Introduction
The incidence of gastric cancer (GC) has decreased
over the last decade. However, GC remains the second leading cause
of cancer-related mortality and the fourth most common malignant
tumor type worldwide (1,2). The incidence of GC in elderly
individuals is currently on the increase in Japan due to the
extended life span of the general population. It is estimated that
GC in patients 65 years of age or older accounts for approximately
70% of total GCs (3). GC risk is
affected by H. pylori virulence factors, a family history of
GC, host genetics and environmental factors (cigarette smoking, in
particular) (4–7). A high mortality rate has been noted
worldwide, with a 5-year survival rate of approximately 20%
(8). In Japan, the 5-year survival
rate is over 50% (9). One of the
key factors that negatively affect the survival rate is the late
detection of tumors. To achieve a complete cure, primary tumors
should be detected at an early stage. It is therefore crucial to
acquire a better understanding of the clinicopathological and
patient characteristics in early gastric neoplasia.
GC develops through the accumulation of genetic and
epigenetic alterations (10). In
sporadic GC, the frequency of DNA methylation of MLH1 is
20–30% (11–13), and that of the P53 gene
mutation is 25–50% (12,14,15).
MLH1 is a DNA mismatch repair (MMR) gene and
hypermethylation of the promoter region of MLH1 is the main
cause of microsatellite instability (MSI) in primary GCs (16). Fleisher et al reported that
immunohistochemical staining of the Mlh1 protein may be included in
routine diagnostic methods used to predict hypermethylation of the
MLH1 promoter (17). This
suggests that the simple detection of MSI caused by the DNA
methylation of MLH1 is achieved by immunohistochemical
analysis of tumor specimens. A number of clinicopathological
characteristics of GC with MMR-gene deficiency or MSI were
previously reported (18–21). However, the relationship between MMR
abnormality and the clinicopathological characteristics in
early-stage gastric neoplasia, particularly in endoscopically
resected samples, has yet to be elucidated.
Mucin-based histochemical and immunohistochemical
examinations showed that gastric and intestinal phenotypic cell
markers are widely expressed in GCs, irrespective of histological
type (22–24). Moreover, it was reported that GCs
with a predominantly gastric phenotype have a pronounced tendency
toward invasion, metastasis and poor prognosis compared to GCs that
have an intestinal phenotypic expression (25–28).
Elucidation of the relationship between the
clinicopathological and patient characteristics and the molecular
events in early gastric neoplasia may improve the early detection,
treatment and surveillance of GC. The relationship between Mlh1
expression and the clinicopathological characteristics, such as
age, gender, alcohol consumption, smoking, oncological family
history, P53 expression and phenotypic expression in early stages
of gastric neoplasias, were evaluated using tumor tissues removed
by endoscopic resection (ER).
Materials and methods
Patients and tissue samples
Tumor specimens were obtained from 140 patients (89
males and 51 females) who underwent ER at Tottori University
Hospital, Japan, between 1994 and 2007. Patients with familial
adenomatous polyposis or hereditary non-polyposis colorectal cancer
were excluded. The histological examination showed that of the 140
tumors, 31 were gastric adenomas (GAs) and 109 early gastric
cancers (EGCs) (Table I).
Macroscopic and histological evaluations were performed according
to the classifications of the Japanese Gastric Cancer Association
(29). Macroscopic features were
divided into two major types: elevated and flat or depressed. The
depth of invasion and histological grade were classified according
to the predominant features of the tumors. In this study, the
adenoma samples corresponded to moderate or high-grade
adenoma/dysplasia and the cancer samples corresponded to
non-invasive or intramucosal carcinoma according to the Vienna
classification system (30), with
the exception of seven submucosal invasive cancers. The EGCs
comprised histologically differentiated carcinomas, with the
exception of four poorly differentiated and/or signet-ring cell
carcinomas. H. pylori positivity was not checked. Two
experienced pathologists (K.Y. and H.I.) verified the pathological
diagnoses. Smoking habits, alcohol consumption and oncological
family history of the patients were recorded by medical doctors on
admission. Alcohol consumption and smoking were defined as regular
intake when the respective consumption was >35 g of ethanol and
5 cigarettes per day. Positivity for family history of cancer was
defined as a history of cancer within first-degree relatives.
