Introduction
Hemangioma appear in various parts of the human
body, including the liver, spleen, colon, retroperitoneum, adrenal
glands, soft tissues, extremities, central nervous system and
mediastinum (1). Gastric
hemangioma, a rare tumor occurring mostly in the antrum, was first
described by Lammers in 1893 (2).
It represents approximately 1.7% of all gastric benign tumors and
20% of unknown hemorrhage. In this case report, we present the
clinical presentation, diagnosis and treatment of a cavernous
hemangioma that arises from the gastric fundus.
Case report
The patient is a 65-year-old Chinese female who
suffered a sudden onset of hematemesis with approximately 800 ml
blood loss in a total of 10 h. When she was admitted to hospital,
she was in a state of pre-shock with the complaint of muscle
weakness and dizziness. She had no past history of diabetes,
hypertension, hepatitis or bronchial asthma. The patient did not
take any medication, including over-the-counter non-steroidal
anti-inflammatory drugs (NSAIDs), and was not taking herbal
supplements. On abdominal examination she felt discomfort only in
the upper abdomen, without tenderness, and she was hemodynamically
unstable. Routine blood tests, including hepatic and renal function
tests, revealed a hemoglobin level of 56 g/l, a platelet count of
93×109/l, elevated blood urea nitrogen (BUN) of 10.25
mmol/l, total protein of 53.9 g/l, albumin of 30.8 g/l, serum
creatinine of 60.9 μmol/l, lactate dehydrogenase (LDH) of 199 U/l,
uric acid of 121.0 μmol/l, and an erythrocyte sedimentation rate
(ESR) of 31 mm/h. Based on the patient’s clinical appearance and
the laboratory analysis, she was diagnosed with acute upper
gastrointestinal bleeding and hemorrhagic shock.
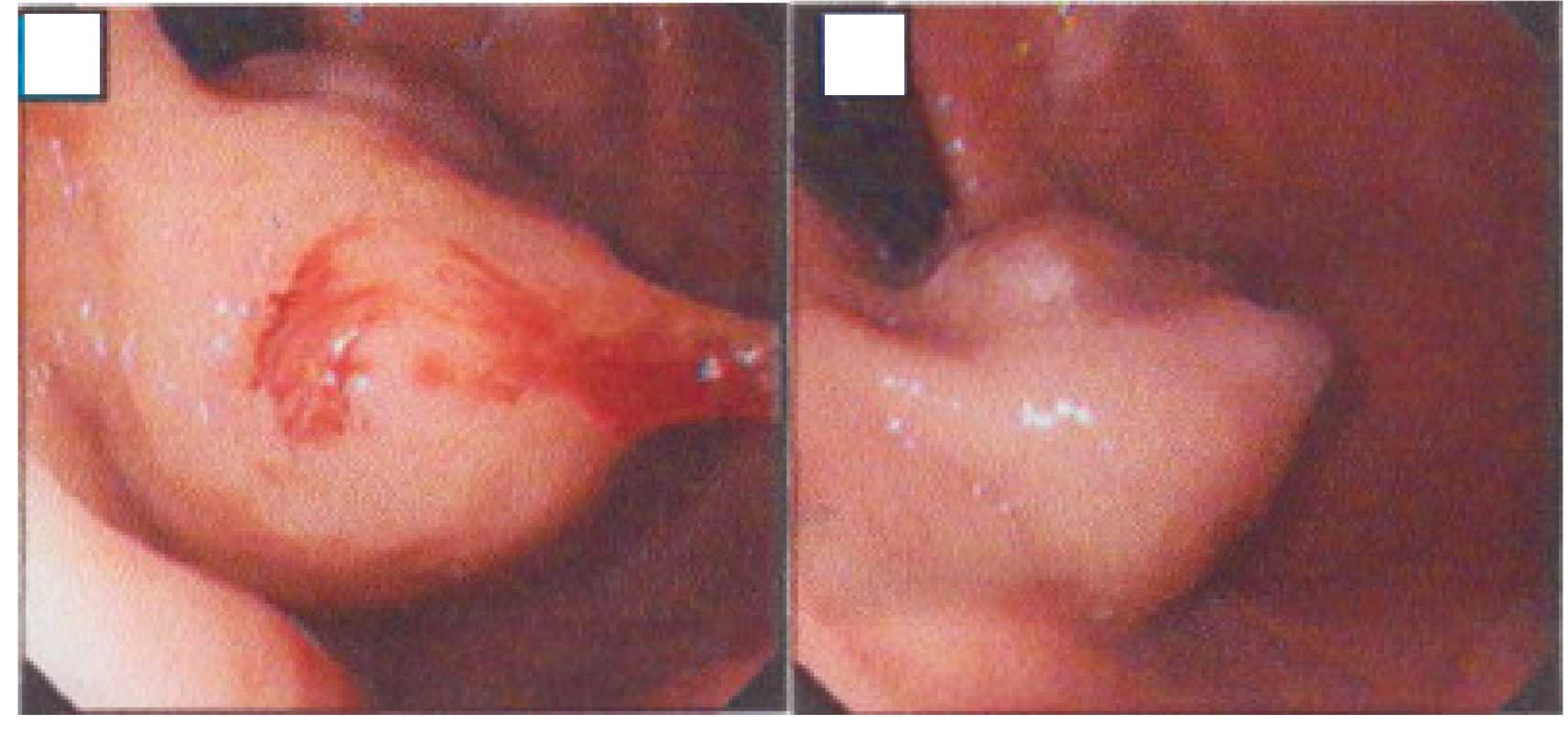
An urgent endoscopy following active resuscitation
revealed a 4×3 cm mass located in the gastric fundus with active
hemorrhage at the surface (Fig.
