Introduction
The successful treatment of Hodgkin’s lymphoma (HL)
is regarded as one of the most significant accomplishments in
cancer therapy over the last century. The introduction of extended
field radiotherapy and mechlorethamine, vincristine, procarbazine
and prednisone (MOPP) combination chemotherapy has resulted in a
cure for more than 60% of patients (1). Further progress in prognostic
definition has been made over the last decade (2) and a number of randomized trials
compared innovative treatments to a doxorubicin, bleomycin,
vinblastine and dacarbazine (ABVD)-based approach (3–6) in
order to increase the number of cured patients and to reduce short-
and long-term secondary toxic effects. The standard treatment for
patients with advanced-stage HL involves 6–8 courses of ABVD.
The optimal treatment strategy for early-stage HL
remains a subject of intense debate (1,7).
During the period between 1950 and 1980, radiotherapy (RT) was
preferentially employed, since it was considered to be a less toxic
curative approach as compared to MOPP. Later trials revealed that
the risk of relapse in non-irradiated sites was approximately
20–30% and that a number of these relapsed patients were rescued by
chemotherapy (CT). This warranted investigation to determine
whether combined modality therapy (CT + RT) improves the results as
compared to RT alone (8–12). A number of trials have assessed the
possibility of treating HL patients with CT alone (13–15).
Mounting evidence suggests that early-stage HL patients are likely
to be cured by 4–6 courses of ABVD alone, thus avoiding RT
altogether (1,7,16,17).
In the last 10 years, the issues addressed by large
controlled trials regard mainly the role of high-dose CT with
autologous stem-cell transplant (18), new CT regimens for relapsing
patients (19–21) and the reduction of RT in early
stages to avoid long-term secondary effects (8,22).
However, since treatment of HL is regarded as
relatively straightforward, patients are not usually referred to
hemato-oncologically specialized centres and are treated locally
instead. Therefore, comprehensive findings beyond prospective
controlled trials are lacking. Clinical data of patients who
received a diagnosis of HL in a northern Italian region (Liguria)
from 1995 to 2010 were collected. These data were used to evaluate
the application of novel therapeutic concepts and to verify the
outcome of HL patients in comparison to what is reported by the
specialized literature.
Patients and methods
The clinical data were retrospectively collected
through an analysis of the records of HL patients diagnosed and
treated in nine centres, including six peripheral non-specialized
hospitals, from 1995 to 2010. The physicians in charge in various
hospitals were interviewed to review the charts. The staging
procedures included a physical examination, a total body CT scan, a
bone marrow biopsy and positron emission tomography (PET).
Only patients with available follow-up information
on the response to therapy, long-term outcome and toxicity were
included in the study. Histological type was reassessed according
to the current World Health Organization Classification (23). Bulky disease was defined as a nodal
tumor mass of >10 cm for advanced stages. Patients with stage
I-II, without symptoms and bulky disease, were regarded as having
favorable disease. Response to therapy was previously assessed by
physical and radiological imaging (ultrasonography and CT), since
the use of FDG PET in defining early and final response is a recent
development.
The median follow-up was 89 months (range 12–232).
Overall survival (OS) was calculated from the time treatment
commenced to June 30th, 2010, or at such time as patients succumbed
to any cause. Event-free survival (EFS) was calculated from the
date of response evaluation to June 30th, 2010, or the first event.
Absence of complete remission following first-line and salvage
therapy, relapse and patients succumbing to any cause were
considered events. Overall EFS and OS curves were calculated
according to the Kaplan-Meier method. Univariate comparisons
between patients in complete response (CR) vs. non-CR were
performed using the Chi-square analysis or the Fisher’s exact test.
The impact of the variables studied was assessed by multivariate
analysis according to the Cox regression model for OS and EFS,
while the logistic regression model was used to evaluate the CR
rate. A two-tailed p-value of ≤0.05 was considered to be
statistically significant.
Patient characteristics and
treatment
Table I shows the
key clinical, laboratory and histological characteristics of the
whole cohort of 209 adult patients. Briefly, median age was 33
years (range 14–80), with 151 patients <45 years of age. The
majority of patients (80%) had classical nodular sclerosis. A total
of 96 patients (45%) had limited-stage disease (I-IIA) and the
remaining 113 had advanced HL. A total of 88 patients (42%) had
favorable disease (stage I-IIA without bulky disease).
 | Table IPatient clinical and histological
characteristics. |
Table I
Patient clinical and histological
characteristics.
