Introduction
Oral squamous cell carcinoma (OSCC) accounts for
approximately 90% of cases of oral cancers and is currently ranked
sixth among malignant human cancers (1). Although advances have been made in
various treatment methods for oral cancer, the five-year survival
rate of OSCC is only 55–60% after standard therapy (1,2).
Long-term clinical observations have shown that a number of OSCCs
are derived from various precancerous lesions (1–3).
However, no effective treatment currently exists to prevent or
block the development of malignant precancerous lesions. Therefore,
reversal of the precancerous lesions or protection from the
malignant transformation through effective intervention is likely
to have a great impact on the prevention and treatment of oral
cancer.
Recent studies (3–5) have
shown that the initiation of OSCC is a multi-staged complex
pathological process involving the changes of numerous genes. The
toxic effect of chemical substances is one of the major causes of
OSCC. Due to its similarity to the malignant transformation process
of human oral mucosal epithelia, the DMBA
(7,12-dimethylbenz[a]anthracene)-induced squamous cell carcinoma
model with buccal mucosa from a hamster cheek pouch is currently
considered to be the most ideal animal model of oral mucosal cancer
(6,7). However, no studies are available at
the whole genome level regarding the changes in genes and pathways
during the DMBA-induced malignant transformation of oral mucosa
from precancerous lesions to OSCC. Subsequently, a DMBA-induced
Syrian hamster model of malignant transformation of cheek pouch
mucosa from precancerous lesions to squamous cell carcinoma was
established. Additionally, the differentially expressed gene
profile at the whole genome level was analyzed during this
transformation process using an Agilent rat whole genome
microarray, which contains 41,000 genes/ESTs. Oral mucosa malignant
transformation-related genes and pathways were examined at the
whole genome level using bioinformatics methods such as gene
ontology (GO) functional classification and pathway analysis. These
methods are crucial to the study of molecular mechanisms involved
in the initiation and progress of the malignant transformation of
oral mucosa precancerous lesions, reversal of the precancerous
lesions, and intervention methods which block the malignant
transformation.
Materials and methods
Animals and reagents
Twelve Syrian hamsters were purchased from the
Chengdu Institute of Biological Products (Chengdu, China). The
reagents used were: DMBA (Sigma, St. Louis, MO, USA), PCR
instrument (PTC-100, MJ Company, USA), hybridization oven (G2545A,
Agilent Technologies, Santa Clara, CA, USA), Agilent scanner
(G2565AA, Agilent Technologies), a spectrophotometer (ND1000,
NanoDrop Technologies Inc., Wilmington, DE USA), an RT-PCR kit
(Bioflux, Japan), and an Agilent rat genome cDNA microarray
(Agilent Technologies).
Experimental methods
To establish the hamster model of cheek pouch
precancerous lesions and squamous cell carcinoma, a total of 12,
6–8-week old Syrian hamsters, weighing 90–120 g, were randomly
divided into groups I and II, with six animals in each group. The
cheek pouch was opened, and 5% DMBA in acetone solution was applied
to the bilateral cheek pouch every Monday, Wednesday and Friday.
The animals were sacrificed after 6 weeks (precancerous lesion
group) and 12 weeks (squamous cell carcinoma group), respectively,
following the DMBA treatment. Samples of the cheek pouch tissues
were removed. Half of the sample was stored immediately in liquid
nitrogen, and the remaining half was fixed with 10% formalin,
paraffin-embedded, sectioned, stained with hematoxylin and eosin
(H&E), and observed under a light microscope. The experimental
procedures were approved by the Laboratory Animal Management
Committee of Chongqing Medical University.
