Introduction
Lung cancer has the highest incidence and mortality
rate of all malignancies. Non-small cell lung cancer (NSCLC)
accounts for approximately 80% of all lung cancers, the majority of
which are at an advanced stage and unresectable when diagnosed
(1). As the main approach for
NSCLC, radiotherapy was adopted exclusively until the 1990s when
the advantage of radiotherapy combined with chemotherapy was
established by CALGB8433 (2). In
addition, Furuse et al concluded the superiority of the
concomitant combination over the sequencing one (3), a theory that was supported by a recent
meta-analysis (4). Nine to eleven
months of the median survival time (MST) for patients with advanced
NSCLC treated by radiotherapy alone has been improved to
approximately 16 months for those treated with radiotherapy
combined with chemotherapy (2–4). A
total of 60 Gy was considered to be the standard dose in
conventional fractionation radiotherapy, but only a 5-year survival
rate of approximately 5% has been achieved since its clinical
application (5). A higher radiation
dose is required to improve the local control rate of tumors, and
it has been suggested in the US RTOG (Radiation Therapy Oncology
Group) trials that the radiation dose in the concomitant
radiochemotherapy may be safely escalated to 70–74 Gy for
3-dimensional conformal radiotherapy (3DCRT) (6,7).
However, due to the absence of reports on high-dose tolerance of
concomitant radiochemotherapy in Chinese patients with NSCLC, a
prospective trial was required to assess the safety of high-dose
radiation. One of the key factors that limited dose escalation was
pulmonary volume radiation dose. The decrease in radiation dose of
the two lungs made it possible to escalate the dose to 70 Gy in
NSCLC. In our prospective small-sample exploratory study, 3DCRT was
used to limit pulmonary radiation dose through a pulmonary
dose-volume histogram (DVH) to treat NSCLC patients with 70 Gy
high-dose radiation combined with concomitant NC chemotherapy
[vinorelbine (NVB) plus carboplatin (CBP)] under the conditions of
V20≤30% and V30≤20%, based on previous reports (8,9,10). The
primary endpoint was to evaluate the tolerance of this regimen, and
observe its preliminary efficacy.
Patients and methods
Patient characteristics
Between February 2008 and January 2010, 37
sequential untreated patients with pathologically or cytologically
confirmed NSCLC were treated with high-dose 3DCRT with concomitant
NC chemotherapy. The 37 patients comprised 22 males and 15 females
aged between 42 and 70 years with a median age of 64.
Eligibility
Hospitalized patients (aged ≥18 and ≤70 years)
diagnosed pathologically or cytologically as stage III or IV
(UICC1997) were recruited into the trial. Patients had at least one
evaluable lesion, an expected survival time of >3 months and no
other severe internal diseases that required hospitalization.
Patients were requested to have a Karnofsky performance status of
≥60. The required laboratory tests included a neutrophil count of
≥2.0×109/l, a platelet count of ≥100×109/l, a
hemoglobin count of ≥100 g/l, and serum creatinine, aspartate
aminotransferase, alanine aminotransferase, and total serum
bilirubin ≤upper limits of normal. The exclusion criteria were:
cases at stage IIIb with effusion; cases with superior vena cava
syndrome; pregnancy; lactation; a history of other malignancies,
with the exception of carcinoma in situ of the cervix,
non-melanomatous skin cancer, or cancer from which the patient had
not been disease-free for five years; a general medical condition
preventing combined modality therapy; and a known hypersensitivity
to NVB or CBP; as well as any use of concurrent other
antineoplastic therapy.
Pretreatment evaluation
Pretreatment evaluation included medical history,
complete physical examination, chest and abdominal helical computed
tomography (CT) scan, electrocardiography, bronchoscopy, bone
marrow scan (if clinically indicated), complete blood count, and a
biochemical profile. These pretreatment tests were performed in the
week prior to treatment initiation. Patients received physical
examinations, and blood counts were obtained once a week or more
often if deemed necessary. A biochemical profile was obtained and
electrocardiography was performed prior to each chemotherapy
cycle.
