Introduction
Lung cancer is the most common malignant disease
worldwide, and it is a major cause of death from cancer,
particularly among males. Lung cancer almost exclusively involves
carcinomas, arising from epithelial tissue of the trachea, bronchi
or lung (1). Principal histological
types of lung cancer are squamous cell carcinoma, adenocarcinoma,
large cell carcinoma (collectively referred to as ‘non-small cell’
lung carcinomas) and small cell carcinoma (2). In addition to smoking, a positive
familial history of lung cancer has been identified as a risk
factor (3). Genetic alterations
associated with lung cancer include frequent mutations in
p53 gene, activating point mutations in the KRAS
oncogene, frequent loss of heterozygosity and aberrant transcripts
of the Fragile Histidine Triad (FHIT) gene, homozygous
deletions and transcriptional silencing of the CDK inhibitor p16.
Increased risk of lung cancer has been associated with certain
polymorphisms of cytochrome P450 genes and with deficiencies in DNA
repair capacity including DNA base excision repair genes XRCC1,
PARP-1 and ERCC4 (4–6).
DNA damage response emerges as a biological
tumourigenesis barrier in the early stages of cancer development,
and exerts a selective pressure that favours outgrowth of malignant
clones with defects in the genome maintenance machinery (7). Findings of previous studies showed
that although wild-type mice rarely develop carcinomas, defective
DNA repair in PARP-1 mutant mice leads to malignant epithelial
tumours such as those of the lung, liver and mammary gland
(8–10). To gain a better understanding of the
role of DNA repair defects in lung carcinogenesis and to establish
a primary culture method for anti-cancer drug screening primary
mouse lung carcinoma cells were isolated from PARP-1 mutant
mice.
Materials and methods
Primary cultures
For primary cell-culture experiments, lung tumours
obtained from PARP-1 deficient mice (from the Jackson laboratory,
Bar Harbor, ME, USA) at various ages were processed within 30 min
after biopsy removal. Tumour specimens were cut into 1-mm slices in
order that single cells as well as cell clumps (organoids) be
released into the washing medium. Larger clumps were separated by
allowing them to gravity settle for a few minutes. The clumps were
reduced in size by gentle rotation in a test tube for 30–60 min.
Cells and organoids isolated in this way were directly used for
primary cultures, as described below. The remaining tissue slices
were routinely subjected to enzymatic digestion with 0.3 mg/ml
collagenase (Sigma, St. Louis, MO, USA) by gentle agitation for 1
to 2 h at 37°C in DMEM containing 5% FCS, 200 U/ml penicillin, 200
μg/ml streptomycin, 50 μg/ml gentamycin and 50 U/ml nystatin. The
procedure involved for the establishment of cell cultures was as
previously described (11–13), and was adapted for the processing of
mouse lung carcinoma.
Isolated cells and cell clumps were routinely seeded
onto round glass coverslips in growth medium I containing 10% FCS,
4.0 mM glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml
streptomycin, 50 μg/ml gentamycin, 50 U/ml nystatin, 1 μg/ml
hydrocortisone (Sigma), 0.2 U/ml insulin (Sigma), 2 μg/ml
transferring (Sigma) and 5 nM sodium selenite (Sigma). After 1 week
the medium was replaced by growth medium II (as above, without
gentamycin and nystatin). Only those cultures in which fibroblast
contamination was low, and in which overgrowth of epithelial cells
by fibroblasts became unlikely, were processed further. Growth
medium III was used (DMEM plus 10% heat-inactivated FCS, 50 U/ml
penicillin, 50 μg/ml streptomycin, 4.0 mM glutamine and 10 mM
HEPES). All cell culture media and reagents were purchased from
Life Sciences Technologies (Blowing Rock, NC, USA).
