Introduction
Prostate cancer is the most frequently diagnosed
invasive cancer and the leading cause of cancer-related death among
males in a number of countries (1).
Mounting evidence indicates that neuroendocrine (NE) cells play a
role in prostatic disease. NE cells populate both normal and
malignant prostate tissues (2), and
can synthesize, store and release growth factors, as well as
biogenic amines, serotonin 5-hydroxytryptamine (5-HT), dopamine and
other neurotransmitter-related substances (3–5). An
increase in the number of NE cells is related to tumor progression
(6). However, whether an increase
in NE cell secretory products as well as their synthesis and
metabolism contribute to tumor progression remains to be
determined.
Serotonin is a well-known neurotransmitter which
exhibits multiple non-neural functions involved in essential
hypertension (7), early
embryogenesis (8), follicle
maturation (9) and behavior
(10). Serotonin acts as a growth
factor in various types of non-tumor cells and has been associated
with oncogenes (11,12). The essential amino acid tryptophan
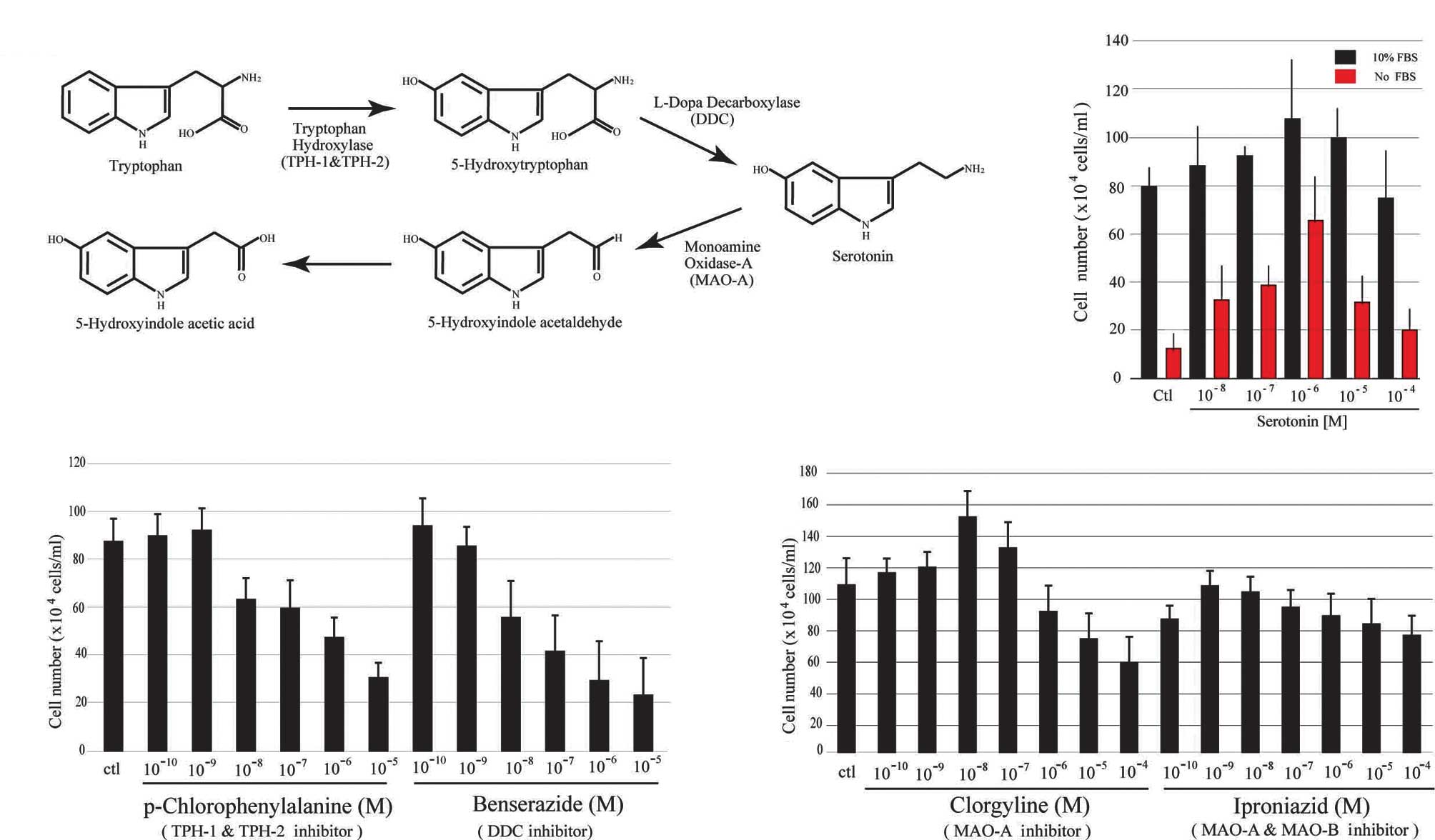
is the precursor of 5-HT, and is synthesized in two steps catalyzed
by the enzymes, tryptophan hydroxylase (TPH) and dopa decarboxylase
(DDC). The major metabolic product of 5-HT is 5-hydroxyindoleacetic
acid (5-HTIAA) via degradation by monoamine oxidase A (MAO-A)
(Fig. 1A). TPH is the rate-limiting
enzyme in 5-HT synthesis and exists in the forms of TPH-1 and
TPH-2. TPH-1 is generally found in the pineal body and gut, and
TPH-2 is selectively expressed in brain. DDC is known as a key
molecule for neuronal disease (13). Findings of a recent study indicated
that DDC is a novel co-activator of the androgen receptor (AR) in
prostate tissues (14).