1A). The bleeding was controlled with an injection of 1 ml
1:10,000 adrenaline (Fig. 1B). The
patient underwent prompt treatment with vigorous intravenous (IV)
rehydration, blood transfusion for worsening anemia, and IV
ranitidine therapy. Following stabilization, abdominal computed
tomography with its periphery enhanced by the contrast material in
the delayed phase revealed the shadow of enhancing linear blood
vessels located in the gastric fundus (Fig. 1). No abnormalities were revealed in
the laboratory data of the tumor markers, which are listed as:
CA19-9, 6.39 KU/l (<35.00); CA242, 3.41 KU/l (<20.00); CA125,
2.27 KU/l (<35.00); CA15-3, 2.28 KU/l (<35.00); NSE, <1.0
ng/ml (<13.00); CEA, 3.25 ng/ml (<5.00); ferritin, 20.10
ng/ml (male<322.00, female<219.00); β-HCG, <0.03 MIU/ml
(<3.00); AFP, 5.68 ng/ml (<20.00); HGH, 2.00 ng/ml
(<7.50). In the laparotomy, a soft mass located in the gastric
fundus was found to vibrate when pressure of the hand was applied.
A proximal gastrectomy was performed. The postoperative period was
uneventful and the patient was discharged 10 days after the
surgery. The final diagnosis of cavernous hemangioma arising from
the gastric fundus was confirmed by postoperative pathological
examination.
Discussion
Kaijser has classified gastrointestinal hemangioma
pathologically as multiple phlebectasia, cavernous hemangioma,
capillary hemangioma and angiomatosis (3).
Abdominal cavernous hemangiomas usually originate in
the liver, but occasionally present in the stomach with the same
origin and mechanism as other cavernous angiomas, which have been
considered as a congenital, benign and abnormal development.
Findings of previous studies have shown that cavernous hemangiomas
are congenital hamartomatous lesions that originate from the
mesodermal remnant tissues. These hemangiomas are composed of large
dilated blood vessels and contain large blood-filled spaces that
are caused by dilation and thickening of the walls of the capillary
loops (4). Due to the thin walls of
blood vessels, gastric cavernous hemangiomas (GCH) are prone to
rupture with rapid blood flow. In this case report, we highlight
the rarity of this lesion and the difficulties in diagnosing it
preoperatively. Most gastrointestinal tract hemangiomas are of the
cavernous type and upper gastrointestinal bleeding is the most
common symptom (5). Gastric
hemorrhages are common clinical emergencies, which often directly
involve the surgeon in diagnosis and treatment; among these, rare
cavernous hemangiomas deserve particular attention.
Endoscopy may serve to establish the diagnosis in
those cases where, due to the hemangioma’s small size or
unfavorable location, radiological examination fails to detect them
(2). Usually, emergency endoscopy
is a means of diagnosis and treatment, but this was not effective
in the present case since the delicate tissue tended to bleed.
Furthermore, it is possible that this characteristic did not allow
a correct tissue sample to be collected for pathological
examination, which yielded a negative result. Radiological
examination suggests the possible diagnosis, and the best
definitive diagnostic procedure is CT scanning and MRI, which
demonstrate the location and relationship of the lesion to
neighboring structures as the preoperative reference of
resectability. The lesion appears either as enhancing linear blood
vessels or caputmedusae, a radial orientation of small vessels that
resemble the hair of Medusa from Greek mythology. However, when the
blood vessel signal is weak, CT scanning and MRI are incapable of
distinguishing GCH from mesochymal tumors. However, GCH may be
misdiagnosed as a stromal tumor. Surgical treatment is a definitive
modality, and recurrence following complete resection has not been
reported thus far. The final diagnosis in the present case was
obtained by definitive histopathology.
In conclusion, we report a rare case of cavernous
hemangioma originating from the gastric fundus. It may cause
diagnostic difficulties preoperatively as biopsy is not an option
due to the submucosal location of the tumor and hemorrhagic factor.
However, in the present case, we were able to obtain the result of
enhancing linear blood vessels by contrast-enhanced CT scanning
prior to the surgery, which assisted us in making a primary
diagnosis. Therefore, abdominal images should be examined on a
regular basis. Although this disease is benign with a lower
recurrence following total resection, we nevertheless suggest the
requirement for long-term follow-up to assess treatment
outcome.
Acknowledgements
L. Zong and P. Chen performed the majority of this
study and wrote the manuscript. G.H. Shi and L. Wang provided the
collection of material from the database.
References
|
1
|
Kinoshita T, Naganuma H and Yajima Y:
Venous hemangioma of the mesocolon. AJR Am J Roentgenol.
169:600–601. 1997. View Article : Google Scholar
|
|
2
|
Bongiovi JJ, Dufly JL and Healdow E:
Gastric hemangioma associated with upper gastrointestinal bleeding.
Arch Surg. 95:93–95. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaijser R: Diagnosis of cavernous
hemangiomas in the digestive tract. Acta Radio. 22:665–686.
1941.
|
|
4
|
Boley SJ, Sammartamo R, Adams A, et al: On
the nature and etiology of vascular ectasias of the colon.
Gastroenterology. 72:650–652. 1987.
|
|
5
|
Wan YL, Eng HL, Lee TY, et al: Computed
tomography of an exophytic gastric hemangioma with torsion and
intratumoral hemorrhage. Clin Imaging. 17:210–212. 1993. View Article : Google Scholar : PubMed/NCBI
|