Ethics
The procedures were approved by the Ethics Committee
of Hebei Medical University and were performed in accordance with
the ethical standards of human experimentation, and the Helsinki
Declaration of 1975, as revised in 2000. All of the patients
provided written informed consent.
Recruitment and treatment plan
Patients were prospectively recruited and treated
with concomitant radiochemotherapy. The treatment scheme is shown
in Table I. For all eligible
patients, radiotherapy began on day 1, concurrently with the first
cycle of chemotherapy.
 | Table ITreatment plan. |
Table I
Treatment plan.
| Concomitant
radiochemotherapy regimen |
|---|
| RT regimen: Week 1–7:
2 Gy/f, 1 f/d, 5 f/w. Total dose: 70 Gy. |
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| RT | ||||| | ||||| | ||||| | ||||| | ||||| | ||||| | ||||| |
| Chemotherapy: NVB (25
mg/m2) day 1 and day 8; CBP at AUC of 5
mg/ml−1.min−1 on day 8, repeated every 28
days. |
| NVB | ◆ | ◆ | | | ◆ | ◆ | |
| CBP | | • | | | | • | |
Radiotherapy
The patient was placed in a supine position with
hands on head and fingers interlocked. A vacuum pad was used to fix
the body position and appropriately limit respiratory movement. A
contrast spiral CT (GE LightSpeed Plus 4) was performed, and the
image data was input into the 3D therapeutic planning system. Venus
5014 software (Shanghai Tuoneng Co., Shanghai, China) was used to
design the radiation plan. Delineation of the target volume was
based on the consensus guidelines for the delineation of the
radiotherapy target volume in NSCLC (11): the target of the primary lesion was
delineated in the pulmonary window (1600, −600 HU), and that of
mediastinal lymph nodes was delineated in the mediastinal window
(400, −20 HU). An involved field radiation was adopted, without
prophylactic radiation on the lymph drainage field. The definition
of the target volume was as follows: gross tumor volume (GTV) was
defined as the primary lesion and the lymph node >1 cm in the
short diameter, clinical target volume (CTV) as the GTV enlarged by
6 mm for squamous cell carcinoma, large cell cancer and metastatic
lymph nodes or by 8 mm for adenocarcinoma; and PTV as the CTV
enlarged by 10–15 mm based on the respiratory movement observed
under the X-ray simulator. The definition of GTV was confirmed
jointly by a diagnostic imaging physician, a radiologist and a
medical physicist. The outlines of vital organs and body surface
were drawn by a radiologist. Three to six coplanar or non-coplanar
fields were adopted for the conformal radiation. The therapeutic
plan was optimized by DVH. A total of 100% of the isodose curve
should cover at least 95% of the PTV. The restrictive conditions of
the key organs included: V20≤30% and V30≤20% for the two lungs,
0%>45 Gy for spinal marrow, esophageal length under 60 Gy
radiation ≤10 cm and 0%>70 Gy, and V40≤40 Gy for heart. All of
the cases were irradiated by a Siemens Premus Plus linear
accelerator of 6MV-X, which was equipped with a 27-pair multileaf
collimator (Topslane, Shanghai Tuoneng Co.). CT scans were repeated
after irradiation dose (DT) reached 50 Gy. The radiation was
continued as planned until completion unless an obvious shrinkage
of the target volume was observed, for which a field reduction was
necessary based on another contrast CT scan in the original body
position. The lesions were involved in the same target field as far
as possible, and a second field was defined if there was a large
margin between lesions. Each of the patients was radiated for DT 70
Gy, 2 Gy/fraction, once per day, 5 days per week for 7 weeks. X-ray
combined with electron at a dose of DT 60–70 Gy was adopted in the
case of supraclavicular nodular metastasis.
Chemotherapy
Chemotherapy was started on day 1 of radiation, with
NVB 25 mg/m2 on days 1 and 8 intravenously, and CBP at
AUC of 5 mg/ml−1.min−1 on day 8, which was
repeated every 28 days. Two cycles of concomitant chemotherapy were
carried out during radiotherapy, and another 4 cycles at most were
carried out after the radiation. Anti-emetic, white blood
cell/platelet promotion and support care were used for each patient
as required.
