1. Introduction
The Werner syndrome (WS) is an autosomal recessive
disorder causing symptoms of premature aging (1). The causative gene of WS is the
WRN gene encoding for WRN protein, a DNA helicase (2). Another characteristic feature of this
disorder is a much higher incidence of rare cancers (3). Non-epithelial tumors, including
soft-tissue sarcoma and benign meningioma, are associated with WS,
as shown by 124 case reports of neoplasia of WS patients from
Japan, and 34 case reports from outside Japan between 1939 and
August 1995. Notably, the ratio of epithelial to non-epithelial
cancers was approximately 1:1 in WS patients compared to 10:1 in
the general population. Two telomere maintenance mechanisms,
telomerase activation (4) and
alternative lengthening of telomeres (ALT) (5,6) exist.
In human tumors of the general population over 80% of carcinomas
maintain telomeres by telomerase activation, whereas various types
of sarcomas elongate telomeres by means of ALT in the absence of
telomerase activity (7). In tumors
in WS patients, however, evidence has shown that the telomerase
activation pathway by telomeric crisis is blocked, while the ALT
pathway is enhanced (8–10), supporting the high incidence of rare
cancers in WS.
2. Role of WRN gene in
immortalization by means of telomeric crisis pathway
The repeated replication of cells shortens
telomeres, culminating in their instability, after which most cells
cease to replicate and die. However, a small fraction of the cells
become immortalized by maintaining telomeres with activated
telomerase activity, known as the telomeric crisis pathway (TCP)
(11–13). We showed that lymphoblastoid cell
lines (LCLs) transformed by the Epstein-Barr virus generate
immortalized cell lines by means of TCP (13). Among 50 LCLs from non-WS
individuals, 5 LCLs (10%) were immortalized and the remaining 45
LCLs were mortal. None of the 44 LCLs (0%; P<0.031 against
healthy individuals using the Chi-square test) from WS patients
were immortalized. Among 11 LCLs obtained from a family with a
tendency towards hereditary type 2 diabetes mellitus, 5 LCLs
(45.5%; P<0.004 against healthy individuals, P<0.0001 against
WS patients) were immortalized (8,14).
These results indicated that WRN helicase is crucial to
immortalization by TCP (8,9). These factors together indicate that
WRN helicase is also required for the immortalization of epithelial
cells by TCP and consequent carcinogenesis, suggesting that the
tumorigenesis of epithelial cells by TCP is suppressed in WS
patients lacking the WRN helicase function. Notably, the ALT
pathway in the absence of telomerase activity in WS has been
indicated to be involved in the immortalization and tumorigenesis
of non-epithelial tumors (10).
These considerations were consistent with the above-mentioned
abundance of non-epithelial cancers in WS.
3. Role of WRN gene in
breakage-fusion-bridge cycle at telomeric crisis pathway
In this review, we present and discuss a
hypothetical scheme showing the role of WRN helicase in
immortalization by means of the supposed ‘breakage-fusion-bridge
cycle’ of chromosomes at telomeric crisis proposed by Ishikawa
(11).