| Patients (n=209) | No. |
|---|
| Males/females
(%) | 99/110 (47/53) |
| Median age
(range) | 33 (14–80) |
| Histology |
| Lymphocyte
prevalence (%) | 14 (7) |
| Classical Hodgkin’s
lymphoma (%) | 195 (93) |
| Nodular sclerosis
(%) | 167 (80) |
| Lymphocyte depletion
(%) | 4 (2) |
| Mixed cellularity
(%) | 24 (11) |
| Stage |
| IA (%) | 16 (6) |
| IB (%) | 2 (2) |
| IIA (%) | 78 (37) |
| IIB (%) | 52 (25) |
| IIIA (%) | 11 (5) |
| IIIB (%) | 25 (12) |
| IVA (%) | 5 (3) |
| IVB (%) | 20 (10) |
| Only lymph nodal
involvement (%) | 184/209 (88) |
| Above the diaphragm
(%) | 137/184 (75) |
| Below the diaphragm
(%) | 9/184 (5) |
| Above and below the
diaphragm (%) | 38/184 (20) |
| Bulky disease
(%) | 38/209 (18) |
| Mediastinal bulky
(%) | 30/209 (15) |
| Abdominal bulky
(%) | 6/209 (3) |
| Mediastinal
involvement (%) | 112/209 (53) |
| Hematochemical
parameters |
| Median
leukocytes/mmc (range) | 9,800
(4,000–35,000) |
| Median Hb gr/dl
(range) | 12.8 (7–17) |
| Median PLT/mmc
(range) | 400,000
(86,000–684,000) |
| Albumin gr/dl
(range) | 4 (2.3–5.2) |
| LDH U/l (range) | 414 (132/1,900) |
| Median ESR
(range) | 40 (2–130) |
| Median PCR
(range) | 14 (1–142) |
| Median β2
microglobulin mg/l (range) | 2 (0.5–25) |
RT alone was administered to 7 patients (3%), 75
(35%) were treated with a combination of CT and RT, and 127
patients received CT alone. ABVD was the regimen of choice in the
CT group (95%). A median of six courses was given. In the CT + RT
group, 53% of the patients received ABVD and 42% the Stanford V
regimen. Table II shows more
detailed data on the treatment administered. RT was initiated
within 1 month of the completion of CT. The target volumes for
involved-field RT initially included involved nodal regions, while
those for extended-field RT included the mantle field, spleen and
para-aortic nodes. Patients received a median of 30 Gy.
 | Table IIFirst-line treatment and response. |
Table II
First-line treatment and response.
| No. (%) |
|---|
| Patients treated with
RT only | 7 (3) |
| Patients treated with
CT + RT | 75 (36) |
| Regimen combined
with RT |
| ABVD | 39 |
| Stanford V | 25 |
| MOPP-ABVD | 4 |
| Other | 7 |
| Patients treated with
CT only | 127 (61) |
| ABVD | 114 |
| MOPP-ABVD | 7 |
| Other | 6 |
| Response to
first-line treatment |
| CR | 178 (85) |
| PR | 28 (13) |
| NR | 3 (2) |
| Response after
salvage therapya |
| CR | 195 (93) |
| PR | 8 (4) |
| NR | 6 (3) |
Results
Response to therapy
The detailed responses to therapy are reported in
Table II. CR after first-line
treatment was achieved in 178 patients (85%). The early application
of salvage treatment [CT ± high-dose therapy (HDT) or RT] in 31
patients who failed to obtain CR increased the number of CRs to 195
(93%).
Table III shows
that a number of clinical factors affect the CR rate. Statistical
analysis revealed that favorable disease (p=0.000359),
limited-stage disease (p=0.0003), sub-diaphragmatic lymph node
involvement (p=0.05) and absence of mediastinal bulky tumor
involvement positively affected the CR rate after first-line
therapy. Following the application of salvage treatment, limited
stage maintained a positive effect on the CR rate (p=0.036).
 | Table IIIFactors affecting the complete
response rate. |
Table III
Factors affecting the complete
response rate.
