Introduction
erbB-2 (HER2, Neu) is a receptor tyrosine kinase of
the EGFR family (1,2). erbB-2 is amplified/overexpressed in
approximately 30% of primary human breast cancers, and has been
associated with poor prognosis and therapeutic resistance (3,4).
erbB-2 overexpression and/or activation induces the subsequent
activation of a plethora of signaling pathways, including those
that are mediated by MAP kinase, PI3 kinase, and the STAT family of
transcription factors (5,6). These activated signaling pathways
ultimately increase cell proliferation, reduce apoptosis, and
induce cell transformation (7).
erbB-2-associated carcinogenesis has been extensively studied.
However, since erbB-2 activation elicits signaling in diversified
downstream pathways, the precise mechanisms involved in
erbB-2-mediated carcinogenesis remain unclear.
erbB-2 transgenic mouse models were utilized in
numerous studies in order to understand erbB-2-mediated
carcinogenesis (8). The association
of erbB-2 overexpression with genomic instability is of marked
interest. Montagna et al reported that tumor cell lines from
transgenic mice overexpressing constitutively activated mutant
erbB-2/Neu exhibited recurrent deletions of chromosome 4,
amplification of chromosome 11, and abnormalities in the centrosome
(9). In previous studies, we
detected a number of cytogenetic lesions using mammary tumor cell
lines derived from wild-type (wt) MMTV-erbB-2/Neu transgenic mice.
The most common chromosomal abnormalities were loss of mouse
chromosome 4 and gain of chromosome 10 (10). We also revealed that tumors and
tumor cell lines derived from MMTV-erbB-2 mice treated with E2 or
soy appeared to have more cytogenetic lesions (10). These results suggest that the
chromosomal imbalance in the erbB-2-associated cytogenetic changes
are affected by erbB-2 signaling activity (mutant or activated
erbB-2 induces stronger carcinogenic activity) and hormonal
conditions.
Although previous studies suggest a correlation
between erbB-2 overexpression and genomic instability, and since
the patterns of cytogenetic changes vary with individual model
systems, the effects of erbB-2 overexpression on genomic
instability in mammary tumor development require further
investigation. The purpose of the present study was to examine the
cytogenetic patterns in mammary tumor cells derived from
multiparous and virgin control wt MMTV-erbB-2 mice. Since the MMTV
promoter is sensitive to pregnancy hormones such as prolactin,
multiparity enhances MMTV-mediated transcription (11), and parous MMTV-erbB-2 mice usually
exhibit accelerated mammary tumor development due to the enhanced
overexpression of MMTV-erbB-2 during pregnancy and lactation
(12). Therefore, this model system
allowed us to examine the effects of erbB-2 overexpression and
increased activation on chromosomal imbalance.
Materials and methods
Animals and tumor samples
The animals used in this study were MMTV-erbB-2 mice
(wt erbB-2 mice) from Jackson Laboratory (Bar Harbour, ME, USA).
Following a protocol approved by the university's Institutional
Animal Care and Use Committee (IACUC), the mice were fed a lifelong
AIN-93G diet and housed in the barrier facility at the University
of Oklahoma Health Sciences Center. Mammary tumors developed from
control virgin MMTV-erbB-2 mice and from mice with three full-term
pregnancies. The tumors were harvested once they reached 1
cm3 in diameter. Five primary tumors from five different
mice in each group were collected. For the samples used to evaluate
erbB-2 expression, mammary tissues were obtained from control
virgin mice and from the parous mice one day after parturition in
the third pregnancy (at 24 weeks).
Western blot analysis
Cell lysates were prepared from the mammary tissues
of virgin control and parous mice one day after parturition.
Protein lysates (50 μg) from each sample were separated using a 10%
SDS-PAGE gel and transferred to nitrocellulose membrane. The
membrane was probed with antibodies against erbB-2 and actin (Santa
Cruz Biotechnology, Santa Cruz, CA, USA). Following incubation with
secondary antibodies and subsequent washing, the specific bands
were visualized with an ECL kit (Thermo Fisher Scientific, Miami,
OK, USA).
Immunohistochemistry
Immunohistochemistry was performed as previously
reported (13). Briefly, mammary
tissues from virgin control or parous mice were fixed in
formaldehyde. Tissue sections were deparaffinized and rehydrated.
