Introduction
Insulin-like growth factors, IGF-I and IGF-II,
together with their receptors, IGF-IR and IGF-IIR, and IGF-BP form
a coherent system that is responsible for the growth and division
of cells in the body (1). The
system of insulin-like growth factors includes two receptors: type
I, which transmits signals through tyrosine kinase, and type II,
which is identical to the mannose-6-phosphate receptor that does
not transmit signals but inhibits the auto- and paracrine functions
of IGF-II through its uptake and internalisation from the plasma
(2).
The IGF-IR receptor is a heterotetramer (2α2β). The
α subunits contain an IGF-I binding site, whereas the β subunits
start the process of phosphorylation and synthesis of intracellular
proteins (1). The activated IGF-IR
regulates the cell proliferation processes by transmitting division
signals, protecting cells against apoptosis, regulating adhesion
processes or inducing growth and differentiation of cells (3-6).
Rouyer-Fessard et al found IGF-IR expression in normal
colorectal mucosa (7). When
overexpressed, IGF-IR behaves as a cell oncogene. The presence or
absence of this receptor affects transformation of various viral
and cellular oncogenes (3). A
higher density of IGF-IR has been noted in carcinomas of the colon,
ovary, breast, thyroid and endometrium, Wilms’ tumours and gliomas
(1,8,9).
IGF-I and IGF-II acting on IGF-IR in colorectal
cancer prevent apoptosis, enhance cell proliferation and induce the
expression of vascular endothelial growth factor (VEGF). Results of
experiments conducted on mice showed that IGF-IR, through VEGF
induction, accelerates tumour growth and the formation of
metastases (5,10). However, the role of IGF-IR in cancer
cells of the colon has yet to be fully elucidated. IGF-IR is
frequently overexpressed in human colorectal cells. IGF-IR blockage
results in the inhibition of growth and angiogenesis of colorectal
carcinoma. A decrease in IGF-IR expression causes much apoptosis of
cancer cells in vivo and in vitro. In experimental
animal studies, IGF-IR hypoexpression is manifested as the
inhibition of tumour and metastasis formation (6).
Taking the above into consideration, this study
aimed to assess the expression of IGF-IR in colorectal cancer cells
in correlation with certain clinico-morphological factors.
Patients and methods
Patients
The study included 88 patients with primary
colorectal carcinoma treated surgically at the Second Department of
General and Gastroenterological Surgery, Medical University of
Bialystok, Poland, in the years 1998-2003. The study group
comprised 48 (54.6%) males and 40 (45.4%) females with an average
age of 64.78 years (range 36-87). Twenty-eight (31.8%) patients
were <60 years of age, and the remaining 60 (68.2%) patients
were >60 years of age. As regards tumour location, the patients
were divided into two groups. The first group included 43 (48.9%)
patients with rectal carcinoma. The patients with carcinoma
elsewhere in of the colon (45; 51.1%) constituted the other group.
Patients underwent scheduled surgery. Eighteen (20.5%) patients had
abdominoperineal excision of the rectum by the Miles method. In 27
(30.7%) patients, low anterior resection of the rectum was
performed with an end-to-end anastomosis. Hartmann’s operation was
carried out in 19 (21.6%) patients. Right-side hemicolectomy was
performed in 17 (19.3%) patients, whereas left-side hemicolectomy
was performed in 4 (4.5%) patients. A further 3 (3.4%) patients
underwent segmental excision of the transverse colon.
Routine histopathological investigations were
performed to analyse tumor node metastasis (TNM) and Dukes’ staging
of anatomo-clinical advancement, histological type and malignancy
grade (G).
Lesions in the pT3 and pT4 stages were found to
predominate in the TNM classification. There were no pT1 patients.
The pT2 group included 8 (9.1%) individuals. Sixty-one (69.3%)
patients had tumours in the pT3 stage and 19 (21.6%) in the pT4
stage. Forty (45.4%) patients had no local lymph node involvement
(group N0). Group N1 comprised 24 (27.3%) patients; group N2, 22
(25%) patients and group N3 had a further 2 (2.3%) patients. Liver
metastases (stage M1) were observed in only 6 (6.8%) patients. The
remaining 82 (93.2%) patients had no distant metastases (stage M0).
Advancement of the neoplastic process was estimated in the Dukes’
classification as modified by Astler-Coller. There were no patients
in group A, 5 (5.7%) patients in group B1, 34 (38.6%) in group B2,
13 (14.8%) in group C1, 30 (34.1%) in group C2 and 6 (6.8%) in
group D.
