol. 170:2004–2008. 2003.PubMed/NCBI
|
|
13
|
Tan BT, Limpens J, Koken M, Valster H and
Scheper RJ: Local administration of various cytostatic drugs after
subcutaneous immunization enhances delayed-type hypersensitivity
reaction to sheep red blood cells in mice. Scand J Immunol.
23:605–609. 1986. View Article : Google Scholar
|
|
14
|
Zitvogel L, Apetoh L, Ghiringhelli F and
Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev
Immunol. 8:59–73. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nowak AK, Robinson BW and Lake RA:
Gemcitabine exerts a selective effect on the humoral immune
response: implications for combination chemo-immunotherapy. Cancer
Res. 62:2353–2358. 2002.PubMed/NCBI
|
|
16
|
Balemans LT, Vegt PD, Steerenberg PA, et
al: Effects of sequential intravesical administration of mitomycin
C and bacillus Calmette-Guerin on the immune response in the guinea
pig bladder. Urol Res. 22:239–245. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Witjes JA, Caris CT, Mungan NA, Debruyne
FM and Witjes WP: Results of a randomized phase III trial of
sequential intravesical therapy with mitomycin C and bacillus
Calmette-Guerin versus mitomycin C alone in patients with
superficial bladder cancer. J Urol. 160:1668–1672. 1998. View Article : Google Scholar
|
|
18
|
Kaasinen E, Rintala E, Pere AK, et al:
Weekly mitomycin C followed by monthly bacillus Calmette-Guerin or
alternating monthly interferon-alpha2B and bacillus Calmette-Guerin
for prophylaxis of recurrent papillary superficial bladder
carcinoma. J Urol. 164:47–52. 2000. View Article : Google Scholar
|
|
19
|
Kaasinen E, Wijkstrom H, Malmstrom PU, et
al: Alternating mitomycin C and BCG instillations versus BCG alone
in treatment of carcinoma in situ of the urinary bladder: a nordic
study. Eur Urol. 43:637–645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Stasi SM, Giannantoni A, Giurioli A, et
al: Sequential BCG and electromotive mitomycin versus BCG alone for
high-risk superficial bladder cancer: a randomised controlled
trial. Lancet Oncol. 7:43–51. 2006.PubMed/NCBI
|
|
21
|
Ratliff TL: Role of animal models in
understanding intravesical therapy with bacille Calmette-Guerin.
Clin Infect Dis. 31(Suppl 3): 106–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan CW, Shen ZJ and Ding GQ: The effect of
intravesical instillation of antifibrinolytic agents on bacillus
Calmette-Guerin treatment of superficial bladder cancer: a pilot
study. J Urol. 179:1307–1311. 2008. View Article : Google Scholar
|
|
23
|
Brandau S and Suttmann H: Thirty years of
BCG immunotherapy for non-muscle invasive bladder cancer: a success
story with room for improvement. Biomed Pharmacother. 61:299–305.
2007.PubMed/NCBI
|
|
24
|
DiPaola RS and Lattime EC: Bacillus
Calmette-Guerin mechanism of action: the role of immunity,
apoptosis, necrosis and autophagy. J Urol. 178:1840–1841. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasaki A, Kudoh S, Mori K, Takahashi N and
Suzuki T: Are BCG effects against urinary bladder carcinoma cell
line T24 correlated with apoptosis in vitro? Urol Int. 59:142–148.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langford LA, Cooksley CS and DeMonte F:
Comparison of MIB-1 (Ki-67) antigen and bromodeoxyuridine
proliferation indices in meningiomas. Hum Pathol. 27:350–354. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asakura T, Takano Y, Iki M, et al:
Prognostic value of Ki-67 for recurrence and progression of
superficial bladder cancer. J Urol. 158:385–388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santos L, Amaro T, Costa C, et al: Ki-67
index enhances the prognostic accuracy of the urothelial
superficial bladder carcinoma risk group classification. Int J
Cancer. 105:267–272. 2003. View Article : Google Scholar : PubMed/NCBI
|