Introduction
Renal cell carcinoma (RCC) is the most common
malignant neoplasm of the kidney, accounting for 5% of all
malignancies in the USA (1). RCC is
one of the most lethal genitourinary malignancies, exhibiting a 67%
5-year survival rate (1). Kidney-
confined RCC, however, has a relatively favorable prognosis with a
70–90% 5-year survival rate, whereas advanced disease with systemic
metastasis results in a poorer prognosis with a 0–10% 5-year
survival rate (2). As systemic
metastasis usually occurs 3.5–4 asymptomatic years after curative
surgery, the precise prediction of RCC prognosis either
preoperatively or in the immediate postoperative period may aid in
the selection of the optimal postoperative treatment modality
(3). The tumor stage and grade are
the most widely accepted prognostic factors (4); however, an integrative approach that
combines various clinicopathological and molecular prognostic
factors appears to improve predictive accuracy (5).
According to the cancer stem cell (CSC) model, tumor
cells are heterogeneous. Typically, a small population of tumor
cells, termed CSCs, are capable of initiating tumor development due
to their self-renewal capacity, which gives rise to more CSCs and
multipotency, generating the bulk of advanced differentiated tumor
cells (6–8). Disseminated CSCs may induce metastatic
disease and high levels of CSCs within a tumor, resulting in a poor
patient outcome (9). However, the
proportion of CSCs and their prognostic significance have yet to be
defined in malignant tumors of the kidney.
The pentaspan membrane glycoprotein CD133
(Prominin-1), initially identified as a cell surface antigen
specific to hematopoietic stem cells, has been used as a stem cell
marker in various normal and neoplastic human tissues, including
the brain, skin, prostate, pancreas and kidney (10,11).
Recent studies have identified CD133 expression as a poor
prognostic factor, particularly in colorectal cancer patients
(12–14). In the normal kidney, it has been
well established that CD133-positive (CD133+) cells
exhibit stem cell characteristics, including self-renewal,
multipotency and involvement in renal repair (15). By contrast, the expression of CD133
in malignant kidney disease requires further clarification. In this
regard, only one previous study described the expression of CD133
in RCC. CD133 expression was shown in two out of three cases of
clear cell renal cell carcinoma (ccRCC), the most common malignant
neoplasm of the kidney, which accounts for approximately 83% of RCC
cases (16,17).
Oct-4 (also known as OCT3 and POU5F1) is a
POU-domain octamer-binding transcription factor (18). Typically, Oct-4 expression is
confined to the pluripotent cells of embryo and embryonic stem
cells (ESCs). Oct-4 acts as a key regulator for the self-renewal of
normal stem cells and the maintenance of pluripotency in ESCs. The
aberrant expression of Oct-4 in pluripotent cells results in the
initiation of differentiation and the progressive loss of potency
(18–20).
In the present study, in an attempt to define the
prognostic significance of CD133 expression in ccRCC, we evaluated
its expression in 140 cases and examined the relationship between
these findings and clinicopathological prognostic factors. The
proliferative activity and stemness of CD133-expressing cells was
evaluated by double immunohistochemical staining with Ki-67 and
Oct-4, respectively.
Materials and methods
Patients and pathological
examination
This study was approved by the Asan Medical Center
Institutional Review Board. We reviewed the pathological materials
of 167 RCC patients treated by surgical tumor removal during the
year 2005 at the Asan Medical Center, Seoul, Republic of Korea.
Histological slides from all patients were examined for the
diagnostic reassessment of tumor type according to the 2004 World
Health Organization tumor classification and the Fuhrman nuclear
grading system (4,21). A total of 27 patients with non-ccRCC
tumors (chromophobe, 10; unclassified, 8; papillary, 7; and
multilocular cystic, 2) were excluded. In total, 140 patients with
ccRCC were selected for this retrospective study. During the slide
review, the proportion of macro- and microcystic patterns,
sarcomatoid region and necrosis were recorded for each tumor. The
clinical information was obtained through a review of the patient
medical records and radiological findings. Tumors were staged
according to the 2002 Tumor Node Metastasis (TNM) staging system
proposed by the American Joint Committee on Cancer (22).
