Introduction
Although the prognosis for patients with bone or
soft tissue sarcomas has shown marked improvement over the past
three decades, those who develop local recurrence or metastatic
disease continue to have high mortality rates. Of all patients
diagnosed with malignant musculoskeletal tumors, 5–30% (1–3) have a
recurrence and 10–38% of patients present with clinically
detectable metastases (4–6). A number of studies have reported the
survival of patients who have undergone either a lung
metastasectomy or chemotherapy (4,5,7–9).
However, little is known about the clinical course of patients with
bone or soft tissue sarcomas who have succumbed to the disease. For
example, only a few reports are available regarding the clinical
course, such as the metastatic site, which led to the cause of
death or the survival time following initial detection of
metastases (10). Thus, this study
aimed to analyze the metastatic patterns of sarcoma patients and to
describe the clinical course following the detection of distant
metastasis.
Patients and methods
We retrospectively reviewed the medical records of
newly diagnosed patients with either bone or soft tissue sarcoma
referred to Mie University Hospital between January 1999 and
December 2008. Well-differentiated liposarcomas were excluded from
this study. During this period, 255 patients with a diagnosis of
sarcoma were referred to our institution. We reviewed the clinical
records of these patients and found 63 patients who died of
metastasis.
We examined the clinical features of the initially
detected distant metastases, the subsequent clinical course up to
the patient’s death and the survival time of the patients who
succumbed to lung metastasis.
Statistical analysis
Overall survival was estimated using the
Kaplan-Meier method. Factors affecting patient survival were
examined based on the log-rank test. The StatMate III program (ATMS
Co., Tokyo, Japan software) was used to conduct statistical
analysis. Post-metastatic survival was defined as the duration
between the first detection of distant metastases and patient
death. The post-treatment survival time was defined as the duration
between the final treatment [surgery, lung radiofrequency (RF)
ablation and chemotherapy] for metastases and death.
Results
Patient and tumor characteristics and
follow-up
A total of 34 male and 29 female patients were
included in our study. The average age was 51 years (range 6–80),
including 17 patients who were ≥70 years of age. The follow-up
periods ranged from 1 to 82 months (average 16) after the initial
detection of metastasis.
The histological diagnoses of the primary bone tumor
were osteosarcoma (n=10), malignant fibrous histiocytoma (MFH) of
the bone (n=3), chondrosarcoma (n=2), Ewing’s sarcoma (n=2) and
others (n=2). Soft tissue tumor included malignant peripheral nerve
sheath tumors (n=9), MFH (n=9), leiomyosarcoma (n=6), myxoid
liposarcoma (n=4), synovial sarcoma (n=3), alveolar soft part tumor
(ASPS) (n=3), extra-skeletal myxoid chondrosarcoma (n=2),
epithelioid sarcoma (n=2) and others (n=6). According to the
American Joint Commission on Cancer classification and stage
grouping of bone sarcomas, 9 were classified as stage 2B, 6 as 3A
and 1 as stage 3B. Of the soft tissue sarcomas, 3 were classified
as stage 2, 26 as stage 3 and 18 as stage 4.
Post-treatment follow-up was performed at 3-month
intervals for 2 years, then every 4–6 months for 5 years, and every
year subsequently. At follow-up, a chest X-ray was routinely
carried out, and an examination using computed tomography (CT)
(X-Vigor or Aquilion; Toshiba, Tokyo, Japan; or HiSpeed Advantage
Qx/I; GE Healthcare, USA; or Asteion; Toshiba) was performed at 3-
to 6-month intervals to detect distant metastasis. A CT scan of the
abdomen and pelvic cavity was performed at 6-month intervals for
the patients with myxoid liposarcoma.
Treatment of the primary tumor
A surgical resection was performed in 56 of the 63
patients. Local recurrence occurred in 26 of the 56 patients. In 10
of the 26 patients, the local recurrence had developed prior to the
first detection of distant metastasis. Local recurrence was treated
with surgical resection (n=20), radiotherapy (n=4) and chemotherapy
(n=3). In total, 7 of the 63 patients were not treated with surgery
for their primary tumor. Four patients underwent radiotherapy due
to their advanced age (n=2), or an unresectable tumor size and
location (n=2). One patient with post-radiation MFH at a
sacro-iliac lesion underwent carbon ion radiotherapy since it was
considered to be less invasive. One patient received only
palliative therapy for multiple metastatic lesions. A 6-year-old
female with osteosarcoma in her femur was treated only with
chemotherapy as her family rejected surgical excision.
