Introduction
Estrogens are steroid hormones that play a crucial
role in the growth, differentiation and function of sexual and
reproductive organs. Estrogens are produced naturally by the body
as three biochemically distinct hormones, estrone, 17β-estradiol
and estriol, with 17β-estradiol being the key and most potent
estrogen in human tissues. Estrogens are metabolized to
2-hydroxyestrone (2-OHE1) and 16-αhydroxyestrone (16-OHE1), and
their relative concentrations in the female body, based on their
estrogen agonist 16-OHE1 or antagonist 2-OHE1 activity, were
reported to increase a female individual’s risk for breast, uterine
and other hormonally induced cancers (1). The cellular activity of estrogen is
known to be mediated by its interaction with its receptors, either
estrogen receptor (ER)-α or ER-β, resulting in an induction of the
growth regulator signal transduction pathway (2–4).
Estrogen mediates cell proliferation via the genomic pathway by
inducing the transcription of various genes, such as c-jun, c-myc
and c-fos, and growth factors, as well as having a direct impact on
cyclins that regulate the cell cycle (5). Furthermore, the non-genomic pathways
cause the binding of estrogen to either membrane-bound estrogen
receptor, resulting in the activation of a number of intracellular
signaling pathways, such as PI3K/Akt and ERK, which upon activation
lead to anti-apoptotic signals (6–8).
Malignant transformation of cells may lead to the dysregulation of
ER signaling, resulting in evasion of apoptosis, self-induced
growth signals and clonogenicity, and ultimately malignant
transformation (2,6).
Apart from epithelial cell proliferation and growth,
estrogen also modulates endothelial cell growth, tubulogenesis and
angiogenesis (9). Angiogenesis is
an indispensable process for tumor growth and metastasis involving
the sprouting of new blood vessels from pre-existing capillaries
(9). On the other hand,
neo-vascularization involves the generation of new blood vessels
from endothelial progenitor cells. Bone marrow-derived endothelial
progenitor cells (BM-EPCs) which normally reside in the bone marrow
are significant mediators of neo-vascularization (10). BM-EPCs have the potential of
proliferating, mobilizing and differentiating into mature
endothelial cells in response to pro-angiogenic factors, such as
vascular endothelial growth factor (VEGF) and matrix
metalloproteinases (MMPs) (10–15).
Under normal conditions, the generation of new vessels is regulated
by a complex interaction network of activators and inhibitors of
angiogenesis. However, in case of inflammation, injury or cancer,
this balance between pro- and anti-angiogenic factors is tilted
towards the pro-angiogenic factors. This leads to mobilization,
homing and incorporation of BM-EPCs at the diseased site,
remodeling of the extracellular matrix and anastomoses of
surrounding pre-existing and new vessels, resulting in
neo-vascularization (10–15). Recently, estrogen was found to act
as a mobilizing agent for breast cancer-responsive
neo-vascularization (16).
Increased angiogenesis, a major process crucial for
the development of new blood vessels, was extensively observed in
thyroid proliferative disease including Graves’ disease,
hyperplastic goiter and thyroid cancer (17). Over 200 million people worldwide are
affected by thyroid proliferative disease (TPD), which includes
hypothyroidism, hyperthyroidism, adenoma, goiter and cancer, and
their incidences have been on the increase, particularly among
women (18). Notably, according to
the American Thyroid Association, one in every eight female
individuals are likely to develop some type of thyroid disorder in
their lifetime (19). Observational
studies indicate that pregnancy, oral contraceptives and estrogen
replacement therapy increase the risk of TPD with a decrease in the
risk of thyroid malignancies following menopause (20). This marked gender bias warrants
investigation into the factors, most obviously estrogen, that make
‘being female’ such a high risk factor for TPD. A recent study from
our laboratory, as well as studies from the literature provide
evidence that thyroid cells express the functional estrogen
receptor and are estrogen-responsive (21–23).
Estrogen potentially modulates the metastasis of thyroid cancer
cells, although the role of estrogen in thyroid neo-vascularization
remains to be determined (23).