Immunohistochemistry
Representative formalin-fixed and paraffin-embedded
tumor sections were immunostained using antibodies against CD133
[CD133/1 (AC133); 1:50 dilution; Miltenyi Biotec, Auburn, CA, USA]
and Ki-67 (1:100 dilution; Thermo Scientific, Fremont, CA, USA) or
Oct-4 (OCT3/4, C-10, 1:200 dilution; Santa Cruz Biotechnology,
Santa Cruz, CA, USA). Double immunostaining was feasible, as CD133
exhibits distinct cell membrane expression, and both Ki-67 and
Oct-4 are expressed in the nucleus. Immunohistochemical staining
was performed as previously described, with minor modifications
(23). Endogenous peroxidase
activity and non-specific binding of antibodies were blocked using
hydrogen peroxide and an Ultra-V block kit (LabVision, Fremont, CA,
USA). Antigen retrieval was achieved by steaming the samples in 10
mM citrate buffer (pH 6.0) for 15 min in a microwave apparatus. For
the CD133 and Ki-67 staining, sections were incubated with
anti-Ki-67 antibody for 1 h, followed by incubation in anti-CD133
antibody at 4°C overnight. The incubation of secondary antibodies
and chromogenic detection were performed using the UltraVision LP
large-volume detection system (Thermo Scientific). Double staining
for CD133 and Oct-4 was performed on 45 cases of CD133-expressing
tumors using the Bond Polymer Intense Detection System (Leica
Microsystems, Wetzlar, Germany) as previously described (23). The anti-OCT3 antibody was applied
for the first 15 min, followed by incubation with anti-CD133
antibody for another 15 min at room temperature. Sections were then
treated with post-primary and polymer reagents. Diaminobenzidine
was used, as the chromogen and tissues were counterstained with
hematoxylin. Normal human kidney and testicular embryonal
carcinomas were used as positive controls for CD133 and Oct-4,
respectively. Negative controls omitting primary antibodies were
included.
During the microscopic examination of the
immunostained slides, the sub-cellular localization of CD133 in
tumor cells was recorded and the percentage of CD133+
cells was measured by manual counting. Even a low percentage of
CD133+ tumor cells were regarded as CD133-expressing
tumors since CSCs usually constitute only a small proportion of
cancer cells, although the frequency differs with the tumor type
and cell assay methods employed (7,8,24). The
Ki-67 labeling index was measured by calculating the mean
percentage of Ki-67+ cells in three representative
fields at a high magnification (x400). Strong nuclear staining was
regarded as positive for Oct-4 expression. Oct-4 expression was
analyzed in 40 cases of CD133-expressing ccRCC, as 5 of the tissue
samples were lost during immunohistochemical staining.
Statistical analysis
Data were analyzed using the SPSS 12.0K software
(SPSS, Inc., Chicago, IL, USA). Crosstabs, the Pearson’s
Chi-square, Fisher’s exact, and the Kruskal-Wallis tests, as well
as a calculation of Pearson’s correlation coefficient were used as
appropriate. The Cox’s proportional hazard model was employed in a
multivariate analysis. P<0.05 was regarded as statistically
significant.
Results
Clinicopathological characteristics
The clinicopathological characteristics of the 140
ccRCC cases are shown in Table I.
The ages ranged from 21 to 80 years (median 54), with a
male-to-female ratio of 2.5:1. Seven cases had previously undergone
contralateral nephrectomy due to RCC. Among the 7 cases, 1 case
with cerebellar hemangioblastoma was diagnosed as von Hippel-Lindau
disease. The mean tumor size was 4.7 cm in the grexatest dimension
(range 0.6–17 cm). Most tumors (112 cases, 80%) were confined to
the kidney. The remaining 28 cases revealed direct invasion to the
perinephric fat tissue (24 cases) and/or gross extension into the
renal vein (15 cases). Regional lymph node dissection was performed
in 50 cases and lymph node metastasis was identified in 3 cases.