Non-specific binding sites were blocked with 10% normal horse serum
and incubated overnight with anti-erbB-2 monoclonal antibody.
Following incubation with biotinylated goat anti-mouse antibody,
the signals were visualized using the ABC kit (Vector Lab,
Burlingame, CA, USA).
Primary cell culture and sample
preparation
Tumors were aseptically removed from the animals and
immediately processed during the tumor harvest (10,13).
Tumor tissues were minced with scissors and then washed with PBS.
The tissue explants were cultured in a flask containing DMEM/F12
media supplemented with 10% FBS and penicillin/streptomycin.
Outgrowth from the explants was trypsinized and isolated as primary
cell lines. After removing the explants, cells in the second
passage were used for cytogenetic analysis.
Chromosome preparation and karyotype
analysis
Primary mammary tumor cells were arrested in
metaphase by adding colcemid (final concentration of 0.02 μg/ml)
(Gibco, Carlsbad, CA, USA) to the culture media for 1 h. The cells
were harvested according to the standard protocols in our
laboratory. Chromosomes were treated and stained using
trypsin-Giemsa banding (GTG-banding). At least 15 metaphase cells
were analyzed and karyotyped from each cell line.
Fluorescent in situ hybridization
(FISH)
The whole chromosome painting (WCP) probe for
chromosome 5 was purchased from commercial sources (Cambio). FISH
was performed according to the manufacturer's instructions. Twenty
metaphase cells were analyzed for each cell line.
Results
Enhanced overexpression of erbB-2 in
parous mammary tissues
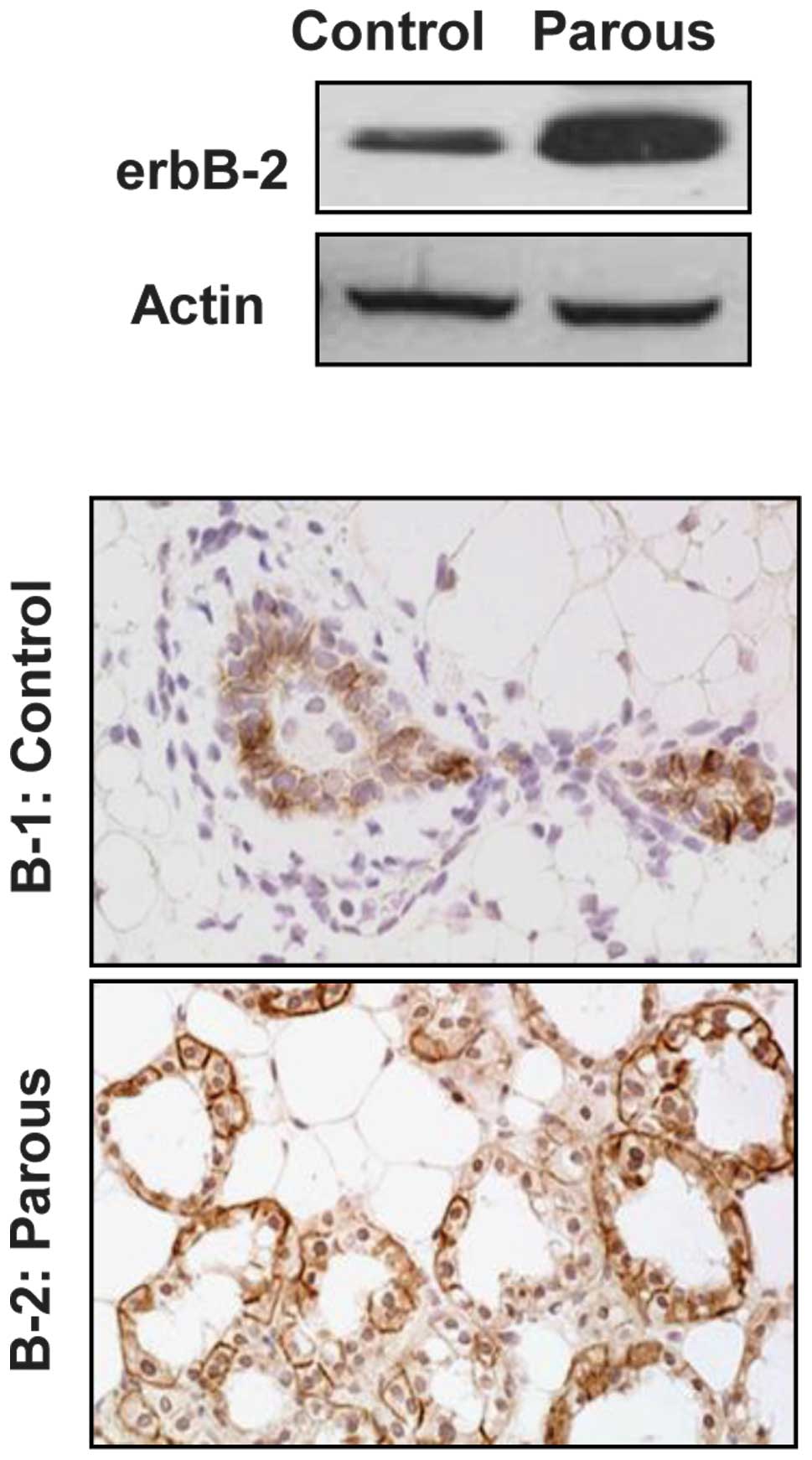
