Introduction
Globally, breast cancer is the most common form of
malignant neoplasia in females, contributing to 23% of all types of
cancer (1). Breast cancer accounts
for 10–18% of all cancer-related deaths and is the most common
cause of cancer-related death in industrialized countries, and the
third in developing countries (1–5).
Breast cancer incidence is on the increase
worldwide, but it varies from areas of low incidence (Japan and
other Asian countries, and Latin American and African countries) to
areas of high incidence (US, Western Europe, Northern Europe and
Australia). For example, in the US less than 0.9 new cases per 1000
women were reported in the 1990s, and more than 1.4 new cases per
1000 were reported in 2006 (1,2,5). The
incidence of breast cancer has also shown a steady increase during
the last 30 years in the Nordic countries. The incidence in Finland
rose from 63.3 per 100,000 female individuals in 1989 to 87.6 in
2007 (6). In the UK, approximately
one in nine female individuals is likely to develop the disease
during her lifetime (7). An
increase has even been noted in the low incidences of breast cancer
in Eastern Europe and Japan (8,9).
Various studies in the US found that female
individuals of African descent have a lower breast cancer incidence
but higher breast cancer mortality rates than Caucasian women. Poor
survival may be related to the fact that female individuals of
African descent are more likely to be diagnosed at an early age but
with an advanced stage of disease (3,5,10,11).
In the sub-Saharan African population low rates of breast cancer
exist, and the majority of patients are premenopausal and present
at an advanced stage (2).
In Arabic countries studies are not comprehensive.
In Morocco, the most frequently occurring cancer in females is
cervical uterine neoplasia (35%) followed by breast cancer (22.3%),
which is also diagnosed at advanced stages (12). In Egypt, approximately 35% of all
female cancer is breast cancer (4).
The background of Arabic patients may be related to a greater
extent to other African breast cancer patients as compared to
European breast cancer patients, although demographic and
environmental differences, e.g,. between Libyan, Nigerian and
European (Finnish) populations, are prominent (Table I). In this study, various
demographic and clinicopathological factors associated with breast
cancer in Libya were identified. The Libyan population and breast
cancer patients were compared with the Nigerian and the Finnish
population and patients as noted in previous studies (13). To the best of our knowledge, such
comparisons have not previously been published. An estimate of the
incidence of female breast cancer in Western Libya was made based
on the Sabratha cancer Registry Database.
 | Table IPopulation data for Libya, Nigeria and
Finland. |
Table I
Population data for Libya, Nigeria and
Finland.
| Libya | Nigeria | Finland |
|---|
| Total population |
6,342,000(07)a | 144,077,000(07)
a | 5,286,000(07)
a |
| Age structure
(%) |
| 0–14 years | 30%a |
44.4%d |
17.1%e |
| 15–64 years |
66.2%a |
52.6%d |
66.4%e |
| >64 years | 3.8%a | 3.0%d |
16.5%e |
| Population growth
rate (%) |
2.3b,f | 2.67d | 0.46e |
| Birth rate
(births/1.000 population) | 26.8b | 41.3a | 11.8e |
| Death rate
(deaths/1.000 population) |
3.5b,f | 17.2a | 9.1e |
| Gender ratio (Total
population; m/f) | 1.05b | 1.05a | 0.95d |
| Infant mortality
(deaths/1.000 population) |
24.6b |
74.18d |
3.82d |
| Life expectancy
(years) |
76.6b,f |
52.0c |
79.3e |
| Female |
78.8b,f |
52.0c |
82.8e |
| Male |
74.3b,f |
52.0c |
75.8e |
| Total fertility
rate |
3.34b |
5.9a |
1.8e |
| Literacy |
84.1%b |
76.3%a |
100%a,c,d,e |
| GDP (in US$) |
8,298b,f |
970d |
21,000d |
Materials and methods
Libyan data
Clinical and pathological
characteristics
A retrospective pathologic study was conducted on
234 patients (Table II). The
patients were treated at the African Oncology Institute (AOI)