Clinical features of the initially
detected distant metastases and the clinical course until patient
death
The location of the initially detected distant
metastases was investigated. In 53 of the 63 patients, lung
metastasis developed (83%) and in 21 patients (33%) extra-pulmonary
metastasis developed. The average size of the maximum diameter of
the lung metastases was 10 mm (3–40 mm), and the average number of
lung metastases was 5 (1–20) upon detection.
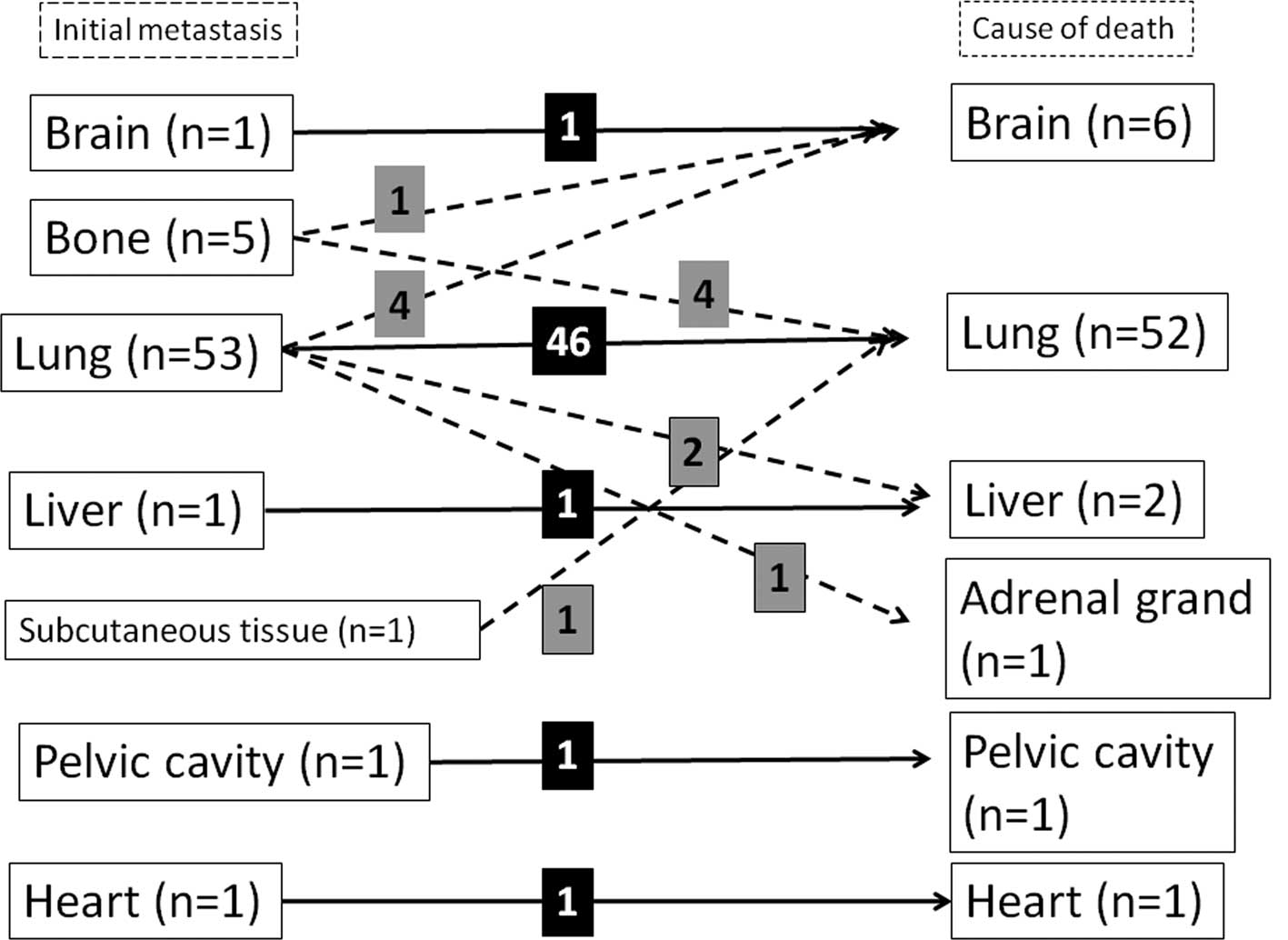
The sites of extra-pulmonary metastasis were bone
(n=10), liver (n=5), soft tissue (n=3), brain (n=2), heart (n=2),
pelvic cavity (n=1) and stomach (n=1). The occurrence of lung and
extra-pulmonary metastases was observed in 11 patients. Ten
patients had only extra-pulmonary metastasis without any
co-existing lung metastasis in the following sites: bone (n=5),
liver (n=1), brain (n=1), heart (n=1), subcutaneous tissue (n=1)
and pelvic cavity (n=1) (Fig.
