Introduction
In 2002, almost 360,000 men and women were newly
diagnosed with bladder cancer (BC) worldwide, with a total
mortality exceeding 145,000 patients (1). Only limited therapeutic options exist
for the treatment of advanced BC (>pT1), including radical
cystectomy, which is used for the majority of patients. After
cystectomy, the overall survival rate is reported to be 66% at 5
years and 43% at 10 years after surgery (2). As with other tumour entities,
attention in advanced BC is increasingly focusing on targeting
intracellular signalling pathways involved in cell survival, the
cell cycle, angiogenesis and invasion (3). Previous studies identified the
phosphatidylinositol-3-kinase (PI3K)-protein kinase B (PKB/Akt)
signalling pathway as being a significant component of the
regulatory pathways affecting cancer (4,5).
Activated Akt protein is reported to play a central
role in an outermost complex network of cell growth modulation
affecting protein biosynthesis, cell cycle arrest and apoptosis.
The enzyme phosphatase and tensin homologue (PTEN) is reported to
inhibit Akt activation (phosphorylation). Activated Akt affects
various downstream molecules causing increased cell proliferation
and acts as a transcriptional activator, an inhibitor of cell cycle
arrest or a promoter of protein synthesis. The protein
p27Kip1 belongs to these molecules and is reported to
cause cell arrest in the G1 phase via a cascade of other mediators
(6). Deregulation of Akt signalling
appears to play a key role in the pathogenesis of cancer.
Understanding the effect of the individual up- and downstream
molecules and presumably existing deregulation of their cascades in
tumour cells may shed light on prognosis, suggesting different
therapeutic options and possibly targeted therapy depending on the
individual changes in the signalling network (3,7).
Understanding the exact individual changes in the
pattern of up- and downstream molecules may allow for the
prediction of the effect of molecular inhibitors of tyrosine
protein kinase as shown for treatment with trastuzumab
(Herceptin®) in patients presenting with breast cancer
tissue overexpressing HER2 (8).
Such findings facilitate the use of tumour-specific as well as
cost-effective treatment. Similarly, studies on various tumour
entities were carried out to investigate the role of PKB/Akt
signalling in prostate (9) and
renal cell cancer (10). Findings
of these studies showed that the prevalence and extent of
phosphorylation, i.e., activation of Akt, its downstream target
p27Kip1 and its inhibitory protein PTEN were determined.
However, the findings of these studies were controversial (11,12).
Data regarding Akt signalling in advanced BC are
limited (3). This study aimed to
determine the prevalence and inter-action of the three signalling
molecules in advanced BC.
Materials and methods
Patients
For this study, paraffin-embedded tissue samples
obtained from 86 patients suffering from muscle-invasive BC were
used. Tissue samples were obtained from patients undergoing radical
cystectomy between May 1993 and December 2002 at the Department of
Urology, University of Tuebingen, Germany. The study was approved
by the local ethics committee (no.383/2010BO2). TNM classification
was re-validated by the Institute of Pathology at Tuebingen
University Hospital in order to guarantee that pure tissue samples
were obtained with muscle-invasive BC. Tumour stage was described
according to the 2002 UICC TNM classification system. Tumour
grading was determined using the WHO grading system of 1973.
Table I shows the characteristics
of the patients included in this study.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | No. of patients
(%) |
|---|
| Gender |
| Male | 58 (67.4) |
| Female | 28 (32.6) |
| Age (years) |
| Median | 71 |
| Range | 45–91 |
| Pathological
stage |
| pT2 | 51 (59.3) |
| pT3/pT4 | 35 (40.7) |
| Tumour grade |
| G1 | 1 (1.2) |
| G2 | 34 (39.5) |
| G3 | 51 (59.3) |
| Lymph node
metastasis |
| N0 | 51 (59.3) |
| N+ | 32 (37.2) |
| Nx | 3 (3.5) |
| Distant
metastasis |
| M0 | 61 (70.9) |
| M+ | 21 (24.4) |
| Mx | 4 (4.7) |
| N0/M0 | 49 (57.0) |
Tissue microarray
In order to obtain representative tumour cores for
tissue microarray (TMA) construction, the specimens obtained were
stained with H&E and suitable areas were selected for TMA. TMA
preparation was conducted as described by Kallioniemi et al
(13). Two cores from each patient
were integrated into the TMA. In total, the TMA contained 172
tissue cores.
