Introduction
Follicular dendritic cell (FDC) sarcoma, first
reported in 1986, is a rare neoplastic proliferation of FDCs
(1). It originates from follicular
dendritic cells of the lymphoid tissue that affect mainly lymph
nodes. The diagnosis of FDC sarcomas is based on node-based spindle
cell lesions, and the expression of CD21, CD35 and clusterin. FDC
sarcoma with an extranodal origin is extremely rare. The most
common sites of extranodal FDC sarcoma are the oral cavity, tonsil,
gastrointestinal tract and liver (2). The clinical and pathological
characteristics of extranodal FDC sarcoma remain under-recognized,
mainly due to limited reported cases in the literature. This case
report focuses on one more case of this rare tumor occurring in the
mesentery, and its diagnosis and treatment are discussed.
Case report
A 43-year-old Chinese male presenting with an upper
abdominal painless mass for approximately 3 months was admitted to
our hospital. A physical examination revealed a firm and palpable
tumor, predominantly in the right superior abdominal quadrant.
There was no evidence of regional lymph node enlargement. The
laboratory examination revealed that all parameters were within
normal levels, with the exception of serum CA125, which was
elevated to 76.9 U/ml. The patient had a 20-year history of tobacco
intake of one packet per day. An ultrasound scan revealed a number
of hypoechoic or anechoic placeholders in the abdomen. The axial
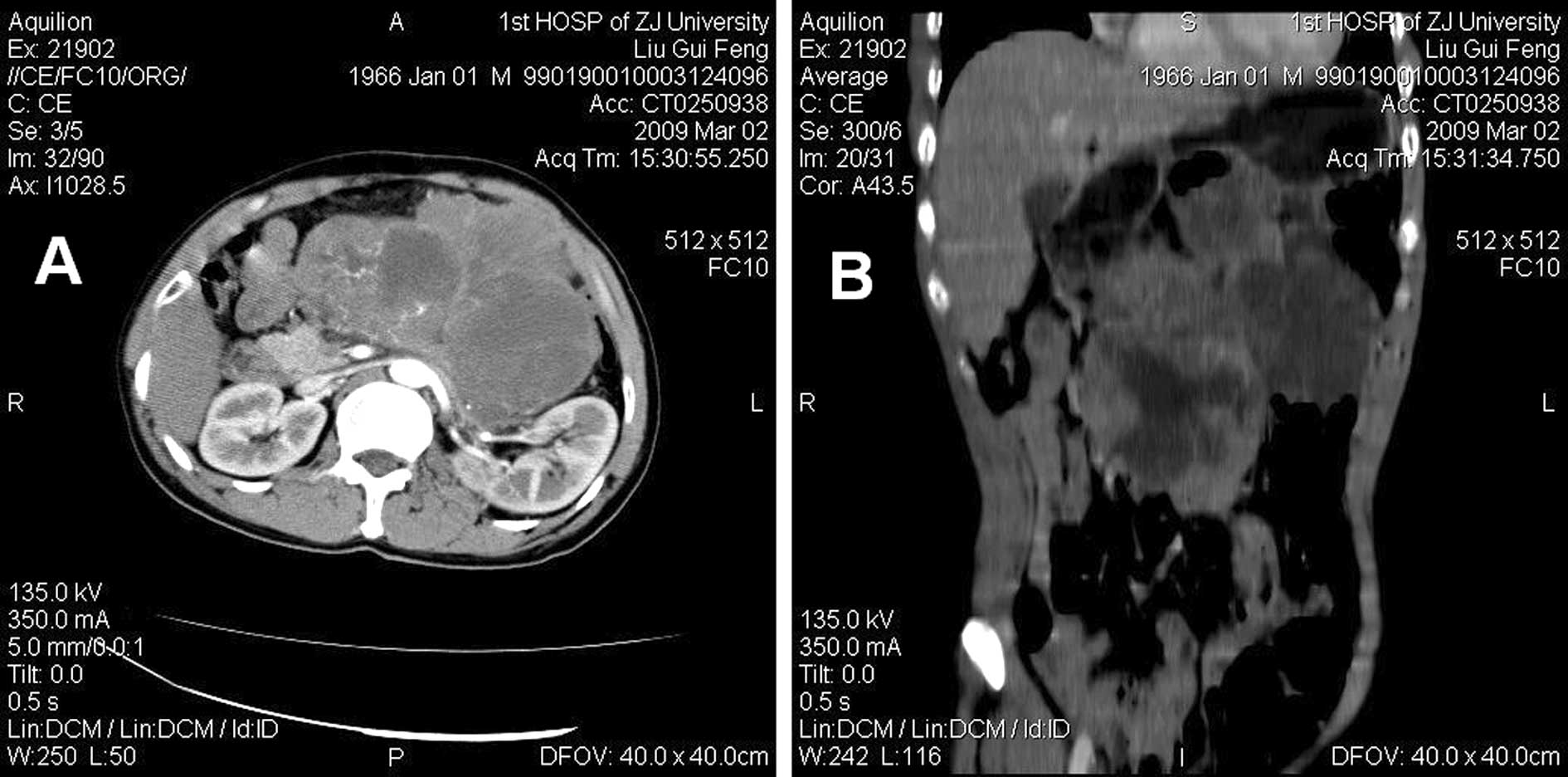
contrast-enhanced computed tomographic (CT) scan showed a large
multilobulated intra-abdominal mass, exhibiting heterogeneous
enhancement and marked necrosis (Fig.
