Introduction
Of all the gynecologic cancers, ovarian cancer has
the highest mortality rate. Even though the survival rate following
early detection of the disease is relatively high, a diagnosis of
ovarian cancer often occurs at a late stage in the disease. The
mechanisms responsible for ovarian cancer are not completely known,
and research is minimal due to a lack of suitable animal models
(1).
The laying hen is a suitable animal model for human
ovarian cancer due to the similarities between human and chicken
ovarian cancers. Most human ovarian cancers arise spontaneously in
cells derived from the ovarian surface epithelium (2,3). This
is consistent with chicken ovarian cancer, which originates from
ovarian epithelial cells (4).
Support for the hypothesis regarding incessant ovulation that
explains ovarian cancer in humans (2,3) is the
fact that hens ovulate almost daily, resulting in genomic damage to
the ovarian surface epithelium and increasing the likelihood of
mutations that lead to the development of spontaneous ovarian
adenocarcinoma (5). Moreover,
anti-tumor antibodies common to human and chicken cancer carcinomas
include cancer antigen 125, cytokeratin, pan cytokeratin,
proliferating cell nuclear antigen (PCNA), carcinoembryonic
antigen, cytokeratin AE1/AE3, epidermal growth factor receptor,
ERBB2, Lewis Y, selenium-binding protein 1 and tumor-associated
glycoprotein 72 (6–8). Despite these similarities, further
characterization of chicken ovarian cancer is crucial for a
comparative study of ovarian cancers in humans and chickens.
Proteases are involved in controlling multiple
biological processes and multiple diseases, including cancer
(9). It was recently reported that
cysteine proteases, known as cathepsins, were involved in chicken
ovarian cancer (10). One of the
protease groups, matrix metalloproteinases (MMPs), is involved in
the degradation of the extracellular matrix and basement membranes.
Due to their function, MMPs have long been considered to play an
essential role in cancer progression by promoting tumor cell
invasion, angiogenesis and metastasis of cancer cells (11–13).
In human ovarian cancer, certain MMPs are abundantly expressed in
epithelial ovarian cancer cells (14,15).
However, the expression of MMPs in cancerous chicken ovaries has
yet to be investigated. We therefore examined the expression
patterns of MMPs in cancerous and normal chicken ovaries, with a
particular emphasis on MMP3.
Materials and methods
Animals
The care and experimental use of White Leghorn (WL)
hens (Gallus gallus domesticus) was approved by the
Institute of Laboratory Animal Resources, the Seoul National
University (SNU-070823-5), Korea. The hens were maintained in a
standard management program at the University Animal Farm at Seoul
National University. The procedures used for animal management,
reproduction and embryo manipulation followed the standard
operating protocols of our laboratory.
Tissue samples
Cancerous (n=5) ovaries were obtained from 2- and
3-year-old WL hens with spontaneously developed ovarian cancer.
Normal (n=3) ovaries were obtained from 2- and 3-year-old healthy
WL hens without any histological changes in the ovaries. Sections
of these ovaries were frozen or embedded in paraffin for further
analysis. For diagnosis, paraffin-embedded tissues were sectioned
at 5 μm and stained with hematoxylin and eosin.
RT-PCR analysis
Total RNA was extracted from frozen tissues by
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was
synthesized using AccuPower® RT PreMix (Bioneer,
Daejeon, Korea). Specific primer sets were used for RT-PCR
(Table I). PCR amplification was
performed as follows: 95°C for 3 min; followed by 30 cycles of 95°C
for 20 sec, 60°C for 40 sec and 72°C for 1 min; and a final
extension of 72°C for 5 min. PCR products were analyzed on a 1%
agarose gel stained with ethidium bromide.
 | Table IPrimer sequences used for RT-PCR and
cloning. |
Table I
Primer sequences used for RT-PCR and
cloning.
