Introduction
Sinomenine (SIN;
7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinane-6-one) is
a biomonomer alkali derived from the Chinese medicinal plant
Sinomenium acutum. Traditionally, SIN has been used in the
treatment of rheumatoid arthritis due to its anti-inflammatory
effect (1). Previous studies
demonstrated that SIN has cardioprotective (2) and immunosuppressive effects (3,4). In
vitro studies indicated that the suppression of
cyclooxygenase-2 (COX-2) expression is one of the possible
mechanisms for the anti-inflammatory characteristic of SIN
(5). Furthermore, in the pioneer
experiment conducted by Zhang et al SIN was found to inhibit
the proliferation of HeLa cells, possibly by inhibiting the
expression of COX-2 (6).
COX is a key enzyme mediating the conversion of
arachidonic acid to prostaglandins. Two distinct COX enzymes have
been identified: COX-1, a constitutive enzyme, and COX-2, an
inducible form (7). COX-1 is a
housekeeping molecule that can be detected in most cells and
tissues under normal conditions and is involved in maintaining
homeostasis by regulating normal physiological functions, such as
immune response, acid secretion and blood supply. The expression of
COX-2 is rapidly induced by growth factors, oncogenes, carcinogens,
mitogens and lipopolysaccharides (8). The majority of the data from animal
and human studies indicate that COX-2 is crucial to inflammation
and oncogenesis. COX-2 is up-regulated in transformed cells and in
a variety of solid tumors such as lung, colorectal, pancreatic and
breast cancers (9–12). COX-2 inhibitors induce apoptosis in
various cancer cells both in vitro and in vivo
(13). COX-2 is considered to be a
potential preventive and therapeutic target for malignancies
(14).
Gastric cancer is one of the most common causes of
cancer-related mortality in China and other Asian countries
(15). At present, surgery and
chemotherapy are the standard treatment modalities utilized in
gastric cancer (16). However, the
5-year survival of gastric cancer patients is estimated to be only
30%. To improve the prognosis of GC, the development of novel
strategies based on its molecular alterations is required. The
majority of gastric adenocarcinomas have a high-level expression of
COX-2 (17–19). Both angiogenesis and Helicobacter
pylori infection have been reported to be associated with the
COX-2 expression in gastric cancer patients (20). The knockdown of COX-2 in a SGC-7901
gastric adenocarcinoma cell line by RNA interference inhibits
proliferation and induces apoptosis (21), indicating that suppression of COX-2
may be developed into an effective approach for the treatment of
gastric cancer. The majority of selective COX-2 inhibitors have
pronounced side effects that limit the administration of these
drugs. In the present study, the inhibitory effect of SIN on the
proliferation of SGC-7901 gastric adenocarcinoma cells was
observed. Additionally, the question of whether the suppression of
COX-2 expression is a potential mechanism for SIN on the
proliferation of SGC-7901 cells was investigated.
Materials and methods
Cell cultures and reagents
SGC-7901 gastric adenocarcinoma cells were cultured
with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island,
NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100
U/ml penicillin and 100 μg/ml streptomycin. Cultures were
maintained at 37°C in a humidified incubator in an atmosphere of 5%
CO2. Cells were passaged at 1:3 every 3 days. SIN and
celecoxib (Sino-American Biotech, Henan, China) were dissolved in
dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA), stored at
−20°C and diluted in DMEM in different proportions (DMSO density of
<0.1%). The morphological and growth patterns of the cells were
dynamically observed under an inverted microscope (Olympus IX-50;
Olympus Optical, Tokyo, Japan).
MTT assay
Following the MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay, 5×103 cells were seeded in 96-well plates and
cultured for 24 h at 37°C and 5% CO2. Media containing
various concentrations of SIN were added to the wells 24 h later to
reach final concentrations of 125, 250, 500 and 1,000 μmol/l.