during the 5-year period from 2002 to 2006. The estimated clinical
or pathological characteristics included age at presentation, age
at first pregnancy, parity, age at menarche, menopausal status at
diagnosis, size of tumor, LN status, stage, histological grade,
histological type and follow-up history of the patient. Patients
were followed-up until they succumbed to the disease or to the end
of the observation period in the middle of January 2007. Some
patients were lost during follow-up prior to January 2007. For
these patients, the last date of contact was defined as the date
for the end of follow-up. The follow-up period ranged from 1 to 74
months with an average of 22.0 months. Table II shows all of the breast cancer
patients during the years 2002–2006 admitted to the AOI. Table III shows that the number of
admissions during the study period varied, and that a complete
follow-up record could not be found for all patients. This reflects
the situation in local hospitals before the Sabratha Cancer
Registry program was initiated. Many patients entered the hospital
for a preliminary diagnosis, after which they consulted their
families and decided on a treatment. Many patients remained in the
hospital until the histopathological diagnosis, usually after the
primary surgery was carried out, and then decided on further
treatment. Some patients commenced therapy at the AOI, but
interrupted the treatment and were lost from follow-up. Finally,
approximately half of the patients were diagnosed, treated,
followed up, and received further therapy at the Institute. The
patients lost to follow-up were generally treated elsewhere (other
Libyan hospitals or abroad).
 | Table IIBreast cancer patients admitted to
the African Oncology Institute, Sabratha, Libya, during the years
2002–2006. |
Table II
Breast cancer patients admitted to
the African Oncology Institute, Sabratha, Libya, during the years
2002–2006.
| Year | No. of breast
cancer patients admitted | No. of breast
cancer patients with available history and follow-up
information | No. of new breast
cancer patients in the Sabratha Regional Registry |
|---|
| 2002 | 99 | 23 | 41 |
| 2003 | 101 | 54 | 42 |
| 2004 | 70 | 31 | 44 |
| 2005 | 95 | 40 | 54 |
| 2006 | 108 | 86 | 50 |
| Total | 473 | 234 | 231 |
 | Table IIILibyan Cancer Registries initiated in
2007a. |
Table III
Libyan Cancer Registries initiated in
2007a.
| Location of cancer
registry | Regions and cities
covered by the registry |
|---|
| 1. Tripoli | Tripoli, Aljafara,
Almergaib, Aljabal Algarbi |
| 2. Benghazi | Benghazi, Albatnan,
Darna, Aljabal Alakhader, Almarg, Alwahat, Alkufra, Ejdabiya |
| 3. Sabha | Sabha, Morzuk, Wadi
Alhiya, Wadi Shatee, Ghat |
| 4. Musrata | Musrata, Sirt,
Aljufra |
| 5. Sabratha | Zawia, Alnikat,
Nalut |
Cancer registration in Libya
The precise number of cancer cases diagnosed each
year in Libya is unknown since a complete cancer registry has not
yet been established in a number of areas. In 2007, the Ministry of
Health created the National Cancer Registry Program, following the
commencement of the Sabratha Cancer registry in 2006. As part of
this program, Libya currently has five Cancer Registries (14) (Table
III).
Evaluation of breast cancer
incidence
Evaluation of breast cancer incidence was based on
the 2006 data obtained from the Sabratha Cancer Registry. The
histological diagnoses were based on available pathology reports.
Two male patients and three non-epithelial malignant cases were
excluded. Two patients did not have evidence of a histological
diagnosis and were excluded. The incidence data were consequently
based on the histologically confirmed cases for the year 2006, when
the Sabratha Registry commenced its first year of operation.
Data from Finland and Nigeria
A portion of the data was previously presented in
the study by Ikpatt et al (13). Updated data were used, when
available (15–19).