| Gene | Sequence (5′-3′):
forward and reverse | Gene bank accession
no. | Product size
(bp) |
|---|
| MMP1 |
CTAATGGGCTGCTGGCTCA
GACCTCTCAGGATGTTTGCG | XM_417176 | 406 |
| MMP2 |
GTGGCAATGGTGATGGACAG
TCCTGAGAAAGGCGGAAGTT | NM_204420 | 460 |
| MMP3 |
CACTGGGATAGGAGGGGATG
TCTGTGGGTGCCATTTCTGT | XM_417175.2 | 348 |
| MMP7 |
CCTCCTACTTTGTGCTGCCA
ATGATGTCTGCCTGTCCCG | NM_001006278.1 | 453 |
| MMP9 |
CGGCTTAGAGGTGAAGACCC
GGAAGGTGAAGGGGAAGACA | NM_204667.1 | 416 |
| MMP11 |
CCAGCCAGACCTTGAAACAA
CAATCTCCTGTGGGACACCA | XM_001232776 | 491 |
| MMP13 |
CGGGTGCTGTGGAAGAAATA
TTGGTGTAGTTGGGGCAGAC | XM_001235204 | 407 |
| MMP15 |
GACGCTGGAAAACACGGAC
ACCACTTGCCCTTGAACACA | XM_413995 | 422 |
| MMP16 |
CAACTGACCCCAGAATGTCG
AAAAATCCTCCCTCCCCATC | NM_205197 | 454 |
| MMP17 |
TTTGGGTATCTGCCTCCTCC
CCTGCTGTGTGATGGTCTCC | XM_415092 | 482 |
| MMP23B |
CGTAGTGGCTTTGCTGGCTA
CAAGTTCCCCTGTTGTTCCA | XM_417569 | 425 |
| MMP24 |
CGACTCTTCCTGTTCGCAGA
TCGCTCTCTTGTCCTCGTTG | XM_417326 | 498 |
| MMP27 |
CAGGAAAACCAGACACCGAG
GAGCAGCAACCAGGAACAAA | NM_205000 | 423 |
| MMP28 |
CAGCACCTACTACTGCCACTCC
AATAGCGGTCATCCCGAAAG | XM_415771 | 500 |
| GAPDH |
CACAGCCACACAGAAGACGG
CCATCAAGTCCACAACACGG | NM_204305 | 443 |
Quantitative RT-PCR analysis
Quantitative RT-PCR was performed using SYBR-Green
(Sigma, St. Louis, MO, USA) and a StepOnePlus real-time PCR system
(Applied Biosystems, Foster City, CA, USA). Relative quantification
of gene expression was calculated using the formula
2−ΔΔCt, where ΔΔCt= (Cttarget gene −
CtGAPDH)cancerous tissue −
(Cttarget gene − CtGAPDH)normal
tissue. The information for the primer sets is shown in
Table II.
 | Table IIPrimer sequences used for quantitative
RT-PCR. |
Table II
Primer sequences used for quantitative
RT-PCR.
| Gene | Sequence (5′-3′):
forward and reverse | Gene bank accession
no. | Product size
(bp) |
|---|
| MMP3 |
ACCTGGGCTTTCCCAGAAGT
CTGAAGGGCAGCATCAACGA | XM_417175.2 | 194 |
| GAPDH |
ACACAGAAGACGGTGGATGG
GGCAGGTCAGGTCAACAACA | NM_204305 | 193 |
In situ hybridization
In situ hybridization was conducted as
previously described (16). For
hybridization probes, PCR products were generated from ovarian
cancer cDNA with the primers used in RT-PCR analysis. Products were
then gel-extracted and cloned into pGEM-T Easy Vector (Promega,
Madison, WI, USA). Following the verification of sequences, a
DIG-labeled RNA probe was prepared using a DIG RNA labeling kit
(Roche Applied Science, Indianapolis, IN, USA). Frozen sections (10
μm) were mounted on slides pretreated with
3-aminopropyltriethoxysilane (Sigma), dried on a 50°C slide warmer,
fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS),
treated with 1% Triton X-100 in PBS for 20 min, washed three times
in PBS and incubated with a prehybridization mixture [50% formamide
and 5X standard saline citrate (SSC)] for 15 min at room
temperature. Following prehybridization, sections were incubated in
a hybridization mixture (50% formamide, 5X SSC, 10% dextran sulfate
sodium salt, 0.02% bovine serum albumin, 250 μg/ml yeast tRNA and
denatured DIG-labeled cRNA probes) for 18 h at 55°C in a humidified
chamber. Sections were then washed for stringency in a series of
solutions containing formamide and SSC. Following blocking with 1%
blocking reagent (Roche), sections were incubated overnight with
sheep anti-DIG antibody conjugated to alkaline phosphatase (Roche).