| No. | CR after first-line
therapy | CR after salvage
therapy |
|---|
| |
|
|
|---|
| | n (%) | p-value | n (%) | p-value |
|---|
| Stage | | | 0.0500 | | 0.1587 |
| I | 18 | 18 (100) | | 18 (100) | |
| II | 130 | 113 (86) | | 122 (93) | |
| III | 36 | 29 (80) | | 33 (91) | |
| IV | 25 | 18 (72) | | 21 (84) | |
| Favorable Hodgkin’s
lymphoma | 88 | 84 (95) | 0.000359 | 85 (97) | 0.0700 |
| Unfavorable
Hodgkin’s lymphoma | 121 | 94 (77) | | 109 (89) | |
| Early stage
(I-IIA) | 96 | 91 (94) | 0.0003 | 93 (96) | 0.0360 |
| Advanced stage
(II-B-IVB) | 113 | 87 (77) | | 101 (89) | |
| Axillary nodes
involvement | 29 | 27 (93) | 0.1900 | 28 (96) | 0.4000 |
| No axillary nodes
involvement | 180 | 151 (83) | | 166 (92) | |
| Exclusive lymph
nodal involvement |
| Above the
diaphragm | 137 | 124 (90) | 0.0500 | 131 (95) | 0.2900 |
| Below the
diaphragm | 9 | 7 (77) | | 8 (88) | |
| Below and above
the diaphragm | 38 | 29 (76) | | 34 (89) | |
| Mediastinal
involvement | 110 | 91 (82) | 0.2900 | 100 (90) | 0.2500 |
| No mediastinal
involvement | 99 | 87 (87) | | 94 (94) | |
| Patients with
mediastinal bulky involvement | 30 | 21 (70) | 0.0300 | 26 (87) | 0.3400 |
| Patients with
non-bulky mediastinal involvement | 80 | 70 (87) | | 74 (92) | |
| Stage IV with
marrow infiltration | 11 | 11 (100) | 0.0120 | 11 (100) | 0.0500 |
| Stage IV without
marrow infiltration | 14 | 8 (57) | | 10 (71) | |
| Gender | | | 0.1500 | | 0.5500 |
| Male | 99 | 88 (88) | | 93 (93) | |
| Female | 110 | 90 (81) | | 101 (91) | |
| Age | | | 0.8100 | | 0.3500 |
| ≤45 years | 151 | 127 (84) | | 139 (92) | |
| >45 years | 58 | 48 (82) | | 51 (87) | |
Patient relapse, long-term outcome and
late toxicity
Out of the 194 patients who achieved CR following
first-line ± salvage treatment, 31 relapsed. Among the relapsed
patients, the first CR lasted a median of 24 months (range 3–130).
Treatments at first relapse are shown in Table IV. Briefly, 23 patients (74%)
obtained a second CR and 22 of them remain alive and disease-free
thus far.
 | Table IVRelapse and subsequent treatment. |
Table IV
Relapse and subsequent treatment.
| Relapses (%) | 31/194 (16) |
| CR length in
relapsed patients (range in months) | 24 (3–130) |
| Therapy of relapse
(%) |
| CT + RT | 4 (14) |
| CT | 15 (48) |
| CT+ HDT | 9 (29) |
| RT alone | 3 (9) |
| Response to therapy
at relapse (%) |
| CR | 23/31 (74) |
| PR | 3/31 (9) |
| NR | 4/31 (13) |
| Not evaluated | 1/31 (4) |
| Alive without
disease | 22/31 (71) |
Table V shows that
male gender (p=0.043) and age >45 years (p=0.047) were
significantly associated with an increased incidence of relapse.
Neither advanced stage (or unfavorable disease) nor mediastinal
bulky tumor involvement negatively affected the relapse rate.
 | Table VFactors affecting the relapse
rate. |
Table V
Factors affecting the relapse
rate.
| Patients | Relapses (%) | p-value |
|---|
| Stage |
| I | 18 | 5 (27) | 0.185 |
| II | 122 | 15 (12) | |
| III | 33 | 8 (24) | |
| IV | 21 | 3 (14) | |
| Favorable Hodgkin’s
lymphoma | 85 | 12 (14) | |
| Unfavorable
Hodgkin’s lymphoma | 109 | 19 (17) | |
| Early stage disease
(I-IIA) | 93 | 13 (14) | 0.460 |
| Advanced stage
disease (IIB-IVB) | 101 | 18 (17) | |
| Axillary node
involvement | 28 | 7 (25) | 0.160 |
| No axillary node
involvement | 145 | 21 (14) | |
| Exclusive lymph
nodal involvement |
| Above the
diaphragm | 131 | 18 (13) | 0.190 |
| Below the
diaphragm | 8 | 1 (12) | |
| Below and above
the diaphragm | 34 | 9 (26) | |
| Mediastinal
involvement | 100 | 13 (13) | 0.240 |
| No mediastinal
involvement | 94 | 18 (19) | |
| Patients with
mediastinal bulky involvement | 26 | 3 (11) | 0.740 |
| Patients with
non-bulky mediastinal involvement | 74 | 10 (13) | |
| Stage IV with
marrow infiltration | 11 | 3 (27) | 0.070 |
| Stage IV without
marrow infiltration | 10 | 0 (0) | |
| Gender | | | 0.043 |
| Male | 93 | 20 (21) | |
| Female | 101 | 11 (10) | |
| Age | | | 0.047 |
| ≤45 years | 139 | 17 (12) | |
| >45 years | 55 | 13 (25) | |
A total of 191 patients (91%) remained alive after a
median follow-up of 89 months. Table
VI shows cause of death, which was mostly disease-related. A
second neoplasia was diagnosed in 8 patients. The development of a
tumor in four cases (breast, lung and thyroid cancer) was possibly
RT-related. Only 1 patient not receiving RT developed acute myeloid
leukemia (AML). The 2 patients who developed AML had received
MOPP-ABVD and ABVD as induction treatment. One of the patients
achieved CR after RT and was treated with dexamethasone, Ara-C and
cisplatin (DHAP), ifosfamide, gemcitabine, vinorelbine and
prednisone (IGEV) and HDT for HL relapse, while the second patient
received CR after ifosfamide, epirubicin and etoposide (IEV) and
HDT.