Evaluation criteria of adverse events and
short-term treatment efficacy
NCI common toxicity criteria 2.0 (NCI CTC 2.0) were
used to grade the toxicity (12).
The short-term response was evaluated 4 weeks after the completion
of radiotherapy, based on the objective criteria by WHO (13): complete response (CR): disappearance
of all observable lesions, maintained for >4 weeks; partial
response (PR): a decrease of at least 50% in the largest diameter
or the product of the largest two vertical diameters, maintained
for >4 weeks; stable disease (SD): a shrinkage of the product of
two diameters, or <50% or an increase of the product ≤25%;
progressive disease (PD): an increase of >25% in at least one
lesion, or appearance of new lesions. CR and PR were considered as
a response, while SD and PD were considered as no response.
Dose attenuation
Dose modifications were based on the most serious
toxicities that occurred on any day after the treatment plan
commenced.
The irradiation dose was not allowed to be modified.
However, radiotherapy was withheld for Grade III or higher
toxicities until they were no longer present. Radiotherapy was
continued, but chemotherapy was withheld in cases where Grade III
or higher toxicities, unrelated to radiotherapy, occurred, such as
peripheral neuritis. Chemotherapy was resumed when those toxicities
were eliminated.
Chemotherapeutic doses were modified in the manner
mentioned below. If Grade III–IV thrombocytopenia, Grade III–IV
anemia, Grade IV neutropenia, or Grade III–IV non-hematological
toxicity occurred (with the exception of Grade III nausea, vomiting
and anorexia), both RT and NVB with CBP were withheld until the
Grade III or IV toxicities were no longer present. If the
toxicities were not eliminated within 2 weeks, the patient was
withdrawn from the study. The NVB and CBP doses of the following
chemotherapy cycle were reduced by 25%. Prophylactic recombinant
human granulocyte colony-stimulating factor was used following that
chemotherapy cycle. If Grade III neutropenia or Grade II
thrombocytopenia alone occurred, chemotherapy was stopped and
radiotherapy continued. The NVB and CBP doses of the following
chemotherapy cycle were the same as those in the original regimen.
Prophylactic recombinant human granulocyte colony-stimulating
factor was used following that chemotherapy cycle.
Subsequent treatment
A second-line regimen based on docetaxel was used in
the case of PD with consideration for the general status of the
patient.
Follow-up
Following treatment, patients were followed up at
every 3 months for the first year, every 6 months for the second
year, and annually thereafter. Each follow-up included history,
physical examination, complete blood count, blood biochemical
examination and chest CT.
Death from any cause was calculated from the date of
treatment until the patient succumbed or until the last follow-up
evaluation.
Statistical analysis
Statistical analysis was performed using the
SPSS13.0 software package. The accumulated overall survival rate
was calculated using the Kaplan-Meier method.
Results
Patient characteristics
A total of 37 sequential untreated patients with
pathologically or cytologically confirmed NSCLC were treated with
high-dose 3DCRT with concomitant NC chemotherapy. The 37 patients
included 22 males and 15 females, aged between 42 and 70 years,
with a median age of 64. Of the 37 patients, 20 patients had
squamous cell carcinoma, 16 had adenocarcinoma and one had large
cell cancer. Seven had stage IIIa disease, 17 had stage IIIb
disease and 13 had stage IV disease.
The patients were followed up until they succumbed
to the disease or until the time of the last follow-up evaluation.
Until May 31, 2010, no patients were lost to follow-up. The median
follow-up time for all patients was 12 months (range 4–16).
Treatment compliance
All 37 patients completed the 70 Gy radiotherapy and
at least 2 cycles of concomitant NC chemotherapy. Toxicity and
response were evaluated in all 37 patients.
Adverse events
No treatment-associated death (Grade V toxicity)
occurred in any of the patients.
