Introduction
Osteosarcoma originates from primitive bone-forming
mesenchymal cells and is the most prevalent primary bone
malignancy. It ranks eighth in general incidence among childhood
cancers (1). The overall 5-year
survival rate for osteosarcoma is 68%. Certain genetic
predispositions have been observed to correlate with osteosarcoma,
including hereditary retinoblastoma and Li-Fraumeni syndrome, which
are characterized by a high risk of developing osteosarcoma
(2,3). Genetic aberrations that accompany
osteosarcoma have been identified; however, osteosarcoma is
characterized by karyotypes which exhibit a high degree of
complexity (4,5). Understanding these mechanisms is
clearly crucial to osteosarcoma therapy.
Cell fusion plays a crucial role in homeostasis,
such as fertilization, formation of placenta, bone and muscle
tissues and tissue repair and regeneration (6). It was first thought to be involved in
tumorigenesis by Otto Aichel in the early 1900s, who posited that
fusion between somatic cells may result in chromosomal
abnormalities in cancer. Recent discoveries of cell fusion in
tissue homeostasis and regeneration have revitalized the interest
in cell fusion as one of the driving forces of cancer progression
(7). Stroma surrounding cancer
cells plays a supportive role in tumor development and progression.
The osteosarcoma stroma histopathologically comprises various
supportive components, including fibroblasts, inflammatory cells,
immune cells, smooth muscle cells and endothelial cells.
Fibroblasts form a significant component of the stromal
compartment. A number of these fibroblasts are differentiated into
carcinoma-associated fibroblasts (CAF), as with so-called
‘myofibroblasts’. Myofibroblasts are thought to contain both
fibroblast and smooth muscle cell characteristics, which are
defined by positive expression of both stromal cell type markers
and smooth muscle cell markers (8).
α-smooth muscle actin is one of the markers that is widely used to
detect myofibroblast.
When investigating the expression of α-smooth muscle
actin in osteosarcoma tissues, we observed that it was excessively
expressed in multinucleated cells in osteosarcoma tissue. This
expression indicates the involvement of cancer cell fusion with
myofibroblast. However, little evidence is currently available that
is of any relevance to osteosarcoma. This is therefore the first
study to demonstrate that human osteosarcoma cells are capable of
fusing with myofibroblast cells to form hybrid cells in
vitro.
Materials and methods
Patients and specimens
Informed consent was obtained from the patients or
their relatives, as appropriate. This investigation was performed
according to the guidelines approved by the Institutional Animal
Care and Use Committee. A total of 12 paraffin wax-embedded
specimens from patients with osteosarcoma were collected from the
Renmin Hospital of Wuhan University, Wuhan, Hubei, China, between
January 2007 and June 2009. All cases were confirmed by
pathological diagnosis.
Immunohistochemistry
Sections were deparaffinized and rehydrated using
graded alcohols. Antigen retrieval was performed by boiling the
slides in 10 mM citrate buffer (pH 6.0) for 10 min. Cells seeded on
the slides were fixed in 4% paraformaldehyde in phosphate-buffered
saline (PBS) and permeabilized in 0.1% Triton X-100. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide. The
slides or cells were then incubated in a humid chamber with the
rabbit anti-human/mouse α-smooth muscle actin polyclonal antibody
(ProteinTech Group, Chicago, IL, USA) at a dilution of 1:100, at
4°C, overnight. The slides were then incubated for 30 min at 37°C
with horseradish peroxidase-labeled goat anti-rabbit antibody. The
slides were developed using DAB, and then counterstained with
haematoxylin, dehydrated through graded alcohols, air-dried and
mounted in neutral resins. Some slides were stained by AEC, then
directly mounted using aqueous mounting solution. Primary
antibodies were substituted with PBS in the negative controls.