Patients with a family history of gastric cancer and those with an
oncological family history were identified. The cases were analyzed
anonymously; specimens were assigned a number without any personal
information. Approval for the study was obtained from the
Institutional Review Board.
 | Table IClinicopathological characteristics
and patient background in early gastric neoplasias. |
Table I
Clinicopathological characteristics
and patient background in early gastric neoplasias.
| Early gastric
carcinoma (n=109) | Gastric adenoma
(n=31) |
|---|
| Gender (M:F) | 75:34 | 14:17 |
| Age (mean ±
SD) | 69.7±9.1 | 71.1±6.4 |
| Elevated/flat,
depressed | 59:50 | 31:0 |
| Histological
type |
| Tub | 84 | Mild | 1 |
| Pap | 21 | Moderate | 20 |
| Por/Sig | 4 | Severe | 10 |
| Depth of invasion
(mucosa:submucosa) | 102:7 | |
| Regular alcohol
intake (%) | 51 (46.8) | 8 (35.8) |
| Current or ever
smoker (%) | 58 (53.2) | 13 (41.9) |
| Oncological family
history (%) | 61 (60.0) | 20 (64.5) |
| Family history of
gastric cancer (%) | 42 (38.5) | 12 (38.7) |
Immunohistochemical staining
Paraffin-embedded, 4-μm sections were
immunohistochemically stained with anti-Mlh1 mouse monoclonal
antibody (G168-15; PharMingen, San Diego, CA, USA; dilution 1:50),
anti-P53 mouse monoclonal antibody (DO-7; Dakopatts, Copenhagen,
Denmark; dilution 1:50), anti-human gastric mucin (HGM) mouse
monoclonal antibody (45M1; Novocastra Laboratories, Ltd.,
Newcastle, UK; dilution 1:50), anti-MUC2 mouse monoclonal antibody
(Ccp58; Novocastra; dilution 1:100) and anti-CD10 mouse monoclonal
antibody (56C6; Novocastra; dilution 1:50) using the
avidin-biotin-peroxidase complex technique.
Immunohistochemical staining was performed as
described below. In brief, following deparaffinization in xylene
and rehydration in ethanol, the sections were immersed in citrate
buffer (0.01 M, pH 6.0) and heated in a microwave oven for 20–30
min to retrieve antigens. Endogenous peroxidase activity was
blocked by incubation with 3% H2O2 and the
sections were then incubated with the primary antibody overnight at
4˚C. As a negative control, the primary antibody was replaced with
normal serum IgG at a similar dilution. The detection reaction
followed the protocol of Vectastain Elite ABC kit (Vector
Laboratories, Burlingame, CA, USA). Diaminobenzidine was used as a
chromogen and haematoxylin was used as a counterstain. The sections
were incubated with biotinylated anti-mouse IgG and
avidin-biotin-peroxidase, and then visualized using
diaminobenzidine tetrahydrochloride.
Two independent observers (S.S. and K.Y.) evaluated
the expression of the proteins tested and the immunohistochemical
analysis was performed in a blind manner with respect to the
clinical information.