Celecoxib at a final concentration of 50 μmol/l was used as a
positive control. For the DMSO control, DMSO was added to a final
concentration of 1‰ to exclude the possible effect of DMSO on cell
proliferation. For the blank control, no reagent was added. Drug
treatment was continued for another 24, 48, 72 or 96 h, and 5 mg/ml
MTT (Sigma) was added to the wells. All of the groups were
incubated for 4 h at 37°C. The supernatant was removed and crystals
were dissolved in 200 μl DMSO. The absorbance was examined with an
automated microplate reader (Bio-Tek, Winooski, VT, USA) at an
absorption wavelength of 490 nm. Only the medium was added to the
negative control well, which was used to zero the absorbance. Three
wells were set up for each group and three independent experiments
were conducted.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The relative expression of COX-2 mRNA was evaluated
using a semi-quantitative reverse transcriptase PCR kit (Takara,
Otsu, Shiga, Japan). Total RNA was isolated from SGC-7901 cells
using a TRIzol reagent (Promega, Madison, WI, USA). Reverse
transcription of total RNA (2 μg) was performed in 20 μl volume
according to the manufacturer’s instructions. The primers used for
COX-2 were: 5′-CGAGGTGTATGTATGAGTGTG-3′ (forward) and
5′-TCTAGCCAGAGTTTCACCGTA-3′ (reverse). β-actin was amplified as an
internal control using the primers: 5′-GTAA AGACCTCTATGCCATCA-3′
(forward) and 5′-GGACTCAT CGTACTCCTGCT-3′ (reverse), resulting in
products of 550 and 227 bp, respectively. Each PCR product was
visualized by staining with ethidium bromide after electrophoresis
on 2% agarose gels under ultraviolet light. The gel images were
photographed (Olympus) and relative densities were analyzed using
the Bandscan software.
Western blotting
All groups of SGC-7901 cells were collected in
1.5-ml Eppendorf tubes when the cells were treated with drugs for
