Introduction
Colorectal cancer (CRC) is the second leading cause
of death from cancer in industrialized nations, with 140,000 newly
diagnosed CRC cases and 70,000 deaths from CRC in the United States
in 2010 (1).
Cellular migration and invasion are critical
parameters in the metastatic dissemination of cancer cells and the
formation of distant metastasis is the major cause of death in
cancer patients (2). Migratory
cancer cells undergo major changes in their cell-cell and
cell-matrix adhesion. The controlled degradation of the
extracellular matrix (ECM) is a critical component for cell
adhesion and cancer cell migration (2,3).
Matrix metalloproteinases (MMPs), a family of zinc
metallo-endopeptidases, are major proteolytic enzymes responsible
for the degradation of ECM. MMPs have been associated with invasive
tumor growth, metastasis and neovascularization in different types
of tumors, such as CRC (4–6), and are therefore the most prominent
proteases associated with tumorigenesis (7). In particular, MMP-7 and MMP-13 are
expressed in a variety of tumor entities (8–12).
Moreover, MMP-7 is associated with distant metastasis and adverse
outcome in early CRC, whereas the expression of MMP-13 is
correlated with poor survival and the existence of liver metastasis
in CRC (13–16).
Recent in vitro studies in breast, brain and
prostate cancer cells as well as in mesenchymal stem cells showed
an increased expression of MMP-13 following treatment with
2′-deoxyribocytidine-phosphate-guanosine (CpG) oligonucleotides as
specific agonists of toll-like receptor-9 (TLR-9) (17–20).
Furthermore, in vitro TLR-9 agonism leads to an enhanced
MMP-13-mediated cellular invasion that can be inhibited following
treatment with neutralizing anti-MMP-13 antibodies (18–20).
Based on this evidence, we hypothesized that MMP-13 is regulated
via TLR-9 in CRC cells in the same manner, and that the stimulation
of TLR-9 in CRC cells results in an increased MMP-13 expression.
The expression of MMP-13, TLR-9 and associated second messengers of
the TLR signal transduction cascade in the CRC cell lines LS174 and
SW620 was determined, and the effects of CpG oligonucleotides or
non-stimulatory GpC control oligonucleotides on the expression of
MMP-13 in these cells were analyzed. We further hypothesized that a
selective MMP-13 inhibition exhibits the potential to decreases the
migration of CRC cells. Therefore, the effects of selective MMP-13
inhibition in LS174 cells in the Boyden chamber experiments were
analyzed.
Materials and methods
Materials
Culture reagents were obtained from Sigma
(Steinheim, Germany), Gibco (Eggenstein, Germany), Sarstedt
(Berlin, Germany) or PAN Biotech (Aidenbach, Germany). All
chemicals were purchased from Sigma, Pharmacia Biotech (Freiburg,
Germany) or ICN (Meckenheim, Germany). Phosphorothioate-modified,
human-specific CpG-ODNs (type C: 5′-TCG TCG TCG TTC GAA CGA CGT TGA
T-3′) and respective GpC-ODN negative controls were purchased from
InVivoGen (San Diego, CA, USA) and dissolved in endotoxin-free
sterile dH2O according to the manufacturer’s
instructions. A final concentration of 5 μM CpG-ODN or respective
control was used in the cell culture experiments. The selective
MMP-13 inhibitor CL-82198 was purchased from Enzo Life Sciences
(Farmingdale, NY, USA) and dissolved in dH20 according
to the manufacturer’s instructions.
Cell culture
Human colon cancer cell lines SW620 (ATCC, CCL-227)
and LS174 (ATCC, CL-188) were employed for this study. SW620 and
LS174 cells were maintained in RPMI-1640 supplemented with 10% FCS,
streptomycin (10 mg/l), and penicillin (10 U/l). CCD18 fibroblasts
from human colon (ECACC, CCD-18Co) were cultured in Eagle’s minimal
essential medium with 20% FCS, 1% glutamine, streptomycin (10 mg/l)
and penicillin (10 U/l). Cells were grown in 5% CO2 at
37°C in a water-saturated atmosphere. One day prior to experiments,
the cells were seeded to provide a final cell density of 60–70%
confluence.