Assessment of Mlh1 immunostaining
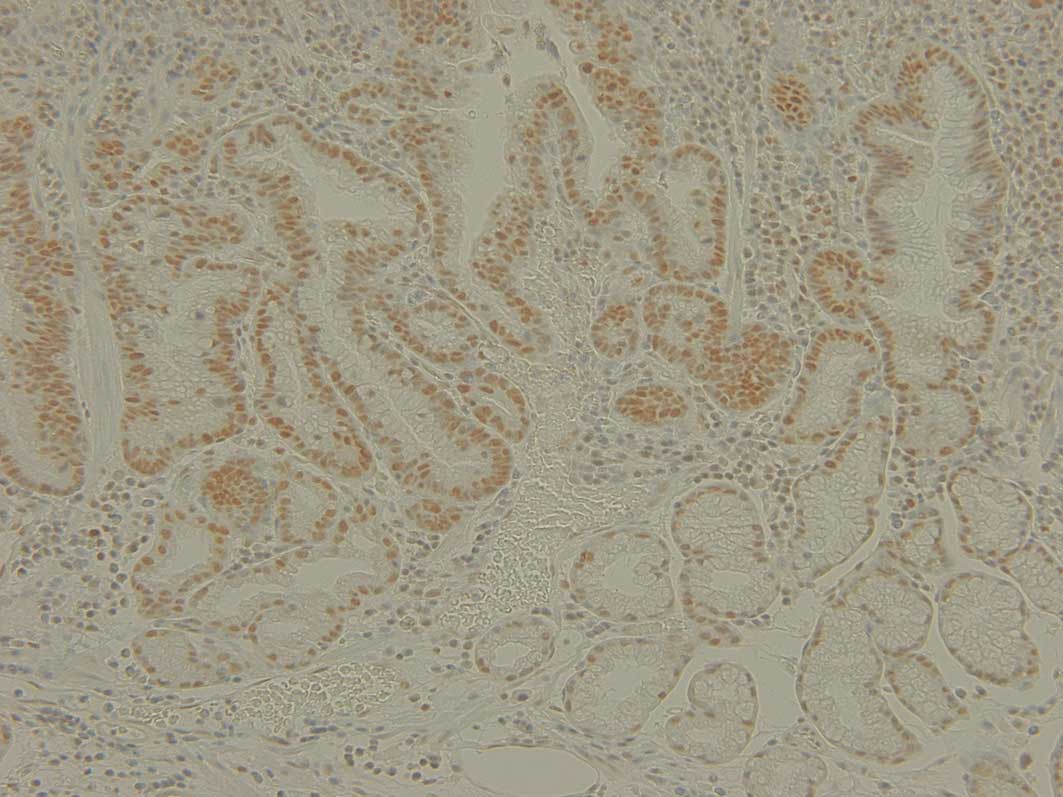
Evaluation of Mlh1 expression was regarded as either
normal or decreased. The cases with definite nuclear staining in
<30% of the tumor cells were identified as decreased (Fig. 1).
Assessment of P53 immunostaining
Five representative fields were examined and a total
of 1,000 tumor cells (200 for each field) were counted under the
microscope using a high-power (x200) objective. Distinct nuclear
immunoreaction was assessed as positive. In this study, the
specimens were regarded as P53-positive when >10% of tumor cells
stained as positive.
Assessment of HGM, MUC2 and CD10
immunostaining
HGM staining was observed in the cytoplasm of the
gastric foveolar epithelium and mucous neck cells. MUC2 staining
was observed in the cytoplasm around the nuclei of the goblet cells
and CD10 staining was observed along the brush border of the
luminal surface of the epithelium. Although CD10 is also expressed
in the apical portion of the cytoplasm of normal gastric mucosa,
only the expression of CD10 along the brush border was studied. The
results from the staining were classified as positive and negative
expression. Staining of >10% of the adenoma and carcinoma cells
was regarded as having a positive expression and <10% as having
a negative expression.
Statistical analysis
Statistical analysis was performed using the
Chi-square test with Yates' correction, Fisher's test, the
Mann-Whitney U test and logistic regression. P<0.05 was
considered to be statistically significant. Statistical
computations were performed using the Stat View 5.0 software (SAS
Institute, Cary, NC, USA).
Results
Clinicopathological and patient
characteristics
Clinico-pathological characteristics and patient
backgrounds in early gastric neoplasias are shown in Table I. The mean age of the EGC cases was
69.7±9.1 years (range 45–85) and that of the GA cases was 71.1±6.4
years (range 51–82). GAs were found more often in female than in
male patients (33.3 vs. 15.7%; P=0.016). Among the 140 gastric
neoplasia cases, 54 (38.5%) had a family history of GC and 81
(57.9%) had a family history of malignancy. A total of 59 patients
(42.1%) consumed alcohol and 71 patients (50.7%) were smokers.