Staining
Specific staining was performed with commercially
available antibodies against p-Akt, PTEN and p27Kip1
(PTEN monoclonal mouse, dilution 1:100; Cell Signaling Technology,
Inc., Beverly, MA, USA; p-Akt polyclonal rabbit, dilution1:50; Cell
Signaling; p27Kip1 monoclonal mouse, dilution1:200; Dako
Cytomation, Glostrup, Denmark). Tissue slides were incubated
overnight at 4°C with corresponding antibody solutions.
Tissue samples were then washed in TBS and incubated
with a secondary biotinylated antibody (Vectastatin Elite ABC kit;
Vector Laboratories, Inc., Burlingame, CA, USA) for 60min.
Visualisation was achieved using the DAB method (Vector
Laboratories), according to the manufacturer’s instructions. After
brief rinsing, counterstaining was performed with Mayer’s
haematoxylin and slides were finally mounted. Breast cancer tissue
was used as a positive control for all three primary antibodies.
For the negative control, the primary antibody was omitted.
For evaluation, two investigators blinded for
patient data and antibody entity evaluated the staining intensity
of each core independently of each other using a semi-quantitative
scale (0, no staining at all; 1, sparse staining; 2, limited, but
considerable intense staining; and 3, strong staining).
Additionally, staining frequency of the cells was obtained from the
percentage of stained tumour cells and intensity, and the frequency
resulted in a staining score described by Theodorescu etal
(14).
Statistical analysis
Using linear regression analysis staining intensity,
the frequency and score of each protein was correlated to detect
possible positive or negative correlations. Staining
characteristics were correlated with tumour characteristics,
including stage, grade and synchronous distant metastases, using
the Wilcoxon-Kruskal-Wallis test. Statistical tests were performed
using JMP software (SAS Inc., Cary, NC, USA). P<0.05 was defined
as statistically significant.
Results
Staining description
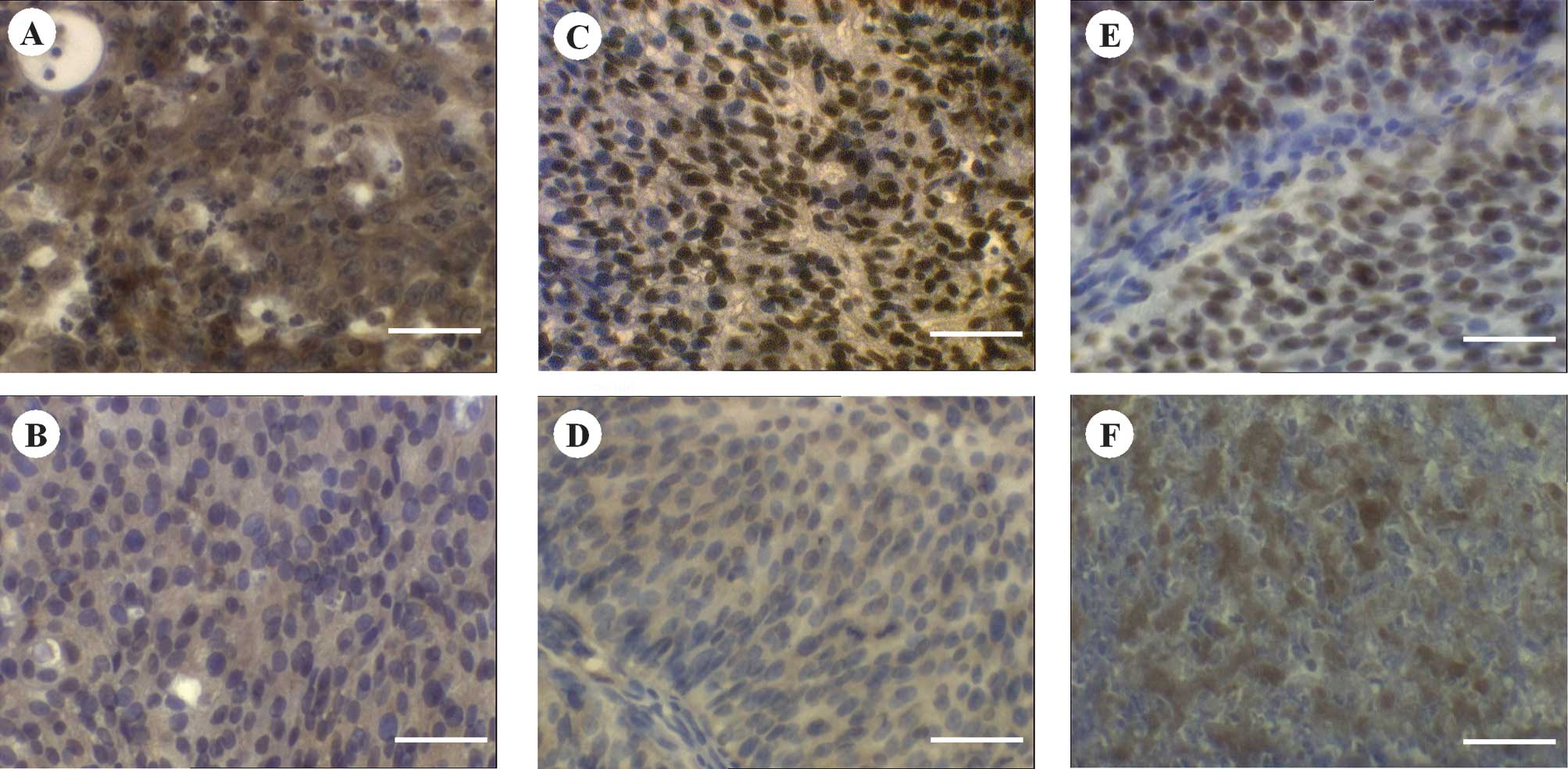
Staining of PTEN was mainly cytoplasmic. However,
the intensely stained tissues showed additional nuclear
localisation of ~20% of the stained cells. p-Akt and
p27Kip1 expression was located predominantly in the
nuclei, whereas a partial cytoplasmic expression was observed.
Cytoplasmic expression was often ubiquitious in the case of PTEN
and p-Akt, whereas that of p27Kip1 revealed a spotted
distribution. Fig. 1 shows the
representative staining results. There was an absence of staining