Statistical analysis
Variables of the Libyan patients were grouped, and
descriptive statistics were calculated for the continuous variables
by using SPSS 16.0 for Windows. For survival analysis, Kaplan-Meier
curves were plotted, and differences between the curves were
analyzed using the log-rank test. Statistical differences among the
three countries were determined by the chi-square test and
Student’s t-test. P<0.05 was considered to be statistically
significant. Statistical analyses and graphs were produced by the
Excel program.
Results and discussion
Breast cancer incidence
There were 101 breast cancer patients among the 299
female cancer patients (33.7%) admitted to the AOI in 2006. This
was in agreement with figures from North African countries; in
Egypt, the corresponding percentage was approximately 35% (2,4). The
Libyan figure also compares favorably with the European and North
American figures (27.3 and 31.3% of cancers in females,
respectively) (1).
Of the above-mentioned 101 patients, 50 were from
the Sabratha Registry region. The population of this region in 2006
was 542,708, as reported by the Libyan Census Committee (14). The male:female ratio in the Libyan
population was 1.05:1 (16). Based
on these data, the incidence was 18.89 new cases per 100,000 Libyan
females.
Evidence shows that breast cancer in western Libya
is on the increase (Fig. 1). This
increase may be attributed to the development in health care,
including improved diagnostic facilities. The breast cancer
incidences in Libya, Nigeria and Finland were 18.9, 33.6 and 87.6
per 100,000 females, respectively. The incidence in Libya is
markedly lower than that in Europe or the US, but is also lower
than the incidence in Nigeria. The breast cancer incidence in
Nigeria has shown a marked increase from 13.7 per 100,000 in the
1970s, to 33.6 per 100,000 in the 1990s (2,13).
Lifestyle differences may be involved, but biological differences
as causes cannot be excluded (5,10). The
incidence in Libya is in concordance with results published from
other North African countries (Tunisia 19.6, Egypt 24.2 and Algeria
23.4) (2,14,20).
The apparently slow increase in incidence may be related to
improved diagnostic practice (mammography, immunostaining) in the
last few years in Libya.
Age at presentation
The occurrence of breast cancer in the female Libyan
population is strongly associated with young age with nearly 70.9%
of cases arising in female individuals who are 50 years or younger.
The median age is 44.0 years, and the mean age, 46.0 years
(Fig. 2).
The difference between the mean ages of Libyan and
Finnish patients was statistically significant (p<0.001),
whereas no statistical difference was noted in age at diagnosis
between Libyan and Nigerian patients (Table IV). The difference between African
and European patients may partly be associated with the age
distribution of populations in the respective countries, but
biological differences may also be involved.
 | Table IVMean age at diagnosis, mean age at
first pregnancy and menopausal status of breast cancer patients in
Libya, Nigeria, and Finland. |
Table IV
Mean age at diagnosis, mean age at
first pregnancy and menopausal status of breast cancer patients in
Libya, Nigeria, and Finland.
| Variable | Libya | Nigeriaa | Finlanda |
|---|
| Mean age at
diagnosis (±SD), in years | 46.0±12.3 | 42.7±12.1 | 58.8±12.5 |
| Mean age at first
pregnancy, in years | 22.1 | 20.8 | 25.6 |
| Menopausal status,
n (%) |
| Premenopausal
patients | 160 (68.4) | 223 (74.3) | 93 (32.6) |
| Postmenopausal
patients | 74 (31.6) | 77 (21.3) | 192 (67.4) |
The variation in genetic marker distribution between
central and north African and European populations is also involved
(21), suggesting that in the
African population, characterized by ‘African’ genomic haplotypes,
the premenopausal type of breast cancer is more common than the
postmenopausal type. In Europe, where the population is
characterized by ‘European’ genomic haplotypes, the contrary is the
case.
Age at first pregnancy
Age at first pregnancy was available from 44 out of
234 Libyan breast cancer patients. The average age at first
pregnancy was 22.1 years. In addition, there were 40 female
individuals without previous pregnancies. The mean age of all of
the women at first pregnancy was not available for the female
population of Libya. The age at first pregnancy in breast cancer
patients in Nigeria was similar to that in the Libyan cases (20,8
years), while Europeans had a higher mean age (25.6 years)
(Table IV). Patients with benign
breast disease were found to have a lower age at first pregnancy
than breast cancer patients (22).