RNA purification, cDNA synthesis and
RT-PCR for MMP analyses
Total cellular RNA was extracted from LS174, SW620
and CCD18 cells using the RNeasy kit (Qiagen, Hilden, Germany)
according to the manufacturer’s instructions. First-strand cDNA was
synthesized from DNA-free total RNA using oligo-dT primers and the
first-strand cDNA synthesis kit for RT-PCR (Roche Diagnostics,
Mannheim, Germany). RNA (1 μg) was utilized for the reverse
transcriptase reaction according to the manufacturer’s
instructions. Real-time PCR was performed using a Platinum
SYBR-Green qPCR kit (Invitrogen, Karlsruhe, Germany) according to
the manufacturer’s instructions. Real-time PCR of each
gene-specific primer pair was optimized prior to the experiment to
confirm the absence of any non-specific amplification product.
Primers were purchased from Eurofins (Ebersberg, Germany). Primer
sequences are shown in Table I.
 | Table ISYBR-Green real-time qPCR primer
sequences. |
Table I
SYBR-Green real-time qPCR primer
sequences.
| Gene | Primer sequence | GenBank accession
no. |
|---|
| Human 18 sRNA | Fw: GAT CAG ATA CCG
TCG TAG TTC C
Rev: TAT CAA TCT GTC AAT CCT GTC C | NR 003286 |
| Human TLR-9 | Fw: CAT CTC AAC CTC
AAG TGG AAC
Rev: CTA GCA TCA GGA TGT TGG TAT | NM 017442 |
| Human MyD88 | Fw: GTA TAT CTT GAA
GCA GCA GCA G
Rev: CAG TCG ATA GTT TGT CTG TTC C | NM 002468 |
| Human IKKγ | Fw: AGA ATA CGA CAA
CCA CAT CAA G
Rev: CAG TTT GCT GTA CTC CCT CTG | NM 001145255 |
| Human NF-κB | Fw: ATT ACA AAA CCA
GCC TCT GT G
Rev: TAT ACC CTG GAC CTG TAC TTC C | NM 001165412 |
| Human MMP-13 | Fw: GCA GTC TTT CTT
CGG CTT AG
Rev: GGA GTT ACA TCG GAC CAA AC | NM 002427 |
qRT-PCR was performed on the Mx3000P (Stratagene, La
Jolla, CA, USA) using 3-stage program parameters as follows: i) 10
min at 96°C; ii) 40 cycles of 10 sec at 95°C, 30 sec at 57°C and 30
sec at 73°C; iii) 10 min at 73°C. The specificity of the PCR was
confirmed by examination of the dissociation reaction plot
subsequent to qRT-PCR. PCR products were separated on a 1.5% TAE
agarose gel and visualized by staining with ethidium bromide to
confirm the appearance of a single band of the correct molecular
size. qRT-PCR data were analyzed using the ΔΔCt model (21).
Boyden chamber experiments
Transwell 8-μm pore membrane inserts (18-mm standard
PCTE filters; Neuro Probe, Gaithersburg, MD, USA) were activated
for 20 min at 50°C with 0.5% acetic acid. After drying (100°C, 1 h
on Whatman paper) the inserts were placed in a blind well
chemotaxis chamber (Neuro Probe). The lower compartment was filled
with FCS-containing medium as a chemoattractant. Prior to seeding,
the cells were cultured in FCS-free medium for 24 h, detached by
trypsin treatment and rinsed with FCS-free medium. Cells
(2×105), with or without CL-82198 or BSA as a reference
protein, were added to the upper chamber. Chambers were placed in a
humified tissue incubator containing 5% CO2 for 24 h at
37°C. Cells on the upper surface of the transwell inserts were
removed using a cotton swab and those on the lower surface of the
membranes were fixed with 10% methanol and stained with crystal
violet. Membranes were rinsed with deionized water, dried and
examined using light microscopy. The number of migrated cells in
five optical fields (magnification, ×400) was averaged.