To assess the effect of pregnancy on MMTV-induced
erbB-2 expression, the expression of erbB-2 was examined in mammary
tissues from MMTV-erbB-2 mice one day after parturition in the
third pregnancy, and compared to that of control virgin mice at the
same age (24 weeks). Fig. 1A shows
that erbB-2 protein levels in the parous mammary tissues were
markedly higher than those in the control. The immunohistochemical
examination indicated that erbB-2 was overexpressed in the luminal
mammary epithelial cells. In contrast to the virgin mice, enhanced
overexpression of erbB-2 was detected in all of the alveolar
epithelial cells of the newly parturated mice (Fig. 1B). The increased erbB-2 levels and
the percentage of positive cells in the parous mammary tissues may
contribute to the cytogenetic alterations detected in the
subsequent experiments.
Cytogenetic alterations in mammary tumor
cells from control and parous MMTV-erbB-2 transgenic mice
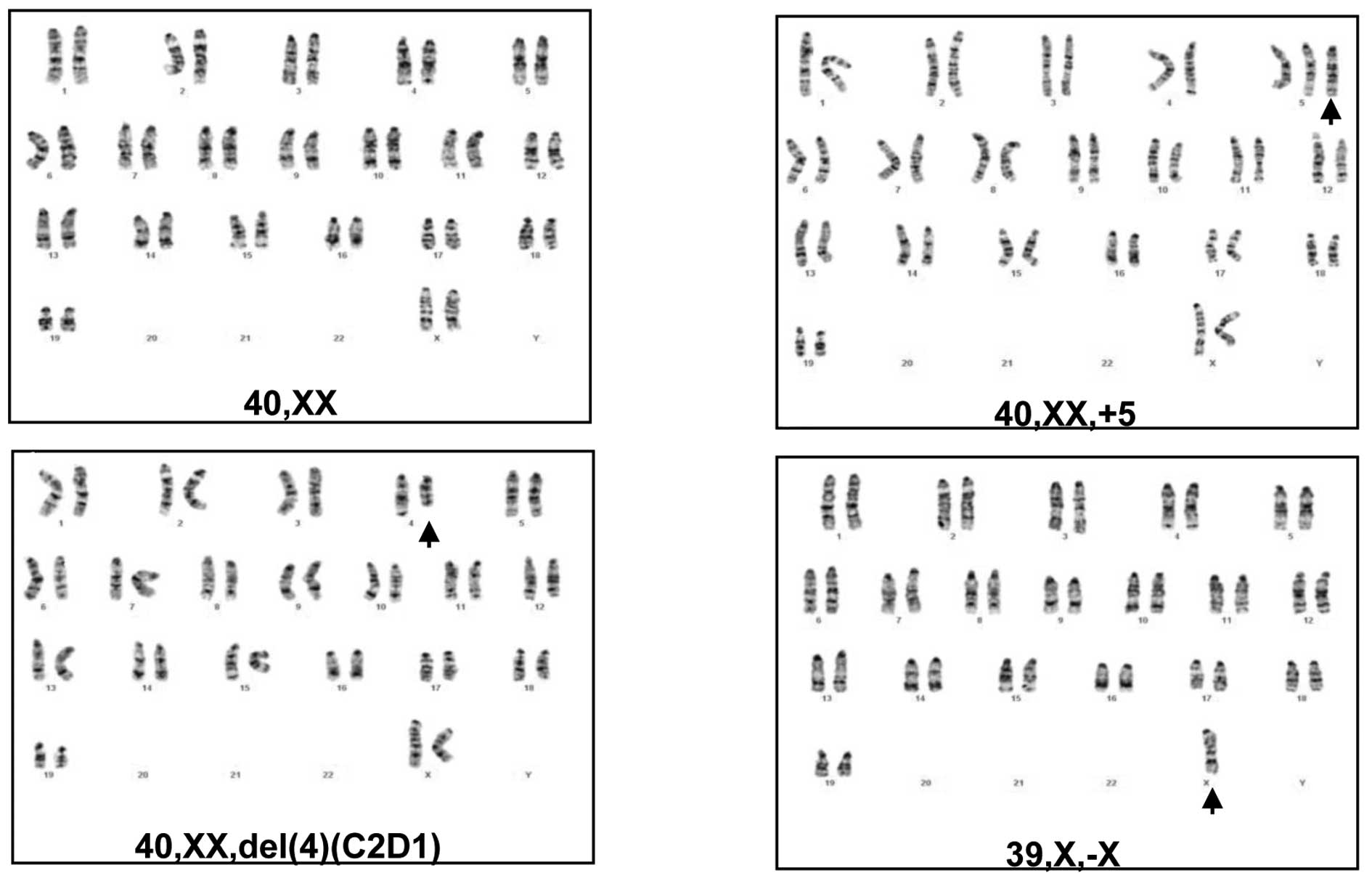
To determine whether the mammary tumors in parous
MMTV-erbB-2 mice acquired more cytogenetic lesions, the chromosomal
changes were characterized in primary mammary tumor cells derived
from control virgin MMTV-erbB-2 mice and parous mice with three
full-term pregnancies. In each group, five tumor cell lines from
five different mice were analyzed using G-banded karyotyping. As
shown in Table I, four of the five
cell lines from the virgin control mice had a ‘normal’ karyotype.
One of these cell lines (VMT-3) contained a chromosome 4 deletion
and trisomy 5. In contrast, each of the tumor cell lines from the