in the negative controls, whereas the positive controls revealed
intense staining reactions (data not shown).
Correlation of the staining
characteristics of PTEN, p-Akt and p27Kip1
Table II shows the
calculated levels of significance correlating the staining
characteristics of PTEN, p-Akt and p27Kip1 antibodies. A
positive correlation was observed in the expression scores for PTEN
and p-Akt, p-Akt and p27Kip1, as well as PTEN and
p27Kip1. Each pair demonstrated a significant
correlation (p<0.02 each, Table
II).
 | Table IILevels of significance of the
staining characteristics and their correlations. |
Table II
Levels of significance of the
staining characteristics and their correlations.
| p-Akt | Intensity | Frequency | Score |
|---|
| PTEN |
| Intensity | 0.0006 | 0.0003 | 0.0330 |
| Frequency | 0.2400 | 0.0361 | 0.2033 |
| Score | 0.0044 | 0.0068 | 0.0059 |
|
|
p27Kip1 | Intensity | Frequency | Score |
|
| p-Akt |
| Intensity | 0.0120 | 0.0378 | 0.0086 |
| Frequency | 0.0116 | 0.0231 | 0.0533 |
| Score | 0.0314 | 0.0760 | 0.0085 |
|
|
p27Kip1 | Intensity | Frequency | Score |
|
| PTEN |
| Intensity | 0.0127 | 0.0179 | 0.0114 |
| Frequency | 0.0797 | 0.5045 | 0.0414 |
| Score | 0.0275 | 0.0734 | 0.0104 |
The positive correlation between PTEN and p-Akt
resulted mainly due to the strong correlation between PTEN
intensity and p-Akt parameters. Highly significant correlations
were observed between PTEN intensity and p-Akt intensity
(p<0.0006), as well as between PTEN intensity and p-Akt
frequency (p=0.0003, Table II).
Positive correlations between the two staining characteristics of
p-Akt and p27Kip1 were found for the three combinations,
with each combination showing a significance of p<0.05 (Table II). Furthermore, positive
correlations were observed between PTEN and p27Kip1
characteristics; these were based on positive correlations between
PTEN intensity and the p27Kip1 characteristics with
significance levels of p=0.0127 for p27Kip1 intensity
and p=0.0179 for frequency. However, no significant correlation was
observed between PTEN frequency and p27Kip1 attributes
(Table II).
No significant correlation was observed between T
and Gstage, and lymphatic and distant metastases and PTEN, p-Akt or
p27Kip1 characteristics. However, a trend was observed
in patients with synchronous distant metastases of a lower
expression of p27Kip1 (mean scores 141 and 116,
respectively; p=0.33) mainly resulting from a lower intensity (mean
1.7 and 1.3, respectively; p=0.16).