Statistical analysis
Differences between groups were assessed using the
Mann-Whitney U-Test. P<0.05 was considered to be statistically
significant. Statistical analysis was performed with SPSS 17.0
(SPSS Inc., Chicago, IL, USA). The values are shown as the mean ±
SEM.
Results
Expression of MMP-13, TLR-9 and
associated second messengers of the TLR signal transduction
cascade
The gene expression of MMP-13 and TLR-9 was assessed
by quantitative RT-PCR in LS174, SW620 and CCD18 cells.
Furthermore, the expression levels of MyD88 and IKKγ as second
messengers and NF-κB as the main effector of TLR-9 signaling were
quantified. The results were compared against 18s RNA, which served
as a housekeeping gene. To calculate the abundant expression in CRC
cells, the expression of the respective genes in colonic
fibroblasts was set as a reference. Determination of gene
expression by RT-PCR was performed from six individually growing
culture plates for each cell line (n=6).
In LS174 cells, MMP-13 gene expression was
significantly enhanced compared to human colon fibroblasts
(84-fold, p=0.016). Concomitantly, LS174 cells exhibited
significantly enhanced mRNA levels of TLR-9, MyD88, IKKγ and NF-κB
(Fig. 1A; TLR-9: 75-fold, p=0.016;
MyD88: 117-fold, p=0.016; IKKγ: 70-fold, p=0.036 and NF-κB:
133-fold, p=0.036).
 | Figure 1Enhanced expression of MMP-13, TLR-9
and second messengers of the TLR signal transduction cascade in CRC
cells. (A) LS174 cells (black bars) showed an enhanced gene
transcription of MMP-13 (84-fold, p=0.016) and TLR-9 (75-fold,
p=0.016), and also of MyD88 (117-fold, p=0.016), IKKγ (70-fold,
p=0.036) and NF-κB (133-fold, p=0.036) as second messengers and
effectors of the signal transduction cascade. (B) In SW620 cells,
the expression of MMP-13, TLR-9 and associated second messengers
was also increased (MMP-13: 587-fold, p=0.008; TLR-9: 26-fold,
p=0.004; MyD88: 264-fold, p=0.036; IKKγ: 255-fold, p=0.008; NF-κB:
64-fold, p=0.036). Abundant expression levels in CRC were related
to the respective gene expression in colon fibroblasts, which were
set as a reference (grey dashed line). The values shown are the
mean ± SEM. |
Similarly in SW620 cells, we observed an increased
gene expression of MMP-13, TLR-9 and associated second messengers
of the TLR signal transduction cascade (Fig. 1B; MMP-13: 587-fold, p=0.008; TLR-9:
26-fold, p=0.004; MyD88: 264-fold, p=0.036; IKKγ: 255-fold, p=0.008
and NF-κB: 64-fold, p=0.036).
TLR-9 agonists induced MMP-13 expression
in human CRC cells, but not in colon fibroblasts
Based on our observation of a simultaneous increase
in MMP-13 and TLR-9 gene expression in CRC cells and on recent
reports of a TLR-9-dependent regulation of MMP-13 (17–20),
we hypothesized that TLR-9 stimulation leads to an enhanced MMP-13
secretion in CRC cells. We used CpG motif containing unmethylated
oligodeoxynucleotides (CpG-ODN) as well-characterized TLR-9 ligands
mimicking the actions of bacterial DNA (22–24),
and non-stimulatory GpC-ODN as a control substance. Cells treated
with CpG-ODN or GpC-ODN are indicated by CpG-ODN-positive and
CpG-ODN-negative, respectively, in Fig.
2. Experiments were performed in triplicate. In LS174 cells
treated with 5 μM of CpG-ODN, MMP-13 gene expression was enhanced
3.6-fold after 12 h in relation to the baseline expression at 0 h,
and increased further to 3.9-fold after 24 h, although the latter
result did not reach the level of statistical significance
(Fig. 2; CpG-ODN-positive: 12 h:
3.6-fold increase, p=0.049 and 24 h: 3.9-fold increase, p=0.12). By
contrast, MMP-13 expression in LS174 cells treated with 5 μM
control substance, which contained GpC dinucleotides instead of
CpGs, did not significantly change in relation to its baseline
expression after 12 and 24 h of culture (Fig. 2; CpG-ODN-negative: 12 h: factor
0.99, p=0.83 and 24 h: factor 1.98, p=0.08).
 | Figure 2TLR-9 agonism leads to increased
MMP-13 gene expression in CRC cells, but not in human fibroblasts.
