Introduction
Colorectal cancer is a common malignancy that is
surgically treated. Radiotherapy has been successfully applied in
rectal cancer as adjuvant therapy and in the treatment of residual
disease. Chemotherapy based on 5-fluorouracil was previously
administered, but without any noteworthy effects (1–3).
Chemotherapy was rendered a viable treatment modality when
leucovorin was introduced into clinical practice as a
5-fluorouracil modulator (4,5). Prior
to this introduction, patients with advanced colorectal cancer
remained untreated, since chemotherapy alone was ineffective. New
drugs were only used in protocol trials. Irinotecan was regarded as
a new drug that was reported to be effective in colorectal cancer
when combined with leucovorin and 5-fluorouracil (6–9).
During the last decade, the cisplatin analogue oxaliplatin was
introduced into clinical practice, mainly for the treatment of
advanced stages of the disease, and was combined with leucovorin
and 5-fluorouracil or with irinotecan. The results were positive
with respect to the response rate, median and overall survival.
(10–16). Currently, no new cytotoxic agent has
been produced for the treatment of colorectal cancer with the
exception of oral capecitabine, which has been used as a substitute
for leucovorin and 5-fluorouracil.
The present study involved a retrospective
evaluation of 40 patients with advanced metastatic colorectal
cancer in order to assess the median and overall survival of
patients. Concurrently, a description was provided of the
biological characteristics of this slow-growing disease as well as
the quality of life of the patients as determined by their
performance status (PS) .
Materials and methods
Patients
The untreated patients were randomly recruited
between 1993 and 1996. Patients were untreated for the reasons that
they refused to undergo cytotoxic therapy or the disease remained
stable and the patients were asymptomatic. Certain patients
required supportive treatment, such as antibiotics in case of
infection, or blood transfusion for anemia.
Eligibility criteria
A patient histological diagnosis of colorectal
cancer and advanced metastatic disease were confirmed by
radiological examinations. None of the patients had under-gone
prior chemotherapy, although 2 (5%) of the patients had received
adjuvant treatment prior to the appearance of metastatic disease.
Physical examination, X-rays, ultra-sound or computed tomography
(CT) were performed prior to disease management. Eligibility
criteria included stage IV disease, performance status of 0–2,
(World Health Organization), an expected survival of ≥12 weeks and
that patients be ≥18 years old. Laboratory examinations were
performed to evaluate disease progression. Patients were required
to have adequate bone marrow reserves (leukocyte count ≥3500/μl,
platelet count ≥100,000/μl and hemoglobin ≥10 g/dl) and adequate
renal function (serum creatinine ≤1.5 mg/dl and serum transaminases
≤3 times the upper limit of normal). Patients exhibiting central
nervous system involvement or secondary malignancy were excluded
from the study. This study was conducted with the approval of the
institutional review board. Written informed consent was obtained
from the patients included in this study.
Following the baseline tests, patients were followed
up via a clinico-laboratory evaluation that included a physical
examination, ECG, full blood count, liver and renal function tests,
and urine analysis. Radiological tests were performed where disease
progression was indicated by clinical signs, otherwise the tests
were performed every six months.