48 h. The total protein was extracted with RIPA lysis buffer
containing proteinase inhibitors. Protein concentration was
determined using the Bradford assay. The protein (100 μg) of each
sample was separated on 10% sodium dodecyl sulfate (SDS)
polyacrylamide gel and transferred to nitrocellulose membranes.
Non-specific binding was blocked by 5% skimmed milk for 2 h at room
temperature. The membranes were incubated with primary antibody
against COX-2 and β-actin (1:1,000 dilution; Sigma) for 4 h at room
temperature or overnight at 4°C. After washing with PBST followed
by incubation with peroxidase-conjugated goat anti-mouse IgG as
secondary antibody (1:2,000 dilution; Sigma) for 1 h at room
temperature, protein was detected using enhanced chemiluminescence
solution, and by exposing membranes to Kodak X-ray film. The
expression of β-actin was detected as an internal control.
Statistical analysis
Statistical analysis was performed using SPSS
software (SPSS13.0). Statistical analyses of the data were
performed using one-way analysis of variance (ANOVA) followed by a
post hoc test. Data were shown as the mean ± standard
deviation. P<0.05 was considered to be statistically
significant.
Results
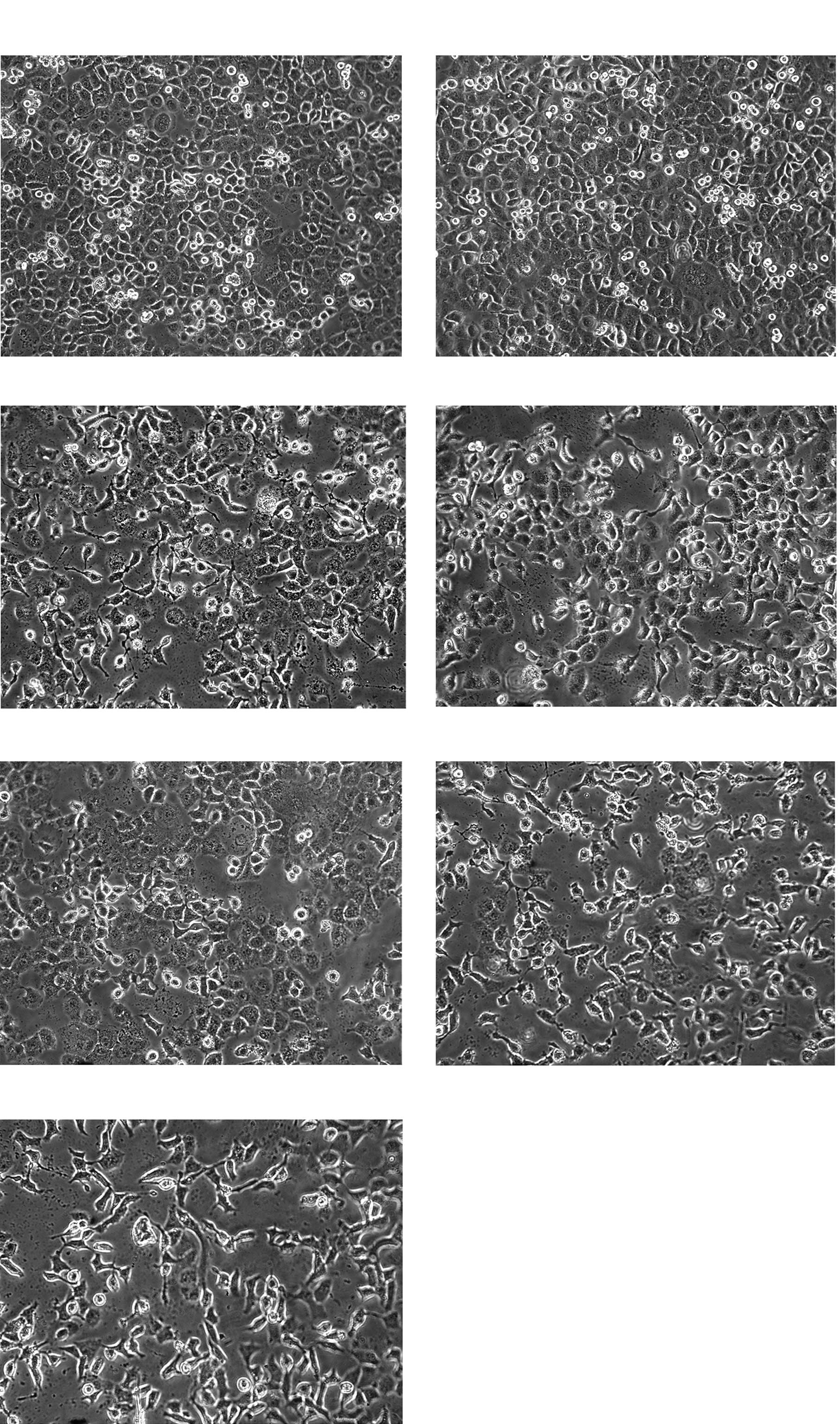
Cell morphology
After SGC-7901 cells were treated with different
concentrations of drugs, proliferation of SGC-7901 cells was
inhibited, the number of cells decreased significantly and cell
growth was retarded. Morphologically, the cells detached from the
bottle and became round. Achromatolysis, deflation and pyknosis of
the nucleus was observed. This phenomenon was most obvious in the
1,000 μmol/l SIN and celecoxib-positive groups. SGC-7901 cells grew
more rapidly in the DMSO control group (Fig. 1).
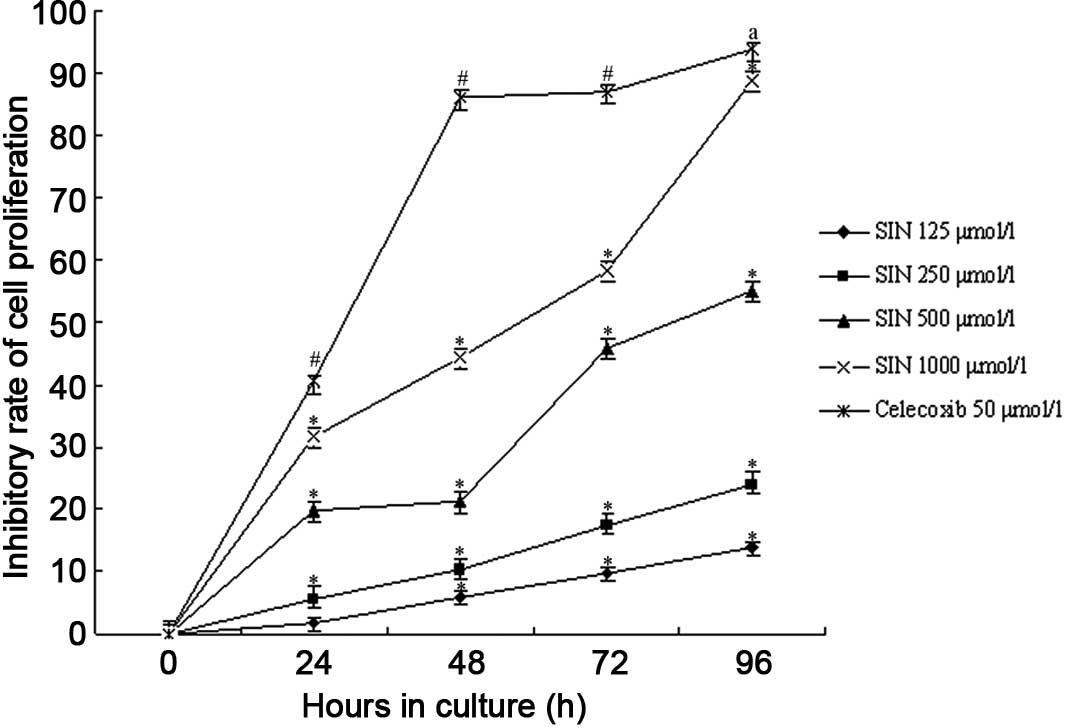
Cell proliferation
We found that the proliferation of SGC-7901 cells