Based on the past literature and our findings, the present study
was designed to examine the contribution of estrogen in BM-EPC
mobilization towards implanted transformed cells using the
well-characterized Tie2/green fluorescent protein (GFP) in
vivo model. In this model, BM-EPCs express GFP under the
transcriptional control of endothelial cell-specific tyrosine
kinase promoter Tek. It was found that estrogen mobilized BM-EPCs
to the tumor site and mediated neo-vascularization in an in
vivo-based experimental system.
Materials and methods
Cell culture
KAT50-TS cells were provided by Dr Kenneth B. Ain
(VA Medical Center, Lexington, KY, USA). This cell line has been
misidentified as being of thyroid origin (24). However, DNA profiling using the
Identifier kit from Applied Biosystems (data not shown) confirmed
that HT-29(50-TS) is not of thyroid cancer origin but matches the
short tandem repeat (STR) profile of the HT-29 colorectal cancer
cell line. Thus, by our own convention, the cell line name
HT-29(50-TS) was designated. This name identifies the parent cell
line, but distinguishes the two cell lines as unique sublines that
may exhibit differential responses compared to similar treatments
of HT-29 in another laboratory. Irrespective of the origin, the
cancer cells grow well in thyroid tissue, and our experimental
results remain validated. Cells were cultured in phenol red-free
RPMI-1640 (Mediatech, Herndon, VA, USA) supplemented with 10% fetal
bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA),
penicillin 10,000 IU/ml, streptomycin 10,000 μg/ml (Mediatech), 2
mM L-glutamine (Mediatech), 100 mM MEM sodium pyruvate solution
(Mediatech) and 10 mM MEM non-essential amino acid solution
(Mediatech).
Western blot analysis
Human tumor cells were routinely maintained in
culture and harvested using trypsin, and then washed with
phophate-buffered saline (PBS) twice. Cytoplasmic and nuclear
lysates were prepared using the NE-PER nuclear and cytoplasmic
extraction reagent kit by Pierce (Rockford, IL, USA). The
cytoplasmic and nuclear fractions were separated according to the
manufacturer’s instructions. Western blot analysis was then
performed for anti-ER-α and anti-ER-β antibodies (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Trypan blue experiment
Cells (1×105) were plated in complete
medium in 6-well culture dishes and were allowed to adhere
overnight. The following day the cells were washed with PBS,
starved using serum- and phenol red-free medium, 10% charcoal
stripped FBS (Sigma Chemical Co., St. Louis, MO, USA) and
penicillin 10,000 IU/ml for 24 h or left in 10% FBS as the control.
Subsequently, cells were treated with 10−8 M estradiol
(E2) (Sigma Chemical Co.) or left untreated as the
control. After 24 h, the cells were harvested and stained using
0.4% trypan blue solution (Sigma Chemical Co.). The number of
viable (unstained) and dead (stained) cells was counted using a
hemocytometer, and the stimulation of cell growth by E2
was calculated as the increase in viable cell count for cells
treated with E2 relative to the control cells.
Animals
Female BALBc/nu/nu mice (aged 8–12 weeks) were
purchased from Charles River Laboratories International, Inc.
(Wilmington, MA, USA). The mice were housed and maintained in
laminar flow cabinets under specific pathogen-free conditions in
facilities approved by the American Association of Laboratory
Animal Care in accordance with current regulations and standards of
the U.S. Department of Agriculture, the U.S. Department of Health
and Human Services, the New York State Department of Health, and
the NIH. The mice were used in accordance with the Animal Care and
Use Guidelines of New York Medical College (Valhalla, NY, USA)
under a protocol approved by the Institutional Animal Care Use
Committee (no. 149-2-1007).
Experimental model
Mice were grouped as follows: non-ovariectomized
(intact), ovariectomized (OVX) and ovariectomized +
estrogen-supplemented (OVX + E2). Estrogen
supplementation was administered by implantation of a 90-day
release pellet containing 1.7 mg 17β-estradiol (Innovative Research
of America, Sarasota, FL, USA). Seven days later, human cancer
cells (100×106 cells per ml) were injected in 5 μl
aliquots into the right thyroid lobe of the OVX and OVX +
E2) mouse. On the other hand, control mice received 5 μl
of PBS as a sham injection. One week following the human cancer
cell injections, bone marrow obtained from the Tek/GFP transgenic
mice was harvested, and bone marrow cells (4×106 cells
in 0.1 ml of saline) were transplanted via tail vein injection to
all of the mice. The donor marrow cells are unique in that they
were populated by BM-EPCs expressing GFP under the transcriptional
control of endothelial cell-specific promoter Tek (formerly Tie2).