Materials and methods
Reagents
Serotonin and all drugs were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Reagents were dissolved in
sterile distilled water or dimethyl sulfoxide.
In vitro proliferation assay
LNCaP and PC-3 cells were seeded in a 96-well plate
and cultured in serum containing media at 37°C. After 24 h, the
cells were washed with phosphate-buffered saline (PBS) twice, and
then replaced with the reagents (serotonin and antagonists)
containing culture media [fetal bovine serum (FBS; +FBS or −FBS)].
Cell proliferation was assessed, and changes in cell number were
quantified using an Alamar blue assay (Invitrogen) and trypan blue
measurement 96 h after adding the reagents. Briefly, after adding
10 μl Alamar blue reagent (Invitrogen) to the well (100 μl media),
the 96-well plates were incubated at room temperature for 1 h. The
optical density of the cells was read at 570 nm using a
spectrophotometric plate reader. To confirm the Alamar blue assay,
the cell numbers were measured using trypan blue staining. Each
experiment was repeated on five separate occasions, each time using
a quadruple sample. Cell viability is expressed as a percentage of
the optical density of the control, defined as 100%. Data were
analyzed, and the statistical significance was accepted at
p<0.05.
Serotonin ELISA assay
The serotonin ELISA kit was purchased from IBL,
Germany. Each cell line was homogenized at 4°C in RIPA buffer [50
mM Tris-HCl (pH.8.0), 150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 50
mM NaF, 1 mM EDTA, 10 μg/ml leupeptin, 10 KIU/ml aprotinin, 1 mM
PMSF, 1 mM DTT] and adjusted to 1 mg/ml using the Protein Assay kit
(BioRad, Japan). The ELISA and protein assays were performed
according to the manufacturer’s instructions and were repeated 3
times each. Data were analyzed, and the statistical significance
was accepted at p<0.05.
Serotonin measurement using high
performance liquid chromatography
Each cell line was homogenized in 0.2 mol/l ice-cold
perchloric acid, and the homogenate was cooled in ice for 0.5 h for
deproteinization. The homogenate was centrifuged at 20,000 × g for
10 min at 4°C. The sample was then filtered through a 0.45-μm
filter (Millipore, USA) at 20,000 × g for 20 min at 4°C. The 30-μl
filtrate was applied to a high performance liquid chromatography
(HPLC) system (Eicom, Kyoto, Japan) with a 150×2.1 mm octadecyl
silane column (SC-50DS, Eicom) and electrochemical detector
(ECD-300, Eicom) at an applied potential of +700 mV versus an
Ag/AgCl reference analytical electrode. Changes in electric current
(nA) were recorded by computer using an interface system (Power
Chrom ver. 2.3.2.J). The mobile phase was composed of aceto-citric
acid buffer (0.1 M), methanol, sodium-1-octane sulfonate (0.46 M)
and disodium ethylenediaminetetraacetic acid (0.015 M)
(830:170:1.9:1) at a flow rate of 0.2 ml/min. The concentrations of
5-hydroxytryptophan, serotonin and 5-HTIAA were determined, and the
cell levels were calculated.
Serotonin detection using
immunohistochemistry
Cells were cultured on 8-well chamber slides, fixed
in 4% paraformaldehyde (Sigma-Aldrich) and permeabilized in 98%
ice-cold ethanol. Donkey serum (10% v/v) was used to block the
non-specific binding of antibodies. The slide was then incubated at
room temperature for 1 h in the primary antibodies. A rabbit
polyclonal antibody against serotonin was used (Sigma) (1:2000).