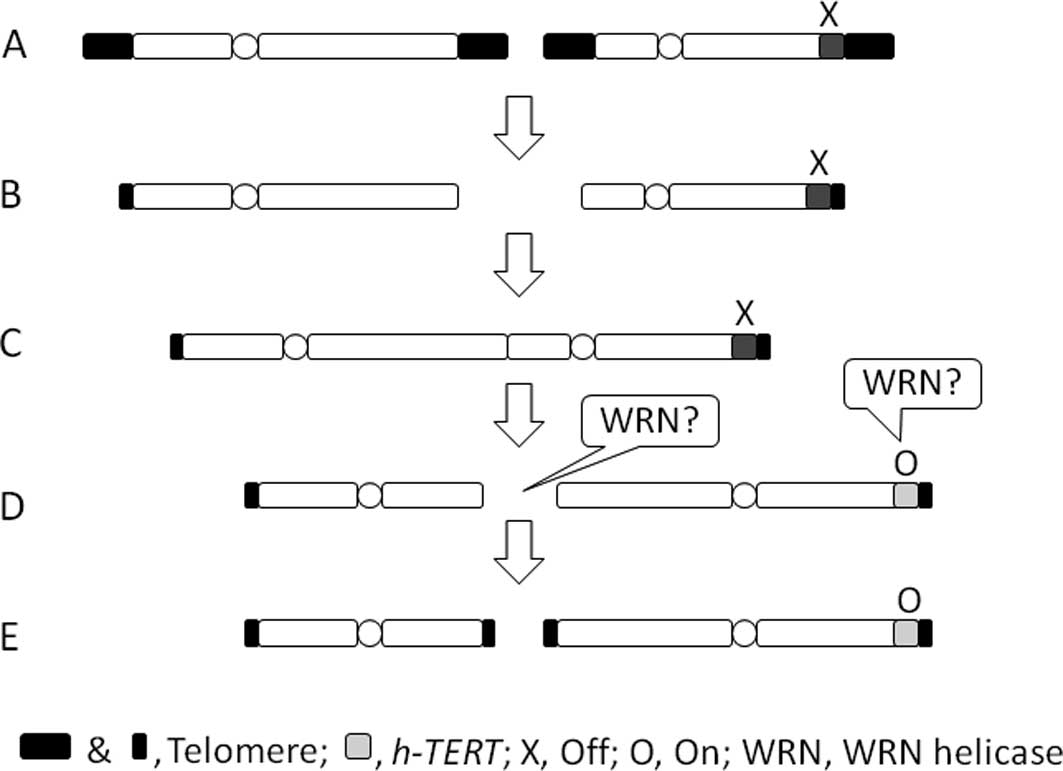
When two chromosomes lose telomeric function by
shortening (Fig. 1A and B) they
fuse, resulting in a dicentric chromosome (Fig. 1C). When the dicentric chromosome is
segregated towards two daughter cells during the M phase, two
spindles from each of the two daughter cells may attach to each of
the two centromeres, leading to the chromosome being pulled apart
(Fig. 1D). Thus, the newly formed
ends of the chromosome are non-telomeric. Abnormal cells with
non-telomeric chromosomes probably do not survive unless the
non-telomeric end is capped again with telomeres.
The de novo addition of telomeres to
non-telomeric ends has been reported (15). For instance, during programmed
chromosomal healing, telomerase adds telomeric repeats directly to
non-telomeric sequences in the protozoan Tetrahymena,
forming de novo telomeres. Therefore, to add a telomere to a
non-telomeric end, initial activation of the telomerase is required
(Fig. 1D and E). Such activation
may occur when the repression of the human telomerase reverse
transcriptase (hTERT) gene is released. Alu-elements and
other human-specific repetitive sequences exist abundantly around
the hTERT gene, probably forming a repressed chromatin
structure in human cells. In this regard, it has been indicated
that chromosomal translocations allow the promoter of hTERT
to escape the repressive chromatin environment at telomeric crisis
(reviewed in 16). One
characteristic of immortalization by telomere crisis in LCLs is
that abnormal chromosomes occur simultaneously with activation of
the telomerase, and all 11 immortalized LCLs were found to possess
abnormal chromosomes (13). Cells
of each immortalized LCL share a set of abnormal chromosomes,
indicating a clonal origin of LCL cells. However, abnormal
chromosomes are not shared among LCLs. During the telomeric crisis
towards immortalization a series of marked changes should occur
accompanied by the reorganization of chromosomes. These changes may
include epigenetic ones around telomeric regions as observed during
the generation of human pluripotent stem cells (17). Such epigenetic changes may
contribute to modification of the hTERT gene region to
activate telomerase.
The above-mentioned process of immortalization by
TCP is summarized in Fig. 1. WRN
helicase may play at least two mutually compatible roles in
immortalization by TCP. First, under the supposed epigenetic
changes WRN helicase may unwind the repressed state of chromatin
DNA that is rich in repetitive elements and resistant to nuclease
digestion, leading to modification and activation of the promoter
region of the hTERT gene by translocation (reviewed in
16) (Fig. 1D). Second, in the
telomerase-mediated de novo addition of telomeres to
non-telomeric sequences (Fig. 1E),
exonuclease activity of WRN helicase may also be involved in this
process to trim the 3′ end to expose a favorable sequence as a
primer for adding a telomere to the non-telomeric end (15). In this regard, a particular
sequence, such as GGGAT in the case of human, is predominantly
added by telomerase onto the 3′ end of non-telomeric primers. Among
the five RecQ helicases, only WRN helicase has exonuclease activity
(reviewed in 18), and the fact
that the remaining four RecQ helicases cannot complement the
function of WRN helicase supports the role of exonuclease activity
of WRN helicase in the immortalization by TCP.