Since fibroblast-conditioned medium appears to
support growth of intestinal epithelial cells, preferential growth
of epithelial cells without fibroblast overgrowth was initiated on
glass coverslips by using mouse primary fibroblast cells (MPFs)
lethally irradiated with 60 Gray (6,000 rads) of γ radiation
(IBL-437C, CIS Bio, Gif-Sur-Yvette, France). MPFs were seeded as a
feeder layer in the tissue-culture plate on which glass coverslips
were placed. Feeder layers were used at 30–40% confluency and were
maintained for 1 week. Alternatively, MPFs-culture supernatants
were mixed 1:1 with fresh growth medium II (conditioned growth
medium). For initial passaging, primary cultures were sub-cultured
only when areas of tumour cell growth became confluent. For the
first passage, all cells from a coverslip were mechanically scraped
off and transferred to a fresh culture plate with conditioned
growth medium. Fibroblast growth had to be continuously suppressed
in cultures by using conditioned growth medium. Cellular morphology
in the primary cultures was evaluated by light microscopy. The
epithelial nature was characterised with electron microscopy and by
immuostaining.
Electron microscopy and
immunostaining
For scanning electron microscopy and transmission
electron microscopy, as well as for immunostaining, cells were
seeded on round glass coverslips. At the end of the culture time
cells were washed in phosphate-buffered saline and fixed for 2 h in
2.5% glutaraldehyde in cacodylate buffer and processed by routine
methods for electron microscopic examination. For immunostaining,
cells were fixed with paraformaldhyde, incubated with monoclonal
antibodies against pan-cytokeratins (NovoCastra, Newcastle, UK) and
β-catenin (Cell Signaling, USA) or Cy3-conjugated anti-IgG antibody
(Cappel, Organon, NC, USA). Glass coverslips were mounted
upside-down in Vectashield mounting medium (Vector, Burlingame, CA,
USA).
Tumourigenicity test
For the tumourigenicity test, cells were cultured to
70% confluence and trypsinised into single cell suspension. LC1 and
LC2 (1×106) cells were inoculated subcutaneously into 5
immunocompromised nude mice (Balb/C) and maintained for two weeks.
Tumours were collected and subjected to histological examination.
The experimental protocols were approved by the Ethics Committee of
the Second Bethune Hospital of Jilin University.
Histology and immunohistochemistry
Histological analysis was carried out on 3-μm