Any lesions were verified by histopathological
examination. Adenocarcinoma was found in 68 (77.3%) cases,
mucogenic adenocarcinoma in 7 (8%) and partly mucogenic
adenocarcinoma in 10 (11.4%) patients. Poorly differentiated
adenocarcinoma (1.1%), ulcerative adenocarcinoma (1.1%) and
mucocellular carcinoma (1.1%) were only identified in single
cases.
In the study group, highly differentiated carcinomas
(G1) were not detected. Moderate differentiation (G2) was observed
in 57 (64.8%) patients, whereas low differentiation (G3) was found
in the remaining 31 (35.2%) patients.
Materials
Two specimens from each tumour were stained using an
immunohistochemical method of evaluation. A standard avidin-biotin
immunoperoxidase method (ABC Staining System, Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was used for the detection of
IGF-IR expression. In all cases, specimens were obtained from the
main mass of the tumour. The specimens were fixed in 40 g/l
formaldehyde, embedded in paraffin and cut into 4-μm sections. The
sections were dewaxed in three changes of xylene and hydrated
through an alcohol series of a decreasing concentration. The
sections were heated for 3 min in citrate buffer (10 mmol/l, pH
6.0) in a pressure cooker to expose the antigen. Endogenous
peroxidase activity was blocked by incubating the sections in a 3%
hydrogen peroxide solution in methanol for 5 min. The slides were
then washed 3 times in phosphate-buffered saline (PBS) and
incubated in normal horse serum for 5 min to reduce non-specific
antibody binding. Following washing with PBS, the slides were
incubated for 24 h at 4°C with monoclonal antibody (Anti-IGF-IR, β
subunit, C-terminal clone CT-3) (H-60, Santa Cruz Biotechnology) at
a dilution of 1:100 for all slides. The antigen-antibody complex
was visualised by DAB chromogen (3′3-diamonobenzidine, Dako,
Denmark). Following rinsing in distilled water, the sections were
stained with hematoxylin, and following dehydration with an alcohol
series of an increasing concentration the sections were mounted on
a Canadian balsam. A light microscope was used for the analysis of
the immunohistochemical reactions in colorectal carcinoma cases.
The expression of IGF-IR was analysed in 10 various fields of
vision, in which the mean percentage of the immunohistochemically
positive cancer cells was determined (positive when >10% of
carcinoma cells were IGF-IR-positive; negative when there was no
reaction or ≤10% of cells were positive). Control
immunohistochemical staining was performed; the positive control
comprised IGF-IR-positive colorectal cancer specimens, in the
negative control primary antibodies were omitted in the staining
procedure. The negative control did not exhibit any specific
immunostaining.
Statistical analysis
The results were subjected to statistical analysis
using the Chi-square test, multivariate analysis and the
Mann-Whitney U test. Differences were considered statistically
significant at p<0.05. Statistical analysis was performed using
the statistical package SPSS 8.0 PL.
Results
Immunoreactivity
IGF-IR expression was assessed in 88 colorectal
tumours. In 44 (50%) of the examined colorectal tumours an
immunohistochemical reaction to IGF-IR was evident. The presence of
an immunohistochemical reaction in at least 10% of cancer cells was
referred to as a positive reaction. In all the tumours, the mean
IGF-IR immunoreactive cell count was found to be 30.79%. The
Mann-Whitney U test and multivariate analysis did not yield
statistical differences.
Correlation between IGF-IR expression and
patient characteristics
The Chi-square test was used to investigate the
relationship of IGF-IR expression with characteristics such as
patient age and gender, tumour location, histological type,
clinicopathological advancement in Dukes’ classification, pTNM and
histological differentiation. Due to the data distribution, the
patients were divided into three groups: i) negatively
immunoreactive against IGF-IR (IGF-IR <10%), ii) moderately
immunoreactive against IGF-IR (IGF-IR 11-50%), iii) highly
immunoreactive against IGF-IR (IGF-IR >50%).