Distant metastasis was present in 24 cases; as single-organ
metastasis in 16 and as multi-organ metastases in 8 cases. Fifteen
cases presented with metastatic disease at the time of initial
diagnosis, and metastasis developed during the postoperative
follow-up period in the remaining 9 cases. The most common
metastatic site was the lung (19 cases), followed by bone (4
cases), liver (3 cases), brain (2 cases), supraclavicular lymph
node (1 case), stomach (1 case), soft tissue (1 case) and adrenal
gland (1 case). During the median follow-up period of 43.6 months
(range 3.7–54.6), 13 patients succumbed and in 4 cases this was due
to ccRCC.
 | Table IClinicopathological characteristics
of 140 patients with clear cell renal cell carcinoma. |
Table I
Clinicopathological characteristics
of 140 patients with clear cell renal cell carcinoma.
| Variable | No. of patients
(%) |
|---|
| Age (years) |
| ≤40 | 18 (12.9) |
| 41–60 | 84 (60.0) |
| ≥61 | 38 (27.1) |
| Gender |
| Male | 100 (71.4) |
| Female | 40 (28.6) |
| Nephrectomy |
| Radical | 104 (74.3) |
| Partial | 36 (25.7) |
| Laterality |
| Right | 69 (49.3) |
| Left | 71 (50.7) |
| Tumor size
(cm) |
| ≤4.0 | 72 (51.4) |
| 4.1–7.0 | 47 (33.6) |
| >7.0 | 21 (15.0) |
| Fuhrman grade |
| 1 | 4 (2.9) |
| 2 | 53 (37.9) |
| 3 | 51 (36.4) |
| 4 | 32 (22.8) |
| T stage |
| 1 | 104 (74.3) |
| 2 | 8 (5.7) |
| 3 | 28 (20.0) |
| N stage |
| X | 90 |
| 0 | 47 (94.0) |
| 1 | 1 (2.0) |
| 2 | 2 (4.0) |
| M stage |
| X | 0 |
| 0 | 116 (82.9) |
| 1 | 24 (17.1) |
Microscopically, the majority of the cases (104
cases, 74.3%) were Fuhrman nuclear grade 2 or 3. The tumors
revealed the characteristic histological features of ccRCC:
alveolar and/or acinar arrangements of tumor cells with a
well-developed network of thin-walled blood vessels and clear to
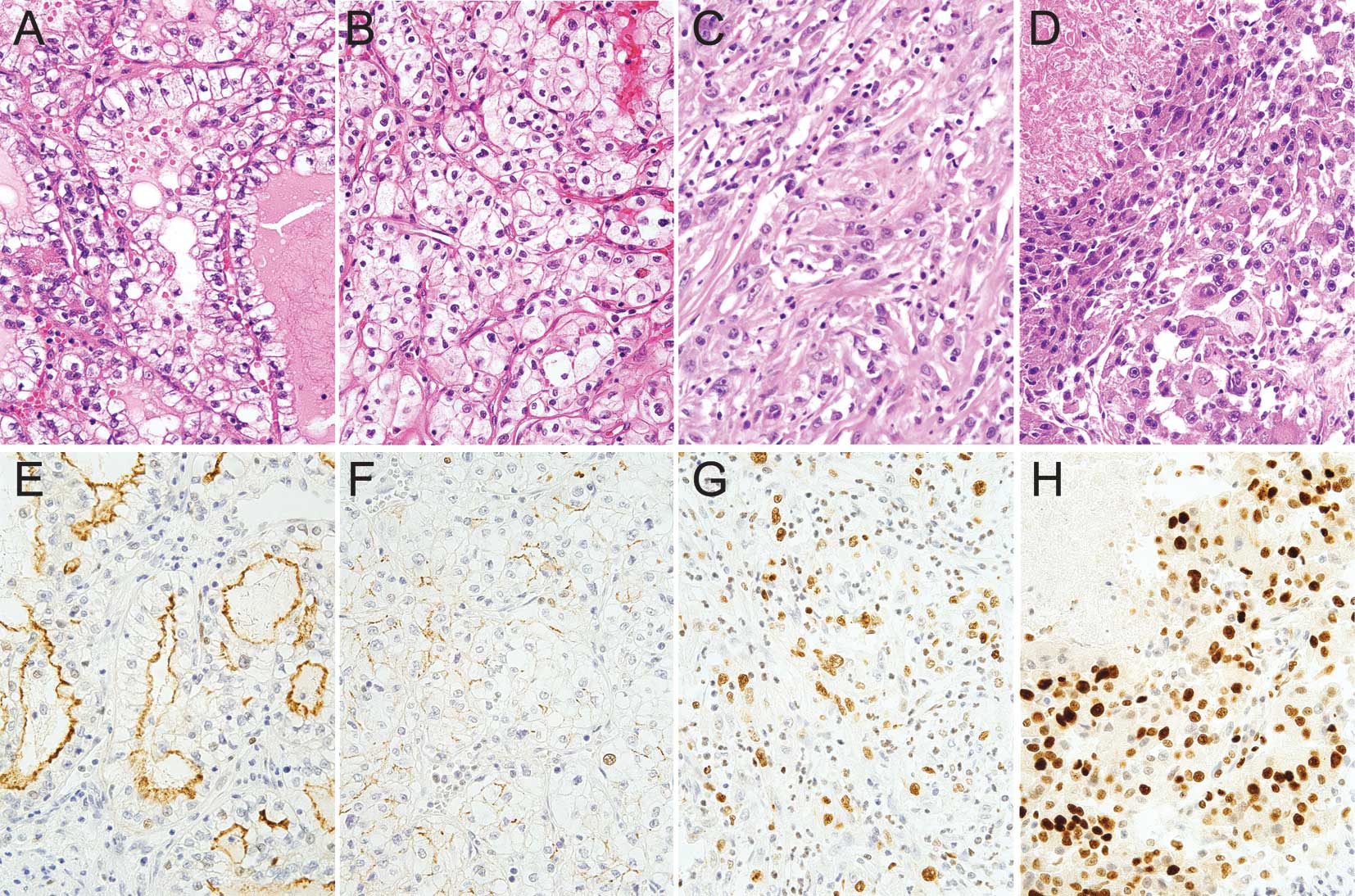
eosinophilic cytoplasm (Fig. 1A). A
macro- or microcystic pattern of dilated alveolar and acinar
structures was noted in 101 patients (72.1%) with a wide range of
the pattern (1–100%; mean 42%). The two poor prognostic
histological features, tumor necrosis and sarcomatoid changes, were
found in 25 (17.9%) and 6 cases (4.3%), respectively. The necrotic
region ranged from 1 to 80% of tumors (mean 24.7%). The sarcomatoid
region constituted 5–90% (mean 40.8%) of the tumor sections