was inhibited to various extents in all of the experimental groups
and the celecoxib-positive control group (Fig. 2). The DMSO control group was not
depressed. SIN inhibited the growth of SGC-7901 cells in a
dose-dependent manner and the number of cells decreased following
the increased concentration of SIN. Compared to that of the blank
control group, the growth of cells treated with SIN decreased
significantly (P<0.05 by ANOVA and Tukey’s post hoc test
to detect significantly different means). A significant difference
was also observed between the celecoxib-positive control group and
the blank control and SIN groups (P<0.05). The DMSO control
group showed no effects on SGC-7901 cells; in a group comparison
between the various densities of SIN, the high-dose group resulted
in a markedly reduced growth of SGC-7901 cells as compared to that
of the low-dose treated group (P<0.05). Concomitantly, SGC-7901
cells treated with SIN for 24–96 h resulted in an obviously
increased inhibitory rate of cell growth. We observed that the
highest inhibitory rate among the SIN groups was 93.89% in the
1,000 μmol/l SIN group at 96 h. Moreover, the inhibitory action of
SIN on SGC-7901 cells occurred in a time-dependent manner
(P<0.05).
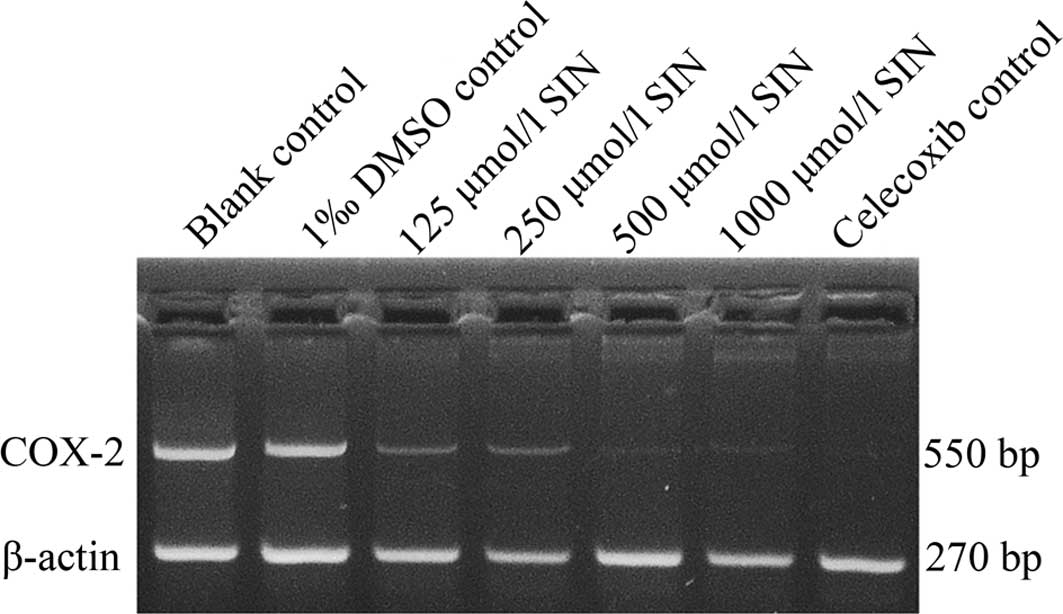
SIN inhibits COX-2 expression in human
gastric adenocarcinoma cells
To determine COX-2 expression in response to SIN
treatment, RT-PCR was performed and SGC-7901 cells were examined
(Fig. 3). SIN at a concentration of
125 μmol/l caused a decrease in the expression of COX-2 mRNA, which
began 48 h after the initial treatment was administered and
occurred in a dose-dependent manner in SGC-7901 cells compared to
the blank control group (P<0.05). The DMSO control group
exhibited no effects on the expression of COX-2 mRNA in SGC-7901
cells. The celecoxib-positive control group was significantly
different from the blank control group (P<0.05).