Cells were treated with CpG oligonucleotides as a TLR-9 ligand
(CpG-ODN-positive) or non-stimulatory GpC-ODN (CpG-ODN-negative).
MMP-13 gene expression was measured after (A) 12 h and (B) 24 h,
and in relation to the respective baseline expression at 0 h
(indicated by the dashed line). In LS174 cells treated with 5 μM of
CpG-ODN, MMP-13 gene expression was enhanced 3.6-fold after 12 h
(CpG-ODN-positive: p=0.049) and increased further to 3.9 fold after
24 h in relation to its baseline expression at 0 h, although the
latter results did not reach the level of statistical significance
(CpG-ODN-positive: p=0.12). By contrast, MMP-13 expression in LS174
cells treated with 5 μM GpC-ODN did not significantly change in
relation to its baseline expression after 12 and 24 h of culture
(CpG-ODN-negative: 12 h: factor 0.99, p=0.83; 24 h: factor 1.98,
p=0.08). In SW620 cells, the presence of 5 μM CpG-ODN increased
MMP-13 gene expression by a factor of 2.7 after 12 h
(CpG-ODN-positive: p=0.049) and by a factor of 2.3 after 24 h
(CpG-ODN-positive: p=0.049), whereas MMP-13 mRNA was not
significantly altered in SW620 cells treated with GpC-ODN
(CpG-ODN-negative: 12 h: factor 1.8, p=0.275; 24 h: factor 0.8;
p=0.827). In human colon fibroblasts (CCD18), however, MMP-13 gene
expression remained unchanged after 12 and 24 h, irrespective of
the presence of CpG-ODN or GpC-ODN (12 h: CpG-ODN-positive:
1.36-fold, p=0.827; CpG-ODN-negative: 0.67, p=0.827; 24 h:
CpG-ODN-positive: 0.25-fold, p=0.513; CpG-ODN-negative: 0.52-fold,
p=0.827). Experiments were performed in triplicate (n=3) for each
group. The values shown are the mean ± SEM. |
A similar result was obtained in SW620 cells: in the
presence of 5 μM of CpG-ODN, MMP-13 gene expression was
significantly increased from the baseline expression by a factor of
2.7 after 12 h (p=0.049) and by a factor of 2.3 after 24 h
(p=0.049), whereas MMP-13 mRNA was not significantly altered in
SW620 cells treated with the control substance (Fig. 2; CpG-ODN-negative: 12 h: factor 1.8,
p=0.275; 24 h: factor 0.8; p=0.827). In human colon fibroblasts,
however, MMP-13 gene expression remained unchanged after 12 and 24
h irrespective of the presence of CpG-ODN or GpC-ODN (Fig. 2; CpG-ODN-positive: 12 h: factor
1.36, p=0.827; 24 h: factor 0.25, p=0.513; CpG-ODN-negative: 12 h:
factor 0.67, p=0.827; 24 h: factor 0.52, p=0.827).