1).
Of the 53 patients who had lung lesions at the first
detection of distant metastasis, 46 died of progression of lung
metastasis, 2 of liver metastasis, 4 of brain metastasis and 1 of
adrenal metastasis. Of the 5 patients who had bone lesions at the
first detection of distant metastasis, 4 died of lung metastasis
and 1 of brain metastasis. One patient who had distant metastasis
in the subcutaneous tissue at the first detection of metastasis
died of lung metastasis. Each patient who had metastasis in the
brain, liver, heart or pelvic cavity at the first detection of the
metastasis died of aggravation of the initial metastasis (Fig. 1). As a result, of the 63 patients,
52 died of lung metastasis, 6 of brain, 2 of liver and 1 of heart,
pelvic cavity or adrenal metastasis. A total of 77% of patients (49
of the 63) succumbed to the primary metastasis.
While all 18 bone sarcoma patients died of lung
metastasis, 11 of the 45 soft tissue sarcoma patients succumbed to
extra-pulmonary metastasis. Seven of these 11 patients died of
other additional distant metastases. The 3 patients with ASPS died
of brain metastases.
The 10 patients that survived >2 years following
the initial detection of distant metastasis developed other
additional distant metastases (Table
I). The sites of additional distant metastasis were:
retroperitoneum (n=2), bone (n=2), soft tissue (n=1), lymph node
(n=1), radial nerve (n=1), lung (n=1), adrenal grand (n=1) and
brain (n=1).
 | Table IPattern of metastases in patients who
survived more than 2 years following the initial metastasis. |
Table I
Pattern of metastases in patients who
survived more than 2 years following the initial metastasis.
| Diagnosis | Initial metastatic
site | Other metastatic
site | Cause of death | Follow-up periods
(months) |
|---|
| ASPS | Lung | Brain | Brain | 45 |
| Synovial sarcoma | Lung | Adrenal gland | Adrenal gland | 32 |
| Ewing’s sarcoma | Lung | Retroperitoneum | Lung | 85 |
| MGCT | Lung | Soft tissue | Lung | 63 |
| MFH | Subcutaneous | Lung | Lung | 43 |
| ESCS | Lung | Lymph node | Lung | 59 |
| Synovial sarcoma | Lung | Radial nerve | Lung | 29 |
| Myxoid sarcoma | Lung | Retroperitoneum | Lung | 25 |
| Osteosarcoma | Lung | Bone | Lung | 35 |