Extraction and purification of total
RNA
Total RNA from the two groups was isolated from
frozen tissues using a TRIzol RNA Extraction kit (Invitrogen,
Carlsbad, CA, USA) and further purified using a RNeasy mini kit
(Qiagen, Valencia, CA, USA) according to the manufacturer's
instructions. The purified RNA was dissolved in RNase-free water
and stored in a freezer at -80°C until required.
cRNA labeling and synthesis
Extracted total RNA in the two groups was
fluorescently labeled with Cy3 through reverse transcription
according to the kit's instructions. Following the synthesis, cRNAs
of each group were labeled with aaUTP and purified with a RNeasy
mini kit (Qiagen). The cRNAs were then quantified with a
spectrophotometer and purified again.
cRNA fragmentation and microarray chip
hybridization
Cy3-labeled cRNA (875 ng) from each group was used
in the experiment. Fragmentation was carried out following the
manufacturer's instructions. The microarray chips were hybridized
with 100 μl of fragmented cRNAs for 17 h (65°C, 10 r/min) and then
washed subsequently in solution 1 and 2 at 37°C for 1 min each
time.
Chip scanning and data processing
Hybridized microarray chips were scanned with an
Agilent G2565AA Microarray Scanner (Agilent Technologies) and the
results were normalized with feature extraction software. Ratio ≥2
and ≤0.5 criteria were used to select differentially expressed
genes.
Bioinformatics analysis
Abnormally expressed genes were functionally
classified based on the GO standard (http://www.ncbi.nlm.nih.gov/). The abnormally changed
pathways were identified by searching pathway databases KECG,
GENMAPP and BIOCARTA (www.biorag.com). P<0.05 was used
to select the pathways that were significantly altered.
Validation of microarray results by
RT-PCR
Up-regulated gene SPARC and down-regulated gene
Casp3 were randomly selected for RT-PCR validation. PCR primers
(Table I) were designed using
Primer3 software.
 | Table IRT-PCR primers. |
Table I
RT-PCR primers.
| Gene name | GenBank
accession | Primer sequence | Product length
(bp) |
|---|
| SPARC | NM_012656 | F:
5′-GTGCCAGGACCCCACCAG-3′
R: 5′-ATGGGAATGAGGGGAGCG-3′ | 504 |
| Casp3 | NM_012922 | F:
5′-ACCCTGAAATGGGCTTGT-3′
R: 5′-GTTTCGGCTTTCCAGTCA-3′ | 348 |
| β-actin | NM_001101.2 | F:
5′-GTAAAGACCTCTATGCCAACA-3′
R: 5′-GGACTCATCGTACTCCTGCT-3′ | 227 |
Results
Pathological examination of precancerous
lesion and squamous cell carcinoma tissues
Routine H&E staining showed that among the six
cases of precancerous lesions in group I, there were five cases of
moderate dysplasia and one case of mild dysplasia. An experiment
was performed with the five moderate dysplasia cases to detect
precancerous lesions. The six cases in group II were all squamous
cell carcinoma tissues (Fig.
1).
Screening the genes that are
differentially expressed in precancerous lesions and squamous cell
carcinoma tissues
A total of 1,981 genes were differentially expressed
during the transformation of hamster cheek mucosa from the
precancerous lesions to OSCC. Of these genes, 1,861 are known, 120
are unknown, 1,033 are up-regulated and 944 are down-regulated.
Functional classification of
differentially expressed genes
The 1,861 differentially expressed known genes were
classified into 14 functional gene categories (Table II) based on the GO functional
classification.
 | Table IIGene ontology (GO) analysis of the
differentially expressed genes. |
Table II
Gene ontology (GO) analysis of the
differentially expressed genes.
| GO functions | Up-regulated
genes | Down-regulated
genes | Total | % |
|---|
| Metabolism | 298 | 185 | 483 | 24.38 |
| Development | 179 | 212 | 391 | 19.74 |
| Cell structure | 133 | 178 | 311 | 15.70 |
| Stress | 72 | 107 | 179 | 9.04 |
| Signal
transduction | 83 | 42 | 125 | 6.31 |
| Immune | 41 | 52 | 93 | 4.69 |
| Transportation | 47 | 15 | 62 | 3.13 |
| Cellular
localization | 19 | 32 | 51 | 2.57 |
| Cell adhesion | 17 | 24 | 41 | 2.07 |
| Transcription | 25 | 9 | 34 | 1.72 |
| Cell growth | 13 | 19 | 32 | 1.62 |
| Cell
differentiation | 23 | 8 | 31 | 1.56 |
| Apoptosis | 6 | 12 | 18 | 0.91 |
| Cell cycle | 7 | 3 | 10 | 0.50 |
| Unknown function | 74 | 46 | 120 | 6.06 |
Pathway analysis
By checking the 1,861 known differentially expressed
genes against three major pathway databases, 9 pathways were
determined to have been significantly alered during the
transformation from precancerous lesions to squamous cell
carcinomas (P<0.05). However, only 14 out of the 1,861 known
genes were enriched in these 9 pathways (Table III).