Since these endothelial cells are GFP-tagged, their migration from
the marrow to tissues were tracked. No animals met the criteria for
sacrifice prior to the end of the treatment period. After a total
of two weeks following the tumor cell injections, the mice were
sacrificed, and the cervical trachea along with the normal
contralateral thyroid gland and thyroid tumors were harvested and
mounted into frozen sections prepared for light microscopy and
immunofluorescence microscopy.
Immunofluorescent analysis
Capillaries were identified as tubular structures
positive for isolectin B4. BM-EPCs were identified as attached
spindle-shaped cells, double-positive for GFP and isolectin B4. The
number of double-positive cells in 15 fields under ×20
magnification was counted per sample in a blinded manner.
Results
Estrogen modulates its effects at cellular levels
via estrogen receptors. As an initial step in the investigation of
the estrogen-mediated tumor responsiveness of BM-EPCs, we
ascertained the presence of estrogen receptors in the human tumor
cells used in the present study. Western blot analysis of the
nuclear and cytoplasmic extracts of the implanted tumor cells
revealed that the two isoforms of estrogen receptors, ER-α and
ER-β, were present in the cell line (Fig. 1A). The presence of estrogen
receptors was also validated by their responsiveness to
estradiol-mediated enhancement of proliferation (Fig. 1B).
Previous studies implicated estrogen in the onset of
angiogenesis and neo-vascularization in estrogen-responsive
tissues, such as breast, including a study from our laboratory.
Results of our previous study showed that estradiol mobilizes
breast cancer-responsive BM-EPCs, initiating neo-vascularization.