sections stained with haematoxylin and eosin, or immunostained
(11–13). Antibodies included
anti-pan-cytokeratin (NovoCastra) and E-cadherin (Chemicon
International, Temecula, CA, USA).
Results
Primary cultures derived from mouse lung
adenocarcinomas
By introducing modifications to previously published
methods (13), i.e., using
collagenase and shorter time periods for enzymatic digestion, as
well as MPFs-conditioned growth medium for maintaining optimal
growth of epithelial cells, viable adherent primary cultures were
successfully established for propagation in 2 out of 5 cases (40%)
of surgically obtained malignant tumours. Cells from 3 cases failed
to passage, although the cells initially attached and started to
form colonies in the first 2 weeks of culture. Tumour samples were
considered to be moderately or well-differentiated. Notably, no
correlation was found between histological grading or staging and
success in establishing primary cultures. In all adherent cultures,
cell migration from organoids was evident within 7 days of
initiation, with 2 cultures proliferating for several months. Two
primary cells (LC1 and LC2) were sub-cultured by mechanical
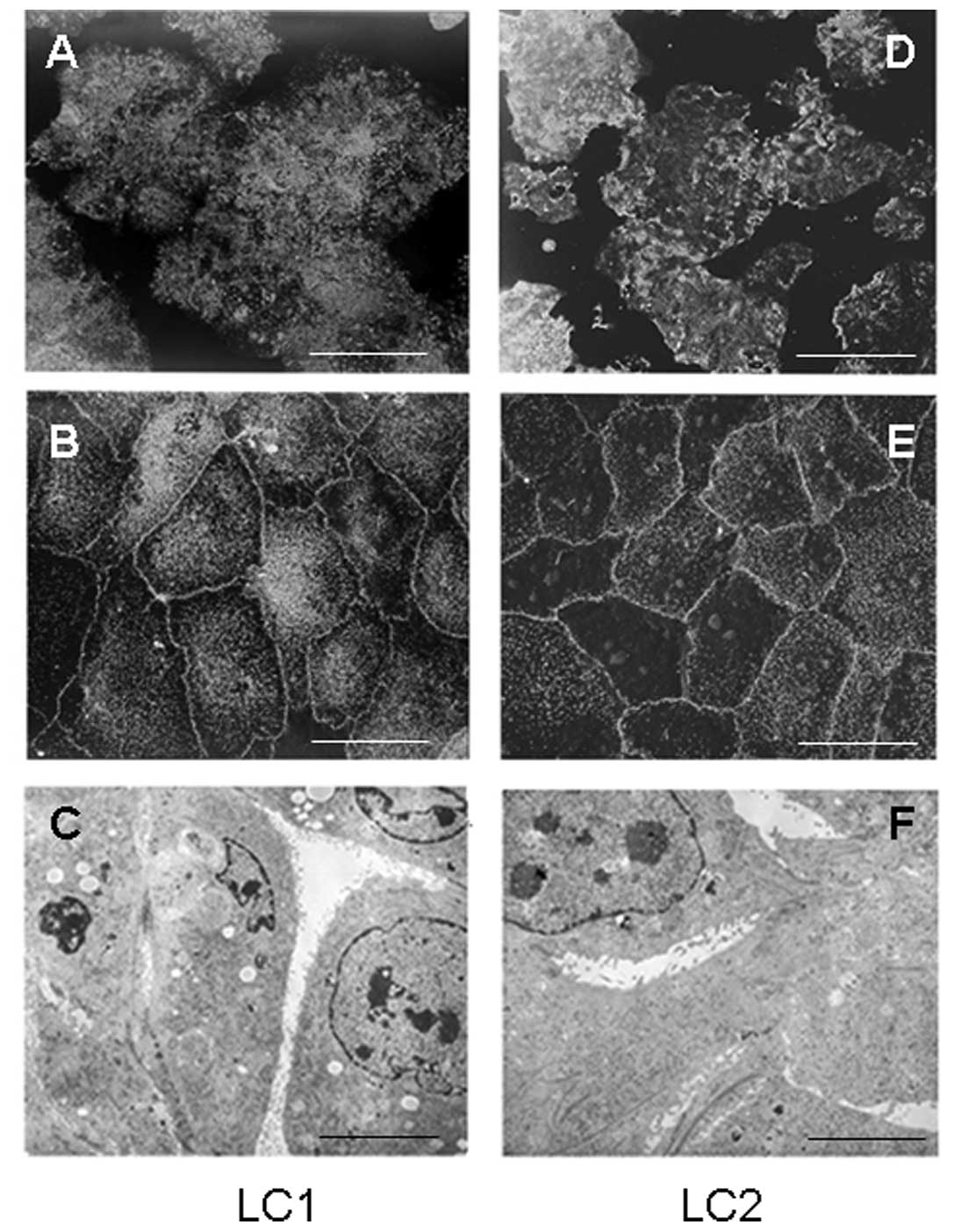
scraping within 1–3 months of the initiation of cultures (Fig. 1A and D), whereas fibroblasts remain
attached. LC1 and LC2 subcultures between passages 3 and 6 were
used to check the epithelial nature of passaged cells by electron
microscopy and by immunostaining of cytokeratin and β-catenin
(Figs. 1 and 2).
Cellular morphology
In two primary cultures, cells grew in monolayers,
though in varying degrees of attachment to the plastic support.
Cells were polarized, exhibiting junctional complexes and
microvilli, albeit with large differences in number and
organisation even between cells in the same culture (Figs. 1 and 2). A scanning electron micrograph of a
primary culture of a mouse lung carcinoma showed that the majority
of polarized cuboidal cells were tightly packed and densely
decorated at the apical aspect with well-developed microvilli
(Fig. 1B). However, the same
culture also contained a number of cells of an epithelial nature
that exhibited few or no microvilli (Fig. 1E). A transmission electron
micrograph of a primary culture of a mouse lung carcinoma showed a
typical epithelial nature as visualized by their decoration to a
greater or lesser extent with microvilli (Fig. 1C and F).