examined. The mean Ki-67 labeling index of the 140 cases was 18%,
with a range from 1 to 90%.
CD133 expression in ccRCC with favorable
clinicopathological characteristics
CD133 expression was present in 45 cases (32.1%),
with a wide range of expression (1–100%; mean 36.9%). CD133 was
significantly expressed in the apical or apicolateral membrane of
tumor cells in the macro-/microcystic region (Fig. 1A and E). The macrocystic region
showed a greater CD133 intensity than the microcystic region (data
not shown). In the alveolar pattern where the luminal space was
indistinct, CD133 was expressed at the cell membrane of tumor cells
with a weaker intensity compared to that in macro-/microcystic
regions (Fig. 1B and F). The CD133
expression revealed a positive correlation with the proportion of
the macro-/microcystic region (p<0.001).
The relationship between CD133 expression and the
clinicopathological prognostic factors are shown in Table II. The high-level expression of
CD133 was more prevalent in males compared to females (p=0.001).
The kidney-confined ccRCC revealed a tendency to express more CD133
than locally advanced cases (p=0.057). Furthermore, non-metastatic
ccRCC revealed higher CD133 expression levels than cases with
distant metastasis (p=0.047), although this correlation was not
significant in the multivariate analysis with regard to stage and
Fuhrman grade. CD133 expression in ccRCC showed a negative
correlation with the proportion of sarcomatoid change (p<0.001).
In ccRCCs containing sarcomatoid change, CD133 expression was not
identified in the sarcomatoid region, and only rare
CD133+ tumor cells were present in non-sarcomatoid
regions (Fig. 1C and G). ccRCC
cases with accompanying necrosis tended to express lower levels of
CD133 than those without necrosis (p=0.081) (Fig. 1D and H). ccRCCs with a significant
tumor necrosis occupying >10% of the tumor volume were observed
in 18 cases, and 14 of these (77.8%) did not express CD133 in the
tumor. The remaining 4 cases showed a low expression of CD133
(3–20%) in the viable tumor, with no increased expression in the
perinecrotic region. No significant correlation was evident between
the CD133 expression levels and the Fuhrman grade, tumor size or
nodal metastasis status (Table
II).