In the present study, the ability of estrogen to mobilize BM-EPCs
towards implanted human cancer cells in the thyroid gland was
investigated. Ovariectomized female BALB/c nude mice were
supplemented with or without estradiol pellets. After 7 days of
estradiol supplementation, mice were injected with human cancer
cells, and at day 14, tail vein injections of BM-EPCs isolated from
Tek/GFP transgenic mice were administered. Mice were sacrificed
after 21 days, and tumors were harvested and analyzed by light
microscopy and immunofluorescence microscopy. The implantation of
5×105 cancer cells/mouse resulted in 100% tumor
incidence for all groups of mice, with the histology of the tumors
being consistent among the groups (Fig.
2).
Tek+GFP+ cells were counted as
a representative population of BM-EPCs. Since these cells were also
GFP-tagged, their migratory pattern in response to tumor growth and
estradiol were easily traced. Capillaries were identified as
tubular structures positive for isolectin B4, a marker for
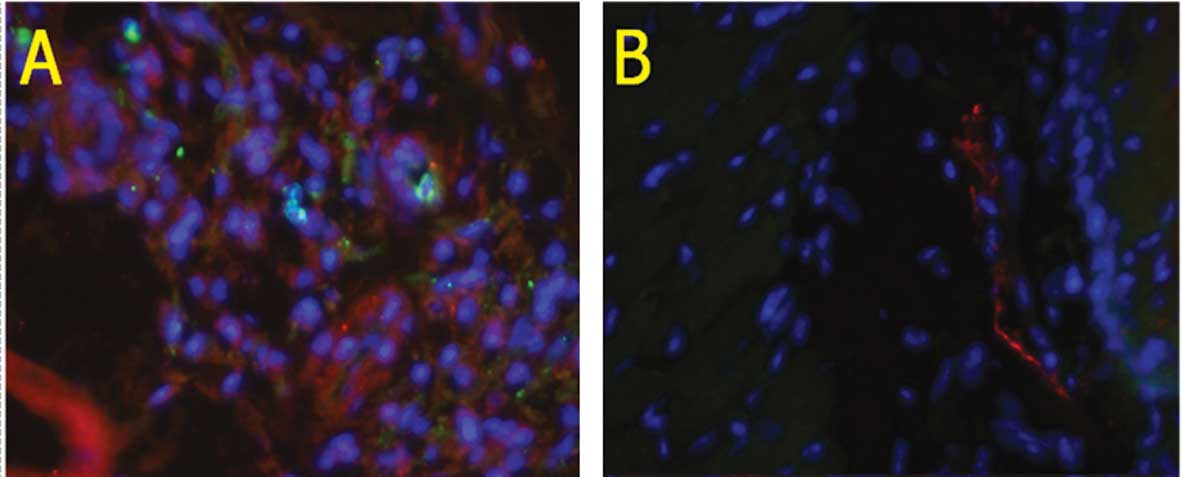
progenitor cells of endothelial origin. Tumor xenografts exhibited
more BM-EPC mobilization in comparison to the sham-injected group,
suggesting that the tumors secreted various paracrine factors that
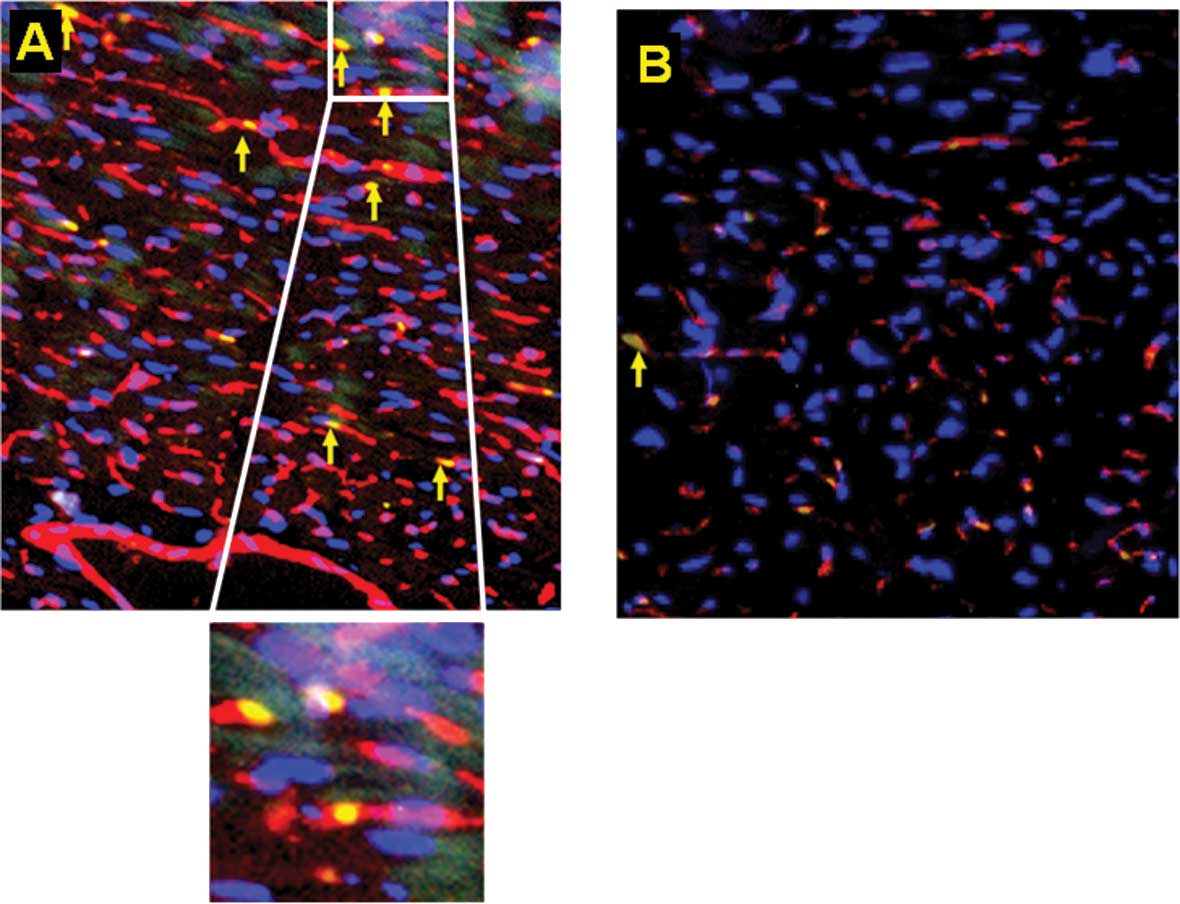
mobilized tumor-responsive BM-EPCS (Fig. 3). Furthermore, increased numbers of
BM-EPCs were incorporated into the tumor neo-vasculature compared
to the sham-injected group, suggesting that not only do EPCs home
to the tumor but physically associate to the blood vessels
(Fig. 4). This homing of the EPCs
to the tumor tissue was considerably enhanced in the
estrogen-supplemented mice compared to the