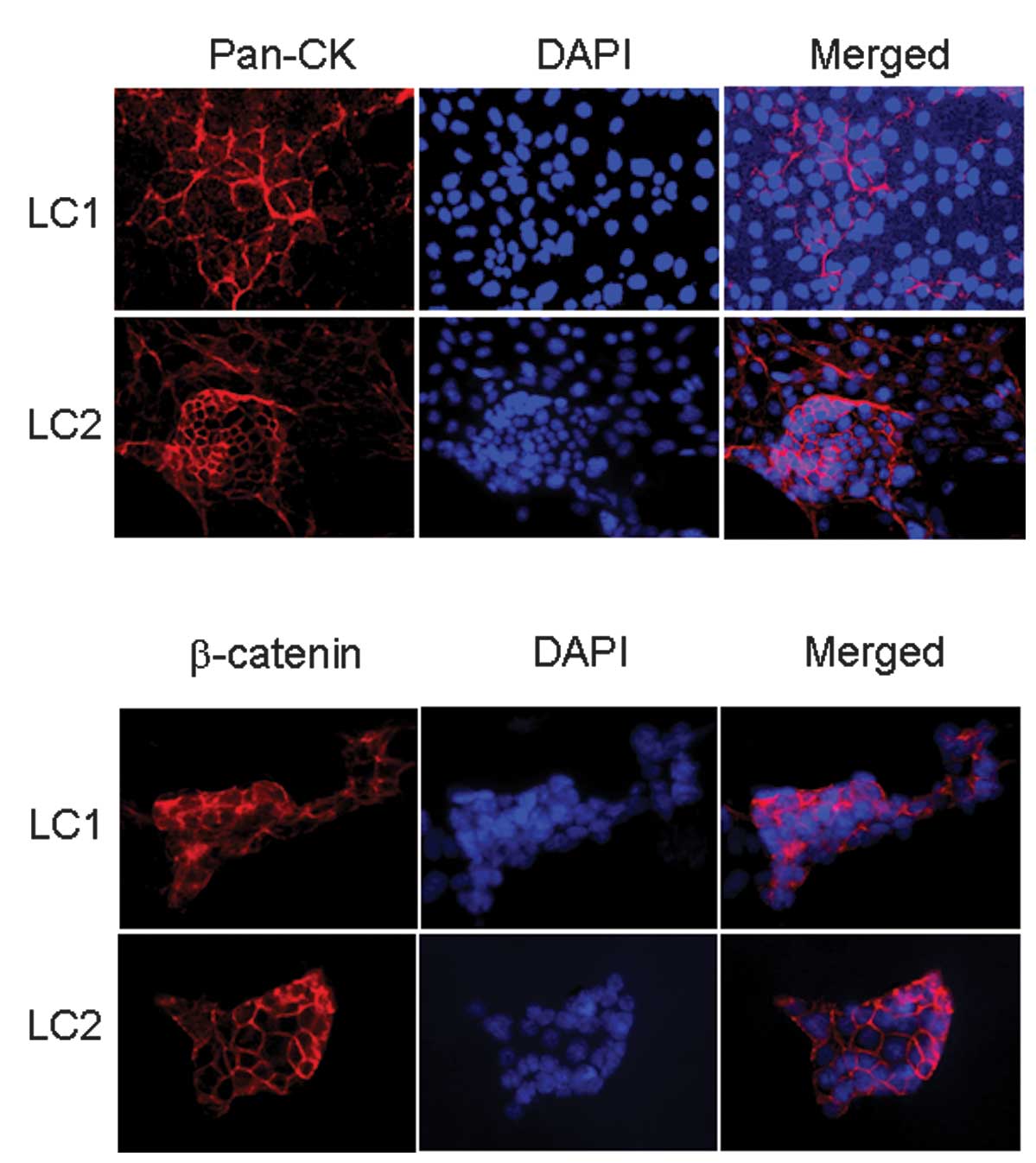
Epithelial characters of primary cultured cells were
further evaluated using antibodies specific to epithelial markers.