Following incubation, a visualization solution (0.4 mM
5-bromo-4-chloro-3-indolyl phosphate, 0.4 mM nitroblue tetrazolium,
and 2 mM levamisole; Sigma) was used. Sections were counterstained
with 1% (w/v) methyl green (Sigma). Images were captured with a
Zeiss Axiophot light microscope equipped with an AxioCam HRc camera
(Carl Zeiss, Inc., NY, USA).
Statistical analysis
Statistical analysis was performed using the
Student’s t-test using the SAS program (SAS Institute, Cary, NC,
USA). P<0.05 was considered to be statistically significant.
Results
Pathological characteristics of chicken
ovarian cancer
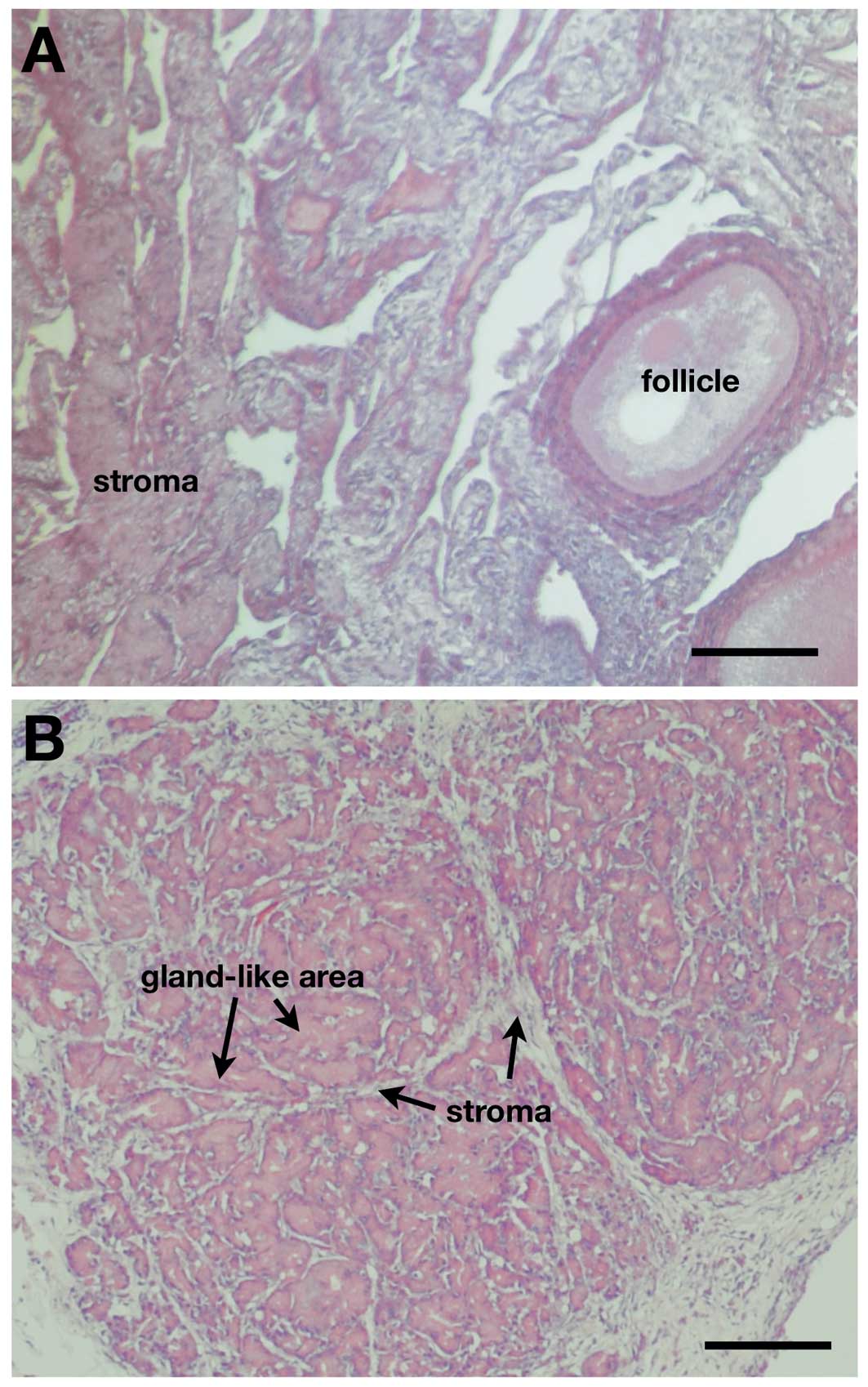
Normal ovaries had typically developing follicles
surrounded by stroma (Fig. 1A). The
morphology of cancerous ovaries was entirely different, showing
gland-like growth of cancer cells invading stromal tissues
(Fig. 1B). The difference between
normal and cancerous ovaries was similar to that reported
previously (17,18).
Increased expression of MMP3 in cancerous
ovaries
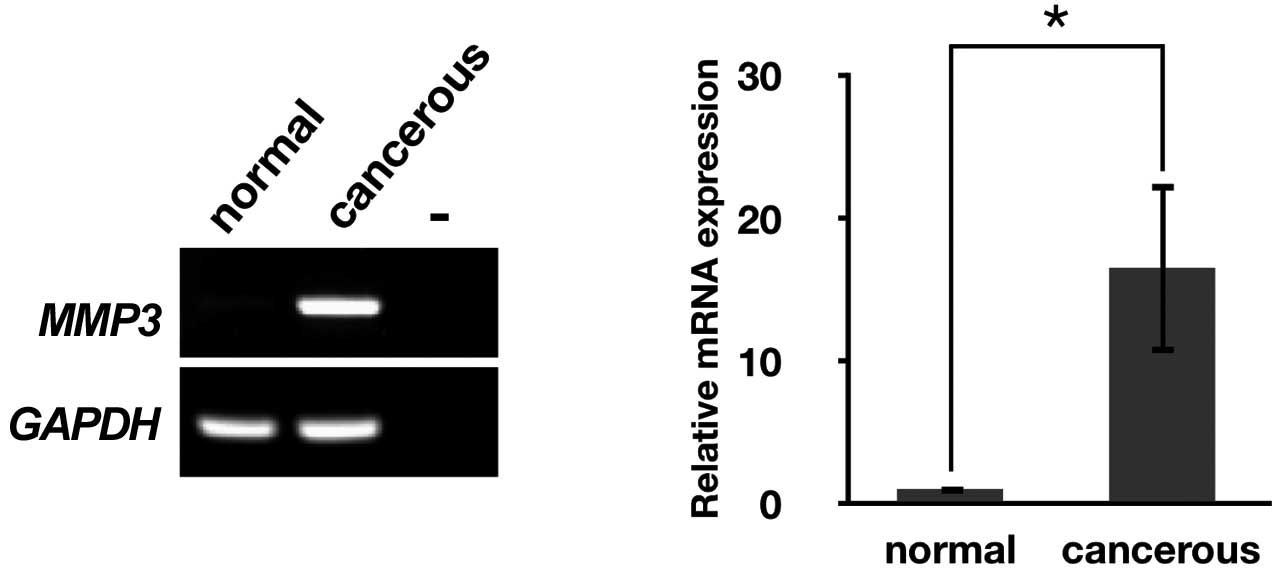
We initially performed RT-PCR analysis to determine
the expression of MMPs that have been identified in chickens.