Hematological toxicity
Less severe hematological toxicity was observed
despite its high incidence. Grade III/IV neutropenia occurred in
18.9% (7/37) of the patients, including 2 patients treated with
antibiotics: Grade III/IV thrombocytopenia was observed in 8.1%
(3/37) of patients, including one patient transfused with
platelets; the incidence of anemia was found to be 32.4% (12/37),
although no incidence was graded III or above, and no blood
transfusion was required (Table
II).
 | Table IIHematological toxicities of
concomitant radiochemotherapy. |
Table II
Hematological toxicities of
concomitant radiochemotherapy.
| 0 | I | II | III | IV | Total |
|---|
|
|
|---|
| Cases (%) | Cases (%) | Cases (%) | Cases (%) | Cases (%) | Cases (%) |
|---|
| Neutropenia | 5 (13.5) | 9 (24.3) | 16 (43.2) | 5 (13.5) | 2 (5.4) | 32 (86.5) |
| Thrombocytopenia | 20 (54.1) | 10 (27.0) | 4 (10.8) | 2 (5.4) | 1 (2.7) | 17 (45.9) |
| Anemia | 25 (67.6) | 6 (16.2) | 6 (16.2) | 0 (0) | 0 (0) | 12 (32.4) |
Non-hematological toxicity
Radiation pneumonitis occurred in 48.7% (18/37) of
the patients including 8.1% (3/37) at Grade III. No cases were
observed at Grade IV or above. The incidence of radiation
esophagitis was 78.4% (29/37), with 64.9% (24/37) at Grade I/II and
13.5% (5/37) at Grade III. No non-hematological toxicity of Grade
IV or above was reported. Nausea was frequently observed in up to
81.1% (30/37) of cases, although generally to a lesser degree
(Grades I/II), and no cases of Grade III or above occurred.
Vomiting occurred in 29.7% (11/37) of cases, all of which were at
Grade I/II with no cases at Grade III or above. No other associated
severe toxicity was observed, such as radiation myelitis or
radiation pericarditis (see Table
III).
 | Table IIINon-hematological toxicities of
concomitant radiochemotherapy. |
Table III
Non-hematological toxicities of
concomitant radiochemotherapy.
| 0 | I | II | III | IV | Total |
|---|
|
|
|---|
| Cases (%) | Cases (%) | Cases (%) | Cases (%) | Cases (%) | Cases (%) |
|---|
| Radiation
pneumonitis | 19 (51.3) | 5 (13.5) | 10 (27.0) | 3 (8.1) | 0 (0) | 18 (48.7) |
| Radiation
esophagitis | 8 (21.6) | 10 (27.0) | 14 (37.8) | 5 (13.5) | 0 (0) | 29 (78.4) |
| Nausea | 7 (18.9) | 8 (21.6) | 12 (32.4) | 0 (0) | 0 (0) | 30 (81.1) |
| Vomiting | 26 (70.3) | 5 (13.5) | 6 (16.2) | 0 (0) | 0 (0) | 11 (29.7) |
Short-term response rate
Complete response (CR) rate of the patients was
13.5% (5/37), partial response (PR) rate was 64.9% (24/37), stable
disease (SD) was 10.8% (4/37), and the progressive disease (PD)
rate was 10.8 (4/37). The short-term response rate (CR+PR) was
78.4% (29/37).