Results
Patients
A total of 40 patients with histologically confirmed
adenocarcinomas of moderate differentiation were included and
evaluated. Table I shows the
patient characteristics at baseline.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. | % |
|---|
| Patients
evaluated | 40 | 100.0 |
| Median age (years)
(range) | 67 (32–87) | |
| Gender |
| Male | 25 | 62.5 |
| Female | 15 | 37.5 |
| Disease stage |
| IV | 40 | 100.0 |
| PS |
| 0–1 | 33 | 82.5 |
| 2 | 7 | 17.5 |
| Histology
adenocarcinoma |
| Moderate
differentiation | 40 | 100.0 |
| Site of
metastasis |
| Liver | 18 | 45.0 |
| Lung | 3 | 7.5 |
| Abdomen | 10 | 25.0 |
| Bone | 1 | 2.5 |
| Multiple | 8 | 20.0 |
Only two patients were administered with adjuvant
chemotherapy (leucovorin and 5-fluorouracil). This treatment had
been administered between 1993 and 1996 when adjuvant chemotherapy
with irinotecan or oxaliplatin was not yet available. All 40
patients had metastatic disease when the evaluation and follow-up
were commenced. Systemic treatment was not administered to the
majority of patients (29 patients, 72.5%). Of the remaining 11, 1
patient underwent 2 courses of chemotherapy, and 2 patients
underwent liver surgery due to liver metastases. The 2 patients
undergoing liver resection showed residual disease within 3 months
after surgery and 1 patient showed liver recurrence, also within 3
months. Intra-arterial chemotherapy was administered once to 2
patients and chemo-embolization was administered to 2 further
patients, also once. Radiotherapy was administered to 2 patients
with temporary liver deposit reduction (Table II).
 | Table IITreatment. |
Table II
Treatment.
| Treatment | No. | % |
|---|
| Chemotherapy (2
cycles) | 1 | 2.5 |
| Liver surgery | 3 | 7.5 |
| Radiotherapy,
skeleton | 1 | 2.5 |
| Radiofrequency | 2 | 5.0 |
| Intra-arterial | 2 | 5.0 |
|
Chemo-embolization | 2 | 5.0 |
| Total | 11 | 27.5 |
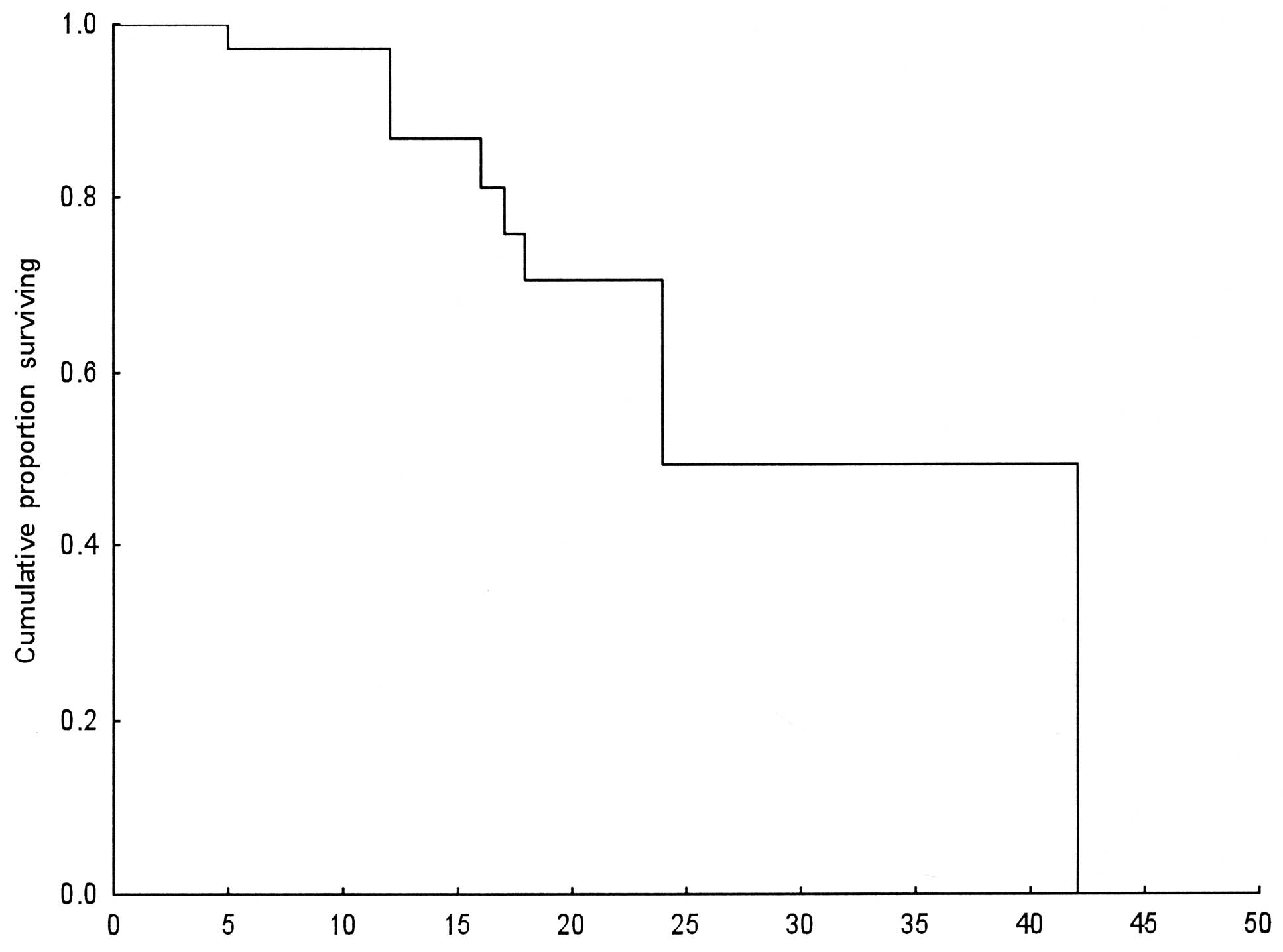
Survival
The median survival for the 40 patients was 24
months (range 5–42). A total of 26 patients (65%) survived for one
year. After 2 years, 10 patients had survived (25%; Table III). The quality of life of these
10 patients was satisfactory based on the PS, which remained at
0–1.
 | Table IIISurvival in months. |
Table III
Survival in months.
| Median survival | 24 (95% CI,
16–42) |
|---|
| Range | 5–42 |
| One-year | 26 patients
(65.0%) |
| 2-year | 10 patients
(25.0%) |
| Alive at the end of
the study | 21 patients