Fertility
Infertility and an unmarried status for females were
associated with an increased risk of breast cancer (23). Since the fraction of unmarried
female individuals (between ages 34 and 40) was higher among the
Libyan breast cancer patients as compared to the North African
population in general (23.8% and 15–21%, respectively) (24), childlessness was a risk factor for
breast cancer.
Menopausal status
In Libya, almost 68.4% of breast cancer patients
were premenopausal, and 31.6% were postmenopausal. The Nigerian
data were similar. African breast cancer was markedly different
from European cases in this respect. In Europe and among US
Caucasian females most patients were postmenopausal (Table IV).
In light of the above findings, breast cancer can
principally be divided into two types. The postmenopausal type is
more common in Europe and North America, and the premenopausal type
is more common in Africa.
Stage
The staging system currently in use for breast
cancer is based on the size of the primary tumor, degree of spread
to lymph nodes and the presence of systemic metastasis. The TNM
classification, adapted by the International Union Against Cancer
(16) can also be utilized. The
Libyan breast cancer staging in our study was based on the TNM
classification of 1997 (25)
(Table V). The majority of Libyan
patients were in stage 2B and 3A, and approximately 51% were
classified in stages 3 and 4.
 | Table VThe frequency of the different stages
determined among the 234 breast cancer patients according to the
staging manual of the International Union Against Cancer (5th
edition). |
Table V
The frequency of the different stages
determined among the 234 breast cancer patients according to the
staging manual of the International Union Against Cancer (5th
edition).
| Clinical stage | Corresponding TNM
categories | Frequency | Percentage |
|---|
| 0 | Tis N0 M0 | 0 | 0 |
| 1 | T1N0 M0 | 12 | 5.1 |
| 2 | T2N0M0 and
T0/1N1M0 | 28 | 12.0 |
| T2N1M0 and
T3N0M0 | 75 | 32.1 |
| 3 | T1/2/3N2M0 and
T3N1M0 | 60 | 25.6 |
| T4NM0 and
TN3M0 | 26 | 11.1 |
| 4 | T0-4N0-3M1 | 33 | 14.1 |
| Total | | 234 | 100.0 |
The large percentage of patients in advanced stages
indicates delayed presentation and late diagnosis, as noted in the
study by Ikpatt et al (13)
on Nigerian breast cancer. Mammography was not performed in
Nigeria, but has been performed in Libya, although not in screening
programs. However, mammography has not been able to improve early
diagnosis. The reason includes the difficulties involved in
obtaining an early mammographic diagnosis in premenopausal breast
cancer (26). The biological
aggressiveness of the premenopausal type also appears to limit the
value of early screening (27).
These results are in agreement with other North African results. In
Egypt, breast cancer is largely responsible for cancer-related
deaths among females (8.2%), and the majority of tumors are
advanced at presentation (2,4). In
Tunisia, breast cancer is associated with poor survival due to late
diagnosis (2,27). Approximately 55% of the breast
cancer patients presenting at the Tunisian Oncology Institute of
Salah Aziiz were found to be characterized by rapid disease
progression, inflammation and edema (2,28).
Tumor size
In Libya, 15 (6.4%) patients had a tumor size
smaller than 2 cm (T1). The average tumor size and tumor range in
the Libyan patients were compared with the Nigerian and Finnish
patients (Table VI). The Libyan
and Nigerian data are similar, whereas the data from Finland
suggest the efficacy of mammography in screening in that
population. This suggests that initiation of mammographic screening
in peri- and postmenopausal patients should be considered in
patients from Libya (26).