 | Table IIIPathway analysis. |
Table III
Pathway analysis.
| Pathway | P-value | Gene changes |
|---|
| Activation of
caspase-3 | 0.0366 | Up: 0
Down: Casp3 |
| Chemokine receptors
bind to chemokines | 0.0406 | Up: CCL5, CXCL12 and
CCL24
Down: 0 |
| E2F-mediated
regulation of DNA replication | 0.0471 | Up: 0
Down: Dhfr and Tyms |
| G1/S transition | 0.0370 | Up: 0
Down: Dhfr and Tyms |
| Integrin cell surface
interactions | 0.0070 | Up: Vcam1, Vtn and
Fn1
Down: 0 |
| Lipoprotein
metabolism | 0.0082 | Up: Apoe and
Alb
Down: 0 |
| Response to elevated
platelet cytosolic Ca | 0.0366 | Up: Alb, Fn1,
Serping1 and Sparc
Down: 0 |
| Arachidonic acid
metabolism | 0.012 | Up: 0
Down: Cyp2b13 |
| Metabolism of
xenobiotics by cytochrome P450 | 0.040 | Up: 0
Down: Cyp2b13 |
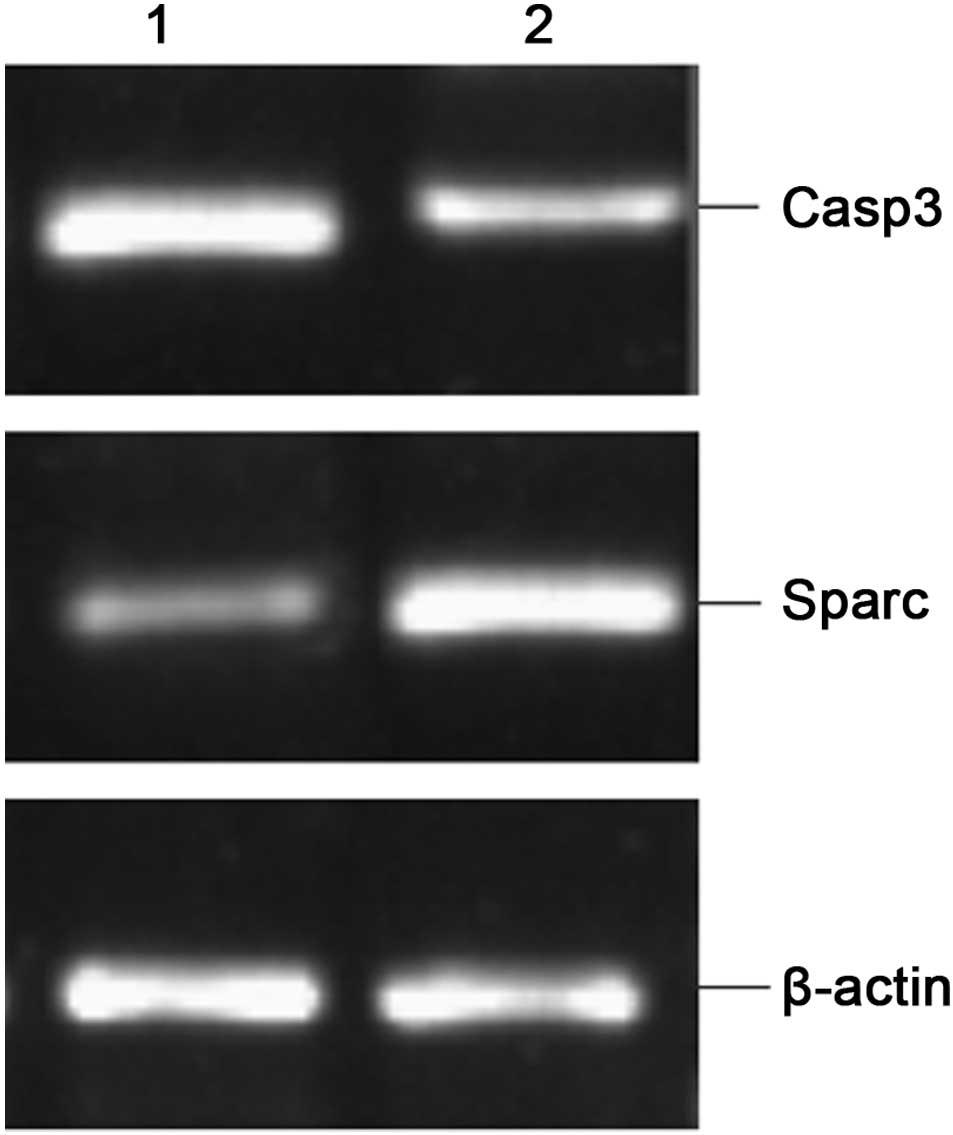
RT-PCR validation
The results from the RT-PCR validation of the
up-regulated gene SPARC and the down-regulated gene Casp3 are shown
in Fig. 2. The gene expression of
Sparc and Casp3, which is shown as integral optical densities of
the PCR products, has been normalized with the expression of
β-actin and compared (Table IV).
The PCR results confirmed the trend of gene expression determined
in the microarray analysis.
 | Table IVValidation of microarray results by
RT-PCR. |
Table IV
Validation of microarray results by
RT-PCR.
| Gene name | Target gene
mRNA/β-actin mRNA (mean ± SD) | B/A | Microarray |
|---|
|
| | |
|---|
| A | B | | |
|---|
| SPARC | 0.1157±0.0031 | 0.5023±0.0131 | 4.34 | 4.70 |
| Casp3 | 0.4401±0.0024 | 0.1467±0.0008 | 0.32 | 0.3a |
Discussion
This study has established a DMBA-induced hamster
model of transformation of buccal mucosa from precancerous lesions
to squamous cell carcinoma. A total of 1,981 differentially
expressed genes were identified through microarray analysis of
whole mouse genome. Of these, 1,861 are known genes. These