1A). The coronal reconstruction CT image revealed the size,
contour and location of the tumor, and indicated no obvious
abdominal organ involved (Fig. 1B).
Fine-needle aspiration cytology showed a low-grade soft tissue
tumor (data not shown).
Intraoperative exploration revealed a large
intra-abdominal mass (20×18×9 cm) located from the transverse colon
to the aortic bifurcation level. The pancreas, kidney and intestine
nearby were compressed to some extent by the mass. The mass
appeared to be well-encapsulated. A radical resection of the tumor
was successfully performed. Gross examination of the resected tumor
revealed that it was an integration of several well-circumscribed,
multilobulated and focally hemorrhagic masses surrounded by a
complete fibrous capsule (Fig. 2A).
The freshly cut surface of the mass was heterogeneous in color,
from light gray to brown-yellow, and exhibited focal irregular
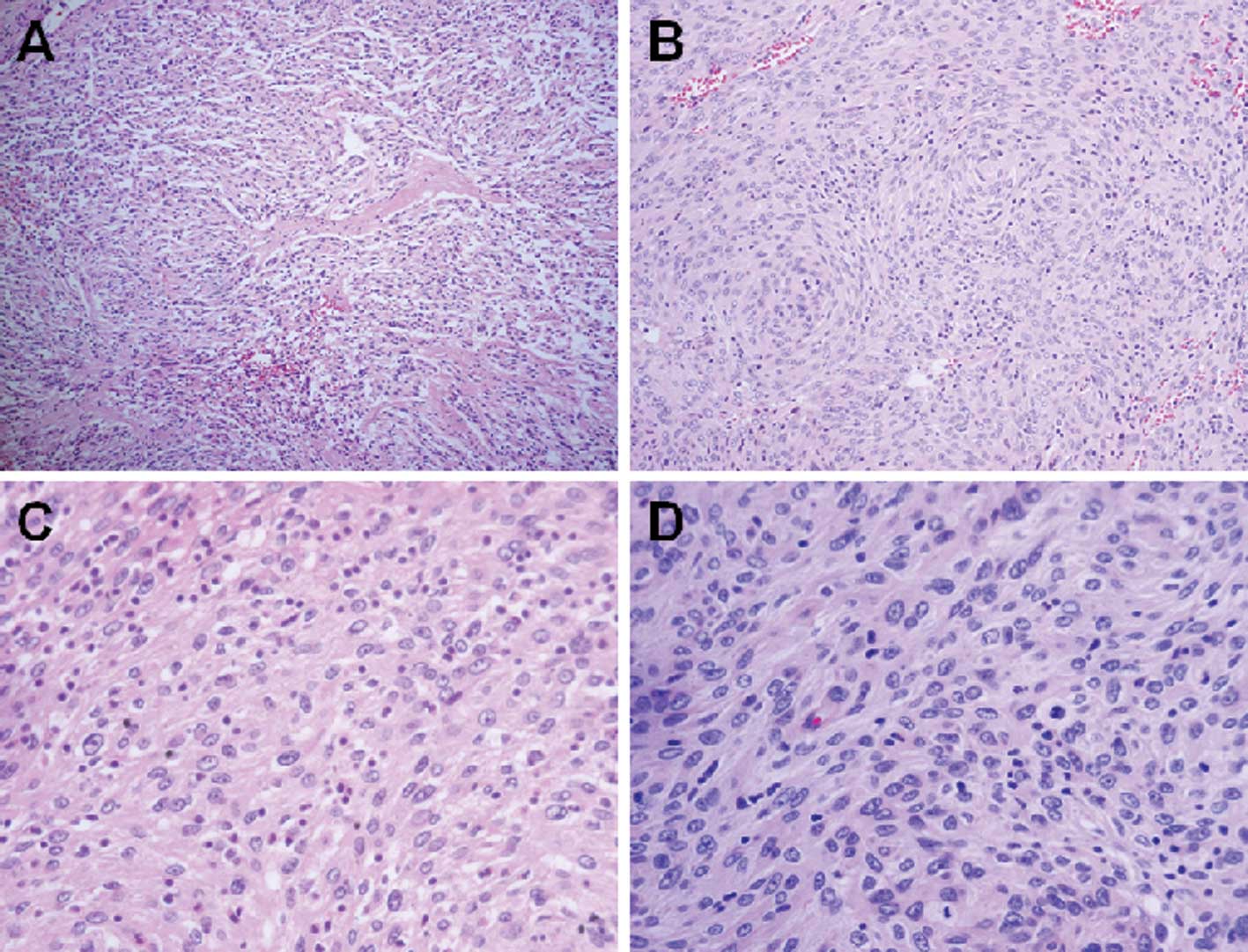
hemorrhagic-cystic changes and necrotic areas (Fig. 2B). A microscopic examination of the
solid tumor revealed that the ovoid to spindle cells were arranged
predominantly in fascicles, storiform and whorls, and formed vague
nodules (Fig. 3A and B). The
individual neoplastic cells exhibited indistinct cell borders
resulting in a syncytial appearance and a moderate amount of
eosinophilic cytoplasm (Fig. 3C).
The nuclei were oval with distinct nucleoli and thin smooth nuclear
membranes. Binucleated and multinucleated tumor cells were
occasionally observed. The tumor was typically infiltrated by small
lymphocytes (Fig. 3D). The admixed
small lymphocytes were mixed with B and T cells. Necrosis and
hemorrhagic-cystic changes were present and filled with necrotic
debris. Immunohistochemical staining revealed that the tumor was
strongly positive for CD21 and CD23 (Fig. 4), and weakly positive for vimentin
(data not shown). No additional adjuvant chemotherapy was
performed. After 18 months of regular follow-up the patient is
asymptomatic.
Discussion
FDCs are found in primary and secondary lymphoid
follicles and play an essential role in antigen presentation for
the B-cell compartment, as well as regulation of the germinal
center reaction. The exact origin of FDCs remains unclear and
hematopoietic lineage origin or stromal-cell derivation has been
proposed. Proliferation of FDCs leads to benign reactive lesions or
generates neoplastic conditions. It was not until 1986 that the FDC