 | Table VIComparison of the distribution of
tumor size, lymph node status, histological grade and stage in
breast cancer patients in Libya, Nigeria and Finland. |
Table VI
Comparison of the distribution of
tumor size, lymph node status, histological grade and stage in
breast cancer patients in Libya, Nigeria and Finland.
| Variable | Libya | Nigeriaa | Finlanda |
|---|
| Tumor size
(cm) |
| Diameter (SD) | 4.8 (2.1) | 4.8 (2.4) | 2.6 (1.9) |
| Range | 1.5–12.5 | 1.0–11.0 | 1.0–15.0 |
| Lymph node
involvement, n (%) |
| Positive | 173 (73.9) | 235 (79.1) | 97 (34.0) |
| Negative | 61 (26.1) | 62 (20.9) | 188 (66.0) |
| Grade, n (%) |
| 1 | 11 (6.6) | 44 (14.8) | 67 (23.5) |
| 2 | 104 (62.3) | 119 (40.1) | 173 (60.7) |
| 3 | 52 (31.1) | 137 (45.1) | 45 (15.8) |
| Stage at
presentation, n (%) |
| 1 | 12 (5.1) | 65 (21.7) | 95 (31.25) |
| 2 | 103 (44.1) | 75 (25.0) | 171 (56.25) |
| 3 | 86 (37.6) | 97 (32.3) | 19 (6.25) |
| 4 | 33 (14.1) | 63 (21.0) | 19 (6.25) |
Lymph node status and distant
metastases
Most Libyan breast cancer patients had regional
lymph node involvement at the time of surgery (Table VI); 21.8% (51 out of 234 patients)
were in category N2. Distant metastases (to bone and liver) were
present in 12.8% of our patients.
In the present study and that by Ikpatt et al
(13), systemic and regional lymph
node metastases were significantly more common in African patients
than in Finnish ones (Table VI).
This suggests a delay in diagnosis in developing countries and a
lack or inefficacy of screening programs. However, the
aggressiveness of biological features (such as the dominance of the
premenopausal type) in African breast cancer should also be
considered.
Histological type
The histological types of the breast cancer cases in
Libya, Nigeria and Finland are shown in Table VII. In all three populations, the
non-specific variety of invasive ductal carcinoma was the
predominant type. Medullary carcinoma was more common in African
populations than in European cases. Genetic factors may be
involved; however, no evidence currently exists that breast cancer
genes (BRCA1 and BRCA2) are more often involved in breast cancer
cases in Africa (2).
 | Table VIIHistological type of breast cancer
among Libyan, Nigerian and Finnish patients. |
Table VII
Histological type of breast cancer
among Libyan, Nigerian and Finnish patients.
| Histological type,
n (%) | Libya | Nigeriaa | Finlanda |
|---|
| Ductal | 191 (81.6) | 252 (84.0) | 244 (85.6) |
| Lobular | 17 (7.3) | 10 (3.3) | 16 (5.6) |
| Medullary | 11 (4.7) | 8 (2.7) | 2 (0.7) |
| Tubular | 5 (2.1) | 8 (2.7) | 8 (2.7) |
| Mucinous | 3 (1.3) | 5 (1.7) | 0 (0.0) |
| Mixed
ductal/tubular | 0 (0.0) | 3 (1.0) | 3 (1.1) |
| Mixed
ductal/lobular | 6 (2.6) | 5 (1.7) | 2 (0.7) |
| Apocrine
carcinoma | 1 (0.4) | 2 (0.7) | 0 (0.0) |
| Metaplastic
carcinoma | 0 (0.0) | 3 (1.0) | 0 (0.0) |
| Total | 234 (100) | 297 (100) | 285 (100) |
Histological grade
Libyan and Nigerian patients had a higher tumor
grade than Finnish ones (Table
VI). Results in African patients are in concordance with those
of African-American patients (5,10). One
explanation for the grade differences may involve the more active
proliferation in the premenopausal type of breast cancer, which is
more common in Africa.