Mlh1, P53 and phenotypic (HGM, MUC2,
CD10) expression in early gastric neoplasias
Loss of Mlh1 expression was found more often in EGCs
than in GAs [30/109 (27.5%) vs. 3/31 (9.6%), P=0.053]. P53
overexpression occurred significantly more often in EGCs than in
GAs [30/109 (27.5%) vs. 2/31 (6.5%), P=0.014]. Significant HGM
expression was found more often in EGCs than in GAs [71/109 (65.1%)
vs. 10/31 (32.3%), P=0.001]. On the other hand, CD10 and MUC2
expression occurred significantly more often in GAs than in EGCs
[21/31 (66.7%) vs. 30/109 (27.5%); P<0.001 and 27/31 (87.1%) vs.
53/109 (27.5%); P=0.001, respectively] (Table II).
 | Table IIMlh1, P53 and phenotype expression in
early gastric neoplasias. |
Table II
Mlh1, P53 and phenotype expression in
early gastric neoplasias.
| Cancer (n=109) | Adenoma (n=31) |
|---|
| Mlh1 (+/−) | 79:30 (27.5%) | 28:3 (9.6%) |
| P53 (+/−) | 30:79 (27.5%) | 2:29 (6.5%) |
| HGM (+/−) | 71:38 (65.1%) | 10:21 (32.3%) |
| CD10 (+/−) | 30:79 (27.5%) | 21:10 (66.7%) |
| MUC2 (+/−) | 53:56 (48.6%) | 27:4 (87.1%) |
Relationship between Mlh1 expression and
the clinicopathological and patient characteristics in early
gastric neoplasias
Female patients presented more often with EGCs
showing a loss of Mlh1 expression than EGCs showing a positive Mlh1
expression [13/30 (43.3%) vs. 21/79 (26.6%), P=0.094]. Adenomas
with a loss of Mlh1 expression were found in only three tumors of
elderly female patients (9.6%) (data not shown). Loss of Mlh1
expression was closely associated with elevated macroscopic
classification (P=0.04) and oncological family history (P=0.03). No
significant difference was noted between the expression of Mlh1 and
other clinicopathological or patient characteristics (Table III).
 | Table IIIRelationship between
clinicopathological characteristics and Mlh1 expression in early
gastric cancer. |
Table III
Relationship between
clinicopathological characteristics and Mlh1 expression in early
gastric cancer.
| Mlh1
expression | P-value |
|---|
|
| |
|---|
| Positive
(n=79) | Negative
(n=30) | |
|---|
| Gender (M:F) | 58:21 | 17:13 | 0.094 |
| Age (mean ±
SD) | 69.7±9.1 | 69.6±9.1 | 0.903 |
| Elevated/flat,
depressed | 38:41 | 21:9 | 0.040 |
| Histologic type
(tub:pap:por/sig) | 64:12:3 | 20:9:1 | 0.216 |
| Depth of invasion
(m:sm) | 76:3 | 26:4 | 0.070 |
| Regular alcohol
intake (%) | 38 (48.1) | 13 (43.3) | 0.660 |
| Current or ever
smoker (%) | 43 (54.4) | 15 (50.0) | 0.683 |
| Oncological family
history (%) | 39 (49.4) | 22 (73.3) | 0.030 |
| Family history of
gastric cancer (%) | 26 (32.9) | 16 (53.3) | 0.051 |
Among the elderly patients (≥65 years), the females
had significantly more EGCs with loss of Mlh1 expression than EGCs
with a positive Mlh1 expression [13/22 (59.1%) vs. 17/57 (29.8%),
P=0.016]. Furthermore, a family history of GC and an oncological
family history were associated more often with EGCs with loss of
Mlh1 expression than EGCs with a positive Mlh1 expression [13/22
(59.1%) vs. 19/57 (33.3%), P=0.037 and 17/22 (77.3%) vs. 29/57
(50.9%), P=0.033, respectively] (Table
IV).
 | Table IVRelationship between Mlh1 expression
and clinicopathological and patient characteristics in elderly (≥65
years of age) patients with early gastric cancer. |
Table IV
Relationship between Mlh1 expression
and clinicopathological and patient characteristics in elderly (≥65
years of age) patients with early gastric cancer.
| Mlh1
expression | P-value |
|---|
|
| |
|---|
| Positive
(n=57) | Negative
(n=22) | |
|---|
| Gender (M:F) | 40:17 | 9:13 | 0.016 |
| Age (mean ±
SD) | 74.4±5.4 | 74.0±5.3 | 0.903 |
| Elevated/flat,
depressed | 33:24 | 17:5 | 0.109 |
| Oncological family
history (%) | 29 (50.9) | 17 (77.3) | 0.033 |
| Family history of
gastric cancer (%) | 19 (33.3) | 13 (59.1) | 0.037 |
However, no correlation was found between P53
expression and phenotypic expression and any of the
clinicopathological or patient data.