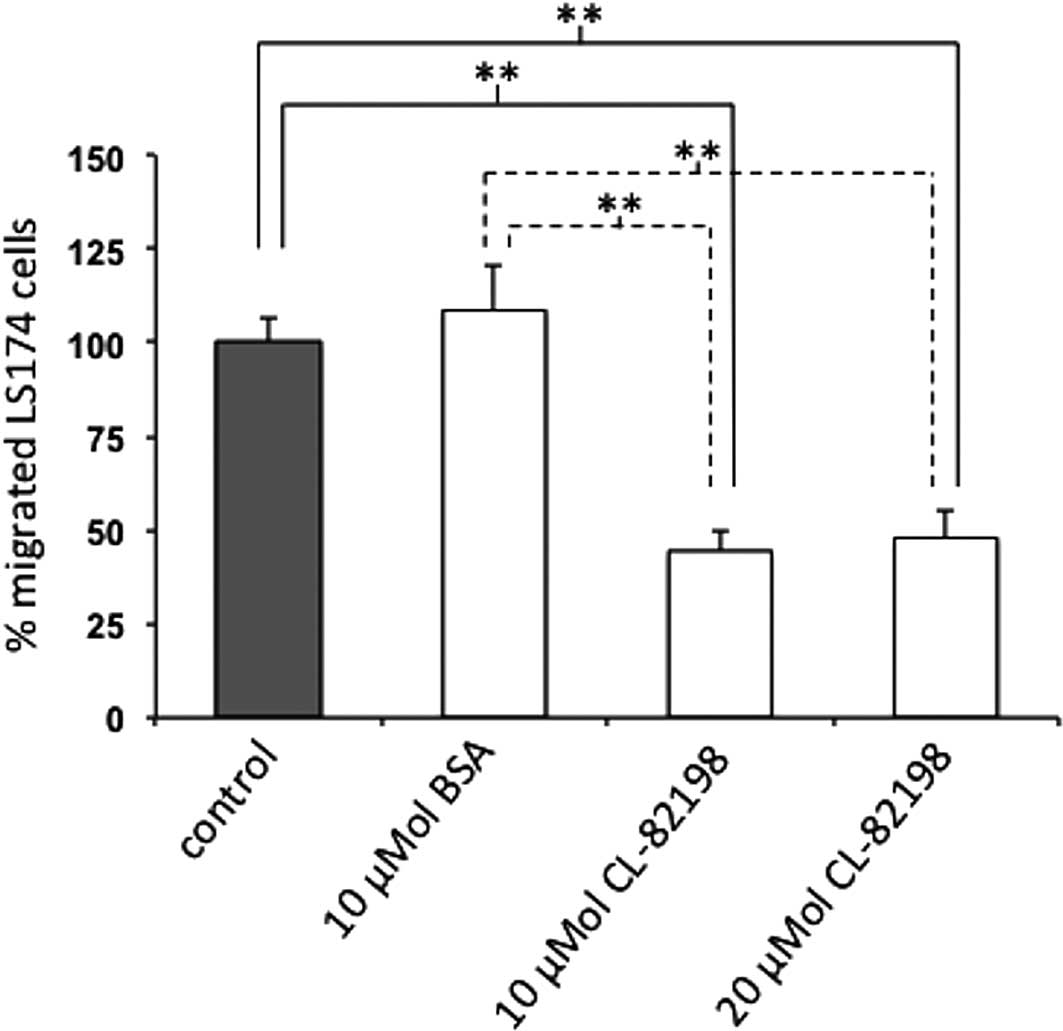
Specific MMP-13 inhibition reduces the
migration of LS174 cells
To investigate whether the inhibition of MMP-13
affects the migration of colon carcinoma cells, we examined the
motility of LS174 cells in Boyden chamber experiments in the
presence or absence of the selective MMP-13 inhibitor CL-82198 or
BSA as a reference protein. For these analyses, we focused on LS174
cells as these cells are primary tumor cells, whereas SW620 cells
are metastasis-derived. CL-82198 binds to the S1’ pocket of MMP-13
leading to 89% enzyme inhibition at a concentration of 10 μg/ml
(25). Following a 24-h incubation,
migrated cells on the underside of the membrane were counted.
Migration experiments were performed with n=6 per group.
Compared to the untreated cells, the addition of the
specific MMP-13 inhibitor CL-82198 at a concentration of 10 μM
resulted in a 45±5.6% reduction in the migration of LS174 cells
(p=0.004). When compared to BSA-treated cells, LS174 cell migration
was significantly reduced as well in the presence of 10 μM CL-82198
(41±5.1%, p=0.004). Similarly, at a concentration of 20 μM
CL-82198, LS174 cell migration was reduced compared to the
untreated (48±7.3%, p=0.004) or BSA-treated cells (44±6.7%,
p=0.002), although this migration was not further reduced compared
to LS174 cells treated with 10 μM CL-82198 (p=0.818). Cellular
migration was unchanged between the untreated and BSA-treated LS174
cells (p=0.818) (Fig. 3).