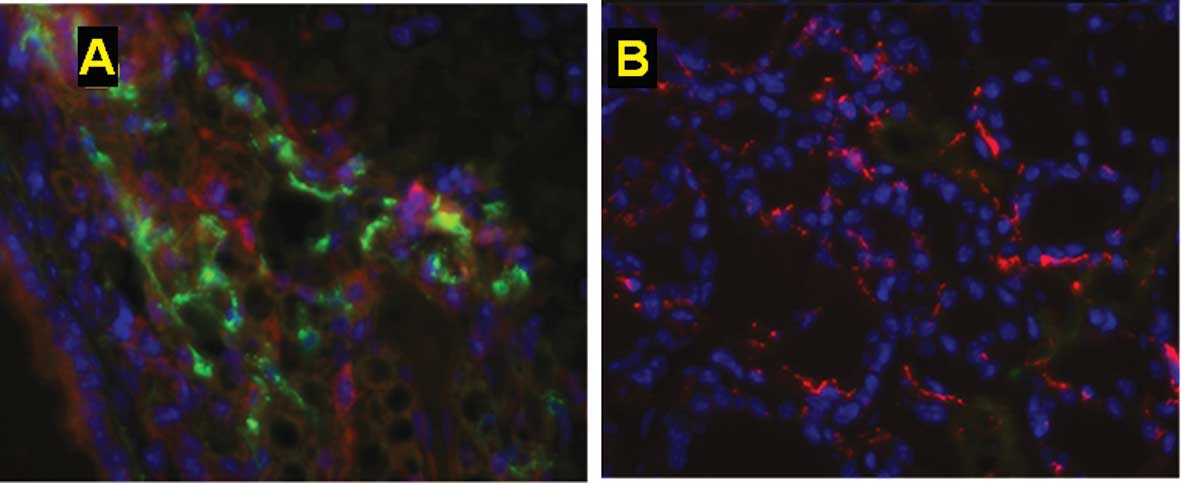
non-estrogen-supplemented tumor-injected group (Fig. 5). In contrast, in the absence of
estrogen supplementation or tumor inoculation, few injected BM-EPCs
mobilized to the tumor tissue (Fig.
3B). These data indicate that the use of estradiol resulted in
an enhanced recruitment of BM-derived cells to the neo-vasculature
of the tumor gland.
Discussion
Angiogenesis is a critical event for normal growth
and development as well as malignant growth and metastasis. The
process occurs throughout our lifespan beginning as early as
embryogenesis and is ongoing throughout postnatal life. Previous
studies found that the mechanism of embryogenic angiogenesis is
different from that which occurs in adults, commonly termed as
postnatal vasculogenesis and neo-vascularization (13). Neo-vascularization is a process
noted in the revascularization of ischemic tissue and wound
healing, as well as a number of diseases, such as diabetic
retinopathy and more recently in various types of cancer (25). Endothelial progenitor cells, which
are mainly located in the bone marrow niche postnatally, play a
crucial role in the process of neo-vascularization. These cells are
phenotypically characterized as CD34+, CD133+
and VEGF receptor 2+ (VEGFR-2+, also known as
KDR or Flk1) (26). The process
involves the release of endothelial progenitor cells from the bone
marrow to the blood circulation in response to various signals,
including stimuli produced by cancer cells, followed by homing to
the source, and in the case of tumor signals, to the tumor bed.
Progenitor stem cells then differentiate into mature endothelial
cells, assisting in ongoing vascular development and
angiogenesis.