differentially expressed genes were grouped into 14 functional
categories: metabolism, development, cell structure, stress, signal
transduction, immune, chemical transportation, cellular
localization, cell adhesion, transcription, cell growth, cell
differentiation, apoptosis and cell cycle. This classification
indicates that the malignant transformation of the oral mucosa
precancerous lesion is a complex pathological process involving
changes in numerous genes.
Further pathway analysis shows that there were 9
significantly altered pathways in the DMBA-induced malignant
transformation of oral mucosa precancerous lesions. However, out of
the 1,861 differentially expressed known genes, only 14 genes were
found to be enriched in the 9 abnormally altered pathways. These
genes were: Casp3, CCL5, CXCL12, CCL24, Dhfr, Tyms, Vcam1, Vtn,
Fn1, Apoe, Alb, Serping1, Sparc and Cyp2b13. Thus, we consider
these 14 genes to play significant pathogenic roles in the
malignant transformation of oral mucosa precancerous lesions.
Studies have shown that Casp3 is an apoptosis regulatory gene; and
that its down- regulation promotes tumor cell proliferation and
inhibits tumor cell apoptosis (8).
CCL5, CXCL12 and CCL24 are all chemokines, whose high expression
promotes cell proliferation and tumor metastasis (9–11).
Dhfr and Tyms are key enzymes of intracellular folate metabolism
(12–14), both of which are the target of the
folic acid antagonist class of anti-cancer drugs and are
responsible for drug resistance. Their expression levels correlate
to the prognosis and drug resistance of the cancer, and a low
expression level indicates a favorable prognosis. Vcam1 belongs to
the immunoglobulin superfamily, and its high expression enhances
the migration of endothelial cells and angiogenesis, thereby
promoting tumor invasion and metastasis (15). A high expression of either Vtn or
Fn1 promotes tumor formation and metastasis (16–18).