Immunocytochemical staining of primary cells revealed strong
positive staining for pan-cytokeratin (Fig. 2A) and β-catenin (Fig. 2B). Cells appeared heterogeneous in
size at the edge of the cell patch (Fig. 2), and various expression levels of
pan-cytokeratin between cells in the same culture were frequently
observed (Fig. 2A).
Primary cultured cells are
tumourigenic
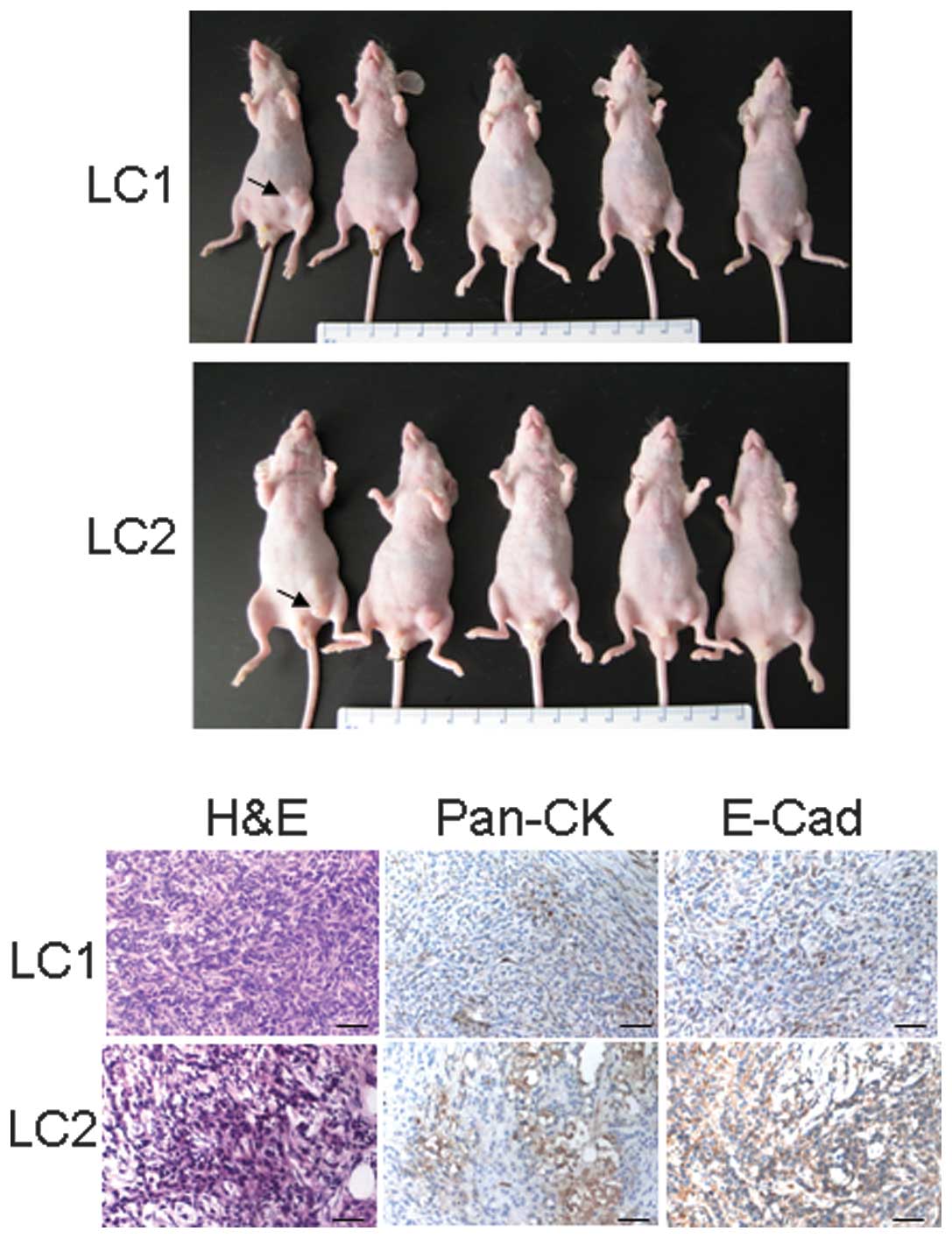
To evaluate whether primary cells were tumourigenic,
LC1 or LC2 (1×106) cells per mouse were inoculated
subcutaneously for two weeks. The mice developed tumours within two
weeks, and tumour size was between 5 and 10 mm in diameter
(Fig. 3A). Histological analysis
revealed the inoculated solid tumours to be more or less dense,
with compacted cells (Fig. 3B)
expressing various levels of pan-cytokeratin and E-cadherin
immunoactivity, indicating their epithelial origin.