Discussion
The positive correlation noted between PTEN and
p27Kip1 may be interpreted in two ways. Firstly, it is
well known that a positive correlation of PTEN and
p27Kip1 results from a decreased expression of
p27Kip1 following the loss of PTEN expression. By
inhibiting PIP3, PTEN appears to inhibit the phosphorylation of
Akt, thus preventing the inhibitory effect of p-Akt on
p27Kip1 (15). Notably,
loss of the protective effect of PTEN, i.e., inhibition of Akt
activation, results in a decrease of p27Kip1. On the
other hand, this correlation may be interpreted as an increase in
p27Kip1 expression being dependent on higher levels of
PTEN expression, as has already been reported for renal cell
carcinoma (26) and breast cancer
by Chiarle et al (16).
However, the present data suggest a reduction of p27Kip1
in M1 patients, suggesting a loss of BC progression, as reported
for other malignancies (9,17).
However, data have indicated an association of PTEN
and p27Kip1 with the Akt protein itself. Considering
this correlation, a positive correlation between the inhibitory
protein PTEN and the phosphorylated (activated) form of Akt was
observed in the present study. Furthermore, a considerable
expression of p27Kip1 was observed in the presence of
activated Akt, indicating a decrease in p27Kip1. Similar
results have already been observed in studies of ovarian cancer
(12). Another example that
contradicts this linear tumourigenic pathway is the study of
Panigrahi etal (18) in
which no correlation was found between PTEN or p-Akt and survival
in breast cancer.
A number of conceivable principal mechanisms may
explain these findings. Cantley and Neel reviewed several inherited
and spontaneous tumours with PTEN deficiency or loss (19). Additionally, numerous studies on
various tumour entities, such as colon and breast cancer, support
this mechanism (20,21). However, the inhibitory effect of
PTEN on the phosphorylation of Akt may be impaired without a
reduction in protein expression. Possible reasons for this
impairment include a structural alteration of the inhibitor itself,
the presence of a PTEN antagonist or an alternative
Pi3K-independent activation of Akt. Yoganathan etal
(22) reported integrin-linked
kinase (ILK) 1-associated Akt phosphorylation at Ser-473. Persad
etal (23) also observed the
phosphorylation of Akt at Thr-308 and Ser-473 by ILK in prostate
carcinoma cell lines PC-3 and LNCaP. Widenmaier etal
(24) reported that glucagon-like
peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide
(GIP) activate Akt independently of PI3K. Later processing, i.e.,
inactivation of p-Akt in the nucleus, as observed by Trotman
etal (25), explains the
simultaneous staining of PTEN and (cytoplasmic) p-Akt. Local
separation of the inhibitor and effector may also explain the
simultaneous presence and positive correlation of the inhibitor and
its downstream target. These explanations are also possible
regarding the positive correlation between p-Akt and
p27Kip1 observed in this study. Various authors have
reported the nuclear export of p27Kip1 (26,27)
preventing its effect on cell cycle regulation. Considering in
particular a local separation as a possible explanation, even high
levels of p27Kip1 would be incapable of inducing a G1
arrest if p27Kip1 was localised in the cytoplasm. This
is also supported by studies observing an impaired nuclear import
of p27Kip1 with a consecutive cytoplasmic accumulation
(28,29).
Another principal mechanism may be an alternative,
yet-unknown activation of p27Kip1 as a compensatory
attempt to inhibit an accelerated cell cycle in tumour cells.
Additionally, an overexpression of PTEN may be conceived as a
compensatory attempt to antagonise an alternatively activated Akt.
Loss of PTEN, which was previously correlated with poor prognosis
by other authors (30,31), may be understood as a loss of this
compensatory means during cancer progression in the sense of a late
event.
Accumulating evidence suggests that inter-individual
molecular differences within the Akt-mTOR signalling of clinically
comparable tumours affect the individual prognosis and response to
different tumour therapies. Several studies support this
observation. Pantuk et al (31) and Azim etal (7) studied the mTOR pathway and its
components and correlated this pathway significantly with
pathological findings and survival in renal cell carcinoma. These
authors also stressed the importance of individual assessment of
distinct molecular markers to predict individual benefit of
targeted therapy. This type of observation results in specifying
individual molecular alterations in cancer.
However, data concerning this correlation with BC
are limited. Chen et al (32) reported molecular data on Akt
signalling and significant correlation with survival, whereas
Shariatetal (33) associated
an increased risk of disease progression and death with alterations
of p27Kip1 in combination with p53, p21 and pRB.