 | Table IICorrelation between CD133 expression
and clinicopathological features in 140 patients with clear cell
renal cell carcinoma. |
Table II
Correlation between CD133 expression
and clinicopathological features in 140 patients with clear cell
renal cell carcinoma.
| Variable | Mean percentage of
CD133 expression | p-value |
|---|
| Gender |
| Male | 16.4 | 0.001 |
| Female | 0.5 | |
| Fuhrman grade |
| 1 and 2 | 9.6 | 0.551 |
| 3 and 4 | 13.5 | |
| Tumor size
(cm) |
| ≤4.0 | 12.9 | 0.833 |
| 4.1–7.0 | 9.6 | |
| >7.0 | 13.6 | |
| T stage |
| T1 and T2 | 12.4 | 0.057 |
| T3 and T4 | 9.8 | |
| LN metastasis |
| Absent | 11.7 | 0.236 |
| Present | 0.0 | |
| Distant
metastasis |
| Absent | 12.6 | 0.047 |
| Present | 8.1 | |
| Necrosis |
| Absent | 13.3 | 0.081 |
| Present | 5.2 | |
| Sarcomatoid
component |
| Absent | 12.4 | <0.001 |
| Present | 0.5 | |
Although the disease-related survival did not show
any significant correlation with CD133 expression due to a short
follow-up period, an analysis of the 4 cases that resulted in death
from ccRCC was performed. These 4 cases were characterized by a
lack of CD133 expression, Fuhrman grade 4, a small amount of
macro-/microcystic region (5–25%), lung metastasis and tumor
necrosis. Tumor extension into perinephric fat tissue and lymphatic
invasion were also noted in 3 of the 4 patients.
Low proliferative activity of
CD133-expressing ccRCC
The Ki-67 labeling index was higher in ccRCCs that
did not express CD133 (mean 19.5%) than in those that did (mean
14.7%), although this difference was not statistically significant
(p=0.097). CD133+ tumor cells lining the macrocystic
spaces rarely expressed Ki-67, whereas sarcomatoid and perinecrotic
regions, where the CD133 expression was low or absent, revealed
high expression levels of Ki-67 (Fig.
1A-H). The Ki-67 labeling index of CD133+ tumor
cells was examined in 45 cases of CD133-expressing ccRCC to measure
the proliferative activity of CD133+ cells. The mean
Ki-67 labeling index of CD133+ cells was 7.7%, which was
lower than that of the average Ki-67 labeling index of all 140
ccRCC cases (18%) and of 95 cases of CD133 non-expressing ccRCCs
(19.5%).
Low expression of Oct-4 in
CD133+ tumor cells in CD133- expressing ccRCC
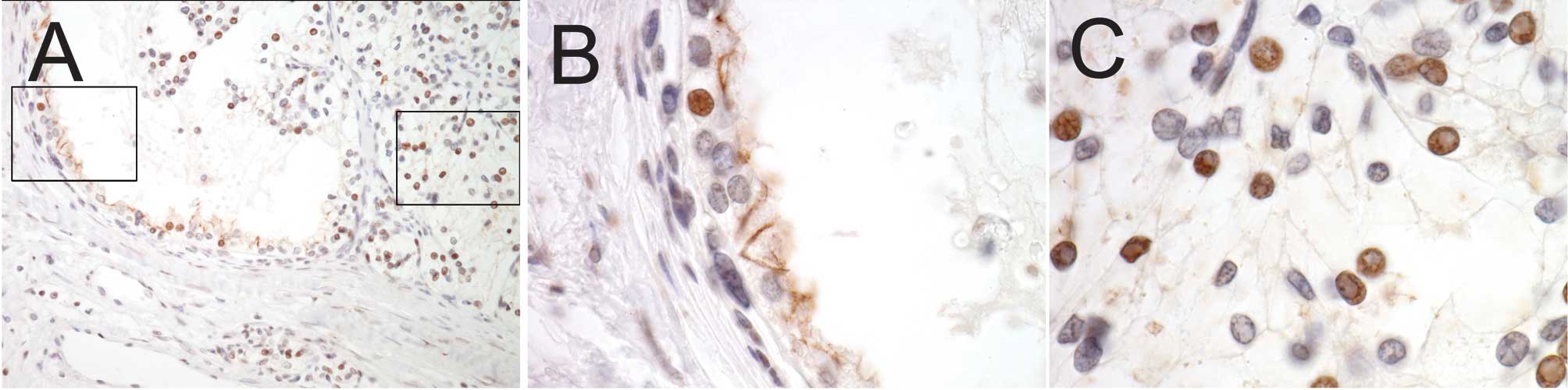
Oct-4 expression was investigated in
CD133-expressing tumors to examine the stemness of
CD133+ cells. Being a transcription factor, Oct-4 was
expressed in the nucleus, as expected (Fig. 2A-C). CD133+ tumor cells
rarely expressed Oct-4 (Fig. 2B),
whereas significant Oct-4 expression was observed in
CD133- tumor cells (Fig.