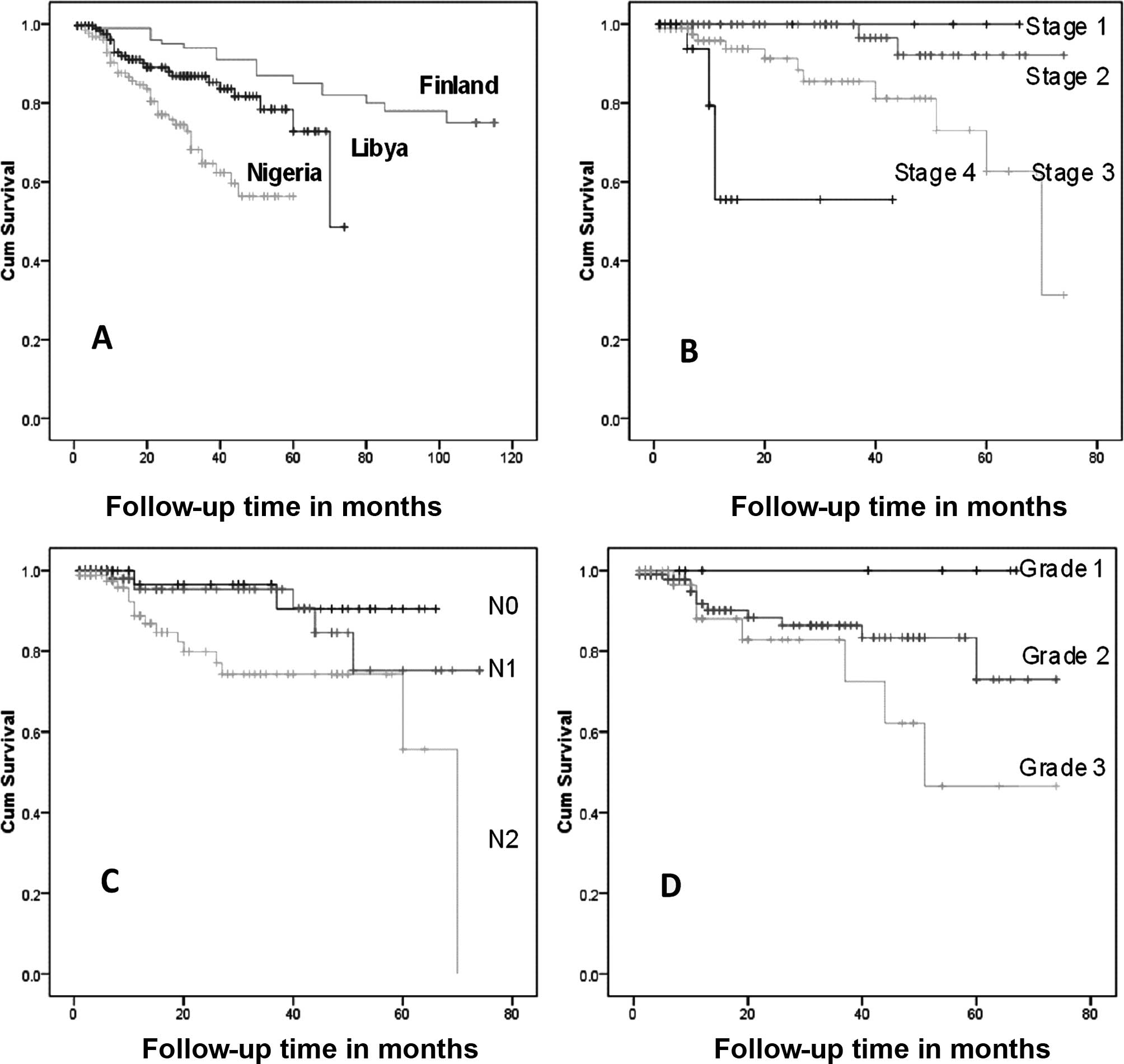
Survival
The African breast cancer cases were associated with
a worse prognosis than the European breast cancer ones (Fig. 3). However, as regards tumor stage,
lymph node involvement and large tumor size, African breast cancer
acts in the same manner as the European breast cancer, as shown by
the Libyan data (Fig. 3B-D)
(29–31). Among Libyan patients the menopausal
status, histological type of tumor, and patient age does not appear
to affect survival.