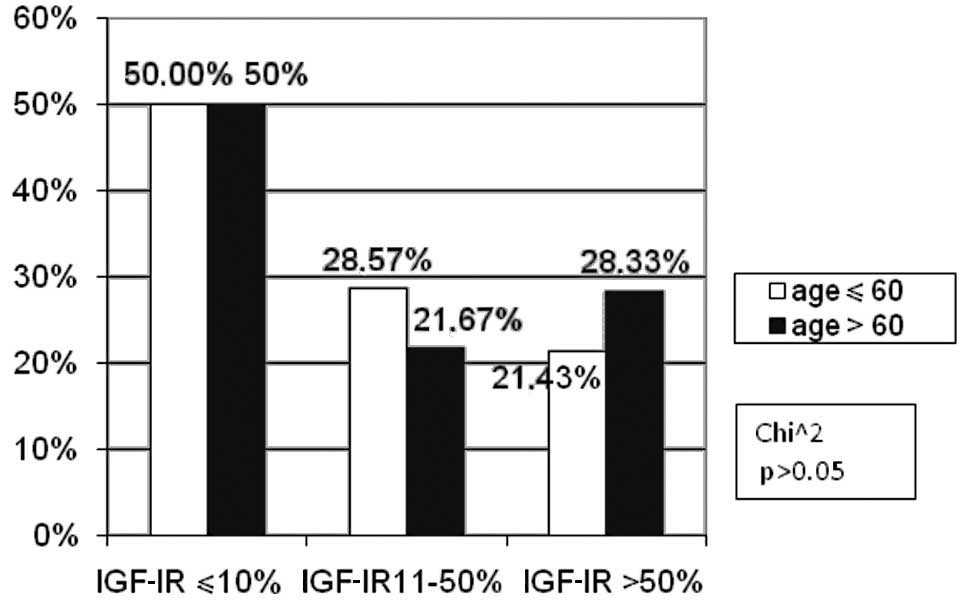
The patients constituted two age groups: <60 and
>60. The number of patients at these ages did not differ between
the subgroups of patients with negative, moderate and high IGF-IR
expression (Fig. 1).
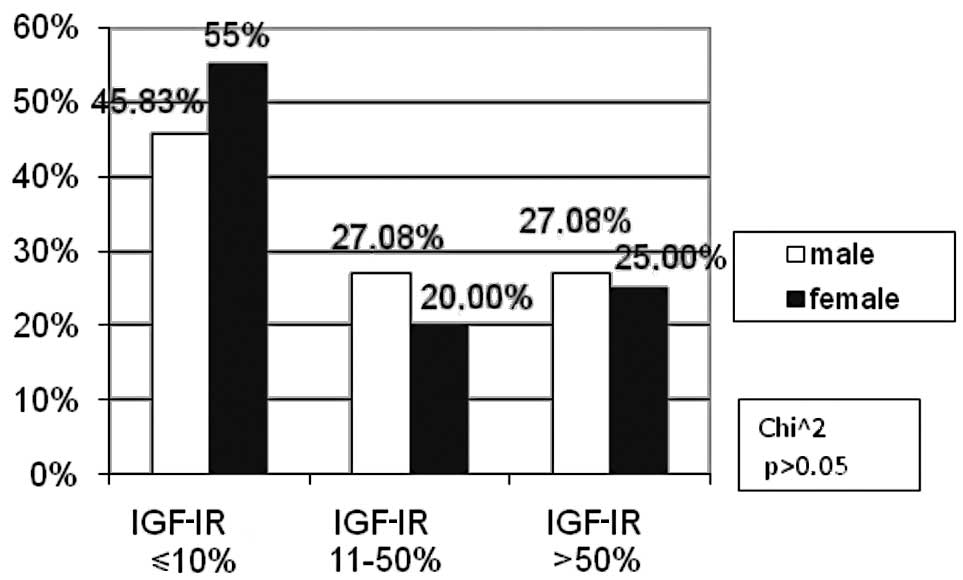
The percentage of IGF-IR negative male patients
(45.83%) was markedly lower in comparison to females (55%).
Conversely, in the moderate IGF-IR subgroup, there were more male
(27.08%) than female (20%) patients. These differences were not
statistically significant (p>0.05, Fig. 2).