Discussion
Mounting evidence supports the hypothesis that
extracellular proteinases, such as MMPs, mediate a number of
changes in the microenvironment during tumor progression and
metastasis. Tumor metastasis is generally considered to be a
multistep process involving attachment to ECM, local matrix
proteolysis and tumor cell migration (26–28).
Although MMPs play diverse biological roles in cancer, a pivotal
role is the degradation and remodeling of ECM, thereby paving the
way through the peripheral tissue for invasion and metastasis
(5–7,29).
Among others, MMP-13 is expressed in a variety of tumor entities
(8,9,30,31)
such as CRC (10–12) and adenomatous polyps (32). Furthermore, MMP-13 expression in CRC
is correlated with poor survival (14) and the existence of liver metastasis
(16).
Recent in vitro studies have shown that
MMP-13 expression is regulated via TLR-9 (17–20).
Furthermore, treatment of TLR-9-expressing cancer cells of various
origin, such as breast, brain and prostate, and mesenchymal stem
cells with TLR-9 ligands stimulates their invasive cell behaviour
in a MMP-13-dependent manner (17–20).
TLRs are evolutionarily well-conserved transmembrane proteins that
identify other conserved structures, particularly microbial
components. TLRs are present in almost all multi-cellular organisms
(33). The mammalian TLR family
constitutes 11 members, each of which identifies a different,
pathogen-derived ligand. TLR-9 responds to unmethylated CpG DNA
motifs that are frequently present in bacteria and viruses, but are
rare in mammalian cells (22–24).
Mounting evidence indicates that TLR-9 expression is not confined
to cells of the immune system, as TLR-9 expression has been
detected in astrocytes, mesenchymal cells and in various normal
epithelial and cancer cells, including breast, brain, lung and
gastric cancer cells (17,19,34–38).
Against this background, we aimed to analyze whether
MMP-13 expression is also regulated by TLR-9 in CRC. In a series of
experiments, we studied the gene expression of MMP-13, TLR-9 and
downstream messengers of the TLR signal transduction cascade in
LS174 and SW620 cells, and compared this expression to the
respective gene expression in human colonic fibroblasts. Using this
approa, we demonstrated that the expression levels of MMP-13 and
TLR-9 and associated second messengers are simultaneously increased
in LS174 and SW620 cells. To determine the TLR-9-dependent
expression of MMP-13, we selectively stimulated TLR-9 using CpG
oligonucleotides and quantified the MMP-13 gene expression after 12
and 24 h compared to the baseline expression at 0 h. Our findings
provide clear evidence that TLR-9 agonism with CpG oligonucleotides
leads to an enhanced MMP-13 gene expression in LS174 and SW620
cells. By contrast, GpC oligonucleotides as a control substance
failed to induce MMP-13 gene expression in these two cell lines.
Notably, although benign human colonic fibroblasts expressed TLR-9
(as confirmed within our RT-PCR studies), they did not exhibit any
TLR-9-dependent expression of MMP-13, suggesting a
carcinoma-specific mechanism. Finally, we showed that the selective
inhibition of MMP-13 via a synthetic inhibitor (CL-82198) reduced
the migration of CRC cells by 56%.
CL-82198 was developed in NMR studies and binds to
the entire S1’ pocket of MMP-13, which is the basis for its
selectivity towards MMP-13 and the lack of inhibitory activities
against other MMPs (25).
Furthermore, CL-82198 was shown to provide an enzyme inhibition of
89% at a concentration of 10 μg/ml (25). This inhibitory ability of almost
complete MMP-13 inhibition at a relatively low concentration is
consistent with our observations, since we were unable to detect a
further reduction of cellular migration when the concentration of
CL-82198 was increased from 10 to 20 μM κ.