MMP3 mRNA was markedly expressed in cancerous ovaries but
almost undetectable in normal ovaries (Fig. 2A), whereas the expression of the
remaining MMPs were weak or undetectable in cancerous and normal
ovaries (data not shown). Therefore, further study was focused on
MMP3.
To measure relative mRNA levels between normal and
cancerous ovaries, we performed quantitative RT-PCR. As expected,
MMP3 mRNA expression in cancerous ovaries was approximately
16-fold higher than that in normal ovaries (P<0.05, Fig. 2B).
Localization of MMP3 mRNA in normal and
cancerous ovaries
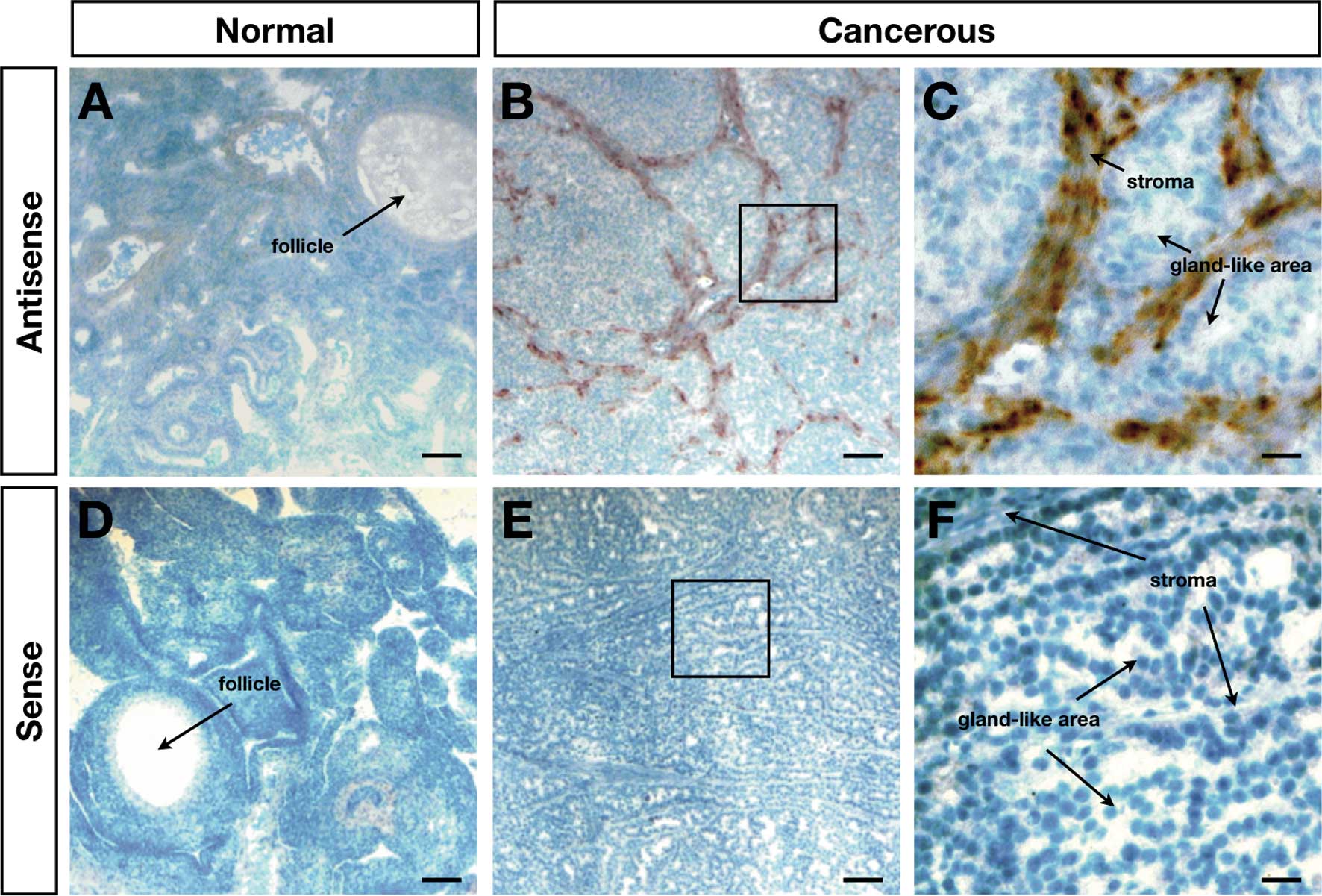
The expression pattern for MMP3 was further
analyzed by the localization of MMP3 mRNA by in situ
hybridization. The results indicated an absence of the
cell-specific expression of MMP3 mRNA in normal ovaries
(Fig. 3A and D), whereas abundant
MMP3 mRNA was observed in the stroma between the gland-like
areas in the cancerous ovaries (Fig.