Cell culture
The human osteosarcoma cell lines U2OS and MG63 were
purchased from the Shanghai Institute for Biological Sciences of
Chinese Academy of Sciences. They were cultured in Dulbecco's
modified Eagle's medium (DMEM; high glucose) supplemented with 10%
fetal bovine serum. The cells were cultured in a 37°C humidified
incubator with a mixture of 95% air and 5% CO2. Primary
mouse embryonic fibroblasts (PMEFs) were established and maintained
according to protocol by Garfield (9). In brief, the mice were sacrificed on
postcoitum day E14.5. Each embryo was removed and placed in
prewarmed complete medium, decapitated and eviscerated, and as much
blood and liver tissue was discarded as possible. Trypsin/EDTA was
added to the remainder after homogenization. Supernatant was
collected following adequate precipitation, and was centrifuged at
1000 rpm for 10 min to obtain the cell pellet. The pellet was
resuspended and 1×106 cells were seeded into a
25-cm2 flask. Cell maintenance and splitting were
manipulated routinely. For induction of myofibroblasts, the cells
were harvested and washed with 0.1% BSA serum-free medium. U2OS or
MG63 cells were seeded on the upper chamber with complete medium as
an inducer. The PMEFs were placed into the bottom chamber of a
transwell unit (Corning Costar, Corning, NY, USA). The
polycarbonate 3 mm pore membrane was precoated with 0.2 mg/ml of
rat tail type I collagen (BD Biosciences, Bedford, MA, USA) to
inhibit the migration of cancer cells. The cells were allowed to
culture at 37°C and 5% CO2 for 28 days and the medium
was changed twice a week. For co-cultures of both osteosarcoma
cells and myofibroblasts, cells were harvested by trypsinization
and equal numbers of cancer and myofibroblast cells were mixed.
Cells were grown on sterile glass slides (Nalge Nunc, Naperville,
IL, USA), fixed in 4% paraformaldehyde in PBS and permeabilized in
0.1% Triton X-100 for immunohistochemistry or in methanol-acetic
acid for fluorescence in situ hybridization (FISH).
Western blot analysis
Cells were washed with ice-cold PBS after
trypsinization and centrifuged at 1000 rpm for 10 min at room
temperature. The pellet containing approximately 1×106
cells was lysed in 100 μl of RIPA cell lysis buffer containing
protease inhibitors and quantified by the BCA method. Protein (100
μg) was separated by SDS-PAGE and transferred to nitrocellulose
membranes. After blocking with 5% skim milk, the membranes were
incubated with the following primary antibodies: polyclonal rabbit
anti-α-smooth muscle actin (1:1000; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and monoclonal mouse anti-β-actin (1:2500; Sigma,
St. Louis, MO, USA). The blots were then incubated with horseradish
peroxidase-conjugated secondary antibody. Enhanced
chemiluminescence was used for detection and developed by X-ray
film.
Fluorescent in situ hybridization
The cells were fixed in methanol-acetic acid and
predenatured, dehydrated, denatured, and hybridized with DNA probes
for mouse 8 and human X chromosomes (ID Labs, London, Ontario,
Canada) overnight at 37°C in a humidified chamber. After a
post-hybridization wash, slides were counterstained with
4,6-diamidino-2-phenylindole and examined using an epifluorescence
microscope (Nikon Eclipse TE2000-U).
Statistical analysis
The Student's t-test was used to compare the means
of the 2 groups. When ≥3 means were compared, one-way ANOVA
followed by multiple comparisons among the means was used.