Various endogenous and exogenous factors have the
potential to mobilize EPCs from the bone marrow to blood
circulation. Growth factors, cytokines, colony-stimulating factor,
VEGF and GM-CSF are significant endogenous factors that mobilize
EPCs and promote angiogenesis as well as neo-vascularization
(27). Cholesterol-lowering drugs
[HMG-COA reductase inhibitors (statins)] and the peroxisome
proliferator-activated receptor-γ (PPAR-γ) agonist are major
exogenous factors that enhance the mobilization of EPCs and induce
angiogenesis (28). Extreme
physical exercise is also reported to increase EPC mobilization and
vascular functions in humans (28).
Counterattacking these pro-angiogenic factors and blocking the
mobilization of BM-EPCs inhibits the growth and metastasis of
tumors. This process indicates a strong potential for cancer
treatment and has been identified as a potential target for
antitumor therapies. Recent therapeutic trials have attempted to
harness this potential for neo-vascularization into treatment
protocols (29,30).
It is believed that the cancer-promoting properties
of estrogen stem from the effects of various pro-survival pathways
that are activated by the binding of estrogen to its receptor. The
correlation of estrogen exposure to the thyroid gland and its
contribution to TPD is lacking. There is an obvious gender bias in
thyroid cancer, alluding to the putative role of estrogen. Our data
aimed to clarify this relationship by utilizing a body of evidence
indicating BM-EPCs as significant factors involved in
neo-vascularization. In this context, our data revealed that
BM-EPCs homed to xenograft tumors inducing neo-vascularization in
our in vivo model. Furthermore, this tumor-responsive homing
of BM-EPCs to the tumor site was greatly enhanced by estradiol by
potentially secreting endothelial and tumor-enhancing factors.
Considering that thyroid vascularization is seminal in thyroid
proliferative disease, our findings may have clinical utility in
using BM-EPCs as a potential ‘Trojan horse’ by which to deliver
bio-molecules that disrupt tumor vasculogenesis and induce the
targeted killing of tumor cells.
Acknowledgements
This study was supported by grants from the National
Cancer Institute 1R01CA131946 and the Department of Otolaryngology,
New York Eye and Ear Infirmary, New York, NY. The authors
gratefully acknowledge the contributions of Dr Raj Kishore
(Northwestern University) for his assistance in the
immunofluorescent analysis and Dr Jeffrey N. Myers and Dr Maria
Gule (The University of Texas M. D. Anderson Cancer Center) for
their aid in refining the protocol for injections in the
thyroid.
References
|
1
|
Lord RS, Bongiovanni B and Bralley JA:
Estrogen metabolism and the diet-cancer connection: rationale for
assessing the ratio of urinary hydroxylated estrogen metabolites.
Altern Med Rev. 7:112–129. 2002.PubMed/NCBI
|
|
2
|
He YY, Cai B, Yang YX, Liu XL and Wan XP:
Estrogenic G protein-coupled receptor 30 signaling is involved in
regulation of endometrial carcinoma by promoting proliferation,
invasion potential, and interleukin-6 secretion via the MEK/ERK
mitogen-activated protein kinase pathway. Cancer Sci.
100:1051–1061. 2009. View Article : Google Scholar
|
|
3
|
Vijayanathan V, Venkiteswaran S, Nair SK,
Verma A, Thomas TJ, Zhu BT and Thomas T: Physiologic levels of
2-methoxyestradiol interfere with nongenomic signaling of
17β-estradiol in human breast cancer cells. Clin Cancer Res.
12:2038–2048. 2006.PubMed/NCBI
|
|
4
|
Lewis-Wambi JS and Jordan VC: Estrogen
regulation of apoptosis: how can one hormone stimulate and inhibit?
Breast Cancer Res. 11:2062009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciocca DR and Fanelli MA: Estrogen
receptors and cell proliferation in breast cancer. Trends
Endocrinol Metab. 8:313–321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song RX and Santen RJ: Membrane initiated
estrogen signaling in breast cancer. Biol Reprod. 75:9–16. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hua K, Feng W, Cao Q, Zhou X, Lu X and
Feng Y: Estrogen and progestin regulate metastasis through the
PI3K/AKT pathway in human ovarian cancer. Int J Oncol. 33:959–967.