 | Table VILong-term outcome and late
toxicities. |
Table VI
Long-term outcome and late
toxicities.
| First CR length,
months (range) | 70 (2–215) |
| Patients alive
(%) | 191 (91) |
| Patients dead
(%) | 18 (9) |
| Overall survival,
months (range) | 89 (7–222) |
| Median follow-up
(range) | 89 (7–222) |
| Patients with
second neoplasia (%) | 8 (4) |
| Rectum | 1 |
| Lung | 1 |
| Breast | 2 |
| Thyroid | 1 |
| AML | 2 |
| Endometrial
carcinoma | 1 |
| Coronary heart
disease | 1 |
| Causes of
death |
| Hodgkin’s
lymphoma | 16 |
| Second
neoplasia | 1 |
| Acute myocardial
infarction | 1 |
Only 1 patient treated with CT + RT developed
coronary heart disease and succumbed to acute myocardial
infarction.
An analysis of the clinical factors affecting EFS
and OS showed that age at diagnosis was the key factor affecting
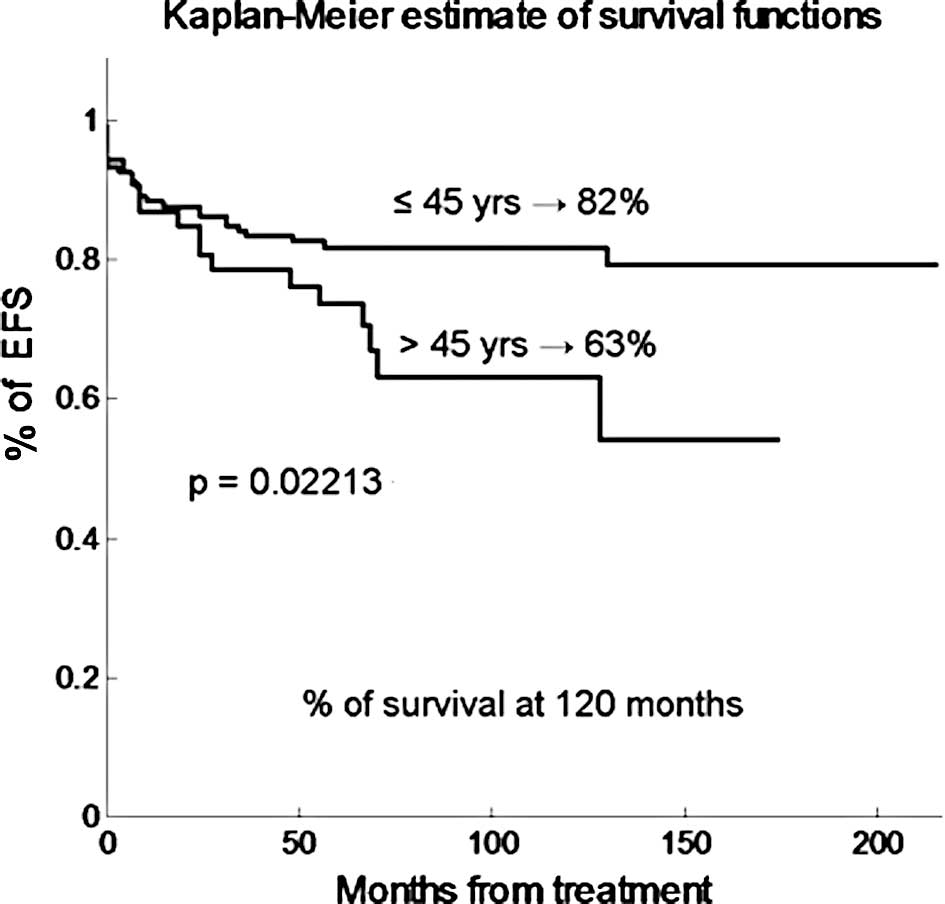
long-term outcome. As shown in Fig.