parous mice exhibited significant chromosomal changes, indicating
that the tumors developing from parous mice acquired more genetic
lesions. Specifically, trisomy 5 (Fig.
2B) was detected in 3 of the 5 cell lines (PMT-3, 4 and 5); the
deletion of chromosome 4 (Fig. 2C)
was detected in 2 of the 5 cell lines (PMT-1 and 2); and the loss
of chromosome X (Fig. 2D) was
detected in 2 of the 5 cell lines (PMT-2 and 5). In the context of
previous studies on cytogenetic changes in mammary tumors induced
by the overexpression of mutant or wt erbB-2 (9,10), we
have identified trisomy 5 as a previously unidentified
characteristic change in tumors from parous mice.
 | Table IKaryotypes of primary mammary tumor
cells derived from virgin and parous MMTV-erbB-2 transgenic
mice. |
Table I
Karyotypes of primary mammary tumor
cells derived from virgin and parous MMTV-erbB-2 transgenic
mice.
| Origin | Cell lines | Karyotype |
|---|
| Control virgin mouse
tumors | VMT-1 | 40,XX |
| VMT-2 | 40,XX |
| VMT-3 |
40,XX,add(4)(E2)[3]/41,XX,+5[2]/40,XX[15] |
| VMT-4 | 40,XX |
| VMT-5 | 40,XX |
| Parous mouse
tumors | PMT-1 |
40,XX,del(4)(C2D1)[3]/40,XX[12] |
| PMT-2 |
39,X,-X[14]/40,XX,del(4)(C2D1)[1] |
| PMT-3 |
41,XX,+5[9]/40,XX[7] |
| PMT-4 |
41,XX,+5[6]/40.XX[9] |
| PMT-5 |
41,XX,+5[2]/39,X,-X[2]/40.XX[14] |
Trisomy 5 confirmation by FISH
The chromosomal status in the tumor cell lines was
analyzed by FISH using a whole chromosome painting probe for
chromosome 5. Consistent with the karyotyping data, trisomy 5
detected using G-banding was confirmed by FISH in the corresponding
mammary tumor cells (Fig. 3).