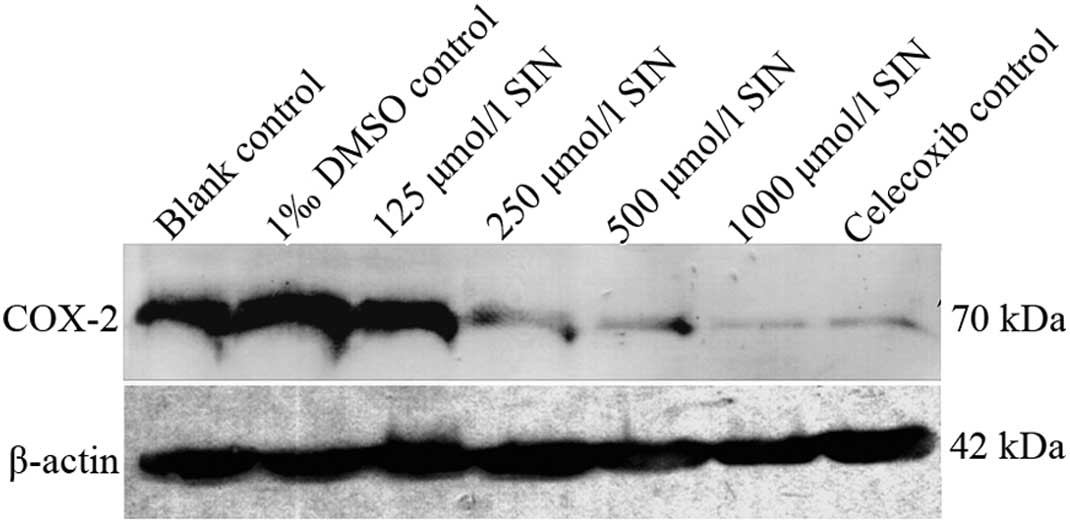
Western blotting verified the expression
of COX-2
Western blot analysis revealed that COX-2 protein
was expressed in gastric cancer cells (Fig. 4). No significant difference was
observed between the blank and DMSO control groups. Compared to the
blank control group, the expression of COX-2 was decreased in
various densities of the SIN group in a dose-dependent manner
(P<0.05). In contrast to the blank control group, the expression
of COX-2 was decreased in the celecoxib-positive control group
(p<0.05).
Discussion
Gastric cancer is the most common cause of
cancer-related mortality worldwide. Numerous molecular studies have
been performed to investigate the developmental mechanism of
gastric cancer and COX-2 expression in the pathogenesis of gastric
cancer. COX-2 was found to play a significant role in gastric
cancer by various pathways. Additionally, the correlation between
COX-2 and clinicopathological characteristics, such as tumor size,
stage, invasion and lymph node metastasis, of gastric cancer have
been identified. COX-2 overexpression protects cancer cells against
various apoptotic stimuli (22).
The up-regulation of COX-2 is closely related to gastric cancer
metastasis through the promotion of lymphangiogenesis and the
angiogenesis of gastric cancer (23). Findings of studies have demonstrated
that COX-2 is constitutively overexpressed in gastric cancer
(24). The relationship between
Helicobacter pylori infection and gastric cancer has also
been demonstrated. Thus, Helicobacter pylori infection is
thought to contribute to the development of gastric cancer via
COX-2, which may be due to the stimulation of tumor growth and
angiogenesis (25). Several
molecular pathways have been hypothesized in the development of
gastric cancer. Previous studies indicated that the
COX-2-PGI2-PPARδ pathway was also involved in
tumorigenesis (26). VEGF is one of
the most significant mediators of the COX-2 pathway (27). COX-2 produced by cancer cells is
correlated with the elevation of Bcl-2 protein and inhibition of
apoptosis in gastric cancer tissue.