The slides were washed and incubated with Alexa Fluor 594 goat
anti-rabbit IgG (Invitrogen) (1:1000) at room temperature for 1 h,
and then washed and incubated with DAPI antibody for DAPI
staining.
RT-PCR analysis
RNA was isolated from the LNCaP, PC-3 and PrEC cell
lines using TRIzol reagent (Invitrogen) according to the
manufacturer’s instructions. cDNA synthesis was performed using
SuperScript II (Invitrogen) for RT-PCR. Amplification of the human
TPH-1 (Gene Bank: NM_004179), human TPH-2 (Gene Bank: NM_173353),
human DDC (Gene Bank: BC008366), human AR (Gene Bank: L29496),
human MAO-A (Gene Bank: NM_000240), human PSA (Gene Bank:
DQ893851), human androgen receptor (AR) (Gene Bank: L29496), and
human GAPDH (Gene Bank: NM_002046) was conducted using specific
primers and PCR conditions. The primers used were: Human TPH-1,
forward: 5′-ATGATTGAAG ACAATAAGGAG and reverse: 5′-AAGTTTTTGAGATACT
CTCTG; human TPH-2, forward: 5′-ATGCAGCCAGCAATGA TGATGT and
reverse: 5′-ACATCCTCTAGCTCTTCTTCCT; human DDC, forward:
5′-ATGAACGCAAGTGAATTCCGA AGG and reverse:
5′-GCCTTTGGTAGTTCCAGCATCTTC; human MAO-A, forward:
5′-AGTATCGCGGGCCACATGTT and reverse: 5′-ACCGCCTAGCAGTCTTTGTC; human
AR, forward: 5′-ATGCAACTCCTTCAGCAACAGC and reverse:
5′-GGACTTGTAGAGAGACAGGGTA; human PSA, forward:
5′-ATGTGGGTCCCGGTTGTCTT and reverse: 5′-GTCCA TGACCTTCACAGCATCC,
and human GAPDH, forward: 5′-GCCTGGTCACCAGGGCTGCTTT and reverse:
5′-GCC AGGGGTGCTAAGCAGTTGG. The PCR conditions were: initial
denaturation of 94°C for 5 min followed by 20 cycles at 94°C for 30
sec, and 51°C (for TPH-1), 52°C (for TPH-2), 56°C (for DDC), 57°C
(for MAO-A), 54°C (for AR), 63°C (for PSA), or 65°C (for GAPDH) for
20 sec, and a final extension of 72°C for 15 sec. Negative controls
with no RT product were routinely performed (data not shown).
Small interfering RNA transfection
siRNAs were purchased for the targeting of human DDC
and TPH-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) which
were transfected to LNCaP cells following the manufacturer’s
instructions. Briefly, cells were seeded in a 6-well plate at
1×105 cells/well in antibiotic-free media containing 10%
FBS. Following a 20-h incubation, the siRNA solution was added to
the well, and incubation was carried out at 37°C for 5 h. Cells
were washed 3 times with PBS, and the media were replaced with
fresh media supplemented with antibiotics (penicillin/streptomycin)
and 5-HT (final volume of 10 μM). Cell proliferation was assessed,
and changes in the cell numbers were quantified using the Alamar
blue assay and trypan blue measurement after 3, 5 and 7 days of
incubation.
Results
Serotonin 5-HT acts as a cell growth factor, as
previously reported (9). As shown
in Fig. 1B, 5-HT promoted the
proliferation of a prostate cancer cell line, LNCaP, in a
dose-dependent manner. The PC-3 and DU145 cell lines also provided
similar results (data not shown). To further confirm this activity,
the same experiments were conducted again using serum-free media
(red bar, Fig. 1B). 5-HT was found
in serum and was circulated in the entire body for the maintenance
of homeostasis (15). The role of
5-HT as a cell growth factor was indicated when the results of our
experiments were compared with those using serum-containing media.