Results
α-smooth muscle actin expression in
osteosarcoma specimens
Table I shows the
patient characteristics. To assess the state of α-smooth muscle
actin expression in clinical osteosarcoma samples,
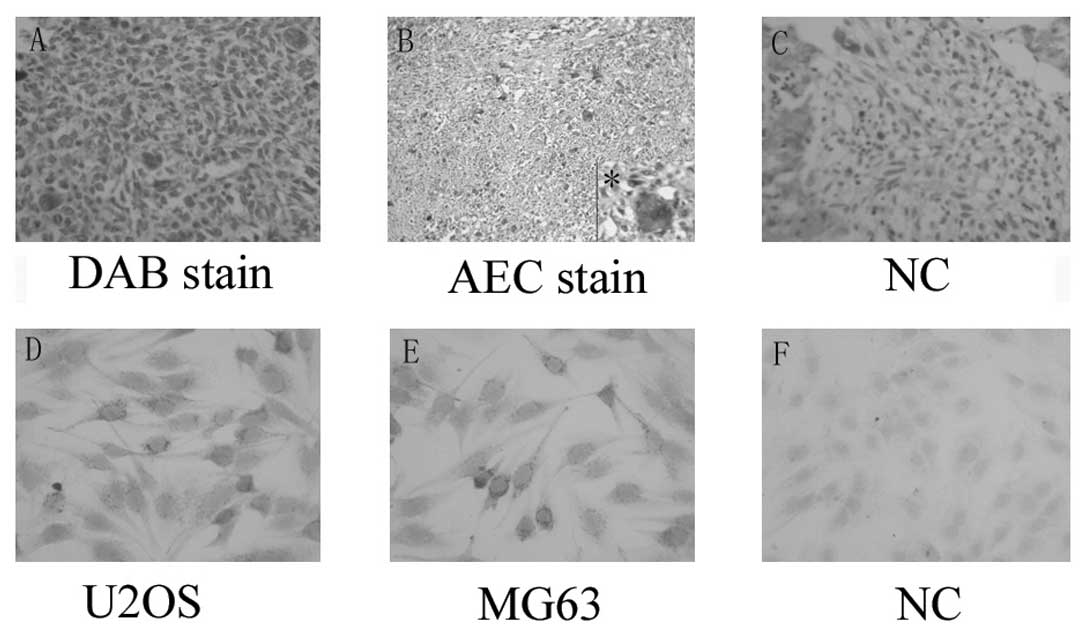
immunohistochemical staining was performed. Marked α-smooth muscle
actin expression was observed in specimens from osteosarcoma
patients compared with normal bone tissue. Marked staining was
observed in 90% (9/10) of the primary osteosarcoma. Slight staining
was detected in 30% (3/10) of normal bone tissue. Most notably, a
distinctive staining pattern was observed depending on the nuclei
status of the cells. Numerous multinucleated cells were observed in
the osteosarcoma tissue (4/10), 3 of which were from histologically
low-differentiated osteosarcoma patients. These cells were markedly
cytoplasmic-positive for α-smooth muscle actin (Fig. 1).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Total | 10 |
| Gender |
| Male | 6 |
| Female | 4 |
| Age |
| Mean | 28.6 |
| Range | 9–57 |
| Location |
| Humerus | 5 |
| Femur | 2 |
| Scapula | 1 |
| Skull | 1 |
| Iliac | 1 |
| Pathological
variables |
| Types of tumor |
| Osteoblastic | 5 |
| Chondroblastic | 2 |
| Fibroblastic | 3 |
| Histological
grade |
| Well | 2 |
| Moderate | 1 |
| Poor | 7 |
α-smooth muscle actin expression in
osteosarcoma cell lines
Expression of α-smooth muscle actin was examined in
osteosarcoma cell lines MG63 and U2OS to confirm the above result.
α-smooth muscle actin was found to be widely expressed in the
osteosarcoma cell line, was likely to form particles, and was
mainly observed in cytoplasm (Fig.