The identification of molecular markers would allow
for the calculation of the individual response rate, and the
prediction of treatment modalities, as well as molecularly targeted
and individually adjusted therapy. Determining the individual
expression levels and the correlation of signalling molecules, as
examined in this study, may result in a better understanding of
transitional cell carcinoma and individualised targeted therapy.
Mass etal (8) observed a
benefit in treating metastatic breast cancer with trastuzumab in
patients overexpressing HER2. Based on these findings, Laé et
al (34) evaluated HER2
expression in muscle-invasive BC and observed HER2 gene
amplification in 5.1% of the BCs. Another example of biomarkers in
BC is found in the study by Havaleshkoetal (35), who combined microarray data and
phosphoprotein profiling to predict lapatinib sensitivity in BC. In
non-invasive BC, Miyakeetal (36) profiled the possible benefit of the
tyrosine kinase inhibitor PD173074 by analysis of fibroblast growth
factor receptor-3 mutations.
The results of the current study in muscle-invasive
BC suggest that neither a reduced expression of PTEN nor
p27Kip1 are reliable biomarkers for the activation of
the Akt pathway. The staining intensity of PTEN appears to be
superior to its frequency in terms of its potential use as a marker
for targeted therapy or a predictor of progression. This finding
may also indicate the various mechanisms of Akt activation within
different tumour cell subpopulations that are partially independent
of PTEN.
As a study predominantly addressing signalling,
neither a reduced nor an increased expression of the parameters in
BC tissue could be determined, nor could their role in the
progression sequence be examined.
The semi-quantitative analysis appears to be a
suitable method for this pilot study; however, a quantitative
method is required to confirm the findings presented in this study.
Consequently, future studies should involve benign bladder tissues
as well as non-invasive BC and correlate patient follow-up with the
expression pattern of parameters investigated in the present study.
The semi-quantitative approach should be extended with quantitative
methods, including Western blotting and RT-PCR, and analysis of
cellular allocation should be conducted.
The findings of Akt signalling in BC observed in
this study are predictable. However, unforeseen correlations of its
mediators were additionally detected, suggesting a yet-unknown
regulatory effect on Akt and p27Kip1 and mechanisms in
cancer, bypassing cellular mechanisms of cell cycle restriction.
Further studies are required to correlate the effect of different
antiproliferative agents with the individual expression patterns of
these signalling molecules.
References
|
1
|
Parkin M, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
3
|
Netto G and Epstein J: Theranostic and
prognostic biomarkers: genomic applications in urological
malignancies. Pathology. 42:384–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Altomare D and Testa J: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherr C and Roberts J: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azim H, Azim H and Escudier B: Targeting
mTOR in cancer: renal cell is just a beginning. Target Oncol.
5:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mass R, Press M, Anderson S, et al:
Evaluation of clinical outcomes according to HER2 detection by
fluorescence in situ hybridization in women with metastatic breast
cancer treated with trastuzumab. Clin Breast Cancer. 6:240–246.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graff JR, Konicek BW, McNulty AM, et al:
Increased AKT activity contributes to prostate cancer progression
by dramatically accelerating prostate tumor growth and diminishing
p27Kip1 expression. J Biol Chem. 275:24500–24505. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merseburger A, Hennenlotter J, Kuehs U, et
al: Activation of PI3K is associated with reduced survival in renal
cell carcinoma. Urol Int. 80:372–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hennenlotter J, Ohneseit P, Simon P, et
al: PTEN and p27Kip1 are not downregulated in the majority of renal
cell carcinomas – implications for Akt activation. Oncol Rep.
19:1141–1147. 2008.
|
|
12
|
Kurose K, Zhou X-P, Araki T, Cannistra S,
Maher E and Eng C: Frequent loss of PTEN expression is linked to
elevated phosphorylated Akt levels, but not associated with p27 and
cyclinD1 expression, in primary epithelial ovarian carcinomas. Am J
Pathol. 158:2097–2106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kallioniemi OP, Wagner U, Kononen J and
Sauter G: Tissue microarray technology for high-throughput
molecular profiling of cancer. Hum Mol Genet. 10:657–662. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theodorescu D, Broder SR, Boyd JC, Mills
SE and Frierson HF: Cathepsin D and chromogranin A as predictors of
long term disease specific survival after radical prostatectomy for
localized carcinoma of the prostate. Cancer. 80:2109–2119. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blain S, Scher H, Cordon-Cardo C and Koff
A: p27 as a target for cancer therapeutics. Cancer Cell. 3:111–115.