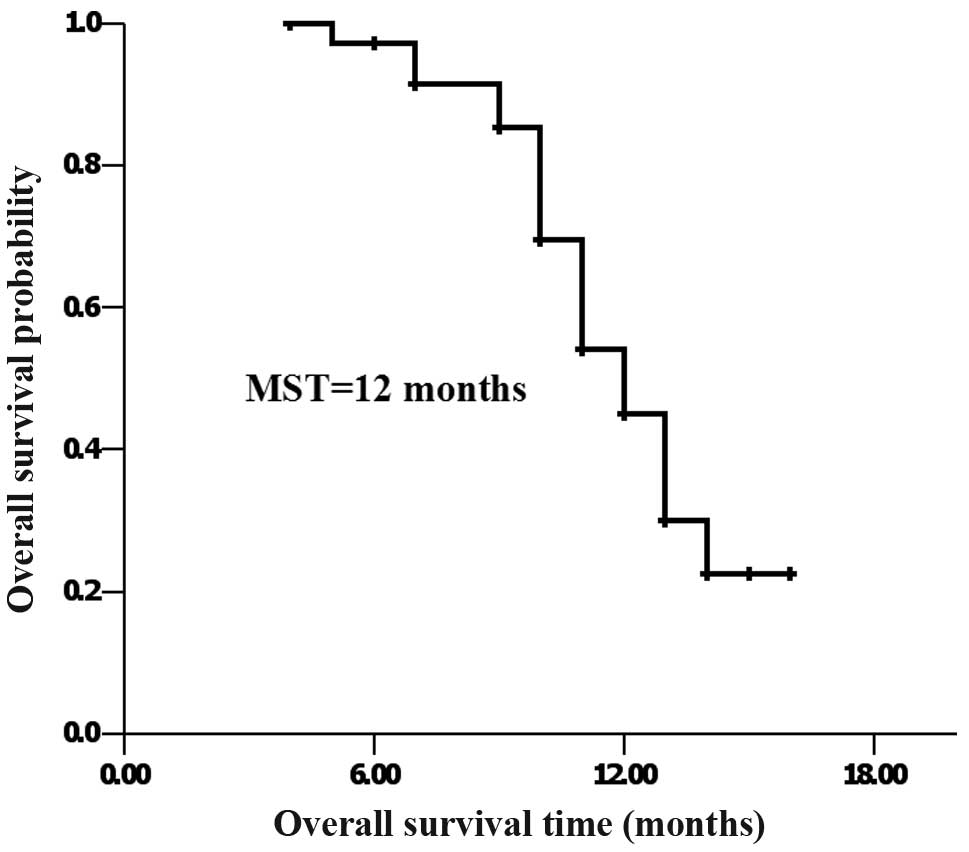
Survival
At the last follow-up, 17 patients had survived and
20 patients had succumbed to the disease. MST was 12 months, and
the 1-year accumulated survival rate was 45.5% (Fig. 1).
Discussion
An increasing number of clinical trials have proven
that concomitant radiochemotherapy is significantly superior to the
sequencing modality in locally advanced unresectable NSCLC
(4,14), although toxicity increases. A lower
dose of radiation (55–60 Gy) has therefore been used in standard
radiochemotherapy regimens (2,15,16).
Fletcher has suggested that a radiation dose of 80–100 Gy is
required to cure lung cancer (17),
validated by stereotactic hypofractionated radiotherapy with the
fact that a BED no less than 100 Gy achieved a better efficacy than
a BED of less than 100 Gy (18).
Therefore, the radiation dose should be escalated in the
concomitant combination regimen to improve both the local control
rate of the tumor and the survival rate (19).
It is feasible to administer high radiation doses
with 3DCRT in NSCLC, since the dose on healthy tissue can be
precisely calculated in 3DCRT, thus providing better protection of
normal tissues. Results of RTOG suggest that a 74 Gy dose is safe
in radiation combined with concomitant chemotherapy in NSCLC
(6). Due to the differences in
physique, the American regimen of concomitant chemo-radiation
cannot be applied to Chinese patients. Our previous dose escalation
research study in Chinese esophageal cancer patients showed that
the tolerable dose for Chinese patients is significantly lower than
that recommended by RTOG (20,21).
Due to the absence of reports on dose tolerance of concomitant
radiochemotherapy in NSCLC in China, a prospective exploratory
trial was necessary in order to evaluate the tolerance of a
high-dose radiotherapy of 70 Gy with concomitant chemotherapy, as
well as to determine an effective method to decrease the incidence
of severe complications.
One of the key factors limiting dose escalation in
concomitant radiochemotherapy is radiation pneumonitis. Findings of
studies both in China and worldwide have suggested that concomitant
radiochemotherapy is safe provided that the dose volume parameters
are controlled under the conditions: V20≤30% and V30≤20% (6,8–10).
Therefore, we limited the pulmonary radiation dose during the
high-dose radiotherapy to prevent severe radiation pneumonitis.