1).
Osteosarcoma cells activated the
PMEFs
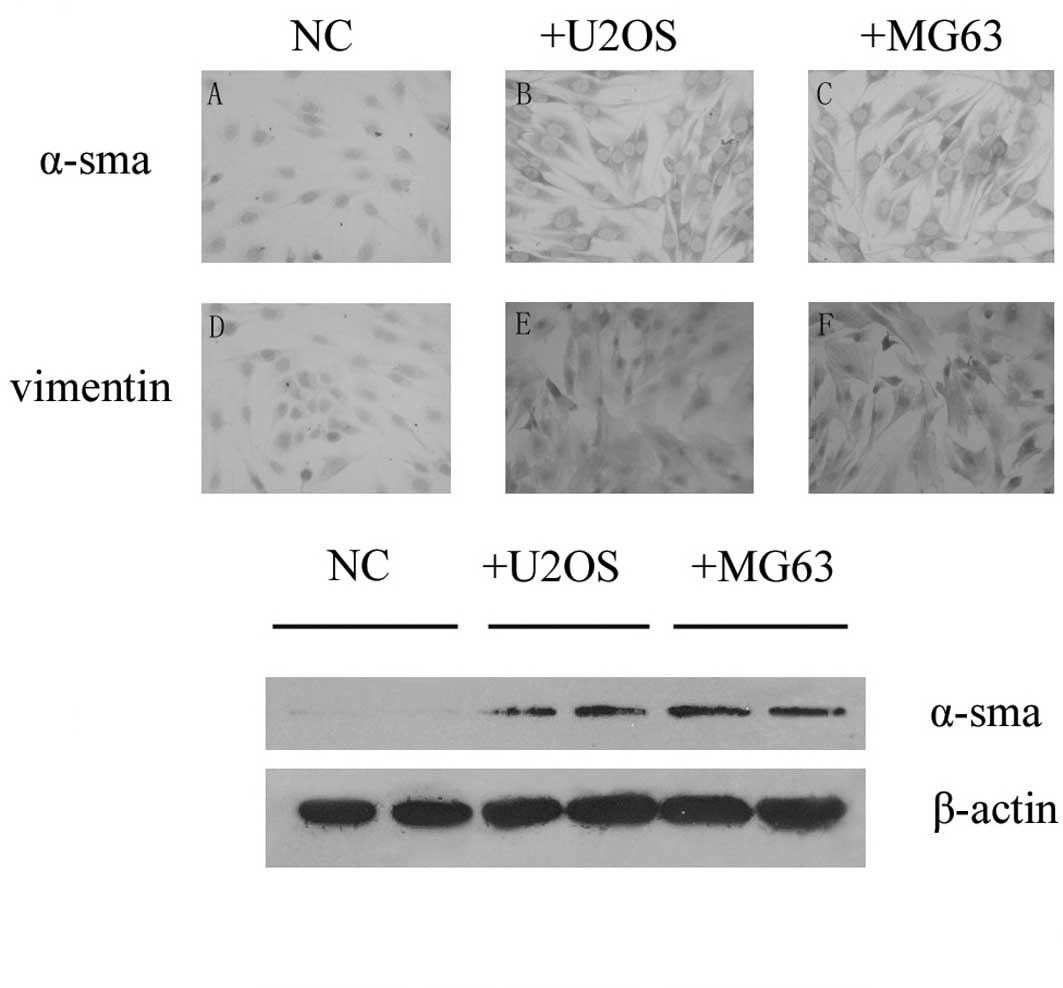
We investigated the expression of α-smooth muscle
actin in PMEFs and noted that there was no staining in PMEFs using
immunohistochemistry and Western blotting methods. To observe
whether osteosarcoma was capable of transforming PMEFs to
myofibroblasts, U2OS or MG63 cells were co-cultured with PMEFs
using a transwell unit. Expression of α-smooth muscle actin in
PMEFs was detected 28 days after placing into the bottom chamber by
both immunohistochemistry and Western blotting, and vimentin was
also induced in the co-culture groups. Both α-smooth muscle actin
and vimentin were found to be expressed in cytoplasm (Fig. 2).
Spontaneous fusion between osteosarcoma
and myofibroblast cells in vitro
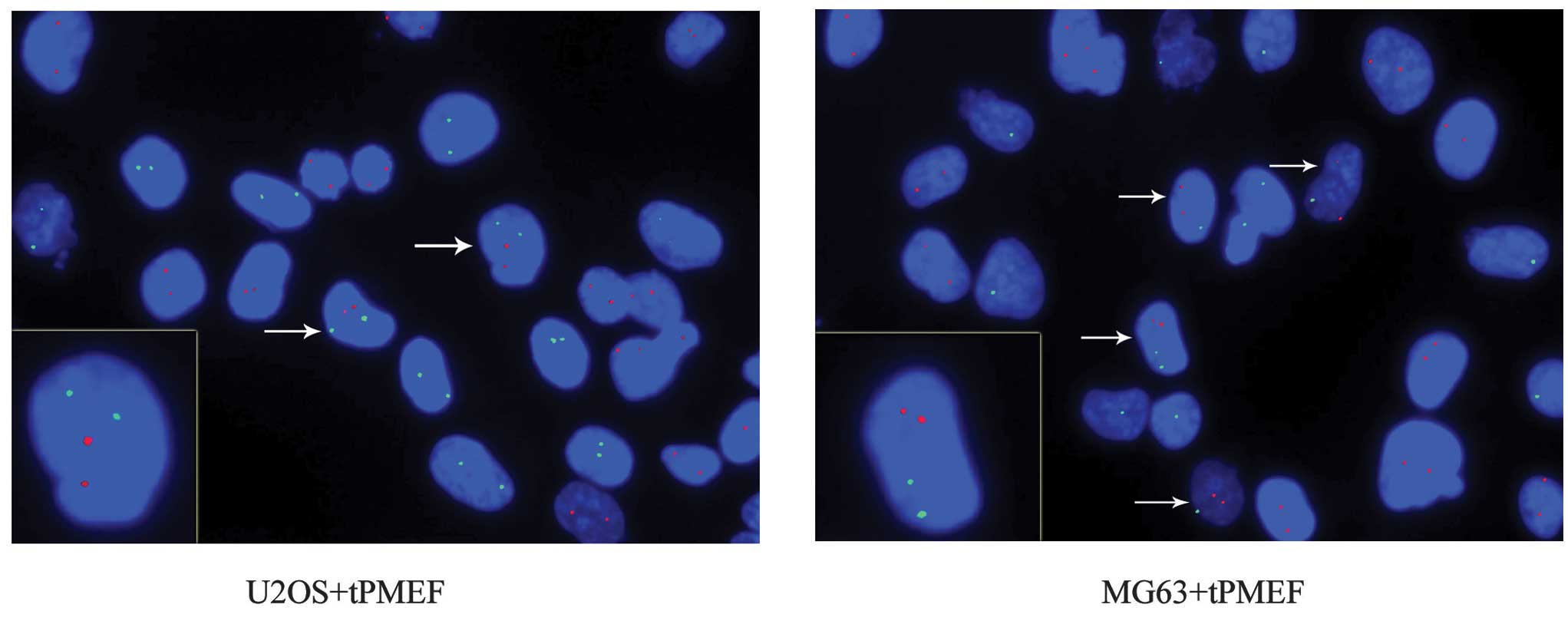
Equal numbers of human osteosarcoma cancer (U2OS and
MG63) cells and PMEFs were co-cultured. FISH was performed using
point FISH probes specific to human chromosome X (hCX) and mouse
probes specific to mouse chromosome 8 (mC8), labeled with
fluorochrome delivering green and red fluorescence, respectively.
This process allowed for the detection of hCX and mC8 in
contrasting colors. Double fluorescence for species-specific
chromosomal markers revealed that mC8 did not occur in U2OS
cultured alone, and hCX did not appear in mouse myofibroblast cells
cultured alone. However, in co-cultures, 3.0±2.3% of all
hCX-positive cells were also mC8-positive and 2.9±2.3% of all
mC8-positive cells were also hCX-positive, with a total fusion rate
of 1.4±0.98%. Co-cultures of MG63 cells and mouse myofibroblast