MMP inhibition as a therapeutic target in cancer
treatment is an area of intense investigation. The first drug
development programs, based on compelling evidence of MMP-mediated
angiogenesis and metastasis in different tumor models, were
initiated approximately 25 years ago and eventually led to
small-molecule metalloproteinase inhibitor (MPI) drugs in phase III
clinical trials. The effects of MPIs in these trials turned out to
be disappointing as they failed to increase the survival of cancer
patients (39). However, a number
of these trials were conducted on patients with advanced stages of
cancer, whereas in murine tumor models MMP inhibition was generally
initiated at an early stage of the disease and maintained
throughout tumor progression (39).
Consequently, whether MPIs may have been more effective if used at
an earlier stage of the disease remains to be determined.
Furthermore, many of the utilized MPIs, such as marimastat or
batimastat, were broad-spectrum MMP inhibitors. Given the growing
evidence of an essential role of a number of MMPs in various
physiological functions, such as growth, cytokine signaling, innate
immunity (40) and inflammatory
conditions (40–42), it seems inevitable to selectively
inhibit single MMPs in further trials to comprehensively evaluate
their specific therapeutic potential.
In conclusion, the results of this study provide
evidence of a TLR-9-dependent regulation of MMP-13 in CRC cells,
but not in human colonic fibroblasts, suggesting a
carcinoma-specific mechanism. It was further demonstrated that the
specific inhibition of MMP-13 reduces the migration of CRC cells.
Therefore, selective MMP-13 inhibition may be a promising
therapeutic strategy in CRC.
Acknowledgements
This study was supported by grants from the Deutsche
Forschungsgemeinschaft (RO 957/7-1 and RO 957/8-1) and from the
ZooMAP (Bundesministerium für Bildung und Forschung, BMBF). Dr Timo
Rath has received grants for young researchers
(‘Anschubfinanzierung’) from the Justus-Liebig-University
Giessen.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Yilmaz M and Christofori G: Mechanisms of
motility in metastasizing cells. Mol Cancer Res. 8:629–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Decock J, Paridaens R and Ye S: Genetic
polymorphisms of matrix metalloproteinases in lung, breast and
colorectal cancer. Clin Genet. 73:197–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fingleton B: Matrix metalloproteinases:
roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Airola K, Karonen T, Vaalamo M, et al:
Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and
-3 correlates with the level of invasion in malignant melanomas. Br
J Cancer. 80:733–743. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heppner KJ, Matrisian LM, Jensen RA and
Rodgers WH: Expression of most matrix metalloproteinase family
members in breast cancer represents a tumor-induced host response.
Am J Pathol. 149:273–282. 1996.PubMed/NCBI
|
|
10
|
Hilska M, Roberts PJ, Collan YU, et al:
Prognostic significance of matrix metalloproteinases-1, -2, -7 and
-13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in
colorectal cancer. Int J Cancer. 121:714–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori D, Nakafusa Y, Miyazaki K and
Tokunaga O: Differential expression of Janus kinase 3 (JAK3),
matrix metalloproteinase 13 (MMP13), heat shock protein 60 (HSP60),
and mouse double minute 2 (MDM2) in human colorectal cancer
progression using human cancer cDNA microarrays. Pathol Res Pract.
201:777–789. 2005. View Article : Google Scholar
|
|
12
|
Roeb E, Arndt M, Jansen B, Schumpelick V
and Matern S: Simultaneous determination of matrix
metalloproteinase (MMP)-7, MMP-1, -3, and -13 gene expression by
multiplex PCR in colorectal carcinomas. Int J Colorectal Dis.
19:518–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang YJ, Lu ZH, Wang GQ, et al: Elevated
expressions of MMP7, TROP2, and survivin are associated with
survival, disease recurrence, and liver metastasis of colon cancer.
Int J Colorectal Dis. 24:875–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leeman MF, McKay JA and Murray GI: Matrix
metalloproteinase 13 activity is associated with poor prognosis in
colorectal cancer. J Clin Pathol. 55:758–762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masaki T, Matsuoka H, Sugiyama M, et al:
Matrilysin (MMP-7) as a significant determinant of malignant
potential of early invasive colorectal carcinomas. Br J Cancer.