2C).
Discussion
In the present study, CD133 expression was increased
in non-metastatic ccRCC and in those without a sarcomatoid
component. CD133-expressing ccRCC exhibited a lack of necrosis and
low proliferative activity.
The high expression of CD133 in ccRCC with favorable
clinicopathological characteristics was an unexpected result, as
CD133 has been applied as a putative CSC marker in numerous
malignant tumors, and its high expression has been considered a
poor prognostic factor. CD133 is currently applied as a
cell-surface marker to isolate CSCs in malignant tumors of the
colon, brain, lung, prostate, pancreas, liver, stomach and uterus
(11,25–27). A
CD133+ sub-population in colon cancer was highly
enriched with CSCs that were capable of forming tumors in serial
xenotransplantation (self-renewal) and reproducing the
heterogeneous morphological and antigenic patterns of the original
tumor (multipotency) (28,29). Previous studies have shown that CSC
has poor prognostic significance and therapeutic relevance. The
proportion of CD133+ cells in the glioma of the brain
was an independent risk factor for tumor regrowth and was thus
negatively correlated with progression-free and overall survival
(30). In colon cancer, high levels
of CD133 expression were associated with a shorter relapse-free
interval and poor overall survival (31). Furthermore, CSCs appear to be highly
resistant to chemoradiation therapy (26,32–34).
In an atypical teratoid/rhabdoid tumor of the brain, high levels of
CD133+ cells were positively correlated with
radioresistance (35). A high-level
expression of CD133 in residual colon cancer tissue following
radiation therapy predicted poor disease-free and overall survival
rates (13). These findings suggest
that the estimation of the CSC proportion within a tumor has
clinical relevance for CSC-targeted therapy. In addition, these
studies indicate that successful tumor control requires the
eradication of CSCs during cancer treatment. This issue has been
actively studied to develop targeted therapy for CSCs. For example,
an anti-CD133 antibody-drug complex has been shown to conjugate
effectively and inhibit the growth of the Hep3B hepatocellular
carcinoma cell line in vitro and in vivo (36).
However, it appears that CD133 expression does not
predict poor prognosis for every tumor type. In ovarian cancer and
malignant melanoma patients, no differences were observed in the
overall survival between tumors with negative CD133 and those
expressing the marker (37,38). In the present study, CD133
expression was increased in ccRCC patients showing favorable
clinicopathological characteristics, although disease-related
survival was not significantly correlated with CD133 levels, mainly
due to a short follow-up period. The indolent nature of
CD133+ RCC cells is supported by previous studies
demonstrating that CD133+ cells isolated from human RCCs
were incapable of forming tumors when transplanted independently
into severe combined immunodeficiency (SCID) mice, although these
cells significantly increased tumor development and growth when
co-transplanted with RCC cells (39). Taken together, these data indicate
that CD133 expression levels may vary with regard to prognostic
significance depending on the tumor type.
In contrast to the widely held theory that CSCs are
relatively undifferentiated, our results demonstrate that CD133 is
highly expressed in the more differentiated macro-/microcystic
region of ccRCCs with a low expression of Oct-4 in
CD133+ tumor cells, whereas CD133 expression was absent
in the dedifferentiated sarcomatoid region. CD133 expression was
found to be reduced in those regions with an alveolar growth
pattern, where the luminal structure was inconspicuous. The high
expression of CD133 in the macro-/microcystic tumor structures and
the favorable prognosis for ccRCC may be explained in three ways.
First, CD133 is a cell membrane protein that is usually expressed
in plasma membrane protrusions, including microvilli (40–44).