3B, C, E and F).
Discussion
MMP3 (also known as stromelysin-1) plays significant
roles in regulating extracellular matrix remodeling and in
activating other MMPs (19).
Over-expression of MMP3 has also been demonstrated in
various types of cancer (20). In
human cancer, MMPs are also commonly expressed in the stromal cells
rather than in the tumor cells: expression of MMPs in stromal cells
has been reported in breast, colorectal, lung, prostate, and
pancreatic cancers (20). Our
results also show that MMP3 mRNA is localized in stromal
cells in chicken ovarian cancer suggesting that the roles of MMP3
in cancer are conserved between chickens and humans, and the
expression of MMP3 may be regulated by similar mechanisms in
the two species. In humans, cancer cells induce MMP1, 2 and
3 expression by secreting signaling molecules known as
extracellular matrix metalloproteinase inducers (21,22).
However, further studies are necessary to determine the detailed
mechanism(s) of tumorigenesis in chicken ovaries.
Although the role of MMP3 in cancer has not been
fully elucidated, certain studies have revealed its tumorigenic
functions. Sternlicht et al showed that MMP3 over-expression
in transgenic mice leads to enhanced mammary carcinogenesis
(23), and Witty et al
showed that MMP3 targets relevant substrates to promote apoptosis
in neighboring epithelial cells, which is relevant for cancer
(24). In addition to their ability
to degrade major protein components of the extracellular matrix or
the basement membrane, MMPs play a role in the early stages of
tumorigenesis by stimulating cell proliferation and modulation of
angiogenesis (25). A focus on the
early stages of ovarian cancer in chickens may reveal new roles for
MMPs in the early developmental stages of cancer and thus improve
the ability of medical professionals to make an early
diagnosis.
In conclusion, this study has demonstrated the
over-expression of MMP3 in chicken ovarian cancer. The
expression pattern of MMP3 is relatively similar to that of
MMPs in human cancer. The cell type-specific expression of the
MMP3 gene renders this gene a unique marker for epithelial
chicken ovarian cancer and suggests the crucial role MMP3 plays in
its development.
Acknowledgements
This study was supported by the Mid-career
Researcher Program through an NRF grant funded by the MEST (No.
2009-0083687) and the World Class University program (R31-10056)
through the National Research Foundation of Korea funded by the
Science and Technology Ministry of Education. The authors would
like to express their appreciation to Dr Fuller W. Bazer (Texas
A&M University, USA; and Seoul National University, Korea) for
assistance with manuscript preparation and helpful discussions.
References
|
1
|
Orsulic S: Ovarian cancer. Mouse Models of
Human Cancer. Holland EC: Whiley-Liss, Inc; New Jersey: pp.
171–187. 2004
|
|
2
|
Auersperg N, Edelson MI, Mok SC, Johnson
SW and Hamilton TC: The biology of ovarian cancer. Semin Oncol.
25:281–304. 1998.
|
|
3
|
Fathalla MF: Incessant ovulation-a factor
in ovarian neoplasia? Lancet. 2:1631971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fredrickson TN: Ovarian-tumors of the hen.
Environ Health Perspect. 73:35–51. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murdoch WJ, Van Kirk EA and Alexander BM:
DNA damages in ovarian surface epithelial cells of ovulatory hens.