cells revealed a slightly higher total fusion rate of 1.8±1.5%.
Mouse myofibroblast cells consistently had two copies of mC8. Due
to different gender origins, the U2OS cells each possessed two
copies of hCX, while MG63 cells had one. When MG63 cells and mouse
myofibroblast cells were co-cultured, it was observed that certain
cells contained two copies of hCX and mC8, indicating fusion
between two MG63 cells and one mouse myofibroblast cell. These data
revealed that cancer and myofibroblast cells are able to fuse to
form hybrid cells (Fig. 3).
Discussion
High-grade conventional osteosarcoma cells are
marked by nuclear pleiomorphism, conspicious chromatin
abnormalities and prominent nucleoli. (10). In our study, osteosarcoma also
presented multinucleated osteoclast-like giant cells, which are
more likely to exhibit low differentiation. However, the origin and
mechanisms of multinucleated giant cells in osteosarcoma are poorly
understood. Spontaneous cell fusion in tissue culture or in animal
models has been reported in a wide variety of tumor cells. Similar
to the formation of multinuclear osteoclasts for bone resorption,
cell fusion is likely to be the origin of the multinucleated
osteoclast-like giant cells in osteosarcoma. α-smooth muscle actin
expression was investigated in osteosarcoma and was found to be
markedly expressed in multinucleated osteoclast-like giant cells in
osteosarcoma specimens. α-smooth muscle actin is commonly used as a
marker of myofibroblasts. We therefore speculate that the giant
cells were the result of fusion between osteosarcoma cancer cells
and myofibroblasts.