Another severe treatment-associated toxicity in the
NSCLC concomitant regimen is myeloid suppression. Neutropenia and
thrombocytopenia not only lead to treatment interruption, but may
also be life-threatening in severe cases. Moreover, the incidence
of radiation pneumonitis may be increased in concomitant
radiochemotherapy due to the sensitizing effect of chemotherapeutic
drugs. Therefore, it is also crucial to select an appropriate
chemotherapeutic regimen. CALGB9431 (22) analyzed the superiority by combining
cisplatin with paclitaxel, gemcitabine and NVB, respectively, in
concomitant radiochemotherapy. By comparing results, it was found
that there was significantly less associated toxicity with NVB than
with paclitaxel or gemcitabine with Grade III/IV neutropenia (27,
53 and 51%, respectively), and notably fewer cases of vomiting and
anorexia (16, 15, 8% and 22, 27, 12%, respectively). Therefore, an
NP regimen has been more frequently adopted for the concomitant
treatment in China and in other countries. Given the strong emesis
inducibility of cisplatin and the inconvenient hydration required
with high dosages, we substituted carboplatin with cisplatin to
reduce those side effects.
In our small-sample exploratory study, patient
tolerance of 3DCRT combined with concomitant NC chemotherapy was
the primary endpoint. The results showed that all patients
tolerated the 70-Gy high-dose radiotherapy well, with minor
treatment-associated side effects. Incidence of severe toxicity was
comparatively low as the involved field radiation was adopted
without preventive radiation on the drainage area of lymph nodes
(11). Grade III radiation
pneumonitis occurred in only 8.1% (3/37) of patients, with no cases
at Grade IV or above. Most cases of radiation esophagitis were
Grade I/II; only 13.5% (5/37) were Grade III, and none were Grade
IV or above. Severe nausea, emesis and anorexia were rare.
Peripheral venous infusion was deemed necessary for only a few
patients and was successful for a short period of time, with no
need for nasal gastric feeding or parenteral hyperalimentation.
A Chinese study also adopted the concomitant NVB
plus carboplatin regimen (NC) during radiotherapy in NSCLC.
However, no 3DCRT, but rather conventional radiation technology was
used in that study, and a preventive radiation treatment on
ipsilateral pulmonary hilar and mediastinum was carried out.
Despite the total dose being only 60 Gy, the side effects were
severe with Grade III radiation esophagitis in up to 42.6% of
patients and Grade III radiation pneumonitis in up to 30.4% of
patients (23). Another
small-sample investigation on conventional radiation combined with
concomitant NVB plus cisplatin (NP) reported a treatment-associated
mortality rate of 3.8% (1/26) due to esophageal-mediastinal fistula
(27).
Although a relatively high radiation dose (70 Gy)
was adopted in our study and the dose of concomitant chemotherapy
remained constant throughout the study, the incidence of severe
hematological toxicity was low, with 18.9% (7/37) of patients
experiencing Grade III/IV neutropenia, 8.1% (3/37) Grade III/IV
thrombocytopenia and only one patient requiring platelet
transfusion, with no cases of anemia at Grade III or above.
However, a higher incidence of Grade III/IV neutropenia (35 and
33%, respectively) was observed in other studies that combined
radiation with concomitant NP (25,26),
which may be explained by the fact that the AUC adopted for
carboplatin calculation in this study better satisfied the
pharmacokinetics. Moreover, the incidence of severe radiation
esophagitis of up to 25–30.8% in the previous concomitant NP study
(25,27,28)
compared with 13.5% in the present study suggested that the
preliminary advantage of concomitant NC during radiotherapy was
that this type of radiotherapy is well-tolerated.
The short-term response rate of NC combined with
concomitant radiotherapy was up to 78.4%, which was comparative to
the NP regimen (24,25,27).
However, the 1-year accumulated survival rate (45.5%) was a little
lower than the results of over 60% observed in the abovementioned
studies. This discrepancy may be due to the different inclusion
criteria; in the above three studies only stage III patients were
recruited, whereas 35% of the patients in this study were stage
IV.