(52.5%) |
Statistical analysis
The Kaplan-Meier method was used for survival
distribution estimation (Fig.
1).
Discussion
Colorectal cancer is a slow-growing malignancy. In
the majority of patients, the result of chemotherapy in advanced
metastatic disease is stabilization (17). According to the RECIST criteria,
stable disease is defined as a tumor reduction of less than 30% and
tumor progression of less than 20% (18). Where evaluation by CT after
treatment showed stable disease, the result may be attributed to
either: i) the effect of chemotherapy, which inhibited disease
progression, or ii) the biological characteristics of the tumor,
which define how rapidly it develops. It is difficult to verify
which interpretation applies in routine clinical practice. Relevant
data are required to determine whether chemotherapy plays a
significant role in regulating disease progression. One example of
how data may be gathered involves a comparison of treated versus
non-treated patients in a randomized trial. A study by Cunningham
et al, which compared chemotherapy with irinotecan plus
supportive care versus supportive care alone, showed that overall
survival was significantly higher in the irinotecan group (p=0.001)
(18). Results by these authors
showed that one-year survival for the irinotecan group was 36.2%
compared with 13.8% for the supportive care group. These
percentages of one-year survival for each group are lower than
those obtained in our study. The reason for this discrepancy is
that the previous study included the time period after the
administration of second-line treatment (18). On the other hand, the results of the
study by Cunningham et al (18) indicate the advantage of chemotherapy
in colorectal cancer. The aim of our study was to contribute to a
better understanding of the biology of colorectal cancer. Our
findings indicate that this is a slow-growing disease with a 65%
survival rate after 1 year, and a 25% survival rate after 2 years
(10/40 patients). Local treatment administered to 11/40 patients
(27.5%) contributed little to overall survival.
The long-term survival of patients with metastatic
advanced colorectal cancer without systemic treatment may be due to
the biological characteristics of this tumor, indicating that it is
a slow-growing malignancy. These findings are useful in obviating
long-term or unstoppable chemotherapy when patients are in stable
disease.
References
|
1
|
Gastrointestinal Tumor Study Group.