The tumor microenvironment contains multiple types
of cells, among which myofibroblasts are attracting increasing
attention. Communication between cancer cells and the
microenvironment appears to be a significant determinant of disease
outcome. In colorectal cancer, both α-smooth muscle actin and FAP
expression are associated with poor prognosis, and the two proteins
are expressed in CAF or myofibroblasts (11,12).
Similarly, elevated FAP or SPARC expression correlates with poor
outcome in patients with pancreatic cancer (13,14).
Myofibroblasts play a crucial role in carcinogenesis as recipients
and producers of pro-tumorigenic signals (15,16).
Myofibroblasts form the source of many well-known tumor-promoting
factors, including EGF, TGFβ or HGF. Previous studies have
demonstrated that myofibroblasts affect sensitivity of malignant
cells to chemo- or radiotherapy (17,18)
and have a direct pro-metastatic effect (19).
Since α-smooth muscle was found to express in the
osteosarcoma cell lines U2OS and MG63, largely due to its
mesenchymal origin, it is difficult to identify in cell cultures
using lineage-specific tracking markers. Although previous studies
have demonstrated that myofibroblasts are derived from bone
marrow-derived (20,21), epithelial (22) or endothelial cells (23), local fibroblasts or fibroblast
precursors have been considered to be the major source of
myofibroblasts. Activated primary mouse embryonic fibroblasts were
therefore co-cultured with osteosarcoma cells and the fusion rate
was investigated. This fusion was examined using probes tagging
different chromosomes according to their different genus. Mouse
myofibroblasts were successfully induced by culturing osteosarcoma
and PMEFs in separate chambers of a transwell unit. Increased
expression of α-smooth muscle actin and vimentin in PMEFs was noted
28 days post-induction, while the naïve or negative control
exhibited little or no expression. Our data are in agreement with
those of Mishra et al (24),
who exposed bone marrow-derived mesenchymal stem cells to
tumor-conditioned medium and succeeded in transforming the cells
into activated CAF.
Experimental and clinical studies suggest a
potentially multifaceted involvement of cell fusion in tumor
initiation and progression. Spontaneous cell fusion in vitro
or in vivo has been reported in a variety of tumors. The
frequency of cell fusion can be up to 1% in vivo in
experimental tumor models. Furthermore, fusion efficiency is
proportional to the malignant level of tumor cells (25,26).
Andersen et al (27)
examined the karyotype of renal-cell carcinoma patients who
received allogeneic bone-marrow transplantation from the opposite
gender. The results showed that they had adopted chromosomes from
the bone marrow donors. This is the most definitive and direct
evidence for involvement of cell fusion in human cancer. Despite
the rarity of direct evidence of cell fusion in human cancer,
increasing experimental evidence has indicated a broad involvement
of cell fusion. In non-programmed accidental fusion, the two nuclei
may fuse and lead to aneuploidy and potentially cancer, given the
existence of other genetic alterations. Binuclear and multinuclear
cells are frequently observed in many types of tumors and cell
fusion is likely to be one of several mechanisms generating such
cells. In the present study, equal numbers of osteosarcoma and
mouse myofibroblast cells were mixed and plated in the same flask.
The fusion rate was investigated using FISH 2 days after
co-culture. We provide definite evidence that human ostesosarcoma
cells fuse with myofibroblast cells and result in hybrid cells that
contain chromosome markers characterizing both fusion partners.
Mortensen et al (28)
co-cultured breast cancer and endothelial cells and confirmed the
existence of spontaneous fusion between cancer cells and cells that
may have originated in the microenvironment. Their results are
supported by our data, which indicate the potential significance of
cell fusion in tumor development and progression.
Taken together, we determined that fusion between
cancer cells and myofibroblasts may contribute to observed
multinucleated giant cells in osteosarcoma and we propose that cell
fusion is a novel mechanism for the interaction between cancer
cells and the microenvironment.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Hansen MF, Koufos A, Gallie BL, et al:
Osteosarcoma and retinoblastoma: a shared chromosomal mechanism
revealing recessive predisposition. Proc Natl Acad Sci USA.