Discussion
The majority of mouse lung tumours were well- or
moderately differentiated adenocarcinomas utilized for the
reproducibility and optimal establishment of primary cultures.
Subsequently, short periods of enzymatic digestion and seeding at
high density were used. Since fibroblast contamination presents
severe difficulties in the establishment of primary cultures,
various methods were adopted in this study in order to obtain cells
of a purely epithelial nature. Mouse fibroblast feeder layers and
fibroblast-conditioned media apparently supported the preferential
growth of epithelial cells. Thus, our method of organoid isolation
and selective cultivation of epithelial cells on glass coverslips
in petri dishes with mouse fibroblast feeder cells protected
against fibroblast overgrowth.
Primary cell growth results in the formation of
colonies. For passage initiation, the advantages of mechanical
scraping are two-fold. First, the cell sheets are released,
allowing cells to attach to fresh culture plates more slowly than
single cells. Second, replating the cell clumps a few hours after
culture into fresh culture plates also protects against fibroblast
contamination. Individual mouse lung adenocarcinomas exhibit
pronounced regional phenotypic heterogeneity, resulting from the
accumulation of specific genetic lesions in different parts of the
same tumour.
Although various methods have been used in clinical
anti-cancer drug screening (14–16),
this study simplified the primary culture protocol and most of the
primary cells were cultured and maintained for 2 weeks.
Consequently, if the extent of responsiveness of a given tumour to
a specific anti-cancer drug is studied in an in vitro model,
it should reflect the cellular diversity of the tumour from which
it was derived (11–12,17).
In this respect, it is of note that the primary cultures described
in the present study contain distinct sub-populations of epithelial
cells. For instance, the density and the forms of microvilli on the
apical plasma membrane were not uniform in a single culture. This
may indicate differences in cellular functions as well as
differentiation, and allow investigation of the mechanisms of DNA
damage response in tumourigenesis (7).
Our results demonstrate the usefulness of the
culture model employed in this study for further applications of
personalized medicine of primary cultures of human lung
adenocarcinoma cells in anti-cancer drug screening (18–20),
and for the improvement of personalized drug response.
Additionally, primary cultures may be useful in adjuvant therapy of
human lung cancer.
Acknowledgements
This study was in part supported by grants from the
Natural Science Foundation of China (No. 30870354), Project of
Scientific Innovation and Creative for Jilin Provincial Oversea
Scholars (No. 2010273), and Jilin Provincial Science &
Technology Services (No. 200805120 & No. 20090732).
References
|
1
|
Stewart BW and Kleihues P: World Cancer
Report. IARC Press; Lyon: pp. 1822003
|
|
2
|
Butnor KJ and Beasley MB: Resolving
dilemmas in lung cancer staging and histologic typing. Arch Pathol
Lab Med. 131:1014–1015. 2007.PubMed/NCBI
|
|
3
|
Mulshine JL and Henschke CI: Prospects for
lung-cancer screening. Lancet. 355:592–593. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright GS and Gruidl ME: Early detection
and prevention of lung cancer. Curr Opin Oncol. 12:143–148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Miao X, Liang G, et al:
Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and
risk of lung cancer. Cancer Res. 65:722–726. 2005.PubMed/NCBI
|
|
6
|
Shao M, Ma H, Wang Y, et al: Polymorphisms
in excision repair cross-complementing group 4 (ERCC4) and
susceptibility to primary lung cancer in a Chinese Han population.