In LNCaP cells, inhibitors of 5-HT synthesis, p-chlorophenylalanine
(Sigma, C8655) for TPHs (Fig. 1C)
and benserazide (Sigma, B7283) for DDC (Fig. 1C) had an almost 50% inhibitory
effect on cell proliferation at a concentration of 10 μM at 72 h of
culture incubation compared to the control cells. Other
antagonists, including fenfluramine for tryptophan hydroxylase
(TPH) and carbidopa for dopa decarboxylase (DDC), showed a similar
inhibitory effect on the three cell lines (data not shown). The
effect of the irreversible inhibitor of monoamine oxidase A (MAO-A)
(clorgyline, Sigma, M3778) on LNCaP cells was also investigated
(Fig. 1D). In the presence of 0.01
μM clorgyline, cell growth was increased by 30% when compared to
the controls, although a higher concentration of clorgyline
resulted in 40% inhibition of cell proliferation when compared to
the controls. The non-selective MAO inhibitor, iproniazid (Sigma,
I7627) exhibited no significant inhibitory effect on cell growth
(Fig. 1D). Each proliferation assay
in Fig. 1 was repeated on five
separate occasions. The results strongly indicate that the 5-HT
synthesis and metabolism system plays a regulatory role in the
proliferation of these prostate cell lines.
To investigate the expression of 5-HT, TPH-1, DDC
and MAO-A in normal prostate cells (PrEC, Takara, C2555) and
prostate cancer cell lines (LNCaP and PC-3), we used a combination
of immunohistochemical, ELISA, and HPLC analyses for 5-HT, and
RT-PCR for the remaining molecules. We first analyzed for 5-HT
synthesis and mRNA expression of the metabolic components by RT-PCR
using specific primers in PrEC, LNCaP and PC-3 cells. Moreover, we
analyzed prostate-specific antigen (PSA) and AR, which were already
characterized in the prostate cell lines (Fig. 2A). The expression of DDC in the PC-3
cells was 3 times that of PrEC cells but no significant difference
was noted in the DDC expression between the LNCaP and PrEC cell
lines. On the other hand, a high MAO-A expression correlated with
that in the LNCaP cells. PC-3 cells exhibited the lowest expression
of TPH-1 when compared to the three cell lines. These data indicate
that 5-HT synthesis and metabolic components are expressed in
prostate cell lines. We then investigated whether differences in
the expression of each gene in the PrEC, LNCaP and PC-3 cells
correlated with the production of 5-HT in these cell lines.
Serotonin immunoreactivity was detected in these cells using an
anti-5-HT Ab, and whole cell conditions were confirmed by
DAPI-staining (Fig. 2B, inset
panels). Serotonin content as measured by ELISA showed different
values for the three cell lines (Fig.
2B). Although, a small difference in 5-HT content between the
normal prostate and prostate cancer cell lines was anticipated,
PC-3 cells exhibited almost 3 times the amount of 5-HT compared to
the LNCaP cell line which consisted of half of the 5-HT content of
the PrEC cells. The contents of the 5-HT precursor (5-HTP), 5-HT,
and 5-HIAA were also measured using HPLC (Fig. 2C). The results correlated to a
similar extent with the gene expression levels. Thus, serotonin was
synthesized and metabolized in each cell line and regulated the
identity of cells by its release.
The effect of signaling via 5-HT receptors is
considered to have an essential role in the effects of cell
proliferation of 5-HT (16).
However, our results indicate that both the synthesis and release
of 5-HT in cells plays a role in cell proliferation. Thus, these
effects were investigated using siRNA targeted to DDC and TPH-1 in
LNCaP cells (Fig. 3). Cells treated
with each siRNA inhibited growth almost completely, and the
addition of 5-HT did not induce cell proliferation. These results
show that the serotonin synthesis and metabolism system has an
essential role in cell growth.
Discussion
This is the first study to show that serotonin or
5-HT synthesis and its release are involved in prostate cancer cell
lines. The primary hypothesis regarding serotonin involvement in
prostate cancer was proposed by Lembeck in 1953 (17). On the other hand, a more general
hypothesis termed the APUD (amine precursor uptake and
decarboxylation) cell concept (18)
has been proposed based on the synthesis and metabolism system.
Regarding prostate cancer generation, much evidence from clinical
research can be elucidated in light of the APUD cell concept.
Our results are also in accordance with the APUD
cell concept. 5-HT is found in both normal prostate and cancer cell
lines, and the system of 5-HT synthesis and its release is
functional. Thus, 5-HT is essential for normal cell proliferation.