In conlusion, a high dose of 70 Gy 3DCRT combined
with concomitant chemotherapy of NVB plus carboplatin in the two
lungs (V20≤30 and V30≤20%) was well tolerated and achieved a
favorable short-term response rate, MST and 1-year accumulated
survival rate. The regimen was feasible for Chinese patients with
NSCLC. Further evaluation of this regimen in a prospective
controlled phase II trial is ongoing.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Dillman RO, Herndon J, Seagren SL, Eaton
WL Jr and Green MR: Improved survival in stage III non-small-cell
lung cancer: seven year follow-up of cancer and leukemia group B
(CALGB) 8433 trial. J Natl Cancer Inst. 88:1210–1215.
1996.PubMed/NCBI
|
|
3
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concomitant versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small cell lung cancer. J Clin Oncol. 17:2692–2699.
1999.PubMed/NCBI
|
|
4
|
Liang HY, Zhou H, Li XL, Yin ZH, Guan P
and Zhou BS: Chemo-radiotherapy for advanced non-small cell lung
cancer: concomitant or sequential? It's no longer the question: a
systematic review. Int J Cancer. 127:718–728. 2010. View Article : Google Scholar
|
|
5
|
Perez CA, Pajak TF, Rubin P, Simpson JR,
Mohiuddin M, Brady LW, Perez-Tamayo R and Rotman M: Long-term
observations of the patterns of failure in patients with
unresectable non-oat cell carcinoma of the lung treated with
definitive radiotherapy. Report by the Radiation Therapy Oncology
Group. Cancer. 59:1874–1881. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bradley J: A review of radiation dose
escalation trials for non-small cell lung cancer within the
Radiation Therapy Oncology Group. Semin Oncol. 32:S111–S113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley JD, Moughan J, Graham MV, Byhardt
R, Govindan R, Fowler J, Purdy JA, Michalski JM, Gore E and Choy H:
A phase I/II radiation dose escalation study with concomitant
chemotherapy for patients with inoperable stages I to III
non-small-cell lung cancer: phase I results of RTOG 0117. Int J
Radiat Oncol Biol Phys. 77:367–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329.
1999.PubMed/NCBI
|
|
9
|
Wang S, Liao Z, Wei X, Liu HH, Tucker SL,
Hu CS, Mohan R, Cox JD and Komaki R: Analysis of clinical and
dosimetric factors associated with treatment-related pneumonitis
(TRP) in patients with non-small-cell lung cancer (NSCLC) treated
with concomitant chemotherapy and three-dimensional conformal
radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 66:1399–1407.
2006. View Article : Google Scholar
|
|
10
|
Yang LQ, Wu LP, Chen LJ, Zhang RN, Gao YQ,
Chen GY and Liang NQ: Value of V20 and V30 parametres for
predicting acute radiation lung toxicity after 3D radiotherapy for
lung cancer. Chin J Cancer Prev Treat (In Chinese). 15:280–282.
2008.
|
|
11
|
Zhu GY, Xia TY, Wang LH, Gao XS, Wang JJ,
Li AF, Zhang FQ, Ma L, Li YX and Xu B: Consensus and controversies
on delineation of radiotherapy target volume for patients with
non-small cell lung cancer. Chin J Radiat Oncol (In Chinese).
17:432–436. 2008.
|
|
12
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
Shipley W and Curran W: Common toxicity criteria: version 2.0. An
improved reference for grading the acute effects of cancer
treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys.
47:13–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y and Zhou J: Medical Oncology [M]
(4th edition). People's Medical Publishing House (In Chinese).
Beijing: 106–107. 2004.
|
|
14
|
El-Sharouni SY, Kal HB, Battermann JJ and
Schramel FM: Sequential versus concomitant chemo-radiotherapy in
inoperable stage III non-small cell lung cancer. Anticancer Res.