A comparison of the survival curves of the patients
of 3 countries (Fig. 3A) showed
marked differences in survival. The survival curve of the Libyan
patients was located between that of the Nigerian and Finnish
survival curves.
In conclusion, breast cancer incidence varies
considerably with high rates in Western countries, such as Finland,
and low rates in African countries such as Libya and Nigeria.
African patients are younger than European ones at presentation.
The majority of breast cancer patients were found to be
premenopausal and at advanced stage at presentation in Africa.
African breast carcinoma cases were similar to European ones in
terms of histopathologic parameters that are known to be associated
with high risk, such as advanced stage, large-sized tumors, lymph
node involvement and high grade. The histological predominant types
were also similar in African and European breast carcinomas.
However, medullary carcinoma was found to be more common in African
patients. In Africa, premenopausal breast cancer is more common
than postmenopausal breast cancer, while the opposite is the case
for European patients.
Acknowledgements
The authors acknowledge the African Oncology
Institute in Sabratha, National Cancer Institute in Misurata and
the University of Turku for support in collecting the data and
providing the research facilities. Special thanks are due to Dr
Hussein Hashmi for his positive attitude. We also wish to thank the
Libyan Medical Specialization Board and the Libyan Health Ministry
for financing Dr Abdalla’s visit to the Department of Pathology,
University of Turku, Finland, for facilitating the comparative
study.
References
|
1
|
Parkin D and Fernandez G: Use of
statistics to assess the global burden of breast cancer. Breast J.
12:S70–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiliams C, Olopade O and Falkson C: Breast
cancer in women of African descent. Springer; Netherlands: 2006,
View Article : Google Scholar
|
|
3
|
American Cancer Society. Cancer facts and
figures for african Americans 2007–2008. American Cancer Society;
Atlanta: 2008
|
|
4
|
Nadia M, Iman G and Iman A: Cancer
pathology registry 2003–2004 and time trend analysis. NCI; Cairo:
2007
|
|
5
|
Bray F, Mc Carron P and Parkin D: The
changing global patterns of female breast cancer incidence and
mortality. Breast Cancer Res. 6:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Finnish Cancer Registry Report. http://http://www.cancerregestry.fi/stats/eng/veng001i0.htm.
2007
|
|
7
|
Statistical Information Team, Cancer
Research UK. Breast cancer. 2009, http://info.cancerresearchuk.org/cancerstats/index.htm.
|
|
8
|
Pompe-Kirn V, Japelj B and Primic-Zakelj
M: Future trends in breast, cervical, lung, mouth and pharyngeal
cancer incidence in Slovenia. Cancer Causes Control. 11:309–318.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagata C, Kawakami N and Shimizu H: Trends
in the incidence rate and risk factors for breast cancer in Japan.
Breast Cancer Res Treat. 44:75–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McBride R, Hershman D, Tsai W, Jacobson J,
Grann V and Neugut A: Within-stage racial differences in tumor size
and number of positive lymph nodes in women with breast cancer.
Cancer. 110:1201–1208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
James J: Differences in breast cancer
among African American and Caucasian women. CA Cancer J Clin.
50:50–64. 2000. View Article : Google Scholar
|
|
12
|
Chaouki N and El Gueddari B:
Epidemiological descriptive approach of cancer in Morocco through
the activity of the National Institute of Oncology from 1986 to
1987. Bull Cancer. 78:603–609. 1991.PubMed/NCBI
|
|
13
|
Ikpatt OF, Kuopio T, Ndoma-Egba R and
Collan Y: Breast cancer in Nigeria and Finland: Epidemiological,
clinical and histological comparison. Anticancer Res. 22:3005–3012.