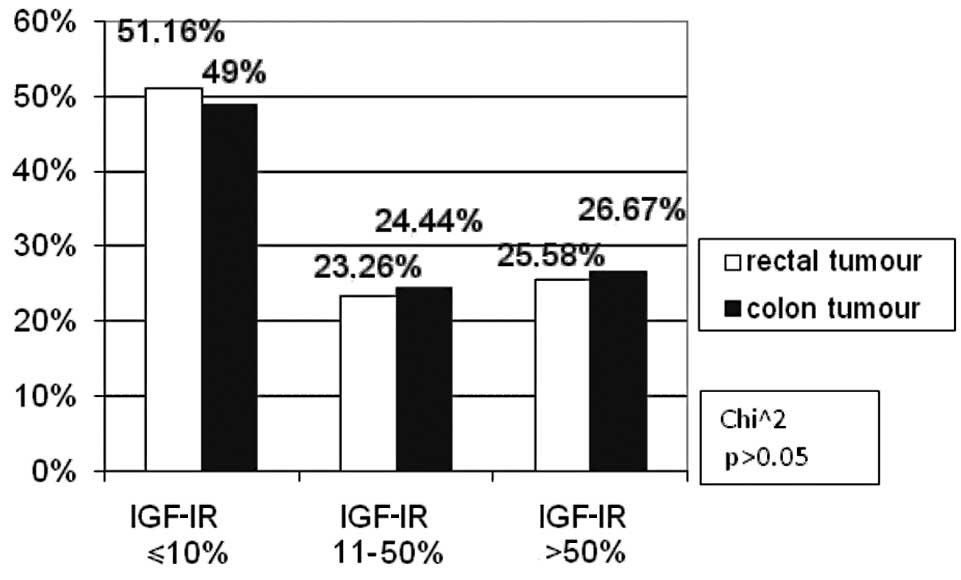
According to tumour location, the patients were
divided into rectal carcinoma and colon carcinoma subgroups. No
difference was observed in the immunohistochemical reaction to
IGF-IR compared to the colorectal cancer location (Fig. 3).
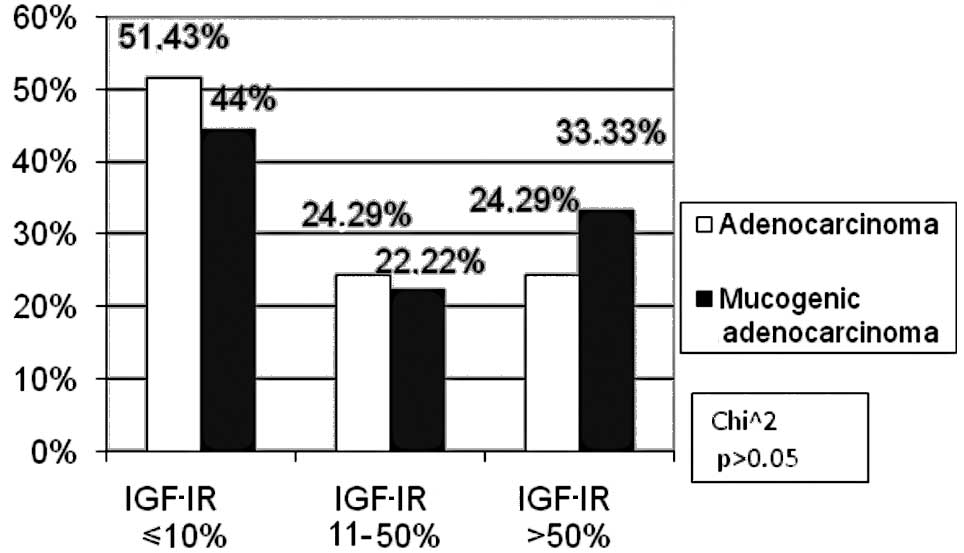
Only adenocarcinomas and mucogenic adenocarcinomas
were considered for the analysis of histopathological type due to
the small number of remaining tumours. In the subgroup exhibiting a
high IGF-IR expression, more patients had mucogenic adenocarcinoma
(33.33%) than those with adenocarcinoma (24.29%), the difference
being statistically insignificant (p>0.05, Fig. 4).