| Rhabdomyosarcoma | Lung | Brain | Brain | 24 |
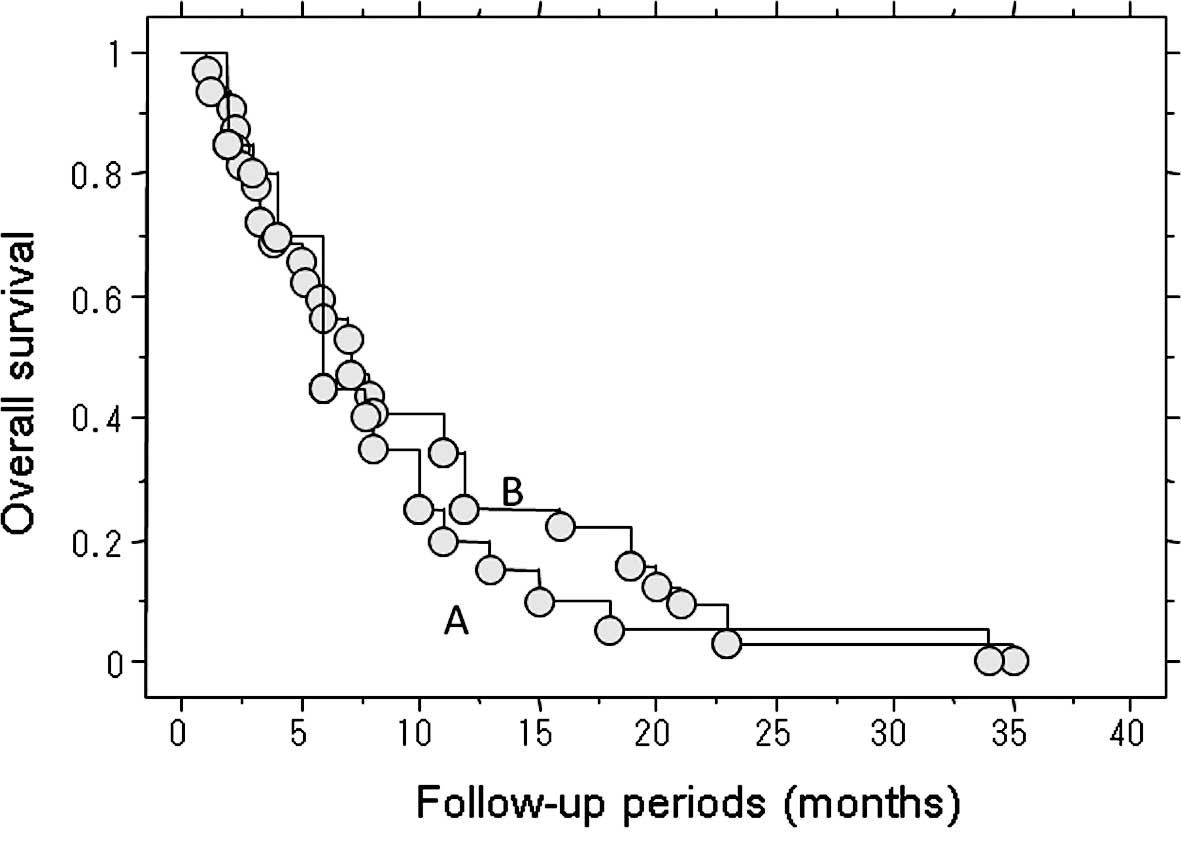
Length of survival of the 52 patients who
succumbed to lung metastasis
The length of survival of the 52 patients who died
of lung metastasis was examined. The median post-treatment survival
of the 20 patients who underwent treatment for metastasis,
including metastasectomy, RF ablation and chemotherapy, was 6
months (Fig. 2). The following
treatments for the lung metastases were performed: lung RF ablation
alone (n=5), lung RF ablation and chemotherapy (n=6),
metastasectomy and chemotherapy (n=4), chemotherapy alone (n=4),
and metastasectomy and lung RF ablation (n=1).
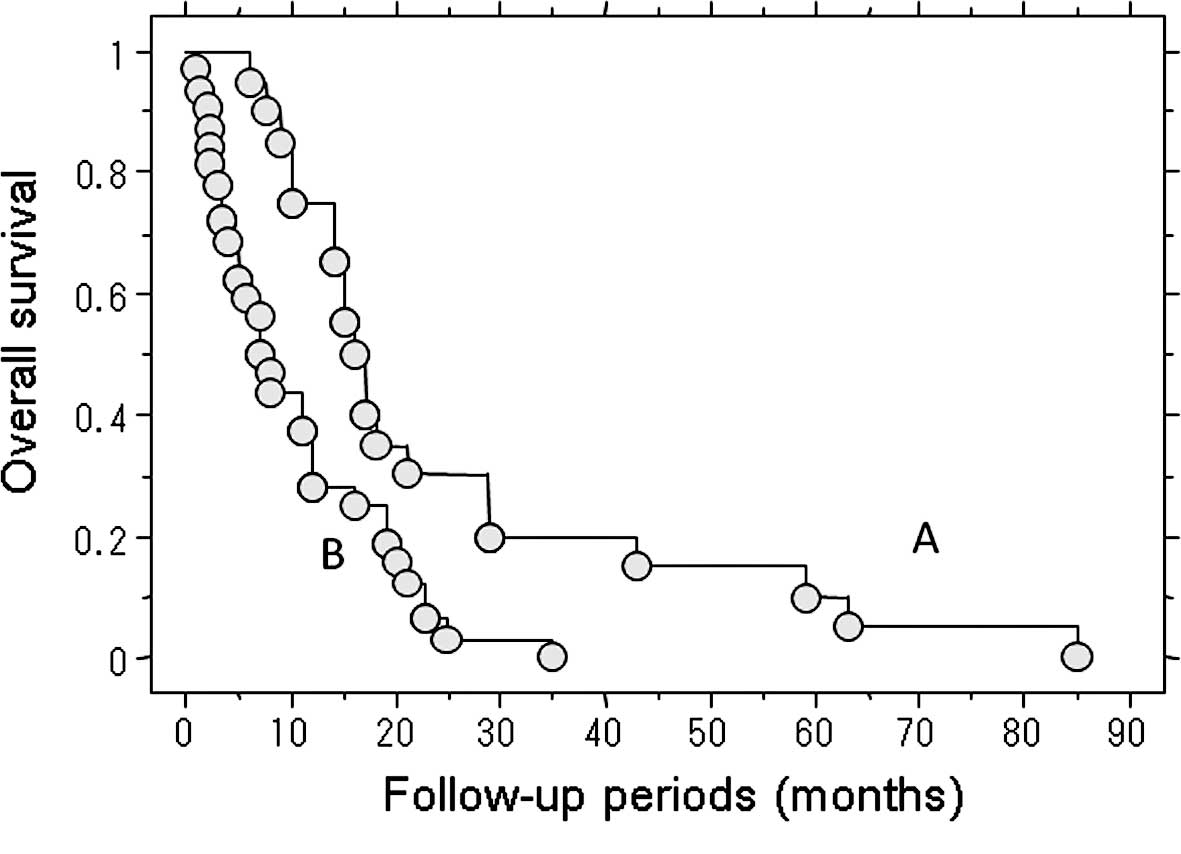
The median post-metastatic survival of the 32
patients who received no treatment for their metastasis was 7.2
months. By contrast, the median post-metastatic survival in the 20
patients who underwent treatment for metastasis was 16 months. This
represents a significant difference in the survival rate (P=0.003)
(Fig. 3).
Discussion
Regarding the location of metastases, 62–82% of
sarcoma patients had lung metastasis and 50–70% had isolated lung
metastases in previous studies (4,11). In
our series, 83% (52 of 63 patients) developed lung metastasis as
the initial metastatic site. Therefore, the present results are
consistent with previous reports. Although numerous reports exist
regarding the initial metastatic site in sarcoma patients and the
treatment of patients with metastasis, few reports are available
regarding the location of the metastatic lesion that ultimately
caused patient death (4,5,7–11). In
the present study, 82.5% of patients succumbed to lung metastasis
and 10% to brain metastasis. Other patients succumbed to liver
(3%), heart (1.5%), adrenal grand (1.5%) and pelvic metastasis
(1.5%).
Brain metastases from soft tissue sarcoma are
believed to occur in a minority of soft tissue sarcoma patients,
with a reported prevalence ranging between 1 and 6% (12,13).
In osteosarcoma, the incidence of brain metastasis is reported to
be 2–6.5% (14). Particularly in
the absence of lung metastases, brain metastasis is thought to be a
relatively uncommon event in the natural history of bone and soft