Discussion
Although carcinogenesis in erbB-2 transgenic mice is
mainly driven by erbB-2 overexpression, accumulating data suggest
that the tumor development in these mice involves the acquisition
of additional genetic defects. For example, the p53 mutation is a
common target of additional genetic defects that facilitate
erbB-2-mediated carcinogenesis (14). Breeding mice that express mutant p53
(p53-172H) with MMTV-erbB-2 transgenic mice causes accelerated
tumor development (14,15). These results suggest that tumors
exhibiting a chromosomal imbalance play a critical role in
erbB-2-mediated carcinogenesis.
Previous studies from our group and other authors
indicate that erbB-2-mediated tumorigenesis in erbB-2 transgenic
mouse models involves a chromosomal imbalance (9,10).
However, it remains unclear whether or not these cytogenetic
changes were complementary to erbB-2 overexpression or were caused
by erbB-2 overexpression and increased activation. This study aimed
to characterize chromosomal changes in mammary tumors that
developed in wt MMTV-erbB-2 transgenic mice with multiparity and to
test whether enhanced overexpression of erbB-2 and hormonal
modulation in these mice induced chromosomal changes. The results
demonstrated that all tumors from the parous mice exhibited a
marked increase in cytogenetic lesions, compared with only one of
the five tumors from virgin mice that contained aberrant
chromosomes. Since tumor development in parous mice involves
hormonal fluctuations that enhance erbB-2 overexpression and
activation, the increase in the number of genetic lesions in the
parous group appears to be caused by the enhanced erbB-2 expression
and activation. Recurrent changes from mice with the same
transgenic background but varying erbB-2 levels support the causal
role of erbB-2 overexpression in the induction of genomic
instability.
The karyotype analysis also revealed that the
chromosomal imbalance in these tumors involves the recurrent
trisomy chromosome 5 (3 of the 5 cell lines), the deletion of the
entire X chromosome (2 of the 5 cell lines), and the partial
deletion of chromosome 4 (2 of the 5 cell lines), suggesting a new
pattern that has not previously been characterized in other
studies. Previous studies that have analyzed transgenic mice
over-expressing mutant or constitutively activated erbB-2 have
shown that the most frequent cytogenetic alterations were deletions
in chromosome 4 and gains in chromosome 11. Trisomy chromosome 5
was only detected in one of the 22 tumors examined (9). In our previous study, using the virgin
wt MMTV-erbB-2 transgenic model, partial or whole chromosome 4
deletion was common but trisomy chromosome 5 was not detected
(10). In the context of these
studies, our current results suggest that cytogenetic changes in
tumors from parous mice, not only occur at a higher frequency, but
also indicate a pattern that is inconsistent with that reported in
previous studies. Trisomy 5 and loss of X chromosome appear to be
associated with enhanced overexpression and activation of
erbB-2.
Notably, although the chromosome 4 deletion was a
common lesion detected in previous studies (9,10),
this deletion is not the most common change (2 in 5 cell lines)
evident in the current study. Nevertheless, repeated detection of
chromosome 4 deletion in various studies underscores its
significance in erbB-2-mediated genomic instability. Previous CGH
analysis indicated that the region frequently lost in mouse
chromosome 4 is mapped to human chromosome 1p35-36, which contains
potential tumor suppressors, including 14-3-3σ. Of note is that
deficiency of 14-3-3σ in numerous primary human breast cancers has
been attributed to loss of the chromosome 1p35-36 (16). Further comparative studies of
erbB-2-associated cytogenetic changes between mouse and human
models may clarify this correlation. Results from this study
support further investigation into the effect of enhanced
overexpression of erbB-2 on genomic instability and the role of
cytogenetic factors in erbB-2-mediated breast cancer development.
Although changes at the chromosomal level cannot reflect
micro-level lesions, the data in this study are fundamental for
future examinations with novel approaches, including a CGH
microarray.