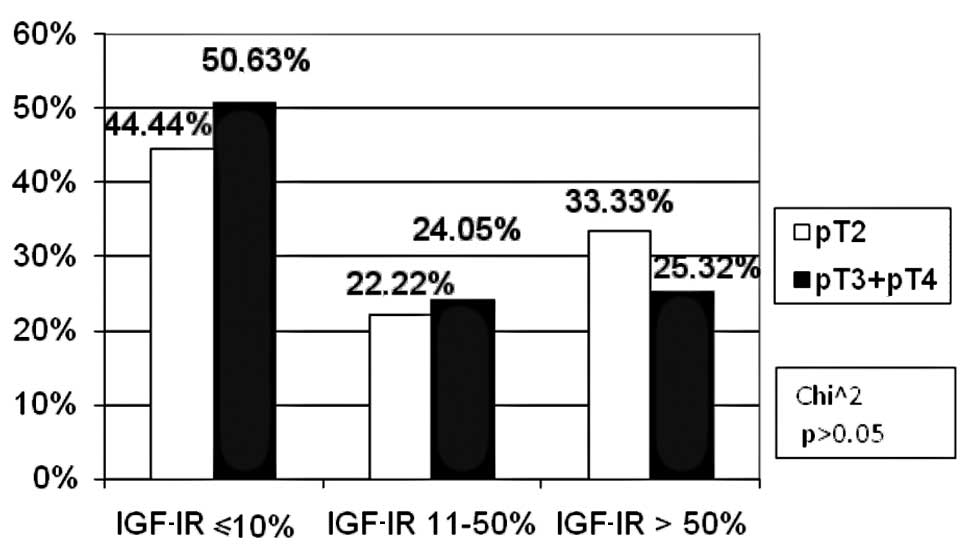
In the TNM classification, with regard to the pT
feature, the patients were divided into two groups: pT2 and
pT3/pT4. No patient was included in the pT1 subgroup. For the pN
and pM features, two groups were distinguished: patients with and
without metastases (pN0, pM0 and pN1 and pM1, respectively). As for
the tumour size, in the high IGF-IR expression subgroup the
percentage of pT3/pT4 (25.32%) patients was statistically
insignificantly lower as compared to pT2 (33.33%) patients. In the
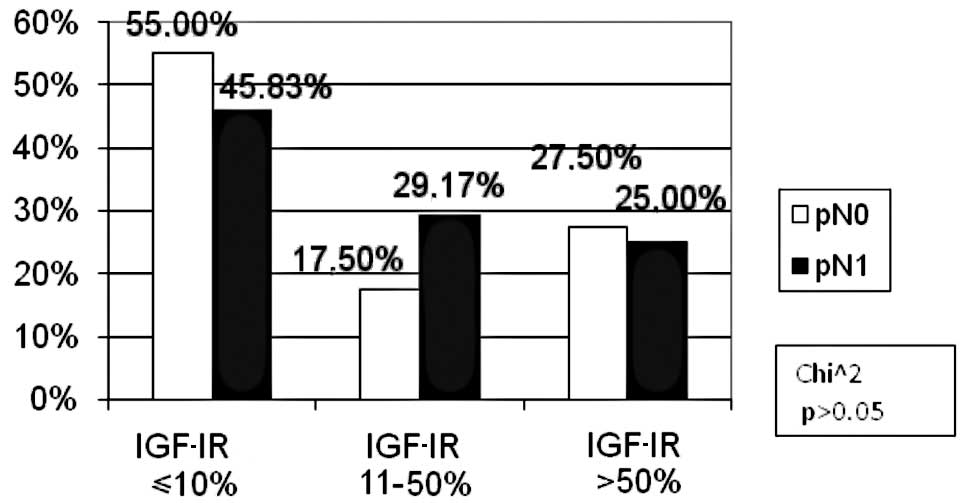
other subgroups, the percentage of patients was similar (Fig. 5). There were fewer patients with
lymph node involvement than those without metastases in the
negative IGF-IR expression subgroup (46 vs. 55%). However, in the
low IGF-IR expression subgroup, the number of patients with lymph
node involvement was higher as compared to the metastasis-free
cases (29.17 vs. 17.5%). However, the difference was not
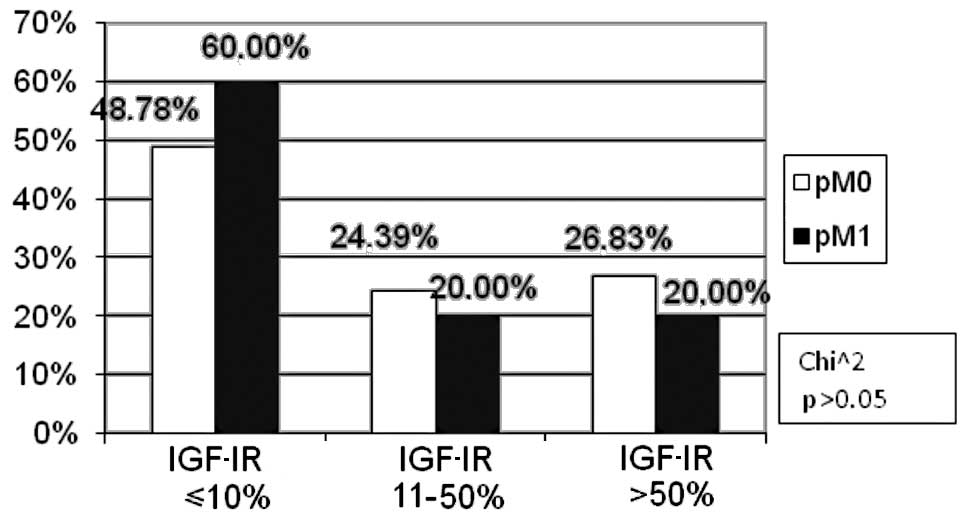
statistically significant (p>0.05, Fig. 6). The percentage of patients with
distant metastases (pM1) was markedly higher (60.00%) as compared
to the metastasis-free subjects (pM0) (48.78%) in the negative
IGF-IR expression subgroup, the difference being statistically
insignificant (p>0.05). No differences were found in the
remaining groups (Fig. 7).