2002.PubMed/NCBI
|
|
14
|
Sabratha Cancer Registry. First annual
report, 2006. African Oncology Institute. Sabratha, Libya: 2008
|
|
15
|
Eltwati A: Libyan healthcare system to
reform or transform? Jamahiria Med J. 7:162–163. 2007.
|
|
16
|
Libyan National Statistics Figures. Annual
Report. Tripoli: 2003, (in Arabic).
|
|
17
|
King T, Turnbull CP, Baver P, Lewis RM,
Pletcher K, Schreiber B, Shephered MC, Sparks KJ, Tikkanen A and
Wolff A: (eds from Encyclopedia Britannica, Inc.). Knauer K:
(contributing ed from Time, Inc.). Countries of the world. Time
Almanac 2009. Encyclopedia Britannica, Inc.; USA: pp. 218–544.
2009
|
|
18
|
Brazier C and Hamed A: The World Guide
2003–2004. An alternative reference to the countries of our planet.
New Internationalist Publications Ltd; Oxford: pp. 1–623. 2003
|
|
19
|
Statistics Finland. Finland in Figures.
http://www.stat.fi/tup/suoluksuoluk_vaesto_en.html.
|
|
20
|
Misurata Cancer Registry. Hospital Cancer
Registry. First annual report, 2008. 1st edition. National Cancer
Institute; Musrata, Libya: 2008
|
|
21
|
Jobling MA, Hurles M and Tyler-Smith C:
Human evolutionary genetics. Origin, peoples and disease. Garland
Science, Taylor and Francis group; New York: 2004
|
|
22
|
MacMahon B: Epidemiology and the causes of
breast cancer. Int J Cancer. 118:2373–2378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helmrich SP, Shapiro S, Rosenberg L,
Kaufman DW, Slone D, Bain C, Miettinen OS, Stolley PD, Rosenshein
NB, Knapp RC, Leavitt T Jr, Schottenfeld D, Engle RL Jr and Levy M:
Risk factors for breast cancer. Am J Epidemiol. 117:35–45.
1983.PubMed/NCBI
|
|
24
|
Rashad H, Osman M and Roudi-Fahimi F:
Marriage in the Arabic World Population reference Bureau.
www.prb.org/pdf05/marriageinarabworld_eng.pdfhttps://www.prb.org/pdf05/marriageinarabworld_eng.pdf.
2005
|
|
25
|
American Joint Committee on Cancer. AJCC
Cancer Staging Manual. 5th edition. Lippincott-Raven; Philadelphia:
1997
|
|
26
|
Smith RA: Mammography screening for breast
cancer. Northeast Florida Medicine; 2005
|
|
27
|
Gao YT, Shu XO, Dai Q, Potter JD, Brinton
LA, Wen W, Sellers TA, Kushi LH, Ruan Z, Bostick RM, Jin F and
Zheng W: Association of menstrual and reproductive factors with
breast cancer risk: results from the breast cancer study. Int J
Cancer. 87:295–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ben Ahmed S, Aloulou S, Bibi M, Landolsi
A, Nouira M, Ben Fatma L, Kallel L, Gharbi O, Korbi S, Khairi H and
Kraiem C: Breast cancer prognosis in Tunisian women: analysis of a
hospital series of 729 patients. Santé Publique. 14:231–241.
2002.PubMed/NCBI
|
|
29
|
Sant M; Eurocare Working Group.
Differences in stage and therapy for breast cancer across Europe.
Int J Cancer. 93:894–900. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galea M, Blamey RW, Elston CW and Ellis
IO: The Nottingham prognostic index in primary breast cancer.
Breast Cancer Res Treat. 22:207–219. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellis IO, Galea M, Broughton N, Locker A,
Blamey RW and Elston CW: Pathological prognostic factors in breast
cancer. II. Histological type. Relationship with survival in a
large study with long-term follow-up. Histopathology. 20:479–489.
1992. View Article : Google Scholar : PubMed/NCBI
|