1, the EFS projected at 120 months was 80 and 57% for patients
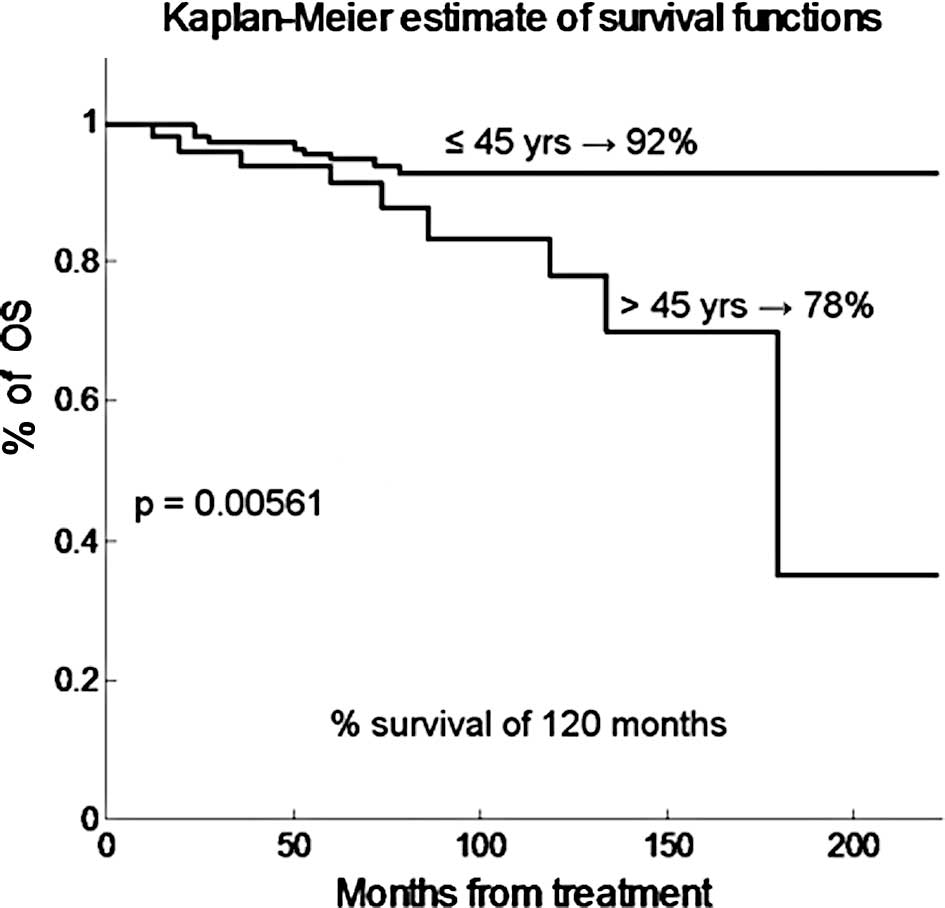
younger and older than 45 years, respectively (p=0.022). Fig. 2 shows that the OS projected at 120
months was 92 and 38% for patients younger and older than 45 years,
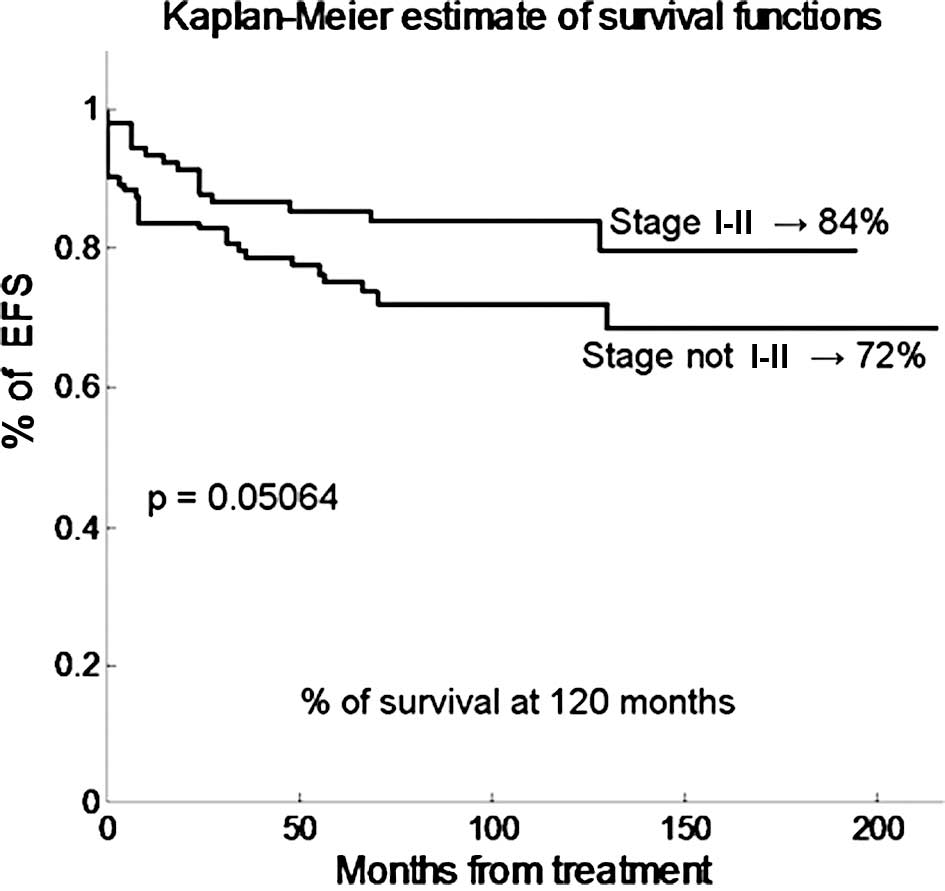
respectively (p=0.00561). Fig. 3
shows that early stage (I and II) has a borderline statistical
effect on EFS (80 compared to 66% in patients with advanced
disease, p=0.05), but not on OS (p=0.26). The EFS and OS of male
and female individuals, of patients with favorable and unfavorable
disease, of patients with or without axillary, mediastinal, marrow
involvement and bulky or non-bulky disease, were not statistically
different.