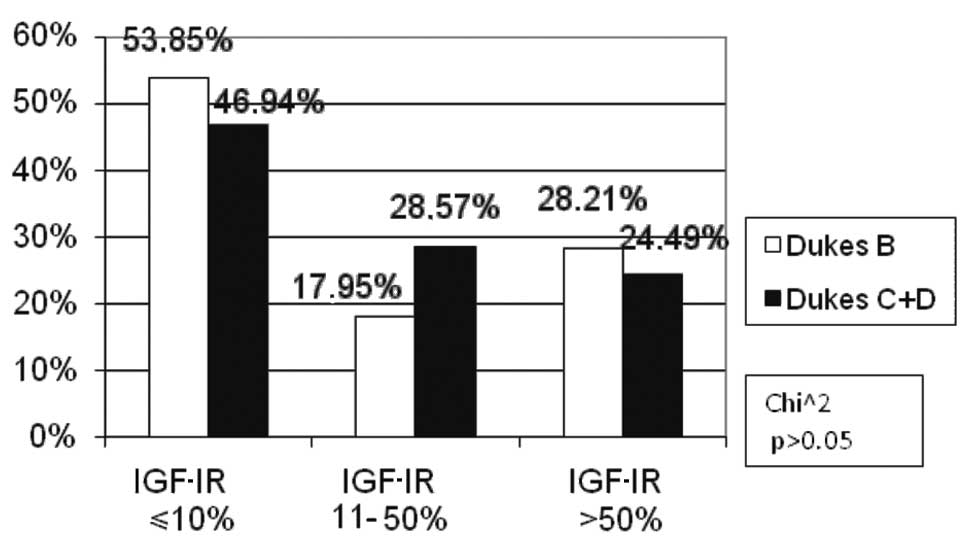
According to Dukes’ classification, two subgroups
were distinguished: Dukes’ B, and Dukes’ C and D. No Dukes’ A
patients were found. This division was determined by the presence
or absence of lymph node involvement. The percentage of Dukes’ C
and D patients was markedly higher (28.57%) than Dukes’ B (17.95%)
in the low IGF-IR expression subgroup; however, there was no
statistical significance (p>0.05). Similar numbers of Dukes’ B,
C and D patients were noted in the other subgroups of IGF-IR
expression (Fig. 8).
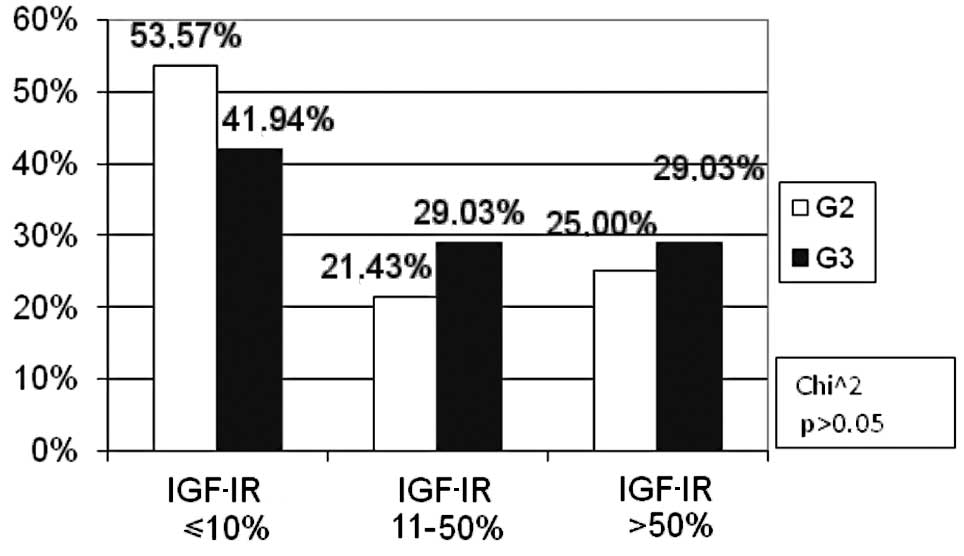
The percentage of patients with a moderate
histological differentiation grade was statistically
insignificantly higher (G2: 53.57%) as compared to those with a low
grade (G3: 41.94%) in the negative IGF-IR expression subgroup
(p>0.05). No difference was found in the percentage of moderate
and low-differentiated tumours in the remaining subgroups of IGF-IR
expression (Fig. 9).