tissue sarcomas. In our cases, only 1 patient with epithelioid
sarcoma had brain metastasis without lung metastasis. ASPS has been
reported to metastasize to the brain more commonly than other types
of high-grade sarcoma, although the majority of studies concerning
ASPS are in the form of case reports and small corrective series
(15–17). In our present series, brain
metastasis developed within 4 years of the primary surgery in the 3
patients with APSP. We therefore recommend the regular use of
intracranial CT imaging for patients with ASPS.
Although the prognosis of sarcoma patients has
improved, extra-pulmonary metastases should be further
investigated. In our series, 11 of the 45 soft tissue sarcoma
patients died of extra-pulmonary metastasis. In addition, 7 of the
11 patients succumbed to other additional distant metastases.
Although follow-up chest CT and physical examinations were
routinely performed at 3- to 6-month intervals to detect distant
metastasis, it was difficult to identify new extra-pulmonary
metastatic lesions without clinical symptoms. Therefore, we
recommend regular screening using a contrast-enhanced CT scan to
detect abdominal or pelvic metastases at 3- to 6-month intervals,
as well as chest CT scans, particularly for soft tissue sarcoma
patients, even when no clinical symptoms occur.
Few reports are available on the metastatic site
causing patient death and the relationship between the metastatic
site and the duration of survival after final treatment (surgery,
lung RF ablation, chemotherapy and radiation) for sarcoma (10). In the present study, the median
post-metastatic survival in the 32 patients who did not receive
radical treatment for metastases was 7.2 months. The median
post-treatment survival in the 20 patients who received treatment
for the metastatic disease was 6 months. This suggests that the
survival of patients with lung metastases is approximately 6 months
in the absence of treatment, regardless of prior treatments. One
limitation of the present study is that it included various
different histological diagnoses which exhibit different clinical
behavior. Therefore, further large-scale investigations are
required to clarify the effects of the metastatic site on
survival.