Relationship between Mlh1 expression and
P53 and phenotypic expression in early gastric cancer
P53 overexpression occurred significantly more often
in EGCs with a positive Mlh1 expression than in EGCs with loss of
Mlh1 expression [28/79 (35.4%) vs. 2/30 (6.7%), P=0.002]. HGM
expression was found significantly more often in GCs with loss of
Mlh1 expression than in GCs with a positive Mlh1 expression [25/30
(83.3%) vs. 46/79 (58.2%), P=0.017] (Table V).
 | Table VRelationship between Mlh1 expression
and P53 and phenotypic expression in early gastric cancer. |
Table V
Relationship between Mlh1 expression
and P53 and phenotypic expression in early gastric cancer.
| Positive
expression | Mlh1
expression | P-value |
|---|
|
| |
|---|
| Positive (n=79,
%) | Negative (n=30,
%) | |
|---|
| P53 | 28 (35.4) | 2 (6.7) | 0.002 |
| HGM | 46 (58.2) | 25 (83.3) | 0.017 |
| CD10 | 25 (31.6) | 5 (16.7) | 0.120 |
| MUC2 | 14 (17.7) | 16 (53.3) | 0.550 |
Multivariate analysis of factors related
to Mlh1 expression
Multivariate analysis using the logistic regression
model showed that female gender (P=0.044; OR=21.1; 95% CI 1.08–408)
and P53 expression (P=0.014; OR=19.6; 95% CI 1.80–211) correlated
with Mlh1 expression in elderly patients (≥65 years of age) with
EGC.
Discussion
The present study focused on endoscopically resected
early gastric neoplasias and showed that loss of Mlh1 expression in
EGCs in elderly individuals occurs more significantly in females
than in males. In addition, our results indicate that the frequency
of aberrant Mlh1 expression in EGCs increases significantly in
patients with an oncological family history and elevated gross
type.
Numerous GCs develop through defects in DNA MMR
genes, such as MLH1 or MSH2, or tumor suppressor
genes, such as P53 (12).
Evidence of MLH1 hypermethylation is noted in approximately
20–30% of differentiated carcinomas (11–13).
Epigenetic methylation-associated inactivation of the MLH1
MMR gene is a potent trigger of MSI, especially high-frequency MSI
(MSI-H) (17). Moreover, a strong
correlation has been noted between MSI and methylation analysis, as
well as MMR protein immunoexpression. Thus, immunohistochemistry is
a rapid and cost-effective method for identifying MMR-gene
alterations (31–34). As previously reported, MLH1
methylation is frequently observed in GCs of elderly patients
(18,19) and MSI-H GCs are identified by their
expanding growth pattern (20).
Similarly, in this study GC with loss of Mlh1 expression was
associated with elevated gross type. In colorectal types of cancer,
Breivik et al found that MSI occurred most frequently in
young male and older female patients (35). In GC, the frequency of the promoter
methylation of MLH1 and the lack of Mlh1 expression
increases with advancing age (9,18,21).
In our study, GCs with loss of Mlh1 expression were statistically
significant in the elderly female patients. In GAs, loss of Mlh1
expression was detected in only three tumors of elderly female
patients. While three studies have indicated a trend of association
between female gender and immunohistochemical negativity for Mlh1
or for the MSI phenotype in GCs, the samples used in these studies
were surgical tissues (13,36,37).
The results from these studies suggest differences between the
genders in the molecular pathways of gastric neoplasia in elderly
patients. Our study is the first to report on the relationship
between gender and Mlh1 expression in early gastric neoplasia
resected by endoscopy in elderly patients. Previous results from
animal experiments have shown a protective effect of female gender
on inflammation-associated cancer, which is attributed to estrogen
(38). This mechanism may account
for the frequent aberrant Mlh1 expression noted in older female
patients with early gastric neoplasias. However, this mechanism has
yet to be elucidated.