82:6216–6120. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter DE, Holden ST, Steel CM, Cohen BB,
Wallace MR and Reid R: A significant proportion of patients with
osteosarcoma may belong to Li-Fraumeni cancer families. J Bone
Joint Surg Br. 74:883–886. 1992.PubMed/NCBI
|
|
4
|
Selvarajah S, Yoshimoto M, Ludkovski O, et
al: Genomic signatures of chromosomal instability and osteosarcoma
progression detected by high resolution array CGH and interphase
FISH. Cytogenet Genome Res. 122:5–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bayani J, Zielenska M, Pandita A, et al:
Spectral karyotyping identifies recurrent complex rearrangements of
chromosomes 8, 17, and 20 in osteosarcomas. Genes Chromosomes
Cancer. 36:7–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogle BM, Cascalho M and Platt JL:
Biological implications of cell fusion. Nat Rev Mol Cell Biol.
6:567–575. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu X and Kang Y: Cell fusion as a hidden
force in tumor progression. Cancer Res. 69:8536–8539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orimo A, Tomioka Y, Shimizu Y, et al:
Cancer-associated myofibroblasts possess various factors to promote
endometrial tumor progression. Clin Cancer Res. 7:3097–3105.
2001.PubMed/NCBI
|
|
9
|
Garfield AS: Derivation of primary mouse
embryonic fibroblast (PMEF) cultures. Methods Mol Biol. 633:19–27.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meister P, Konrad E, Lob G, Janka G, Keyl
W and Stürz H: Osteosarcoma: histological evaluation and grading.
Arch Orthop Trauma Surg. 94:91–98. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujino T, Seshimo I, Yamamoto H, et al:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henry LR, Lee HO, Lee JS, et al: Clinical
implications of fibroblast activation protein in patients with
colon cancer. Clin Cancer Res. 13:1736–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen SJ, Alpaugh RK, Palazzo I, et al:
Fibroblast activation protein and its relationship to clinical
outcome in pancreatic adenocarcinoma. Pancreas. 37:154–158. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Infante JR, Matsubayashi H, Sato N, et al:
Peritumoral fibroblast SPARC expression and patient outcome with
resectable pancreatic adenocarcinoma. J Clin Oncol. 25:319–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki T, Nakamura T, Rebhun RB, et al:
Modification of the primary tumor microenvironment by transforming
growth factor alpha-epidermal growth factor receptor signaling
promotes metastasis in an orthotopic colon cancer model. Am J
Pathol. 173:205–16. 2008. View Article : Google Scholar
|
|
16
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
17
|
Shekhar MP, Santner S, Carolin KA and Tait
L: Direct involvement of breast tumor fibroblasts in the modulation
of tamoxifen sensitivity. Am J Pathol. 170:1546–1560. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koukourakis MI, Giatromanolaki A, Harris
AL and Sivridis E: Comparison of metabolic pathways between cancer
cells and stromal cells in colorectal carcinomas: a metabolic
survival role for tumor-associated stroma. Cancer Res. 66:632–637.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii G, Sangai T, Oda T, et al:
Bone-marrow-derived myofibroblasts contribute to the cancer-induced
stromal reaction. Biochem Biophys Res Commun. 309:232–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Direkze NC, Hodivala-Dilke K, Jeffery R,
et al: Bone marrow contribution to tumor-associated myofibroblasts
and fibroblasts. Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Radisky DC, Kenny PA and Bissell MJ:
Fibrosis and cancer: do myofibroblasts come also from epithelial
cells via EMT? J Cell Biochem. 101:830–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007.PubMed/NCBI
|
|
24
|
Mishra PJ, Mishra PJ, Humeniuk R, et al:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller FR, McInerney D, Rogers C and
Miller BE: Spontaneous fusion between metastatic mammary tumor
subpopulations. J Cell Biochem. 36:129–136. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duelli D and Lazebnik Y: Cell fusion: a
hidden enemy? Cancer Cell. 3:445–448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andersen TL, Boissy P, Sondergaard TE, et
al: Osteoclast nuclei of myeloma patients show chromosome
translocations specific for the myeloma cell clone: a new type of
cancer-host partnership? J Pathol. 211:10–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mortensen K, Lichtenberg J, Thomsen PD and
Larsson LI: Spontaneous fusion between cancer cells and endothelial
cells. Cell Mol Life Sci. 61:2125–2131. 2004. View Article : Google Scholar : PubMed/NCBI
|