Apoe is an arginine-rich basic protein, whose high expression also
promotes tumor cell proliferation and metastasis (19,20).
Alb expression correlates to cancer prognosis; the prognosis of
tumors expressing high levels of Alb protein is better than that of
tumors with a low Alb expression (21). Serping1 gene encodes the C1
inhibitor, which inhibits the activity of coagulation factors XIIa
and XIa, kallikrein and plasmin, thereby playing significant roles
in the anticoagulation system of the body. Although studies have
shown that the expression levels of Serping1 are variable among
different types of tumors (22,23),
Serping1 expression is up-regulated in the present study.
Therefore, it is hypothesized that Serping1 exerts diverse effects
on different types of tumors; however, the underlying mechanisms
should be investigated. A high expression of SPARC enhances the
mobility of tumor cells and promotes tumor invasion and metastasis
(24–26). Cyp2b13 belongs to the second family
of cytochrome P450. It participates in the metabolism of a number
of carcinogens and toxins, induces mutation in a number of genes,
and inhibits certain gene expressions (27). The down-regulation of Cyp2b13 causes
a decrease in carcinogen metabolism and detoxification. The present
study has shown a similar expression pattern of the above-mentioned
genes during the malignant transformation process of oral mucosa
precancerous lesions. The low expression of Dhfr and Tyms and the
high expression of Alb indicate a positive prognosis of the tumor,
which may be related to the fact that the animal model of OSCC in
this study is currently at an early stage.
The present study is a preliminary one, and the gene
expression results should be validated and verified. However, an
overview is provided of the gene and pathway changes occurring
during the transformation of oral buccal mucosa from precancerous
lesions to squamous cell carcinoma at a whole genome level. The
identified abnormally altered genes and pathways in this study
potentially shed light on further in-depth investigations.
Acknowledgements
The authors would like to express their appreciation
to Shanghai Biochip Co., Ltd., for the technical support in this
study. This study was supported by the Medical Foundation of
Chongqing City Health Bureau (no. 03-2-073).
References
|
1
|
Kademani D, Bell RB, Schmidt BL,
Blanchaert R, Fernandes R, Lambert P and Tucker WM: Oral and
maxillofacial surgeons treating oral cancer: a preliminary report
from the American Association of Oral and Maxillofacial Surgeons
Task Force on Oral Cancer. J Oral Maxillofac Surg. 66:2151–2157.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lumerman H, Freedman P and Kerpel S: Oral
epithelial dysplasia and the development of invasive squamous cell
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
79:321–329. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang K, Zhang G, Mei J, Chen D and Wu M:
Screening and analysis of pathogenic genes during DMBA-induced
buccal mucosa carcinogenesis in golden hamsters. Oncol Rep.
23:1619–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carinci F, Lo Muzio L, Piattelli A, et al:
Genetic portrait of mild and severe lingual dysplasia. Oral Oncol.
41:365–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng L and Wang Z: Chemopreventive effect
of celecoxib in oral precancers and cancers. Laryngoscope.
116:1842–1845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gimenez-Conti IB and Slaga TJ: The hamster
cheek pouch carcinogenesis model. J Cell Biochem Suppl. 9:83–90.
1993. View Article : Google Scholar
|
|
8
|
Meggiato T, Calabrese F, De Cesare CM,
Baliello E, Valente M and Del Favero G: C-JUN and CPP32 (CASPASE 3)
in human pancreatic cancer: relation to cell proliferation and
death. Pancreas. 26:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki K, Natsugoe S, Ishigami S, et al:
Expression of CXCL12 and its receptor CXCR4 in esophageal squamous
cell carcinoma. Oncol Rep. 21:65–71. 2009.PubMed/NCBI
|
|
10
|
Vaday GG, Peehl DM, Kadam PA and Lawrence
DM: Expression of CCL5 (RANTES) and CCR5 in prostate cancer.