Exp Biol Med. 230:429–433. 2005.PubMed/NCBI
|
|
6
|
Zhuge Y, Lagman JA, Ansenberger K, et al:
CYP1B1 expression in ovarian cancer in the laying hen Gallus
domesticus. Gynecol Oncol. 112:171–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stammer K, Edassery SL, Barua A, et al:
Selenium-binding protein 1 expression in ovaries and ovarian tumors
in the laying hen, a spontaneous model of human ovarian cancer.
Gynecol Oncol. 109:115–121. 2008. View Article : Google Scholar
|
|
8
|
Rodriguez-Burford C, Barnes MN, Berry W,
Partridge EE and Grizzle WE: Immunohistochemical expression of
molecular markers in an avian model: a potential model for
preclinical evaluation of agents for ovarian cancer
chemoprevention. Gynecol Oncol. 81:373–379. 2001. View Article : Google Scholar
|
|
9
|
Lopez-Otin C and Bond JS: Proteases:
Multifunctional enzymes in life and disease. J Biol Chem.
283:30433–30437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahn SE, Choi JW, Rengaraj D, et al:
Increased expression of cysteine cathepsins in ovarian tissue from
chickens with ovarian cancer. Reprod Biology and Endocrinol.
8:1002010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolf K, Wu YI, Liu Y, et al: Multi-step
pericellular proteolysis controls the transition from individual to
collective cancer cell invasion. Nat Cell Biol. 9:893–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartolome RA, Ferreiro S, Miquilena-Colina
ME, et al: The chemokine receptor CXCR4 and the metalloproteinase
MT1-MMP are mutually required during melanoma metastasis to lungs.
Am J Pathol. 174:602–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergers G, Brekken R, McMahon G, et al:
Matrix metalloproteinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stadlmann S, Pollheimer J, Moser PL, et
al: Cytokine-regulated expression of collagenase-2 (MMP-8) is
involved in the progression of ovarian cancer. Eur J Cancer.
39:2499–2505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kenny HA and Lengyel E: MMP-2 functions as
an early response protein in ovarian cancer metastasis. Cell Cycle.
8:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rengaraj D, Kim DK, Zheng YH, Lee SI, Kim
H and Han JY: Testis-specific novel transcripts in chicken: in
situ localization and expression pattern profiling during
sexual development. Biol Reprod. 79:413–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giles JR, Shivaprasad HL and Johnson PA:
Ovarian tumor expression of an oviductal protein in the hen: a
model for human serous ovarian adenocarcinoma. Gynecol Oncol.
95:530–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hales DB, Zhuge Y, Lagman JAJ, et al:
Cyclooxygenases expression and distribution in the normal ovary and
their role in ovarian cancer in the domestic hen (Gallus
domesticus). Endocrine. 33:235–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kucukali CI, Aydin M, Ozkok E, et al: Do
schizophrenia and bipolar disorders share a common disease
susceptibility variant at the MMP3 gene? Prog Neuro-Psychopharmacol
Biol Psychiatry. 33:557–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
21
|
Kataoka H, DeCastro R, Zucker S and Biswas
C: Tumor cell-derived collagenase-stimulatory factor increases
expression of interstitial collagenase, stromelysin, and 72-kDa
gelatinase. Cancer Res. 53:3154–3158. 1993.
|
|
22
|
Biswas C, Zhang Y, Decastro R, et al:
Human tumor cell-derived collagenase stimulatory factor (renamed
Emmprin) is a member of the immunoglobulin superfamily. Cancer Res.
55:434–439. 1995.PubMed/NCBI
|
|
23
|
Sternlicht MD, Lochter A, Sympson CJ, et
al: The stromal proteinase MMP3/stromelysin-1 promotes mammary
carcinogenesis. Cell. 98:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Witty JP, Lempka T, Coffey RJ and
Matrisian LM: Decreased tumor-formation in
7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice
is associated with alterations in mammary epithelial-cell
apoptosis. Cancer Res. 55:1401–1406. 1995.PubMed/NCBI
|
|
25
|
Egeblad M and Werb Z: New functions for
the matrix metallo proteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar
|