The EFS and OS of 141 early-stage patients treated
with CT + RT (n=62) or with CT alone (n=79) were also not
statistically different.
Discussion
HL is now considered to be a highly curable disease
and patients are frequently diagnosed and treated in medical
divisions or in peripheral oncological and hematological centres.
Only primary refractory younger patients or patients showing an
early relapse following the completion of treatment are referred to
more specialized hematologic centres. Furthermore, the majority of
patients are not enrolled in prospective clinical trials and
receive therapy according to well-established clinical guidelines
(24). The aim of this
retrospective study was to review the current management of HL in a
northern Italian region (Liguria), with special emphasis on
long-term outcome and toxicity. This study confirms the previously
reported clinical factors and histological distribution of HL,
including the young median age of patients at diagnosis, the
frequency of nodular sclerosis subtype of classical HL and the
rarity of sub-diaphragmatic presentation (1). The only notable inconsistency in the
reported data is that in our series female individuals were more
frequently affected than males ones.
The front-line therapeutic approach almost always
included CT, with only 7 patients with limited-stage disease being
treated with RT alone. In stage I-II disease, first-line treatment
included involved- or extended-field RT in 46% of patients.
ABVD was the regimen most frequently utilized in
patients with advanced stage HL, with less than 10% of patients
receiving alternative regimens. Our study shows that CT ± RT
induced CR in the majority of patients (85%) and that early salvage
therapy (frequently including HDT) induced CR in more than half of
the patients failing to achieve CR with the first-line therapy. As
previously reported (2–5), we confirm that early-stage HL and an
absence of bulky tumors are statistically associated with an
increased CR rate following first-line therapy. In our study,
patient characteristics such as gender, age at diagnosis and
mediastinal involvement did not affect the response rate. Patients
with early stage HL showed a more favorable EFS than those with
advanced stage disease, but only with borderline statistical
significance. This observation may be related to the fact that 7
patients with early-stage disease received RT only as first-line
treatment and 3 out of the 7 patients relapsed.
Long-term follow-up analysis showed a relapse rate
of 16%, with 1 patient relapsing after more than 12 years. The
relapse rate was lower than that reported by most recent trials
(4–7,25).
Male gender was associated with an increased relapse rate, but only
age over 45 years was associated with an increased relapse rate and
a worse EFS and OS. Among relapsed patients, we observed a
favorable response to therapy, with 74% of patients achieving a
second CR and more than 70% currently remaining alive and
disease-free.
Overall, 23 patients (11%) with refractory or
relapsed disease underwent HDT with IEV or IGEV mobilized
peripheral stem cells. The low toxicity and high therapeutic
efficacy of HDT contributed to the favorable outcome of our series
of patients and reduced the negative prognostic relevance of
advanced disease. The reduced feasibility of salvage therapy and,
in particular that of HDT, in elderly patients may aid in
elucidating the worse outcome of patients over 45 years of age. On
the other hand, a reduced dose intensity of CT in elderly patients,
mainly related to co-morbidity and toxicity, may also explain a
worse EFS (26,27).
Using PET, an evaluation of early response was
routinely performed in the majority of patients only in the last 2
years. However, the findings did not result in either a
modification of therapy nor an earlier application of HDT. Only
future randomized trials are likely to clarify the significance of
early response assessment and design response-oriented strategies
(28).
Long-term outcome data show that a high cure rate
can be achieved with limited side effects in the vast majority of
early-stage HL patients using RT + CT or CT alone, as recently
reported by our group (29) and
confirmed by a number of recent randomized trials (13–15).
Nonetheless, RT, especially when administered in the
extended field, is associated with an increased risk of second
neoplasia (22,30,31).