Discussion
In this study, a positive IGF-IR expression in 50%
of the colorectal carcinomas examined was observed. The mean
percentage of IGF-IR immunoreactive cells was 30.79% in the whole
study group. Weber et al described a positive
immunohistochemical reaction for IGF-IR in the cells of 91% of the
colorectal cancers studied (11).
Hakam et al also observed a positive immunohistochemical
reaction in 96% of the tumours examined. The authors found the
enhanced IGF-IR expression to correlate with the histopathological
differentiation grade and with the anatomopathological advancement
of colorectal cancer. According to these investigators, a higher
expression of IGF-IR may promote the formation of metastases
(12). On the other hand, neither
Adenis nor Zenilman, who studied the expression of IGF-IR mRNA in
colorectal carcinoma patients, observed an elevated IGF-IR
expression in colorectal cancer cells (13,14).
Nosho et al found an increased IGF-IR expression in 37.8% of
the colorectal carcinomas studied (15). The findings reported by Pollak et
al explain the discrepancies between the present results and
the majority of the literature data (16). These authors have shown that a lower
IGF-IR expression may be observed in high-grade colorectal
carcinomas, including prostate carcinoma (10). In the present study group, almost
20% of patients were classified as pT4 according to the pTNM
classification. A lower percentage of pT3 and pT4 as compared to
pT2 patients in the high IGF-IR expression subgroup were noted.
No statistically significant correlation was found
between the increased IGF-IR expression in colorectal cancer cells
with characteristics such as patient age and gender, tumour
location, histological type, advancement or lymph node involvement.
However, an increased number of immuno-reactive cells for IGF-IR in
low-differentiated colorectal cancers was observed, the differences
being statistically insignificant. Similarly, Nosho et al
observed no correlation of IGF-IR expression in colorectal cancer
cells with clinical and pathomorphological factors (15). No other data concerning the
correlation of IGF-IR expression with clinical and
pathomorphological factors were detected. Results of the present
study suggest that the determination of IGF-IR expression in
advanced colorectal cancer may have a limited prognostic and
diagnostic value.
This study did not include patients with adenomas
and only a few of the patients had early cancer. Teramukai et
al assert that the plasma concentration of IGF-I may change
during the carcinoma sequence of colorectal cancer (17). Estimation of the changes in IGF-IR
expression in normal mucous, adenomas and early cancers of the
large bowel appear to be justified.
Assessment of the number of cells with a positive
immuno- histochemical reaction for IGF-IR may be applied when
quali-fying patients for therapy. As shown in certain publications,
blockage of IGF-IR effectively increases cancer cell apoptosis
(18). Anticancer therapy based on
the action of one of the GH-IGF-IGFR axis links leads to high
expectations, particularly for colorectal carcinoma (5). A number of studies have concluded that
IGF-IR is the most promising target in anticancer therapy (16,18-22).
The majority of data concerning IGFs are based on
in vitro studies. Studies in vivo are rare, and
frequently provide contradictory and unclear evidence. Broader
studies on IGFs are required (23),
as they may aid in the search for an early detection mode and
facilitate the design of effective anti-colorectal cancer
therapy.
Based on the study findings, the following
conclusions have been formulated: i) no correlation was found
between IGF-IR expression and the clinical and pathomorphological
factors studied, ii) a controversial low expression of IGF-IR in
the advanced colorectal cancer cells studied delimits the
usefulness of immunohistochemical assessment of IGF-IR in the
prognosis of the course of the neoplastic process and
simultaneously stimulates further investigations.
References
|
1
|
Mauro L and Surmacz E: IGF-I receptor,
cell-cell adhesion, tumor development and progression. J Mol
Histol. 35:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grimberg A and Cohen P: Role of
insulin-like growth factor and their binding proteins in growth
control and carcinogenesis. J Cell Physiol. 183:1–9. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams TE and Epa VC: Structure and
function of the type I insulin-like growth factor receptor. Cell
Moll Life Sci. 57:1050–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baserga R: The IGF-I receptor in cancer
research. Exp Cell Res. 253:1–6. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinmuth N, Fan F, Liu W, Parikh AA and
Stoeltzing O: Impact of insulin-like growth factor receptor-I
function on angiogenesis, growth and metastasis of colon cancer.