tumor was first characterized by Monda et al (1). Since then, a number of studies have
been reported, expanding the clinical and morphologic spectrum.
Extranodal FDC sarcoma was first reported by Chan
et al in 1994 (3). Since
then, the spectrum of FDC sarcoma in extranodal sites has greatly
expanded to include locations throughout the body, such as head and
neck, liver, spleen, gastrointestinal tract, soft tissue, skin,
lung and breast (3–21). However, due to limited reported
cases in the literature, the clinical and pathological
characteristics of extranodal FDC sarcoma remained
under-recognized. Almost one-third of cases were misdiagnosed at
initial evaluation (3,5–26). The
main cause of misdiagnosis is that it is impossible to initially
consider a poorly differentiated tumor in the extranodal site to be
FDC sarcoma when it is first encountered. Another cause is that FDC
markers are not routinely used for detecting FDC sarcoma in the
extranodal sites.
The diagnosis of FDC sarcoma is established based on
the findings of morphology and immunohistochemistry (7). FDC sarcoma has distinct pathological
characteristics that facilitate an accurate diagnosis. The
histological features of FDC sarcoma tend to be stereotypical
(3). It is composed of spindle or
oval cells arranged in sheets, nets and fascicles, and focally
exhibiting a storiform or whorled growth pattern. Positive
immunohistochemical staining of CD21, CD35 and CD23 was
particularly useful for the final diagnosis of FDC sarcoma. In the
present case, microscopic findings were consistent with those of
the studies mentioned above and a diagnosis of FDC sarcoma
resulted.
In the present case, the laboratory examination
indicated that serum CA125 was elevated to 76.9 U/ml
pre-operatively and decreased to a normal level of 5.5–5.7 U/ml
post-operatively. This result indicated certain intrinsic
associations between serum CA125 levels and FDC sarcoma. However,
elevated serum CA125 levels have not been observed in other
reported FDC sarcomas. Although lymphoma cells do not secrete
CA125, investigators have reported serum elevations of CA125 in as
many as 40% of patients with non-Hodgkin’s lymphoma, particularly
when peritoneal, pleural or pericardial effusions were present
(27). Various investigators have
proposed including serum CA125 level in prognostic indices for
lymphoma (28). The value of
elevated CA125 levels for FDC sarcoma diagnosis remains to be
determined.