Adjuvant therapy for colon cancer: results of a prospectively
randomized trial. N Engl J Med. 310:737–740. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mulcahy HE, Toner M, Patchett SE, et al:
Identifying stage B colorectal cancer patients at high risk of
tumor recurrence and death. Dis Colon Rectum. 40:326–336. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moertel CG: Chemotherapy of
gastrointestinal cancer. Recent Advances in Cancer Treatment.
Tajnon HJ and Staquet MJ: New York: Raven Press; pp. 311–323.
1997
|
|
4
|
Wolmark N, Rockette H, Fisher B, et al:
The benefit of leucovorin-modulated fluorouracil as postoperative
adjuvant therapy for primary colon cancer: results from National
Surgical Adjuvant Breast and Bowel Project. Protocol C-03. J Clin
Oncol. 11:1879–87. 1993.
|
|
5
|
Francini GP and Lorenzini L: Folinic acid
and 5-fluorouracil as adjuvant chemotherapy in colon cancer.
Gastroenterology. 106:899–906. 1994.PubMed/NCBI
|
|
6
|
De Gramont A, Vignoud J, Tournigand C, et
al: Oxaliplatin with high-dose leucovorin and 5-fluorouracil
48-hour continuous infusion in pretreated metastatic colorectal
cancer. Eur J Cancer. 33:214–219. 1997.
|
|
7
|
Rougier P, Van Cutsem E, Bajetta E, et al:
Randomised trial of irinotecan versus fluorouracil by continuous
infusion after fluorouracil failure in patients with metastatic
colorectal cancer. Lancet. 352:1407–1412. 1998. View Article : Google Scholar
|
|
8
|
Sargent DJ, Niedzwiecki D, O’ Conell MJ,
et al: Recommendation for caution with irinotecan, fluorouracil,
and leucovorin for colorectal cancer. N Engl J Med. 345:144–145.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
10
|
Scheithauer W, Kornek GV, Raderer M, et
al: Combined irinotecan and oxaliplatin plus granulocyte
colony-stimulating factor in patients with advanced
fluoropyrimidine/leucovorin-pretreated colorectal cancer. J Clin
Oncol. 17:902–906. 1999.
|
|
11
|
Rothenberg ML: Efficiacy of oxaliplatin in
the treatment of colorectal cancer. Oncology. 14:9–14.
2000.PubMed/NCBI
|
|
12
|
Souglakos J, Mavroudis D, Kakolyris S, et
al: Triplet combination with irinotecan plus oxaliplatin plus
continuous-infusion fluorouracil and leucovorin as first-line
treatment in metastatic colorectal cancer: a multicenter phase II
trial. J Clin Oncol. 20:2651–2657. 2002. View Article : Google Scholar
|
|
13
|
Scheithauer W, Kornek GV, Raderer M, et
al: Randomized multicenter Phase II trial of two different
schedules of capecitabine plus oxaliplatin as first-line treatment
in advanced colorectal cancer. J Clin Oncol. 21:1307–1312. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar
|
|
15
|
Stathopoulos GP, Rigatos SK, Stathopoulos
JG, Xynotroulas JP and Dimou E: Efficacy and tolerability of
oxaliplatin plus irinotecan 5-fluorouracil and leucovorin regimen
in advanced stage colorectal cancer patients pretreated with
irinotecan, 5-fluorouracil and leucovorin. AJ Clin Oncol.
28:565–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stathopoulos GP, Rigatos SK, Malamos N, et
al: Long term survival of patients with advanced colorectal cancer
may not be due to the response to chemotherapy. Oncology Rep.
12:1295–1300. 2004.PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhower E, et
al: New guidelines to evaluate the response to tumor in solid
tumors. JNCI. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cunningham D, Pyrhonen S, James RD, et al:
Randomised trial of irinotecan plus supportive care versus
supportive care alone after fluorouracil failure for patients with
metastatic colorectal cancer. Lancet. 31(353): 1275–1276.
1999.PubMed/NCBI
|