The association of MSI and family history of GC is
controversial. In five studies, correlations between MSI and
GC-positive family history were found (39–43).
In particular, Shinmura et al noted an association between
MSI and GC familial clustering in early GC, but not in
advanced-stage GC (40). Kanemitsu
et al indicated that MSI is induced by a deficiency in the
MMR protein expression (42). Our
analyses using endoscopically resected EGCs showed a significant
association between aberrant Mlh1 expression and GC-positive family
history in elderly patients. The association between MSI and family
history may reflect exposure to environmental or dietary factors
shared by close relatives. Taken together, these causal factors in
familial GC may also be associated with MSI in the early stages of
carcinogenesis.
On the basis of cellular phenotype, obvious
differences occur in the biological behavior of gastric vs.
intestinal phenotype of GCs. Gastric phenotype GCs are considered
to have greater invasiveness and metastatic potential than
intestinal phenotype GCs (28).
Concerning the relationship between genetic/epigenetic alterations
and phenotypes, DNA methylation of MLH1 occurred more
frequently in GCs with gastric phenotype. Furthermore, Endo et
al reported that MSI was detected more frequently in gastric
phenotype GCs than in intestinal phenotype GCs (26). Findings of our previous and current
studies showed that gastric phenotype EGCs were significantly
associated with loss or reduction of Mlh1 expression (44). However, we did not find any
correlation between phenotypic expression and other
clinicopathological or patient data. Thus, our data and those of
previous reports support the correlation between cellular phenotype
and Mlh1 alterations. However, more studies are required to clarify
the mechanisms of this association.
In the present study, P53 overexpression was found
in 27.5% of EGCs and was inversely associated with loss of Mlh1
expression. This finding is consistent with previous reports
(12,45). However, the relationship between P53
expression and clinicopathological factors was not determined. It
has been reported that H. pylori mediated the up-regulation
of activation-induced cytidine deaminase results in the
accumulation of nucleotide alterations in the P53 tumor suppressor
gene in gastric cells in vitro (46). Thus, P53 overexpression may be
directly affected by H. pylori infection and not by
individual characteristics.
In conclusion, this study showed that EGC with Mlh1
silencing exhibits characteristics, such as age, gender, family
history and tumor growth pattern. These data should be used for the
screening and surveillance of GC, particularly in elderly
individuals. Further investigations into the relationship between
MMR methylation and patient and tumor characteristics through in
vitro and/or in vivo studies are required to thoroughly
elucidate the molecular interactions involved.
References
|
1
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar
|
|
2
|
Parkin DM, Brey F, Ferlay J, et al: Global
cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Arai T, Esaki Y, Inoshita N, et al:
Pathologic characteristics of gastric cancer in the elderly: a
retrospective study of 994 surgical patients. Gastric Cancer.
7:154–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eslick GD: Helicobacter pylori
infection causes gastric cancer? A review of the epidemiological,
meta-analytic, and experimental evidence. World J Gastroenterol.
21:2991–2999. 2006.
|
|
5
|
Berinini M, Barbi S, Roviello F, et al:
Family history of gastric cancer: a correlation between
epidemiologic findings and clinical data. Gastric Cancer. 9:9–13.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawasaki K, Kanemitsu K, Yasuda T, et al:
Family history of cancer in Japanese gastric cancer patients.
Gastric Cancer. 10:173–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Cancer Research Fund, American
Institute for Cancer Research, Food, Nutrition, Physical Activity
and Prevention of Cancer. A Global Perspective. AICR; 2007
|
|
8
|
Jemal A, Clegg LX, Ward E, et al: Annual
report to the nation on the status of cancer, 1975–2001, with a
special feature regarding survival. Cancer. 101:3–27. 2004.
|
|
9
|
Arai T and Takubo K: Clinicopathologicalal
and molecular characteristics of gastric and colorectal carcinomas
in the elderly. Pathol Int. 57:303–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yashima K, Sasaki S, Koda M, Kawaguchi K,
Harada K and Murawaki Y: Premalignant lesions in gastric cancer.