Prostate. 66:124–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheadle EJ, Riyad K, Subar D, et al:
Eotaxin-2 and colorectal cancer: a potential target for immune
therapy. Clin Cancer Res. 13:5719–5728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joerger M, Omlin A, Cerny T and Früh M:
The role of pemetrexed in advanced non small-cell lung cancer:
special focus on pharmacology and mechanism of action. Curr Drug
Targets. 11:37–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jensen SA, Vainer B, Witton CJ, Jørgensen
JT and Sørensen JB: Prognostic significance of numeric aberrations
of genes for thymidylate synthase, thymidine phosphorylase and
dihydrofolate reductase in colorectal cancer. Acta Oncol.
47:1054–1061. 2008. View Article : Google Scholar
|
|
14
|
Shimokawa H, Uramoto H, Onitsuka T, Iwata
T, Nakagawa M, Ono K and Hanagiri T: TS expression predicts
postoperative recurrence in adenocarcinoma of the lung. Lung
Cancer. Oct 21–2010.(Epub ahead of print).
|
|
15
|
Nakao S, Kuwano T, Ishibashi T, Kuwano M
and Ono M: Synergistic effect of TNF-alpha in soluble
VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol.
l70:5704–5711. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gubała E, Wiench M, Oczko-Wojciechowska M,
et al: Gene expression analysis by DNA microarray in papillary
thyroid cancer. Endokrynol Pol. 56:752–757. 2005.PubMed/NCBI
|
|
17
|
Heyman L, Kellouche S, Fernandes J, Dutoit
S, Poulain L and Carreiras F: Vitronectin and its receptors partly
mediate adhesion of ovarian cancer cells to peritoneal mesothelium
in vitro. Tumour Biol. 29:231–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heyman L, Leroy-Dudal J, Fernandes J,
Seyer D, Dutoit S and Carreiras F: Mesothelial vitronectin
stimulates migration of ovarian cancer cells. Cell Biol Int.
34:493–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huvila J, Brandt A, Rojas CR, et al: Gene
expression profiling of endometrial adenocarcinomas reveals
increased apolipoprotein E expression in poorly differentiated
tumors. Int J Gynecol Cancer. 19:1226–1231. 2009. View Article : Google Scholar
|
|
20
|
Su WP, Chen YT, Lai WW, Lin CC, Yan JJ and
Su WC: Apolipo-protein E expression promotes lung adenocarcinoma
proliferation and migration and as a potential survival marker in
lung cancer. Lung Cancer. Apr 27–2010.(Epub ahead of print).
|
|
21
|
Chen SS, Chen KK, Lin AT, Chang YH, Wu HH
and Chang LS: Correlation between pre-treatment serum biochemical
markers and treatment outcome for prostatic cancer with bony
metastasis. J Chin Med Assoc. 72:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santin AD, Zhan F, Bignotti E, et al: Gene
expression profiles of primary HPV16- and HPV18-infected early
stage cervical cancers and normal cervical epithelium:
identification of novel candidate molecular markers for cervical
cancer diagnosis and therapy. Virology. 331:269–291. 2005.
View Article : Google Scholar
|
|
23
|
Fernandez-Ranvier GG, Weng J, Yeh RF, et
al: Candidate diagnostic markers and tumor suppressor genes for
adrenocortical carcinoma by expression profile of genes on
chromosome 11q13. World J Surg. 32:873–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar
|
|
25
|
Alonso SR, Tracey L, Ortiz P, et al: A
high-throughput study in melanoma identifies epithelial-mesenchymal
transition as a major determinant of metastasis. Cancer Res.
67:3450–3460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Briggs J, Chamboredon S, Castellazzi M,
Kerry JA and Bos TJ: Transcriptional up-regulation of SPARC, in
response to c-Jun overexpression, contributes to increased motility
and invasion of MCF7 breast cancer cells. Oncogene. 21:7077–7091.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brookman-Amissah N, Mackay AG and Swann
PF: Isolation and sequencing of the cDNA of a novel cytochrome P450
from rat oesophagus. Carcinogenesis. 22:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|