2008.PubMed/NCBI
|
|
8
|
Pietras RJ: Interaction between estrogen
and growth factor receptors in human breast cancers and
tumor-associated vasculature. Breast J. 9:361–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Losordo DW and Isner JM: Estrogen and
angiogenesis: A review. Arterioscler Thromb Vasc Biol. 21:6–12.
2001. View Article : Google Scholar
|
|
10
|
Risau W: Mechanism of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aghi M and Chiocca EA: Contribution of
bone marrow-derived cells to blood vessels in ischemic tissues and
tumors. Mol Ther. 6:994–1005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Young PP, Vaughan DE and Hatzopoulos AK:
Biological properties of endothelial progenitor cells (EPCs) and
their potential for cell therapy. Prog Cardiovasc Dis. 49:421–429.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masuda H and Asahara T: Post-natal
endothelial progenitor cells for neovascularization in tissue
regeneration. Cardiovasc Res. 58:390–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood ‘endothelial progenitor cells’ are derived from
monocytes/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003.
|
|
15
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suriano R, Chaudhuri D, Johnson RS,
Lambers E, Ashok BT, Kishore R and Tiwari RK: 17β-Estradiol
mobilizes bone marrow-derived endothelial progenitor cells to
tumors. Cancer Res. 68:6038–6042. 2008.
|
|
17
|
Ramsden JD: Angiogenesis in the thyroid
gland. J Endocrinol. 166:475–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
19
|
Cook MB, Dawsey SM, Freedman ND, Inskip
PD, Wichner SM, Quraishi SM, Devesa SS and McGlynn KA: Sex
disparities in cancer incidence by period and age. Cancer Epidemiol
Biomarkers Prev. 18:1174–1182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levi F, Franceschi S, Gulie C, Negri E and
Vecchia CL: Female thyroid cancer: the role of reproductive and
hormonal factors in Switzerland. Oncology. 50:309–315. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furlanetto TW, Nguyen LQ and Jameson JL:
Estradiol increases proliferation and down-regulates the
sodium/iodide symporter gene in FRTL-5 cells. Endocrinology.
140:5705–5711. 1999.PubMed/NCBI
|
|
22
|
Manole D, Schildknecht B, Gosnell B, Adams
E and Derwahl M: Estrogen promotes growth of human thyroid tumor
cells by different molecular mechanisms. J Clin Endocrinol Metab.
86:1072–1077. 2001.PubMed/NCBI
|
|
23
|
Rajoria S, Suriano R, Shanmugam A, Wilson
YL, Schantz SP, Geliebter J and Tiwari RK: Metastatic phenotype is
regulated by estrogen in thyroid cells. Thyroid. 20:33–41. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schweppe RE, Klopper JP, Korch C,
Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow L, Copland JA,
Smallridge RC and Haugen BR: Deoxyribonucleic acid profiling
analysis of 40 human thyroid cancer cell lines reveals
cross-contamination resulting in cell line redundancy and
misidentification. J Clin Endocrinol Metab. 93:4331–4341. 2008.
View Article : Google Scholar
|
|
25
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Patore C, Silver M, Marianne K, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar
|
|
26
|
Gehling UM, Ergun S, Schumacher U, Wagener
C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N,
Kluge K, Schafer B, Hossfeld DK and Fiedler W: In vitro
differentiation of endothelial cells from AC133-positive progenitor
cells. Blood. 95:3106–3112. 2000.
|
|
27
|
Khakhoo AY and Finkel T: Endothelial
progenitor cell. Ann Rev Med. 56:79–101. 2005. View Article : Google Scholar
|
|
28
|
Dong F and Ha X-Q: Effect of endothelial
progenitor cells in neovascularization and their application in
tumor therapy. China Med J. 123:2454–2460. 2010.PubMed/NCBI
|
|
29
|
Wu LC and Zhang WD: Clinical trials of
antiangiogenesis therapy on gastric cancer. Gastroenterol Res.
1:14–19. 2008.
|
|
30
|
Kerbel RS: Antiangiogenic therapy: A
universal chemosensitization strategy for cancer? Science.
312:1171–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|