Lab Invest. 82:1377–1389. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valentinis B and Baserga R: IGF I receptor
signaling in transformation and differentiation. J Clin Pahol.
54:133–137. 2001.PubMed/NCBI
|
|
7
|
Rouyer-Fessard C, Gammeltoft S and
Laburthe M: Expression of two types of receptor for insulin-like
growth factors in human colonic epithelium. Gastroenterol.
98:703–707. 1990.PubMed/NCBI
|
|
8
|
Renehan AG, Painter JE, O’Halloran D,
Atkin WS, Potten CS, O’Dwyer ST and Shalet SM: Circulating
insulin-like growth factor II and colorectal adenomas. J Clin
Endocrinol Metab. 85:3402–3408. 2000.PubMed/NCBI
|
|
9
|
Wu Y, Yakar S, Zhao L, Hennighausen L and
LeRoith D: Circulating insulin-like growth factor-I levels regulate
colon cancer growth and metastasis. Cancer Res. 15:1030–1035.
2002.PubMed/NCBI
|
|
10
|
Reinmuth N, Liu W, Fan F, Jung YD and
Ahmed SA: Blockade of insulin-like growth factor I receptor
function inhibits growth and angiogenesis of colon cancer. Clin
Cancer Res. 8:3259–3269. 2002.PubMed/NCBI
|
|
11
|
Weber MM, Fottner C, Liu SB, Jung MC,
Engelhardt D and Baretton GB: Overexpression of the insulin-like
growth factor I receptor in human colon carcinomas. Cancer.
95:2086–2095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hakam A, Yeatman TJ, Lu L, Mora L, Marcet
G, Nicosia SV, Karl RC and Coppola D: Expression of insulin-like
growth factor-1 receptor in human colorectal cancer. Hum Patol.
30:1128–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adenis A, Peyrat JP, Hecquet B, Delobelle
A, Depadt G, Quandalle P, Bonneterre J and Demaille A: Type I
insulin-like growth factor receptors in human colorectal cancer.
Eur J Cancer. 31:50–55. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zenilman ME and Graham W: Insulin-like
growth factor I receptor messenger RNA in the colon is unchanged
during neoplasia. Cancer Invest. 15:1–7. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nosho K, Yamamoto H, Taniguchi H, Adachi
Y, Yoshida Y, Arimura Y, Endo T, Hinoda Y and Imai K: Interplay of
insulin-like growth factor-II, insulin-like growth factor-I,
insulin-like growth factor-I receptor, COX-2, and matrix
metalloproteinase-7, play key roles in the early stage of
colorectal carcinogenesis. Clinical Cancer Res. 10:7950–7957. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pollak MN, Perdue JF, Margolese RG, Baer K
and Richard M: Presence of somatomedin receptors on primary human
breast and colon carcinomas. Cancer Lett. 38:223–230. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teramukai S, Rohan T, Lee KY, Eguchi H,
Oda T and Kono S: Insulin-like growth factor (IGF)-I, IGF-binding
protein-3 and colorectal adenomas in Japanese men. Jpn J Cancer
Res. 93:1187–1194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adachi Y, Lee CT, Coffee K, Yamagata N,
Ohm JE, Park KH, Dikov MM, Nadaf SR, Arteaga CL and Carbone DP:
Effects of genetic blockade of the insulin-like growth factor
receptor in human colon cancer cell lines. Gastroenterology.
123:1191–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassan AB and Macaulay VM: The
insulin-like growth factor system as a therapeutic target in
colorectal cancer. Ann Oncol. 13:349–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moschos SJ and Mantzoros CS: The role of
the IGF system in cancer: from basic to clinical studies and
clinical applications. Oncology. 63:317–332. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khandwala HM, McCutcheon IE, Flyvbjerg A
and Friend KE: The effects of insulin-like growth factors on
tumorigenesis and neoplastic growth. Endocr Rev. 21:215–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandhu MS, Dunger DB and Giovannucci EL:
Insulin, insulin-like growth factor-I (IGF-I), IGF binding
proteins, their biologic interactions, and colorectal cancer. J
Natl Cancer Inst. 94:972–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Durai R, Yang W, Gupta S, Seifalian AM and
Winslet MC: The role of the insulin-like growth factor system in
colorectal cancer: review of current knowledge. Int J Colorectal
Dis May. 20:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|