Given that microvilli are abundant in differentiated cells,
including the proximal tubules of the adult kidney, this may
explain the absent or low expression levels of CD133 in the
undifferentiated sarcomatoid region and less differentiated
alveolar regions. However, this observation does not fully explain
the high expression levels of CD133 in the macro-/microcystic
regions of ccRCC since not all these regions expressed the marker,
and a small number of cells in the proximal tubules expressed CD133
in normal kidney tissue remote from the tumor. Second, since CD133
is a glycoprotein, its expression may reflect aberrant
glycosylation or a variation in glycosylation status according to
the degree of tumor differentiation. It has been well established
that the glycosylation of CD133 varies with cellular
differentiation and malignant transformation, and the anti-CD133
antibody used in this study identifies only the undefined
glycosylated epitopes (10,16,40).
Finally, CD133+ cells may represent a heterogeneous
population of tumor cells that contains a small number of CSCs and
a large number of differentiated non-CSC cells. Our
immunohistochemical staining for CD133 and Oct-4 supports this
hypothesis, as the majority of CD133+ cells did not
express Oct-4, although a small number of cells co-expressed CD133
and Oct-4. Taken together, these data suggest that the proportion
of CD133-expressing cells in ccRCC identified through
immunohistochemical staining may not accurately reflect the overall
proportion of CSCs. Therefore, CD133 as a single marker is not
sufficient for CSC identification in ccRCC, and other, more
specific CSC markers need to be developed.
In the present study, the ccRCCs from male patients
revealed higher CD133 expression levels than those from female
patients. Although the impact of gender on stem cell populations
should be defined, previous studies have shown that the number of
circulating CD133+ endothelial progenitor cells is
higher in pre-menopausal females compared to postmenopausal ones
and age-matched males (45,46). Therefore, the gender difference in
CD133 expression in ccRCC and its clinical significance require
further clarification.
Acknowledgements
This study was supported by a grant (no. 2008-388)
from the Asan Institute for Life Sciences, Seoul, Republic of
Korea.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Wein AJ, Kavoussi LR, Novick AC, Partin AW
and Peters CA: Campbell-Walsh Urology. 9th edition. Saunders
Elsevier; Philadelphia: 2007
|
|
3
|
Levy DA, Slaton JW, Swanson DA and Dinney
CP: Stage specific guidelines for surveillance after radical
nephrectomy for local renal cell carcinoma. J Urol. 159:1163–1167.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eble JN, Epstein JI and Sesterhenn IA:
World Health Organization classification of tumors. IARC Press;
Lyon: 2004
|
|
5
|
Kim HL, Seligson D, Liu X, et al: Using
protein expressions to predict survival in clear cell renal
carcinoma. Clin Cancer Res. 10:5464–5471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells – perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006.
|
|
8
|
Burkert J, Wright NA and Alison MR: Stem
cells and cancer: an intimate relationship. J Pathol. 209:287–297.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells – an
integrated concept of malignant tumour progression. Nat Rev Cancer.
5:744–749. 2005.PubMed/NCBI
|
|
10
|
Miraglia S, Godfrey W, Yin AH, et al: A
novel five-transmembrane hematopoietic stem cell antigen:
isolation, characterization, and molecular cloning. Blood.
90:5013–5021. 1997.PubMed/NCBI
|
|
11
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: Prognostic significance of the cancer stem cell markers
CD133, CD44, and CD166 in colorectal cancer. Cancer Invest.
27:844–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Chen ZG, Du CZ, Wang HW, Yan L and
Gu J: Cancer stem cell marker CD133+ tumour cells and clinical
outcome in rectal cancer. Histopathology. 55:284–293. 2009.
|
|
14
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bussolati B, Bruno S, Grange C, et al:
Isolation of renal progenitor cells from adult human kidney. Am J
Pathol. 166:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Florek M, Haase M, Marzesco AM, et al:
Prominin-1/CD133, a neural and hematopoietic stem cell marker, is
expressed in adult human differentiated cells and certain types of
kidney cancer. Cell Tissue Res. 319:15–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheville JC, Lohse CM, Zincke H, Weaver AL
and Blute ML: Comparisons of outcome and prognostic features among
histologic subtypes of renal cell carcinoma. Am J Surg Pathol.