84:1317–1321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada T, Oshima T, Yoshihara K, et al:
Overexpression of MMP-13 gene in colorectal cancer with liver
metastasis. Anticancer Res. 30:2693–2699. 2010.PubMed/NCBI
|
|
17
|
Ilvesaro JM, Merrell MA, Li L, et al:
Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular
invasion. Mol Cancer Res. 6:1534–1543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ilvesaro JM, Merrell MA, Swain TM, et al:
Toll like receptor-9 agonists stimulate prostate cancer invasion in
vitro. Prostate. 67:774–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merrell MA, Ilvesaro JM, Lehtonen N, et
al: Toll-like receptor 9 agonists promote cellular invasion by
increasing matrix metalloproteinase activity. Mol Cancer Res.
4:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nurmenniemi S, Kuvaja P, Lehtonen S, et
al: Toll-like receptor 9 ligands enhance mesenchymal stem cell
invasion and expression of matrix metalloprotease-13. Exp Cell Res.
316:2676–2682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hemmi H, Takeuchi O, Kawai T, et al: A
Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Latz E, Visintin A, Espevik T and
Golenbock DT: Mechanisms of TLR9 activation. J Endotoxin Res.
10:406–412. 2004. View Article : Google Scholar
|
|
24
|
Takeshita F, Gursel I, Ishii KJ, Suzuki K,
Gursel M and Klinman DM: Signal transduction pathways mediated by
the interaction of CpG DNA with Toll-like receptor 9. Semin
Immunol. 16:17–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JM, Nelson FC, Levin JI, et al:
Structure-based design of a novel, potent, and selective inhibitor
for MMP-13 utilizing NMR spectroscopy and computer-aided molecular
desing. J Am Chem Soc. 122:9648–9654. 2000. View Article : Google Scholar
|
|
26
|
Koblinski JE, Ahram M and Sloane BF:
Unraveling the role of proteases in cancer. Clin Chim Acta.
291:113–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
28
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
30
|
Inoue A, Takahashi H, Harada H, et al:
Cancer stem-like cells of glioblastoma characteristically express
MMP-13 and display highly invasive activity. Int J Oncol.
37:1121–1131. 2010.PubMed/NCBI
|
|
31
|
Yong HY, Kim IY, Kim JS and Moon A:
ErbB2-enhanced invasiveness of H-Ras MCF10A breast cells requires
MMP-13 and uPA upregulation via p38 MAPK signaling. Int J Oncol.
36:501–507. 2010.PubMed/NCBI
|
|
32
|
Rath T, Roderfeld M, Graf J, et al:
Enhanced expression of MMP-7 and MMP-13 in inflammatory bowel
disease: a precancerous potential? Inflamm Bowel Dis. 12:1025–1035.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bowman CC, Rasley A, Tranguch SL and
Marriott I: Cultured astrocytes express toll-like receptors for
bacterial products. Glia. 43:281–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Droemann D, Albrecht D, Gerdes J, et al:
Human lung cancer cells express functionally active Toll-like
receptor 9. Respir Res. 6:12005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Platz J, Beisswenger C, Dalpke A, et al:
Microbial DNA induces a host defense reaction of human respiratory
epithelial cells. J Immunol. 173:1219–1223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmausser B, Andrulis M, Endrich S, et
al: Expression and subcellular distribution of toll-like receptors
TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter
pylori infection. Clin Exp Immunol. 136:521–526. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmausser B, Andrulis M, Endrich S,
Muller-Hermelink HK and Eck M: Toll-like receptors TLR4, TLR5 and
TLR9 on gastric carcinoma cells: an implication for interaction
with Helicobacter pylori. Int J Med Microbiol. 295:179–185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parks WC, Wilson CL and Lopez-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rath T, Roderfeld M, Graf J and Roeb E:
Matrix metalloproteinases in inflammatory bowel disease – from
basic research to clinical significance. Z Gastroenterol.
47:758–769. 2009.
|
|
42
|
Rath T, Roderfeld M, Halwe JM, Tschuschner
A, Roeb E and Graf J: Cellular sources of MMP-7, MMP-13 and MMP-28
in ulcerative colitis. Scand J Gastroenterol. 45:1186–1196.
2010.PubMed/NCBI
|