Although the optimal treatment modality of FDC
sarcoma has yet to be defined due to the limited number of reported
cases, the current therapeutic guidelines refer to treatment
modalities used for soft tissue sarcomas of high grade. Treatment
principles include radical resection, adjuvant radiation and
chemotherapy (2). Radical resection
of the tumor is the primary therapy, although the treatment
modalities for FDC sarcoma vary widely. FDC sarcoma was previously
considered an indolent tumor with low tendency towards recurrence
or metastasis. However, findings of studies with larger patient
cohorts and longer follow-up have shown that FDC sarcoma is a more
aggressive tumor and should be considered an intermediate-grade
malignancy. It has been reported that at least 40% of documented
FDC sarcomas have recurred and 25% have metastasized with a
mortality rate of 16.7% (2,8). Due to this significant recurrent and
metastatic potential, it is reasonable that, following radical
resection of the localized tumor, recurrence may be prevented by
adjuvant radiotherapy or chemotherapy (29–31).
However, the role of radiotherapy and chemotherapy in the treatment
of this neoplasm has yet to be clearly defined since the value of
these adjuvant treatments to effectively improve survival rates
remains to be determined (2,8). The
present case suggested that FDC sarcoma is effectively treated by
surgery and no radiotherapy or chemotherapy after radical excision
is required.
In conclusion, extranodal FDC sarcoma is an
extremely rare tumor. Due to the scarcity of the identified cases,
FDC remains under-recognized and misdiagnosis is common. With the
aid of immunohistochemical analysis and the two most reliable FDC
markers, CD21 and CD35, the diagnostic accuracy has been
significantly improved. Therefore, when FDC sarcoma is suspected
histologically, immunohistochemical stains for FDC differentiation
should be performed to avoid potential misdiagnosis.
Acknowledgements
This study was supported by the State Key Basic
Research and Development Program of China (973 Program, Grant no.
2009CB521704), the National High-tech Research & Development
Program of China (863 Program, Grant no. 2006AA02A245) and the
Zhejiang Provincial Science and Technology Project (Grant no.
2009C13021).
References
|
1
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation. A report of 4 cases. Am J Pathol.
122:562–572. 1986.PubMed/NCBI
|
|
2
|
Shia J, Chen W, Tang LH, Carlson DL, Qin
J, Guillem JG, Nobrega J, Wong WD and Klimstra DS: Extranodal
follicular dendritic cell sarcoma: clinical, pathologic and
histogenetic characteristics of an underrecognized disease entity.
Virchows Arch. 449:148–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JK, Tsang WY, Ng CS, Tang SK, Yu HC
and Lee AW: Follicular dendritic cell tumors of the oral cavity. Am
J Surg Pathol. 18:148–157. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youens KE and Waugh MS: Extranodal
follicular dendritic cell sarcoma. Arch Pathol Lab Med.
132:1683–1687. 2008.PubMed/NCBI
|
|
5
|
Nayler SJ, Verhaart MJ and Cooper K:
Follicular dendritic cell tumour of the tonsil. Histopathology.
28:89–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollowood K, Stamp G, Zouvani I and
Fletcher CD: Extranodal follicular dendritic cell sarcoma of the
gastrointestinal tract. Morphologic, immunohistochemical and
ultrastructural analysis of two cases. Am J Clin Pathol. 103:90–97.
1995.
|
|
7
|
Perez-Ordonez B, Erlandson RA and Rosai J:
Follicular dendritic cell tumor: report of 13 additional cases of a
distinctive entity. Am J Surg Pathol. 20:944–955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan JK, Fletcher CD, Nayler SJ and Cooper
K: Follicular dendritic cell sarcoma. Clinicopathologic analysis of
17 cases suggesting a malignant potential higher than currently
recognized. Cancer. 79:294–313. 1997.