Lung Cancer. 60:332–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartek J, Lukas J and Bartkova J: DNA
damage response as an anti-cancer barrier: damage threshold and the
concept of ‘conditional haploinsufficiency’. Cell Cycle.
6:2344–2347. 2007.PubMed/NCBI
|
|
8
|
Tong WM, Cortes U, Hande MP, et al:
Synergistic role of Ku80 and poly(ADP-ribose) polymerase in
suppressing chromosomal aberrations and liver cancer formation.
Cancer Res. 62:6990–6996. 2002.PubMed/NCBI
|
|
9
|
Tong WM, Yang YG, Cao WH, et al:
Poly(ADP-ribose) polymerase-1 plays a role in suppressing mammary
tumourigenesis in mice. Oncogene. 26:3857–3867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong W-M, Hande MP, Lansdorp PM and Wang
ZQ: DNA-strand break-sensing molecule PARP cooperates with p53 in
telomere function, chromosomal stability and tumour suppression.
Mol Cell Biol. 21:4046–4054. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang CG, Sun M, Zhao XJ and Zhang XY:
Effect of STAT3 siRNA-induced inhibition of STAT3 gene expression
on the growth and apoptosis of Lewis lung cancer cells. Chin J Clin
Oncol. 3:392–399. 2006. View Article : Google Scholar
|
|
12
|
Wang CG, Wang RY, Sun M, et al: In vivo
antitumour effect of siRNA against STAT3 on transplanted Lewis lung
cancer in mice. Chem Res Chin Univ. 24:322–329. 2008.
|
|
13
|
Tong WM, Ohgaki H, Huang H, Granier C,
Kleihues P and Wang ZQ: Null mutation of DNA strand break-binding
molecule poly(ADP-ribose) polymerase causes medulloblastomas in
p53(−/−) mice. Am J Pathol. 162:343–352. 2003.PubMed/NCBI
|
|
14
|
Yang S, Su J, Cao J, Zhang P, Lu J and Xie
W: Establishment of a novel Chinese human lung adenocarcinoma cell
line CPA-Yang1 which produces highly bone metastases in
immunodeficient mice. Zhongguo Fei Ai Za Zhi. 12:753–759.
2009.PubMed/NCBI
|
|
15
|
Yaghi A, Zaman A and Dolovich M: Primary
human bronchial epithelial cells grown from explants. J Vis Exp.
26:pii1789. View
Article : Google Scholar
|
|
16
|
Kalinina T, Gungor C, Thieltges S, et al:
Establishment and characterization of a new human pancreatic
adenocarcinoma cell line with high metastatic potential to the
lung. BMC Cancer. 10:2952010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashiyama M, Oda K, Okami J, et al:
Prediction of chemotherapeutic effect on postoperative recurrence
by in vitro anticancer drug sensitivity testing in non-small cell
lung cancer patients. Lung Cancer. 68:472–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Camps C, Sirera R, Iranzo V, Taron M and
Rosell R: Gene expression and polymorphisms of DNA repair enzymes:
cancer susceptibility and response to chemotherapy. Clin Lung
Cancer. 8:369–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hardwicke MA, Oleykowski CA, Plant R, et
al: GSK1070916, a potent Aurora B/C kinase inhibitor with broad
antitumour activity in tissue culture cells and human tumour
xenograft models. Mol Cancer Ther. 8:1808–1817. 2009. View Article : Google Scholar
|
|
20
|
Powell C, Mikropoulos C, Kaye SB, et al:
Pre-clinical and clinical evaluation of PARP inhibitors as
tumour-specific radiosensitisers. Cancer Treat Rev. 36:566–575.
2010. View Article : Google Scholar : PubMed/NCBI
|