In the present study, we observed that COX-2 was
highly expressed in gastric cancer cells, a result that is
consistent with findings of other studies. COX-2 selective
inhibitors have been shown to induce apoptosis in gastric cancer
(28). Our study found that SIN was
suppressed COX-2 expression in SGC-7901 cells, which grew slowly
and became round. In their study, Zhang et al found that SIN
inhibited the proliferation of HeLa cells as a COX-2 selective
inhibitor (6). This inhibition may
relate to SIN blockage of the cell cycle and induction of
apoptosis, the mechanism of which may constitute the inhibition of
COX-2 expression in a dose- dependent manner. Studies have also
shown that SIN mediated the down-regulation of COX-2 expression and
the production of induced PGE2 in PC-12 cells by
suppressing the activity of NF-κB (5). To assess whether the inhibition of
COX-2 expression is involved in gastric cancer cells, MTT assay,
RT-PCR analysis and Western blotting were performed to test cell
viability, COX-2 mRNA and protein expression, respectively.
The results of this study suggest that SIN has an
inhibitory effect on the growth of gastric cancer. Based on our
observation of cell morphology, we found that SIN effectively
inhibited the growth of SGC-7901 cells. Compared to the control
group, the number of cells decreased significantly in the SIN
groups and the proliferation of SGC-7901 cells was inhibited. The
highest inhibitory rate was 93.89% in the 1,000 μmol/l SIN group at
96 h. The preliminary inhibitory effect of SIN on gastric cancer
cells was demonstrated by this result. We showed that SIN was
capable of reducing up-regulated mRNA and the protein levels of
COX-2. COX-2 mRNA was significantly decreased compared to the blank
control group. SIN down-regulated the COX-2 protein expression in a
dose-dependent manner in gastric cancer cells. The present results
indicate that the inhibitory effect of SIN on gastric cancer cells
may be activated by the COX-2 pathway. COX-2 is a key enzyme in
prostaglandin synthesis. PGE2 may promote the growth of
gastric cancer cells and induce Foxp3 expression independently of
TGF-β and IL-10 in the gastric cancer microenvironment (29). SIN may also inhibit PGE2
synthesis by suppressing the expression of COX-2. Further
investigation is required to identify the signal transduction
pathway of COX-2. Blocking this pathway using SIN may facilitate
tumor therapeutics.
In conclusion, the present study suggests that SIN
is involved in inhibiting the proliferation of gastric cancer cells
in vitro and that its therapeutic mechanism is related to
the inhibition of COX-2 expression. The findings of this study
suggest that SIN has a preliminarily therapeutic effect on gastric
cancer, indicating that SIN is an effective candidate drug for
treating gastric cancer.
Acknowledgements
The authors are indebted to Professor Hongxia Li
(The First Affiliated Hospital of Xi’an Jiao Tong University,
College of Medicine, China) for the kind assistance with cell
cultures, and wish to thank Professor Xinyang Wang (The First
Affiliated Hospital of Xi’an Jiaotong University, College of
Medicine, China) for directing the experimental work.
References
|
1
|
Ke XY, Yu MX and Jiang M: Clinical
observation on treatment of rheumatoid arthritis with sinomenine.
Bei Jing Yi Xue. 8:186–188. 1986.
|
|
2
|
Satoh H: Electropharmacology of sinomeni
caulis et rhizome and its constituents in cardiomyocytes. Am J Chin
Med. 33:967–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vieregge B, Resch K and Kaever V:
Synergistic effects of the alkaloid sinomenine in combination with
the immunosuppressive drugs tacrolimus and mycophenolic acid.
Planta Medica. 65:80–82. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai YB, Huang X and Luo ZG:
Immunosuppressive effect of sinomenine on ICAM-1 expression in rat
renal allograft rejection. Mod J Integr Trad Chin West Med.
12:1358–1363. 2003.
|
|
5
|
Chen W, Shen YD and Zhao GS: Inhibitory
effect of sinomenine on expression of cyclooxygenase-2 in
lipopolysaccharide-induced PC-12 cells. China J Chin Mat Medica.
29:900–903. 2004.PubMed/NCBI
|
|
6
|
Zhang Y, Wu M and Li XG: Experimental
study on the effect of selective COX-2 inhibitor sinomenine on HeLa
cells. Prog Mod Biomed. 6:38–40. 2006.
|
|
7
|
Hla T, Bishop-Bailey D, Liu CH, Schaefers
HJ and Trifan OC: Cyclooxygenase-1 and -2 isoenzymes. Int J Biochem
Cell Biol. 31:551–557. 1999. View Article : Google Scholar
|
|
8
|
Morita I: Distinct functions of COX-1 and
COX-2. Prostaglandins Other Lipid Mediat. 68:165–175. 2002.