In our series, a second tumor was diagnosed in 7 patients,
following exposure to RT, although a clear relationship with RT was
only found in 3 out of the 7 cases. Only one secondary AML among
patients not exposed to RT was noted. In this case, the patient was
treated with various lines of CT and HDT. Only 1 patient who
received mediastinal RT developed coronary disease. However, an
increased incidence of delayed heart complications is anticipated
in the future in patients submitted to mediastinal RT, as
previously reported (30,31). A longer period of observation is
required to reveal small differences in toxicity and
non-lymphoma-related mortality. Hoppe et al showed that the
risk of death from Hodgkin’s disease is 17% at 15 years of
follow-up and increases only slightly in subsequent years, whereas
the risk of succumbing to other causes is also 17% at 15 years, but
increases sharply in the subsequent 25 years (22).
In conclusion, our study indicates that in our
region HL is a well-known and correctly treated disease. Moreover,
an efficient network between local general hospitals and
specialized centres is available, allowing for the administration
of treatment of a high standard throughout the entire region.
References
|
1
|
Diehl V: Hodgkin’s disease, from pathology
specimen to cure. N Engl J Med. 357:1968–1971. 2007.
|
|
2
|
Hasenclever D and Diehl V: A prognostic
score for advanced Hodgkin’s disease. N Engl J Med. 339:1506–1514.
1998.
|
|
3
|
Canellos GP, Anderson JR, Propert KJ, et
al: Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or
MOPP alternating with ABVD. N Engl J Med. 327:1478–1484. 1992.
|
|
4
|
Duggan DB, Petroni GR, Johnson JL, et al:
Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment
of advanced Hodgkin’s disease: report of an intergroup trial. J
Clin Oncol. 21:607–614. 2003.
|
|
5
|
Gobbi PG, Levis A, Chisesi T, et al;
Intergruppo Italiano Linfomi. ABVD versus modified stanford V
versus MOPPEBVCAD with optional and limited radiotherapy in
intermediate- and advanced-stage Hodgkin’s lymphoma: final results
of a multicenter randomized trial by the Intergruppo Italiano
Linfomi. J Clin Oncol. 23:9198–9207. 2005.PubMed/NCBI
|
|
6
|
Diehl V, Franklin J, Pfreundschuh M, et
al: Standard and increased-dose BEACOPP chemotherapy compared with
COPP-ABVD for advanced Hodgkin’s disease. Standard and
increased-dose BEACOPP. N Eng J Med. 348:2386–2395. 2003.
|
|
7
|
Diehl V and Fuchs M: Early, intermediate
and advanced Hodgkin’s lymphoma: modern treatment strategies. Ann
Oncol. 18(Suppl 9): 71–79. 2007.
|
|
8
|
Bonadonna G, Bonfante V, Viviani S, Di
Russo A, Villani F and Valagussa P: ABVD plus subtotal nodal versus
involved-field radiotherapy in early-stage Hodgkin’s disease:
long-term results. J Clin Oncol. 22:2835–2841. 2004.PubMed/NCBI
|
|
9
|
Koontz BF, Kirkpatrick JP, Clough RW, et
al: Combined-modality therapy versus radiotherapy alone for
treatment of early-stage Hodgkin’s disease: cure balanced against
complications. J Clin Oncol. 24:605–611. 2006.
|
|
10
|
Engert A, Franklin J, Eich HT, et al: Two
cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus
extended-field radiotherapy is superior to radiotherapy alone in
early favorable Hodgkin’s lymphoma: final results of the GHSG HD7
trial. J Clin Oncol. 25:3495–3502. 2007.PubMed/NCBI
|
|
11
|
Fermè C, Eghbali H, Meerwaldt JH, et al:
Chemotherapy plus involved-field radiation in early-stage Hodgkin’s
disease. EORTC-GELA H8 Trial. N Engl J Med. 357:1916–1927.
2007.
|
|
12
|
Olweny CL and Ziegler JL: Chemotherapy
plus involved-field radiation in early-stage Hodgkin’s disease. N
Engl J Med. 358:742–759. 2008.
|
|
13
|
Laskar S, Gupta T, Vimal S, et al:
Consolidation radiation after complete remission in Hodgkin’s
disease following six cycles of doxorubicin, bleomycin,
vinblastine, and dacarbazine chemotherapy: is there a need? J Clin
Oncol. 22:62–68. 2004.
|
|
14
|
Straus DJ, Portlock CS, Qin J, et al:
Results of a prospective randomized clinical trial of doxorubicin,
bleomycin, vinblastine, and dacarbazine (ABVD) followed by
radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA
nonbulky Hodgkin disease. Blood. 104:3483–3489. 2004. View Article : Google Scholar
|
|
15
|
Meyer RM, Gospodarowicz MK, Connors JM, et
al: Randomized comparison of ABVD chemotherapy with a strategy that
includes radiation therapy in patients with limited-stage Hodgkin’s
lymphoma: National Cancer Institute of Canada Clinical Trials Group
and the Eastern Cooperative Oncology Group. J Clin Oncol.