In conclusion, the results from this study indicate
that tumors from multiparous mice contain more chromosomal
aberrations. The study also demonstrates that trisomy chromosome 5
is a recurrent cytogenetic lesion in mammary tumors from
multiparous MMTV-erbB-2 transgenic mice. These results identify a
new pattern of cytogenetic lesions that may contribute to
erbB-2-mediated carcinogenesis. Moreover, multiparity is known to
be associated with erbB-2-mediated carcinogenesis in human breast
cancer; however, the underlying mechanisms have yet to be
elucidated. Further characterization of cytogenetic changes
associated with erbB-2 overexpression, and hormonal modulation are
likely to be invaluable in directing the focus of clinical
studies.
Acknowledgements
X. Yang and Z. Ma were supported in part by a
Research Scholar Grant from the American Cancer Society
(RSG-08-138-01-CNE) and by the Oklahoma Center for the Advancement
of Science and Technology.
References
|
1
|
Bargmann CI, Hung MC and Weinberg RA: The
neu oncogene encodes an epidermal growth factor receptor-related
protein. Nature. 319:226–230. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schechter AL, Stern DF, Vaidyanathan L, et
al: The neu oncogene: an erb-B-related gene encoding a 185,000-Mr
tumour antigen. Nature. 312:513–516. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koeplinger KA, Mildner AM, Leone JW, et
al: Caspase 8: an efficient method for large-scale autoactivation
of recombinant procaspase 8 by matrix adsorption and
characterization of the active enzyme. Protein Expr Purif.
18:378–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, et al: Human
breast cancer: correlation of relapse and survival with
amplification of the HER-2/neu oncogene. Science. 235:177–182.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stern DF: Tyrosine kinase signalling in
breast cancer: ErbB family receptor tyrosine kinases. Breast Cancer
Res. 2:176–183. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prenzel N, Fischer OM, Streit S, et al:
The epidermal growth factor receptor family as a central element
for cellular signal transduction and diversification. Endocr Relat
Cancer. 8:11–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muthuswamy SK, Gilman M and Brugge JS:
Controlled dimerization of ErbB receptors provides evidence for
differential signaling by homo- and heterodimers. Mol Cell Biol.
19:6845–6857. 1999.PubMed/NCBI
|
|
8
|
Siegel PM, Ryan ED, Cardiff RD, et al:
Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are
involved in the induction of mammary tumors in transgenic mice:
implications for human breast cancer. Embo J. 18:2149–2164. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montagna C, Andrechek ER, Padilla-Nash H,
et al: Centrosome abnormalities, recurring deletions of chromosome
4, and genomic amplification of HER2/neu define mouse mammary gland
adenocarcinomas induced by mutant HER2/neu. Oncogene. 21:890–898.
2002. View Article : Google Scholar
|
|
10
|
Jeruss JS, Liu NX, Chung Y, et al:
Characterization and chromosomal instability of novel derived cell
lines from a wt-erbB-2 transgenic mouse model. Carcinogenesis.
24:659–664. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagner KU, McAllister K, Ward T, et al:
Spatial and temporal expression of the Cre gene under the control
of the MMTV-LTR in different lines of transgenic mice. Transgenic
Res. 10:545–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anisimov VN, Popovich IG, Alimova IN, et
al: Number of pregnancies and ovariectomy modify mammary carcinoma
development in transgenic HER-2/neu female mice. Cancer Lett.
193:49–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim A, Liu B, Ordonez-Ercan D, et al:
Functional interaction between mouse erbB3 and wild-type rat c-neu
in transgenic mouse mammary tumor cells. Breast Cancer Res.
7:R708–R718. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Rosen JM, McMenamin-Balano J, et al:
Neu/ERBB2 cooperates with p53-172H during mammary tumorigenesis in
transgenic mice. Mol Cell Biol. 17:3155–3163. 1997.PubMed/NCBI
|
|
15
|
Brodie SG, Xu X, Li C, et al: Inactivation
of p53 tumor suppressor gene acts synergistically with c-neu
oncogene in salivary gland tumorigenesis. Oncogene. 20:1445–1454.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hodgson JG, Malek T, Bornstein S, et al:
Copy number aberrations in mouse breast tumors reveal loci and
genes important in tumorigenic receptor tyrosine kinase signaling.
Cancer Res. 65:9695–9704. 2005. View Article : Google Scholar : PubMed/NCBI
|