26:495–505. 2006.PubMed/NCBI
|
|
15
|
Sause W, Kolesar P, Taylor S IV, Johnson
D, Livingston R, Komaki R, Emami B, Curran W Jr, Byhardt R, Dar AR
and Turrisi A III: Final results of phase III trial in regionally
advanced unresectable non-small cell lung cancer: Radiation Therapy
Oncology Group, Eastern Cooperative Oncology Group, and Southwest
Oncology Group. Chest. 117:358–364. 2000. View Article : Google Scholar
|
|
16
|
Schaake-Koning C, van den Bogaert W,
Dalesio O, Festen J, Hoogenhout J, van Houtte P, Kirkpatrick A,
Koolen M, Maat B and Nijs A: Effects of concomitant cisplatin and
radiotherapy on inoperable non-small-cell lung cancer. N Engl J
Med. 326:524–530. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fletcher GH: Clinical dose response curves
of human malignant epithelial tumors. Br J Radiol. 46:1511973.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Onishi H, Shirato H, Nagata Y, Hiraoka M,
Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura
T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada
K and Araki T: Hypofractionated stereotactic radiotherapy
(HypoFXSRT) for stage I non-small cell lung cancer: updated results
of 257 patients in a Japanese multi-institutional study. J Thorac
Oncol. 2:S94–S100. 2007. View Article : Google Scholar
|
|
19
|
Lichter AS and Lawrence TS: Recent
advances in radiation oncology. N Engl J Med. 332:371–379. 1995.
View Article : Google Scholar
|
|
20
|
Lin Q, Gao XS, Qiao XY, Zhou ZG, Zhang P
and Yang XR: Dose escalation of cisplatin with 5-fluororacil in
concomitant chemoradiotherapy for esophageal carcinoma. Chin J
Radiat Oncol (In Chinese). 15:301–304. 2006.
|
|
21
|
Lin Q, Gao XS, Qiao XY, Zhou ZG, Zhang P,
Chen K, Zhao YN and Asaumi J: Phase I trial of escalating-dose
cisplatin with 5-fluorouracil and concomitant radiotherapy in
Chinese patients with esophageal cancer. Acta Med Okayama.
62:37–44. 2008.PubMed/NCBI
|
|
22
|
Vokes EE, Herndon JE II, Crawford J,
Leopold KA, Perry MC, Miller AA and Green MR: Randomized phase II
study of cisplatin with gemcitabine or paclitaxel or vinorelbine as
induction chemotherapy followed by concomitant radiochemotherapy
for stage IIIB non-small-cell lung cancer: cancer and leukemia
group B study 9431. J Clin Oncol. 20:4191–4198. 2002. View Article : Google Scholar
|
|
23
|
Zhang ZM, Zhao HY, Zhang CJ and Mu JG:
Vinorelbine and carboplatin with concomitant radiotherapy in
locally advanced non-small cell lung cancer. Chin J Clin Oncol (In
Chinese). 9:625–627. 2004.
|
|
24
|
Rao CY: Study of concomitant versus
sequential radiochemotherapy with vinorelbine and cisplatin in
stage III non-small cell lung cancer. Chin J Cancer Prev Treat (In
Chinese). 14:942–943. 2007.
|
|
25
|
Liu J, Lv CX, Wang JM, Li HX, Guo JD, Wang
CL, Gao LT and Zhao L: Analysis of two different concomitant
radiochemotherapy regimens in the treatment of locally advanced
stage III non-small cell lung cancer. Chin J Clin Oncol (In
Chinese). 15:226–229. 2010.
|
|
26
|
Wang JP, Zhai XM, Zhang JN and Xu CS:
Clinical study on concomitant radiotherapy in combination with
vinorelbine and cisplatin for advance non-small cell lung cancer.
Acta Med Jiangsu Univ (In Chinese). 16:483–485. 2005.
|
|
27
|
Lu DJ, Wang L, Han C, Gao C and Li XN:
Toxicity analysis for esophagus in locally advanced non-small cell
lung cancer treatment using three dimensional conformal
radiotherapy combined with concomitant chemotherapy. Chin J Radiol
Med Prot (In Chinese). 30:185–188. 2010.
|
|
28
|
Zheng JX, Niu DL and Xu K: Radiotherapy
combined with concomitant chemotherapy for stage III non-small cell
lung cancer. Chin J Cancer Prev Treat (In Chinese). 13:1822–1823.
2006.
|