|
|
9
|
Araujo VC, Martins MT, Salmen FS and
Araujo NS: Extranodal follicular dendritic cell sarcoma of the
palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
87:209–214. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galati LT, Barnes EL and Myers EN:
Dendritic cell sarcoma of the thyroid. Head Neck. 21:273–275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi PC, To KF, Lai FM, Lee TW, Yim AP and
Chan JK: Follicular dendritic cell sarcoma of the neck: report of
two cases complicated by pulmonary metastases. Cancer. 89:664–672.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beham-Schmid C, Beham A, Jakse R, Aubock L
and Hofler G: Extranodal follicular dendritic cell tumour of the
nasopharynx. Virchows Arch. 432:293–298. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fonseca R, Tefferi A and Strickler JG:
Follicular dendritic cell sarcoma mimicking diffuse large cell
lymphoma: a case report. Am J Hematol. 55:148–155. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwarz RE, Chu P and Arber DA: Extranodal
follicular dendritic cell tumor of the abdominal wall. J Clin
Oncol. 17:2290–2292. 1999.PubMed/NCBI
|
|
15
|
Lee IJ, Kim SC, Kim HS, Bang D, Yang WI,
Jung WH and Chi HS: Paraneoplastic pemphigus associated with
follicular dendritic cell sarcoma arising from Castleman’s tumor. J
Am Acad Dermatol. 40:294–297. 1999.PubMed/NCBI
|
|
16
|
Shek TW, Liu CL, Peh WC, Fan ST and Ng IO:
Intra-abdominal follicular dendritic cell tumour: a rare tumour in
need of recognition. Histopathology. 33:465–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shek TW, Ho FC, Ng IO, Chan AC, Ma L and
Srivastava G: Follicular dendritic cell tumor of the liver.
Evidence for an Epstein-Barr virus-related clonal proliferation of
follicular dendritic cells. Am J Surg Pathol. 20:313–324. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moriki T, Takahashi T, Wada M, Ueda S,
Ichien M, Yamane T and Hara H: Follicular dendritic cell tumor of
the mesentery. Pathol Res Pract. 193:629–639. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fisher C, Magnusson B, Hardarson S and
Smith ME: Myxoid variant of follicular dendritic cell sarcoma
arising in the breast. Annu Diagn Pathol. 3:92–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pallesen G and Myhre-Jensen O:
Immunophenotypic analysis of neoplastic cells in follicular
dendritic cell sarcoma. Leukemia. 1:549–557. 1987.PubMed/NCBI
|
|
21
|
Desai S, Deshpande RB and Jambhekar N:
Follicular dendritic cell tumor of the parapharyngeal region. Head
Neck. 21:164–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah RN, Ozden O, Yeldandi A, Peterson L,
Rao S and Laskin WB: Follicular dendritic cell tumor presenting in
the lung: a case report. Hum Pathol. 32:745–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan AC, Chan KW, Chan JK, Au WY, Ho WK
and Ng WM: Development of follicular dendritic cell sarcoma in
hyaline- vascular Castleman’s disease of the nasopharynx: tracing
its evolution by sequential biopsies. Histopathology. 38:510–518.
2001.
|
|
24
|
Chiaramonte MF, Lee D, Abruzzo LV, Heyman
M and Bass BL: Retroperitoneal follicular dendritic cell sarcoma
presenting as secondary amyloidosis. Surgery. 130:109–111. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang KC, Jin YT, Chen FF and Su IJ:
Follicular dendritic cell sarcoma of the colon mimicking stromal
tumour. Histopathology. 38:25–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han JH, Kim SH, Noh SH, Lee YC, Kim HG and
Yang WI: Follicular dendritic cell sarcoma presenting as a
submucosal tumor of the stomach. Arch Pathol Lab Med.
124:1693–1696. 2000.PubMed/NCBI
|
|
27
|
Bairey O, Blickstein D, Stark P,
Prokocimer M, Nativ HM, Kirgner I and Shaklai M: Serum CA 125 as a
prognostic factor in non-Hodgkin’s lymphoma. Leuk Lymphoma.
44:1733–1738. 2003.
|
|
28
|
Gui W, Wang T, Wang J, Wang L, He J, Yang
B, Zhao Z, Zhang H and Zhang Q: An improved prognostic parameter
for non-Hodgkin’s lymphoma based on the combination of three serum
tumor markers. Int J Biol Markers. 23:207–213. 2008.
|
|
29
|
Fonseca R, Yamakawa M, Nakamura S, van
Heerde P, Miettinen M, Shek TW, Myhre Jensen O, Rousselet MC and
Tefferi A: Follicular dendritic cell sarcoma and interdigitating
reticulum cell sarcoma: a review. Am J Hematol. 59:161–167. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashima T, Kuratomi Y, Shiratsuchi H,
Yamamoto H, Yasumatsu R, Yamamoto T and Komiyama S: Follicular
dendritic cell sarcoma of the neck; a case report and literature
review. Auris Nasus Larynx. 29:401–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satoh K, Hibi G, Yamamoto Y, Urano M,
Kuroda M and Nakamura S: Follicular dendritic cell tumor in the
oro-pharyngeal region: report of a case and a review of the
literature. Oral Oncol. 39:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|