View Article : Google Scholar
|
|
9
|
Su JL, Shih JY, Yen ML, Jeng YM, Chang CC
and Hsieh CY: Cyclooxygenase-2 induces EP1-and HER-2/Neu-dependent
vascular endothelialgrowth factor-C up-regulation: a novel
mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res.
64:554–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: a
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tucker ON, Danneberg AJ and Tang EK:
Cyclooxygenase-2 expression is up-regulated in human pancreatic
cancer. Cancer Res. 59:987–990. 1999.PubMed/NCBI
|
|
12
|
Timoshenko AV, Chakraborty C, Wagner GF
and Lala PK: COX-2-mediated stimulation of the lymphangiogenic
factor VEGF-C in human breast cancer. Br J Cancer. 94:1154–1163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meric JB, Rottey S, Olaussen K, Soria JC,
Khayat D and Rixe O: Cyclooxygenase-2 as a target for anticancer
drug development. Crit Rev Oncol Hematol. 59:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dannenberg AJ and Subbaramaiah K:
Targeting cyclooxygenase-2 in human neoplasia: rationale and
promise. Cancer Cell. 4:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
16
|
Wagner AD, Grothe W, Haerting J, et al:
Combination chemotherapies in advanced gastric cancer: an updated
systematic review and meta-analysis. American Society of Clinical
Oncology. 25:45552007.
|
|
17
|
Chen CN, Sung CT, Lin MT, Lee PH and Chang
KJ: Clinicopathologic association of cyclooxygenase-1 and
cyclooxygenase-2 expression in gastric adenocarcinoma. Ann Surg.
233:183–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaca D, Ayyildiz T, Coskun U, et al:
Cyclooxygenase-2 expression and its association with angiogenesis,
Helicobacter pylori, and clinicopathologic characteristics
of gastric carcinoma. Pathol Res Pract. 204:527–536. 2008.
View Article : Google Scholar
|
|
19
|
Tatsuguchi A, Matsui K and Shinji Y:
Cyclooxygenase-2 expression correlates with angiogenesis and
apoptosis in gastric cancer tissue. Hum Pathol. 35:488–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun WH, Yu Q, Shen H, et al: Roles of
Helicobacter pylori infection and cyclooxygenase-2
expression in gastric carcinogenesis. World J Gastroenterol.
10:2809–2813. 2004.PubMed/NCBI
|
|
21
|
Wang BH, Qian W and Gao YJ: Effects of
inhibition of cyclooxygenase-2 by RNA interference on proliferation
and apoptosis of human gastric cancer cells: an experimental study
with human gastric cancer cells and mice. Nat Med J China.
86:266–271. 2006.
|
|
22
|
Tjiu JW, Liao YH, Lin SJ, et al:
Cyclooxygenase-2 over-expression in human basal cell carcinoma cell
line increases antiapoptosis, angiogenesis, and tumorigenesis. J
Invest Dermatol. 126:1143–1151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fosien E: Biochemistry of cyclooxygenase
(COX-2) inhibitors and molecular pathology of COX-2 in neoplasia.
Crit Rev Las Sci. 37:431–502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Willams CS and DuBois RN: Prostaglandin
endoperoxide synthase: why two isoforms? Am J Physiol. 270:393–400.
1996.PubMed/NCBI
|
|
25
|
Konturek PC, Hartwich A, Zuchowicz M, et
al: Helicobacter pylori gastrin and cyclooxygenases in
gastric cancer. J Physiol Pharmacol. 51:737–749. 2000.
|
|
26
|
Yu J, Leung WK, Chen J, Ebert MPA,
Malfertheiner P and Sung JJY: Expression of peroxisome
proliferators-activated receptor δ in human gastric cancer and its
response to specific COX-2 inhibitor. Cancer Lett. 223:11–17.
2005.
|
|
27
|
Da MX, Wu XT, Wang J, et al: Expression of
cyclooxygenase-2 and vascular endothelial growth factor-c
correlates with lymph-angiogenesis and lymphatic invasion in human
gastric cancer. Arch Med Res. 39:92–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tegeder I, Pfeilschifter J and Geisslinger
G: Cyclooxygenase-independent actions of cyclooxygenase inhibitors.
FASEB J. 15:2057–2072. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan XL, Chen L, Li MX, et al: Elevated
expression of Foxp3 in tumor-infiltrating Treg cells suppresses
T-cell proliferation and contributes to gastric cancer progression
in a COX-2-dependent manner. Clin Immunol. 134:277–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|