27:612–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng L, Sung MT, Cossu-Rocca P, et al:
OCT4: biological functions and clinical applications as a marker of
germ cell neoplasia. J Pathol. 211:1–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones TD, Ulbright TM, Eble JN and Cheng
L: OCT4: a sensitive and specific biomarker for intratubular germ
cell neoplasia of the testis. Clin Cancer Res. 10:8544–8547. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lengner CJ, Welstead GG and Jaenisch R:
The pluripotency regulator Oct4: a role in somatic stem cells? Cell
Cycle. 7:725–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greene FL and Fleming ID: AJCC Cancer
Staging Manual. 6th edition. Springer-Verlag; Chicago: 2002,
View Article : Google Scholar
|
|
23
|
Kim K, Lee KM, Han DJ, Yu E and Cho YM:
Adult stem cell-like tubular cells reside in the corticomedullary
junction of the kidney. Int J Clin Exp Pathol. 1:232–241.
2008.PubMed/NCBI
|
|
24
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tirino V, Camerlingo R, Franco R, et al:
The role of CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rutella S, Bonanno G, Procoli A, et al:
Cells with characteristics of cancer stem/progenitor cells express
the CD133 antigen in human endometrial tumors. Clin Cancer Res.
15:4299–4311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
28
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
29
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeppernick F, Ahmadi R, Campos B, et al:
Stem cell marker CD133 affects clinical outcome in glioma patients.
Clin Cancer Res. 14:123–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Artells R, Moreno I, Diaz T, et al: Tumour
CD133 mRNA expression and clinical outcome in surgically resected
colorectal cancer patients. Eur J Cancer. 46:642–649. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiou SH, Kao CL, Chen YW, et al:
Identification of CD133-positive radioresistant cells in atypical
teratoid/rhabdoid tumor. PLoS One. 3:e20902008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith LM, Nesterova A, Ryan MC, et al:
CD133/prominin-1 is a potential therapeutic target for
antibody-drug conjugates in hepatocellular and gastric cancers. Br
J Cancer. 99:100–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrandina G, Martinelli E, Petrillo M, et
al: CD133 antigen expression in ovarian cancer. BMC Cancer.
9:2212009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piras F, Perra MT, Murtas D, et al: The
stem cell marker nestin predicts poor prognosis in human melanoma.
Oncol Rep. 23:17–24. 2010.PubMed/NCBI
|
|
39
|
Bruno S, Bussolati B, Grange C, et al:
CD133+ renal progenitor cells contribute to tumor
angiogenesis. Am J Pathol. 169:2223–2235. 2006.
|
|
40
|
Corbeil D, Roper K, Hellwig A, et al: The
human AC133 hematopoietic stem cell antigen is also expressed in
epithelial cells and targeted to plasma membrane protrusions. J
Biol Chem. 275:5512–5520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fargeas CA, Joester A, Missol-Kolka E,
Hellwig A, Huttner WB and Corbeil D: Identification of novel
Prominin-1/CD133 splice variants with alternative C-termini and
their expression in epididymis and testis. J Cell Sci.
117:4301–4311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giebel B, Corbeil D, Beckmann J, et al:
Segregation of lipid raft markers including CD133 in polarized
human hematopoietic stem and progenitor cells. Blood.
104:2332–2338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weigmann A, Corbeil D, Hellwig A and
Huttner WB: Prominin, a novel microvilli-specific polytopic
membrane protein of the apical surface of epithelial cells, is
targeted to plasmalemmal protrusions of non-epithelial cells. Proc
Natl Acad Sci USA. 94:12425–12430. 1997. View Article : Google Scholar
|
|
44
|
Corbeil D, Roper K, Fargeas CA, Joester A
and Huttner WB: Prominin: a story of cholesterol, plasma membrane
protrusions and human pathology. Traffic. 2:82–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rousseau A, Ayoubi F, Deveaux C, et al:
Impact of age and gender interaction on circulating endothelial
progenitor cells in healthy subjects. Fertil Steril. 93:843–846.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lemieux C, Cloutier I and Tanguay JF:
Menstrual cycle influences endothelial progenitor cell regulation:
a link to gender differences in vascular protection? Int J Cardiol.
136:200–210. 2009. View Article : Google Scholar : PubMed/NCBI
|