4. Conclusion
In this review, we proposed a hypothesis regarding
the role of WRN helicase in immortalization by means of TCP,
indicating that WRN helicase may play an important role in the
‘breakage-fusion-bridge cycle’. This hypothesis may lead to future
studies at molecular levels in order to elucidate the role of WRN
helicase in immortalization and tumorigenesis.
References
|
1
|
Goto M and Miller RW: From Premature Gray
Hair to Helicase – Werner Syndrome: Implications for Aging and
Cancer. Japan Scientific Societies Press; Karger, Tokyo: 2001
|
|
2
|
Yu CE, Oshima J, Fu YH, et al: Positional
cloning of the Werner’s syndrome gene. Science. 272:258–262.
1996.
|
|
3
|
Goto M, Miller RW, Ishikawa Y and Sugano
H: Excess of rare cancers in Werner syndrome (adult progeria).
Cancer Epidemiol Biomarkers Prev. 5:239–246. 1996.PubMed/NCBI
|
|
4
|
Harley CB, Kim NW, Prowse KR, et al:
Telomerase, cell immortality, and cancer. Cold Spring Harb Symp
Quant Biol. 59:307–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bryan TM, Englezou A, Gupta J, Bacchetti S
and Reddel RR: Telomere elongation in immortal human cells without
detectable telomerase activity. Embo J. 14:4240–4248.
1995.PubMed/NCBI
|
|
6
|
Bryan TM and Reddel RR: Telomere dynamics
and telomerase activity in in vitro immortalised human cells. Eur J
Cancer. 33:767–773. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuo T, Shimose S, Kubo T, Fujimori J,
Yasunaga Y and Ochi M: Telomeres and telomerase in sarcomas.
Anticancer Res. 29:3833–3836. 2009.PubMed/NCBI
|
|
8
|
Sugimoto M, Tahara H, Okubo M, et al: WRN
gene and other genetic factors affecting immortalization of human
B-lymphoblastoid cell lines transformed by Epstein-Barr virus.
Cancer Genet Cytogenet. 152:95–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Futami K, Ishikawa Y, Goto M, Furuichi Y
and Sugimoto M: Role of Werner syndrome gene product helicase in
carcinogenesis and in resistance to genotoxins by cancer cells.
Cancer Sci. 99:843–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laud PR, Multani AS, Bailey SM, et al:
Elevated telomere-telomere recombination in WRN-deficient, telomere
dysfunctional cells promotes escape from senescence and engagement
of the ALT pathway. Genes Dev. 19:2560–2570. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikawa F: Telomere crisis, the driving
force in cancer cell evolution. Biochem Biophys Res Commun.
230:1–6. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shay JW and Wright WE: Senescence and
immortalization: role of telomeres and telomerase. Carcinogenesis.
26:867–874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugimoto M, Tahara H, Ide T and Furuichi
Y: Steps involved in immortalization and tumorigenesis in human
B-lymphoblastoid cell lines transformed by Epstein-Barr virus.
Cancer Res. 64:3361–3364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okubo M, Tsurukubo Y, Higaki T, et al:
Clonal chromosomal aberrations accompanied by strong telomerase
activity in immortalization of human B-lymphoblastoid cell lines
transformed by Epstein-Barr virus. Cancer Genet Cytogenet.
129:30–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H and Blackburn EH: De novo telomere
addition by Tetrahymena telomerase in vitro. Embo J. 16:866–879.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Zhao Y and Wang S: Chromatin and
epigenetic regulation of the telomerase reverse transcriptase gene.
Protein and Cell. 1:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yehezkel S, Rebibo-Sabbah A, Segev Y, et
al: Reprogramming of telomeric regions during the generation of
human induced pluripotent stem cells and subsequent differentiation
into fibroblast-like derivatives. Epigenetics. 6:63–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimamoto A, Sugimoto M and Furuichi Y:
Molecular biology of Werner syndrome. Int J Clin Oncol. 9:288–298.
2004. View Article : Google Scholar : PubMed/NCBI
|