23:4634–4642. 2005.PubMed/NCBI
|
|
16
|
Gospodarowicz MK and Meyer RM: The
management of patients with limited-stage classical Hodgkin
lymphoma. ASH 2006 Educational Book. pp. 255–258. 2006, PubMed/NCBI
|
|
17
|
Canellos GP, Abramson JS, Fisher DC and
LaCasce AS: Treatment of favourable, limited-stage Hodgkin’s
lymphoma with chemotherapy without consolidation by radiation
therapy. J Clin Oncol. 28:1611–1615. 2010.
|
|
18
|
Viviani S, Di Nicola M, Bonfante V, et al:
Long-term results of high-dose chemotherapy with autologous bone
marrow or peripheral stem cell transplant as first salvage
treatment for relapsed or refractory Hodgkin lymphoma: a single
institution experience. Leuk Lymphoma. 51:1251–1259. 2010.
View Article : Google Scholar
|
|
19
|
Zinzani PL, Tani M, Molinari AL, Stefoni
V, Zuffa E, Alinari L, Gabriele A, Bonifazi F, Salvucci M, Tura S
and Baccarani M: Ifosfamide, epirubicin and etoposide regimen as
salvage and mobilizing therapy for relapsed/refractory lymphoma
patients. Haematologica. 87:816–821. 2002.PubMed/NCBI
|
|
20
|
Clavio M, Garrone A, Pierri I, Michelis
GL, Balocco M, Albarello A, Varaldo R, Canepa P, Miglino M,
Ballerini F, Canepa L and Gobbi M: Ifosfamide, epirubicin,
etoposide (IEV) and autologous peripheral blood progenitor cell
transplant: a feasible and effective salvage treatment for lymphoid
malignancies. Oncol Rep. 14:933–940. 2005.
|
|
21
|
Santoro A, Magagnoli M, Spina M, Pinotti
G, Siracusano L, Michieli M, Nozza A, Sarina B, Morenghi E,
Castagna L, Tirelli U and Balzarotti M: Ifosfamide, gemcitabine,
and vinorelbine: a new induction regimen for refractory and
relapsed Hodgkin’s lymphoma. Haematologica. 92:35–41.
2007.PubMed/NCBI
|
|
22
|
Hoppe RT: Hodgkin’s disease: complications
of therapy and excess mortality. Ann Oncol. 8(Suppl 1): 115–118.
1997.
|
|
23
|
Jaffe ES, Lee Harris N, Stein H and
Vardiman JW: World Health Organization Classification of Tumors.
Pathology and Genetics of Tumors of Haematopoietic and Lymphoid
Tissues. IARC Press; Lyon: 2001
|
|
24
|
Brusamolino E, Bacigalupo A, Barosi G, et
al: Classical Hodgkin’s lymphoma in adults: guidelines of the
Italian Society of Hematology, the Italian Society of Experimental
Hematology, and the Italian Group for Bone Marrow Transplantation
on initial work-up, management, and follow-up. Haematologica.
94:550–565. 2009.
|
|
25
|
Provencio M, Salas C, Millan I, Cantos B,
Sanchez A and Bellas C: Late relapses in Hodgkin Lymphoma: a
clinical and immunochemistry study. Leuk Lymphoma. 51:1686–1691.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walker A, Schoenfeld ER, Lowman JT,
Mettlin CJ, MacMillan J and Grufferman S: Survival of the older
patient compared with the younger patient with Hodgkin’s disease.
Influence of histologic type, staging, and treatment. Cancer.
65:1635–1640. 1990.
|
|
27
|
Diaz-Pavon JR, Cabanillas F, Majlis A and
Hagemeister FB: Outcome of Hodgkin’s disease in elderly patients.
Hematol Oncol. 13:19–27. 1995.
|
|
28
|
Gallamini A: Positron emission tomography
scanning: a new paradigm for the management of Hodgkin’s lymphoma.
Haematologica. 95:1046–1048. 2010.
|
|
29
|
Olcese F, Clavio M, Rossi E, et al: The
addition of radiotherapy to chemotherapy does not improve outcome
of early stage Hodgkin’s lymphoma patients: a retrospective
long-term follow-up analysis of a regional Italian experience. Ann
Hematol. 88:855–861. 2009.
|
|
30
|
Ghalibafian M, Beaudre A and Girinsky T:
Heart and coronary artery protection in patients with mediastinal
Hodgkin lymphoma treated with intensity-modulated radiotherapy:
dose constraints to virtual volumes or to organs at risk? Radiother
Oncol. 87:82–88. 2008.
|
|
31
|
Travis LB: Evaluation of the risk of
therapy-associated complications in survivors of Hodgkin lymphoma.
Hematology Am Soc Hematol Educ Program. 2007.192–196
|