Clin J Gastroenterol. 3:6–12. 2010. View Article : Google Scholar
|
|
11
|
Koseki K, Takizawa T, Koike M, Ito M,
Nihei Z and Sugihara K: Distinction of differentiated type early
gastric carcinoma with gastric type mucin expression. Cancer.
89:724–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamura G: Alteration of tumor suppressor
and tumor-related genes in the development and progression of
gastric cancer. World J Gastroenterol. 14:192–198. 2006.PubMed/NCBI
|
|
13
|
Hong SH, Kim HG, Chung WB, et al: DNA
hypermethylation of tumor-related genes in gastric carcinoma. J
Korean Med Sci. 20:236–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maesawa C, Tamura G, Suzuki Y, Ogasawara,
Sakata K, Kashiwaba M and Satodate R: The sequential accumulation
of genetic alterations characteristic of the colorectal
adenoma-carcinoma sequence does not occur between gastric adenoma
and adenocarcinoma. J Pathol. 176:249–258. 1995. View Article : Google Scholar
|
|
15
|
Yamazaki K, Tajima Y, Makino R, et al:
Tumor differentiation phenotype in gastric differentiated-type
tumors and its relation to tumor invasion and genetic alterations.
World J Gastrenterol. 28:3803–3809. 2006.PubMed/NCBI
|
|
16
|
Fleisher AS, Esteller M, Wang S, et al:
Hypermethylation of the hMLH1 gene promoter in human gastric
cancers with microsatellite instability. Cancer Res. 59:1090–1095.
1999.PubMed/NCBI
|
|
17
|
Fleisher AS, Esteller M, Tamura G, et al:
Hypermethylation of the hMLH1 gene promoter is associated with
microsatellite instability in early human gastric neoplasia.
Oncogene. 20:329–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima T, Akiyama Y, Shiraishi J, et al:
Age-related hypermethylation of the hMLH1 promoter in gastric
cancers. Int J Cancer. 94:208–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HG, Lee S, Kim DY, et al: Aberrant
methylation of DNA mismatch repair genes in elderly patients with
sporadic gastric carcinoma: a comparison with younger patients. J
Surg Oncol. 101:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceccarelli M, Sera F, Costantini RM, Nesi
G, Palli D and Ottini L: Gastric cancer with high-level
microsatellite instability: target gene mutations,
clinicopathologic features and long-term survival. Hum Path.
39:925–932. 2008. View Article : Google Scholar
|
|
21
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: epigenetics joins genetics.
Trends Genet. 16:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Egashira Y, Shimoda T and Ikegami M: Mucin
histochemical analysis of minute gastric differentiated
adenocarcinoma. Pathol Int. 49:55–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshino T, Shimoda T, Saito A, Nakanishi
Y, Tajima Y, Shirasu T and Miura S: Macroscopic features of
differentiated adenocarcinoma with gastric or intestinal phenotype
expression in early gastric cancer. Stomach Intestine. 34:513–525.
1999.
|
|
24
|
Koseki K, Takizawa T, Koike M, Ito M,
Nihei Z and Sugihara K: Distinction of differentiated type early
gastric carcinoma with gastric type mucin expression. Cancer.
89:724–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koseki K, Takizawa T, Koike M, et al:
Subclassification of well-differentiated gastric cancer with
reference to biological behavior and malignancy, gastric type vs.
intestinal type, and papillary carcinoma vs tubular carcinoma.
Stomach Intestine. 34:507–512. 1999.
|
|
26
|
Endoh Y, Tamura G, Sakata K, et al:
Genetic analysis of differentiated-type adenocarcinomas of the
stomach with gastric phenotype and intestinal phenotype. Stomach
Intestine. 34:539–544. 1999.
|
|
27
|
Tajima Y, Shimoda T, Nakanishi Y, et al:
Gastric and intestinal phenotypic marker expression in gastric
carcinomas and its prognostic significance: immunohistochemical
analysis of 136 lesions. Oncology. 61:212–220. 2001. View Article : Google Scholar
|
|
28
|
Kabashima A, Yao T, Sugimachi K and
Tsuneyoshi M: Relationship between biologic behavior and phenotypic
expression in intramucosal gastric carcinoma. Hum Pathol. 33:80–86.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma - 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schlemper RJ, Riddell RH, Kato Y, et al:
The Vienna classification of gastrointestinal epithelial neoplasia.
Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dietmaier W, Wallinger S, Bocker T,
Kullmann F, Fishel R and Ruschoff J: Diagnostic microsatellite
instability: definition and correlation with mismatch repair
protein expression. Cancer Res. 57:4749–4756. 1997.PubMed/NCBI
|
|
32
|
Mizoshita T, Tsukamoto T, Cao X, et al:
Microsatellite instability is linked to loss of hMLH1 expression in
advanced gastric cancers: lack of a relationship with the
histological type and phenotype. Gastric Cancer. 8:164–172. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thibodeau SN, French AJ, Cunningham JM, et
al: Microsatellite instability in colorectal cancer: different
mutator phenotypes and the principal involvement of hMLH1. Cancer
Res. 58:1713–1718. 1998.PubMed/NCBI
|
|
34
|
Chaves P, Cruz C, Lage P, Claro I, Cravo
M, Leitao CN and Soares J: Immunohistochemical detection of
mismatch repair gene proteins as a useful tool for the
identification of colorectal carcinoma with the mutator phenotype.
J Pathol. 191:355–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breivik J, Lothe RA, Meling GI, et al:
Different genetic pathways to proximal and distal colorectal cancer
influenced by sex-related factors. Int J Cancer. 74:664–669. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
French AJ, Petroni G, Thibideau SN, et al:
Allelic imbalance of 8p indicates poor survival in gastric cancer.
J Mol Diagn. 6:243–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beghelli S, Manzoni G, Barbi S, et al:
Microsatellite instability in gastric cancer is associated with
better prognosis in only stage II cancers. Surgery. 139:347–356.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwartz AG, Whitcomb JM, Nyce JW, Lewbart
ML and Pashko LL: Dehydroepiandrosterone and structural analogs: a
new class of cancer chemopreventive agents. Adv Cancer Res.
51:391–424. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keller G, Rotter M, Vogelsang H, et al:
Microsatellite instability in adenocarcinomas of the upper
gastrointestinal tract. relation to clinicopathologicalal data and
family history. Am J Pathol. 147:593–600. 1995.PubMed/NCBI
|
|
40
|
Shinmura K, Yin W, Isogaki J, et al:
Stage-dependent evaluation of microsatellite instability in gastric
carcinoma with familial clustering. Cancer Epidemiol Biomarkers
Prev. 6:693–697. 1997.PubMed/NCBI
|
|
41
|
Ottini L, Palli D, Falchetti M, et al:
Microsatellite instability in gastric cancer is associated with
tumor location and family history in a high-risk population from
Tuscany. Cancer Res. 57:4523–4529. 1997.PubMed/NCBI
|
|
42
|
Kanemitsu K, Kawasaki K, Nakamura M, et
al: MSI is frequently recognized among gastric cancer patients with
a family history of cancer. Hepatogastroenterology. 54:2410–2414.
2007.PubMed/NCBI
|
|
43
|
Pedrazzani C, Corso G, Velho S, et al:
Evidence of tumor microsatellite instability in gastric cancer with
familial aggregation. Familial Cancer. 8:215–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hara A, Yashima K, Yasugi A, et al:
Expression of Fhit, Mlh1, p16INK4A and E-cadherin in
early gastric neoplasia: Correlation with histological grade and
gastric phenotype. Oncol Rep. 18:553–559. 2007.PubMed/NCBI
|
|
45
|
Martinez R, Schackert HK, Plaschke J,
Baretton G, Appelt H and Schackert G: Molecular mechanisms
associated with chromosomal and microsatellite instability in
sporadic glioblastoma multiforme. Oncology. 66:395–403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsumoto Y, Marusawa H, Kinoshita K, et
al: Helicobacter pylori infection triggers aberrant
expression of activation-induced cytidine deaminase in gastric
epithelium. Nat Med. 13:470–476. 2007. View
Article : Google Scholar
|