In the event of deviation to the 5-HT synthesis process, the
initial step of tumor progression may occur since the gene
knockdown of dopa decarboxylase (DDC) and tryptophan hydroxylase
(TPH-1), using siRNA in the cell lines, was found to inhibit cell
proliferation. Moreover, additional 5-HT stimulation to the gene
did not affect cell proliferation in the cell lines. Evidence
indicates that DDC binds directly with AR, which has an essentia0l
role in prostate tumor generation and prostate cancer progression
(17). LNCaP cells exhibit
AR-dependent cell growth as compared to PC-3 cells. This fact
indicates that DDC is more essential than AR in prostate cancer
progression in that DDC-knockout mouse show a lethal phenotype. No
literature regarding prostate in the mouse is currently available,
but DDC conditional mouse targeted to prostate affects prostate
tissues (unpublished data). The 5-HT synthesis process, mainly
involving DDC, may regulate prostate cell proliferation directly.
Thus, the development of specific DDC inhibitors are crucial for
the future clinical assessment of DDC as an effective cancer
target.
Acknowledgements
This study was supported by grants-in-aid from the
Ministry of Education, Science and Culture of Japan.
References
|
1
|
Chin SN, Wang L, Moore M and Sridhar SS: A
review of the patterns of docetaxel use for hormone-resistant
prostate cancer at the Princess Margaret Hospital. Curr Oncol.
17:24–29. 2010.PubMed/NCBI
|
|
2
|
Sandberg AA: Endocrine control and
physiology of the prostate. Prostate. 1:169–184. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abrahamsson PA: Prostate cancer and active
surveillance. Front Radiat Ther Oncol. 41:1–6. 2008. View Article : Google Scholar
|
|
4
|
Hansson J and Abrahamsson PA:
Neuroendocrine differentiation in prostatic carcinoma. Scand J Urol
Nephrol Suppl. 212:28–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seuwen K and Pouyssegur J: Serotonin as a
growth factor. Biochem Pharmacol. 39:958–990. 1990. View Article : Google Scholar
|
|
6
|
Waguespack SG, Rich T, Grubbs E, Ying AK,
Perrier ND, Ayala-Ramirez M and Jimenez C: A current review of the
etiology, diagnosis, and treatment of pediatric pheochromocytoma
and paraganglioma. J Clin Endocrinol Metab. 95:2023–2037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rapport MM, Green AA and Page IH: Serum
vasoconstrictor (serotonin) IV: Isolation and characterization. J
Biol Chem. 176:1243–1251. 1948.PubMed/NCBI
|
|
8
|
Fukumoto T, Kema PI and Levin M: Serotonin
signaling is a very early step in patterning of the left-right axis
in chick and frog embryos. Curr Biol. 15:794–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buznikov GA, Lambert HW and Lauder JM:
Serotonin and serotonin-like substances as regulators of early
embryogenesis and morphogenesis. Cell tissue Res. 305:177–186.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marino J and Caballero J: IIoperidone for
the treatment of schizophrenia. Ann Pharmacother. 44:863–870. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Julius D, Livelli TJ, Jessel TM and Axel
R: Ectopic expression of serotonin 1c receptor and triggering of
malignant transformation. Science. 244:1057–1062. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siddiqui EJ, Thompson CS, Mikhailidis DP
and Mumtaz FH: The role of serotonin in tumour growth (review).
Oncol Rep. 14:1593–1597. 2005.PubMed/NCBI
|
|
13
|
Feng LR and Maguire-Zeiss KA: Gene therapy
in Parkingson’s disease: rationale and current status. CNS drugs.
24:177–192. 2010.
|
|
14
|
Margiotti K, Wafa LA, Cheng H, Novelli G,
Nelson CC and Rennie PS: Androgen-regulated genes differentially
modulated by the androgen receptor coactivator L-dopa decarboxylase
in human prostate cancer cells. Mol Cancer. 6:38–50. 2007.
View Article : Google Scholar
|
|
15
|
Richard DM, Dawes MA, Mathias CW, Acheson
A, Hill-Kapturczak N and Dougherty DM: L-tryptophan: basic
metabolic functions, behavioral research and therapeutic
indications. Int J Tryptophan Res. 2:45–60. 2009.PubMed/NCBI
|
|
16
|
Siddiqui EJ, Shabbir M, Mikhailidis DP,
Thompson CS and Mumtaz FH: The role of serotonin
(5-hydroxytryptamine 1A and 1B) receptors in prostate cancer cell
proliferation. J Urol. 176:1648–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lembeck F: 5-Hydroxytryptamine in a
carcinoid tumor. Nature. 172:910–911. 1953. View Article : Google Scholar
|
|
18
|
Pearse AG: The diffuse neuroendocrine
system and the apud concept: related ‘endocrine’ peptides in brain,
intestine, pituitary, placenta, and anuran cutaneous glands. Med
Biol. 55:115–125. 1977.
|