2003. View Article : Google Scholar
|
|
16
|
Chiarle R, Pagano M and Inghirami G: The
cyclin dependent kinase inhibitor p27 and its prognostic role in
breast cancer. Breast Cancer Res. 3:91–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seki R, Ohshima K, Fujisaki T, et al:
Prognostic significance of S-phase kinase-associated protein 2 and
p27kip1 in patients with diffuse large B-cell lymphoma: effects of
rituximab. Ann Oncol. 21:833–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panigrahi AR, Pinder SE, Chan SY, Paish
EC, Robertson JFR and Ellis IO: The role of PTEN and its signalling
pathways, including AKT, in breast cancer; an assessment of
relationships with other prognostic factors and with outcome. J
Pathol. 204:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khaleghpour K, Li Y, Banville D, Yu Z and
Shen SH: Involvement of the PI 3-kinase signaling pathway in
progression of colon adenocarcinoma. Carcinogenesis. 25:241–248.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi W, Zhang X, Pintilie M, et al:
Dysregulated PTEN-PKB and negative receptor status in human breast
cancer. Int J Cancer. 104:195–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoganathan TN, Costello P, Chen X, et al:
Integrin-linked kinase (ILK): a ‘hot’ therapeutic target. Biochem
Pharmacol. 60:1115–1119. 2000.
|
|
23
|
Persad S, Attwell S, Gray V, et al:
Inhibition of integrin-linked kinase (ILK) suppresses activation of
protein kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA.
97:3207–3212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Widenmaier S, Sampaio A, Underhill M and
McIntosh C: Noncanonical activation of Akt/protein kinase B in
{beta}-cells by the incretin hormone glucose-dependent
insulinotropic polypeptide. J Biol Chem. 284:10764–10773.
2009.PubMed/NCBI
|
|
25
|
Trotman L, Alimonti A, Scaglioni PP,
Koutcher J, Cordon-Cardo C and Pandolfi PP: Identification of a
tumour suppressor network opposing nuclear Akt function. Nature.
441:523–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blain S and Massagué J: Breast cancer
banishes p27 from nucleus. Nat Med. 8:1076–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Connor M, Kotchetkov R, Cariou S, et al:
CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear
export signal and links p27 export and proteolysis. Mol Biol Cell.
14:201–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang J, Zubovitz J, Petrocelli T, et al:
PKB/Akt phosphorylates p27, impairs nuclear import of p27 and
opposes p27-mediated G1 arrest. Nat Med. 8:1153–1160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin I, Yakes M, Rojo F, et al: PKB/Akt
mediates cell-cycle progression by phosphorylation of p27Kip1 at
threonine 157 and modulation of its cellular localization. Nat Med.
8:1145–1152. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Capodanno A, Camerini A, Orlandini C, et
al: Dysregulated PI3K/Akt/PTEN pathway is a marker of a short
disease-free survival in node-negative breast carcinoma. Hum
Pathol. 40:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pantuck A, Seligson D, Klatte T, et al:
Prognostic relevance of the mTOR pathway in renal cell carcinoma.
Cancer. 109:2257–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen M, Gu J, Delclos G, et al: Genetic
variations of the PI3K-AKT-mTOR pathway and clinical outcome in
muscle invasive and metastatic bladder cancer patients.
Carcinogenesis. 31:1387–1391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shariat S, Ashfaq R, Sagalowsky A and
Lotan Y: Predictive value of cell cycle biomarkers in nonmuscle
invasive bladder transitional cell carcinoma. J Urol. 177:481–487.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laé M, Couturier J, Oudard S, Radvanyi F,
Beuzeboc P and Vieillefond A: Assessing HER2 gene amplification as
a potential target for therapy in invasive urothelial bladder
cancer with a standardized methodology: results in 1005 patients.
Ann Oncol. 21:815–819. 2010.PubMed/NCBI
|
|
35
|
Havaleshko D, Smith SC, Cho H, et al:
Comparison of global versus epidermal growth factor receptor
pathway profiling for prediction of lapatinib sensitivity in
bladder cancer. Neoplasia. 11:1185–1193. 2009.PubMed/NCBI
|
|
36
|
Miyake M, Ishii M, Koyama N, et al:
1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea
(PD173074), a selective tyrosine kinase inhibitor of fibroblast
growth factor receptor-3 (FGFR3), inhibits cell proliferation of
bladder cancer carrying the FGFR3 gene mutation along with
up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther.
332:795–802. 2010.
|