This study provides crucial data regarding the
clinical course of metastatic sarcoma patients. Of the 63 patients
who died of distant metastasis, 52 (83%) developed lung metastasis
as the initial metastatic site, while 22 (35%) developed
extra-pulmonary metastasis. A total of 51 patients succumbed to
lung metastasis, 6 to brain, 3 to liver and 1 to heart, pelvic
cavity and adrenal metastasis. The majority (77%; 49 of 63
patients) died of the primary metastasis. Six patients (10%) died
of brain metastasis, while 11 (24%) of the 45 soft tissue sarcoma
patients died of extra-pulmonary metastasis. It is therefore
crucial to pay particular attention to the appearance of brain
metastasis during treatment. In addition, we recommend that routine
screening using contrast-enhanced CT scans be utilized to detect
abdominal or pelvic metastases. Chest CT scans should also be
performed on a regular basis, even when no clinical symptoms are
noted in patients.
In conclusion, the survival of patients with lung
metastases is only approximately 6 months following the cessation
of treatment, regardless of the type of treatment initially
used.
References
|
1
|
Weiss SW and Goldblum JR: Local
recurrence. Enzinger and Weiss’s Soft Tissue Tumors. 4th edition.
Mosby; St. Louis: pp. 28–32. 2001
|
|
2
|
Zager GK, Ballo MT, Pesters PW, et al:
Prognostic factors for patients with localized soft-tissue sarcoma
treated with conservation surgery and radiation therapy; an
analysis of 1225 patients. Cancer. 97:2530–2543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duffaud F, Digue L, Mercier C, et al:
Recurrence following primary osteosarcoma in adolescents and adults
previously treated with chemotherapy. Eur J Cancer. 39:2050–2057.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kane JM III, Finley JW, Driscoll D, et al:
The treatment and outcome of patients with soft tissue sarcomas and
synchronous metastases. Sarcoma. 6:69–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harting MT, Blakely ML and Jaffe N:
Long-term survival after aggressive resection of pulmonary
metastases among children and adolescents with osteosarcoma. J
Pediatr Surg. 41:194–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollock RE, Karnell LH and Menck HR: The
national cancer data base report on soft tissue sarcoma. Cancer.
78:2247–2257. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiser MR, Downey RJ and Leung DHY: Repeat
resection of pulmonary metastases in patients with soft-tissue
sarcoma. J Am Coll Surg. 191:184–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karavasilis V, Seddon BM, Ashley S, et al:
Significant clinical benefit of first line palliative chemotherapy
in advanced soft-tissue sarcoma. Cancer. 112:1585–1591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D’Adamo DR, Anderson SE and Albritton K:
Phase III study of doxorubicin and bevacizumab for patients with
metastatic soft-tissue sarcomas. J Clin Oncol. 23:7135–7142.
2006.PubMed/NCBI
|
|
10
|
Jeffree GM, Price CHG and Sissons HA: The
metastatic patterns of osteosarcoma. Br J Cancer. 32:87–107. 1975.
View Article : Google Scholar
|
|
11
|
Vezeridis MP, Moore R and Larakousis CP:
Metastatic patterns in soft tissue sarcomas. Arch Surg Oncol.
118:915–918. 1998. View Article : Google Scholar
|
|
12
|
Espat NJ, Bilsky M, Lewis JJ, et al: Soft
tissue sarcoma brain metastases. Cancer. 94:2706–2711. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta T, Laskar S, Gujral S, et al: Brain
metastases in soft tissue sarcomas: case report and literature
review. Sarcoma. 9:147–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yonemoto T, Tatezaki S, Ishii T, et al:
Longterm survival after surgical removal of solitary brain
metastasis from osteosarcoma. Int J Clin Oncol. 8:340–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans HL: Alveolar soft-part sarcoma. A
study of 13 typical examples and one with a histologically atypical
component. Cancer. 55:912–917. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Temple HT, Scully SP, O’Keefe RJ, et al:
Clinical presentation of alveolar soft-part sarcoma. Clin Orthop.
300:213–218. 1994.PubMed/NCBI
|
|
17
|
Portera CA Jr, Ho V, Patel SR, et al:
Alveolar soft part sarcoma: clinical course and patients of
metastasis in 70 patients treated at a single institution. Cancer.
91:585–591. 2001. View Article : Google Scholar : PubMed/NCBI
|